Role of Secretory Mucins in the Occurrence and Development of Cholelithiasis
Abstract
:1. Introduction
2. Literature Search
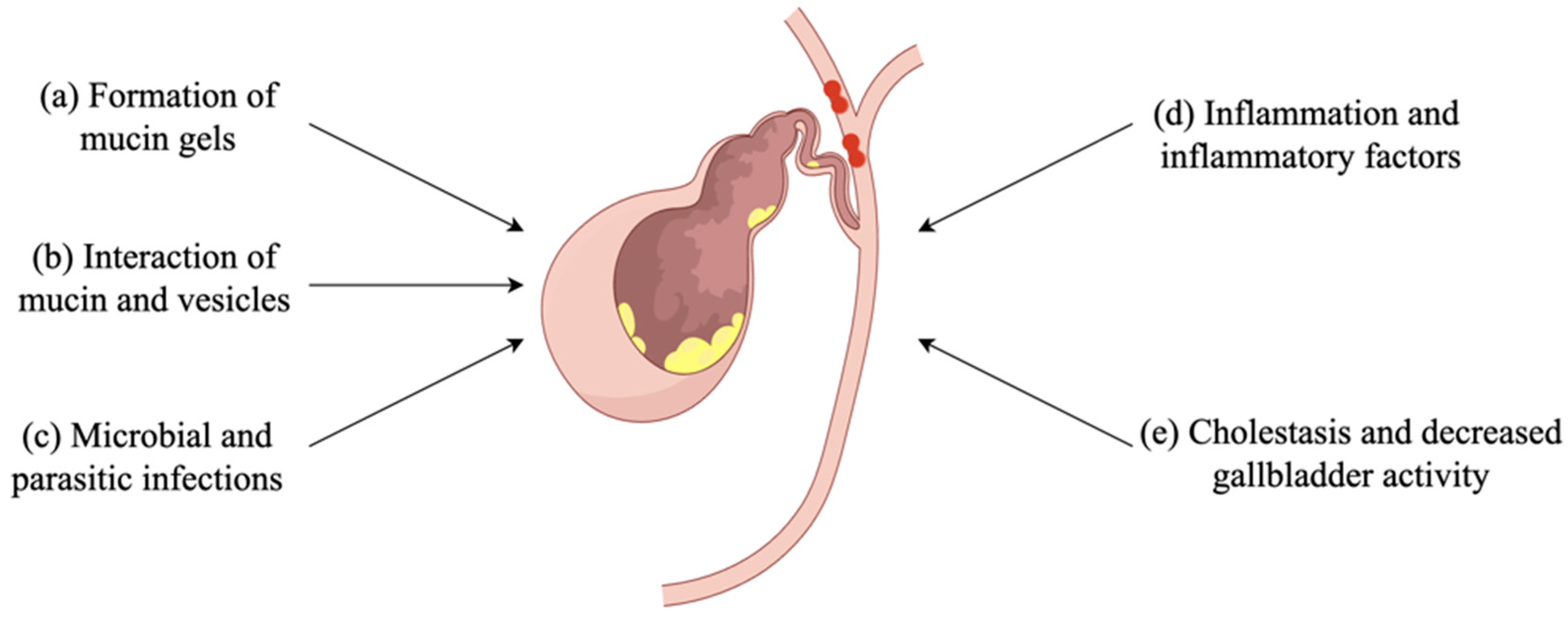
3. Mucin Is Involved in Gallstone Formation
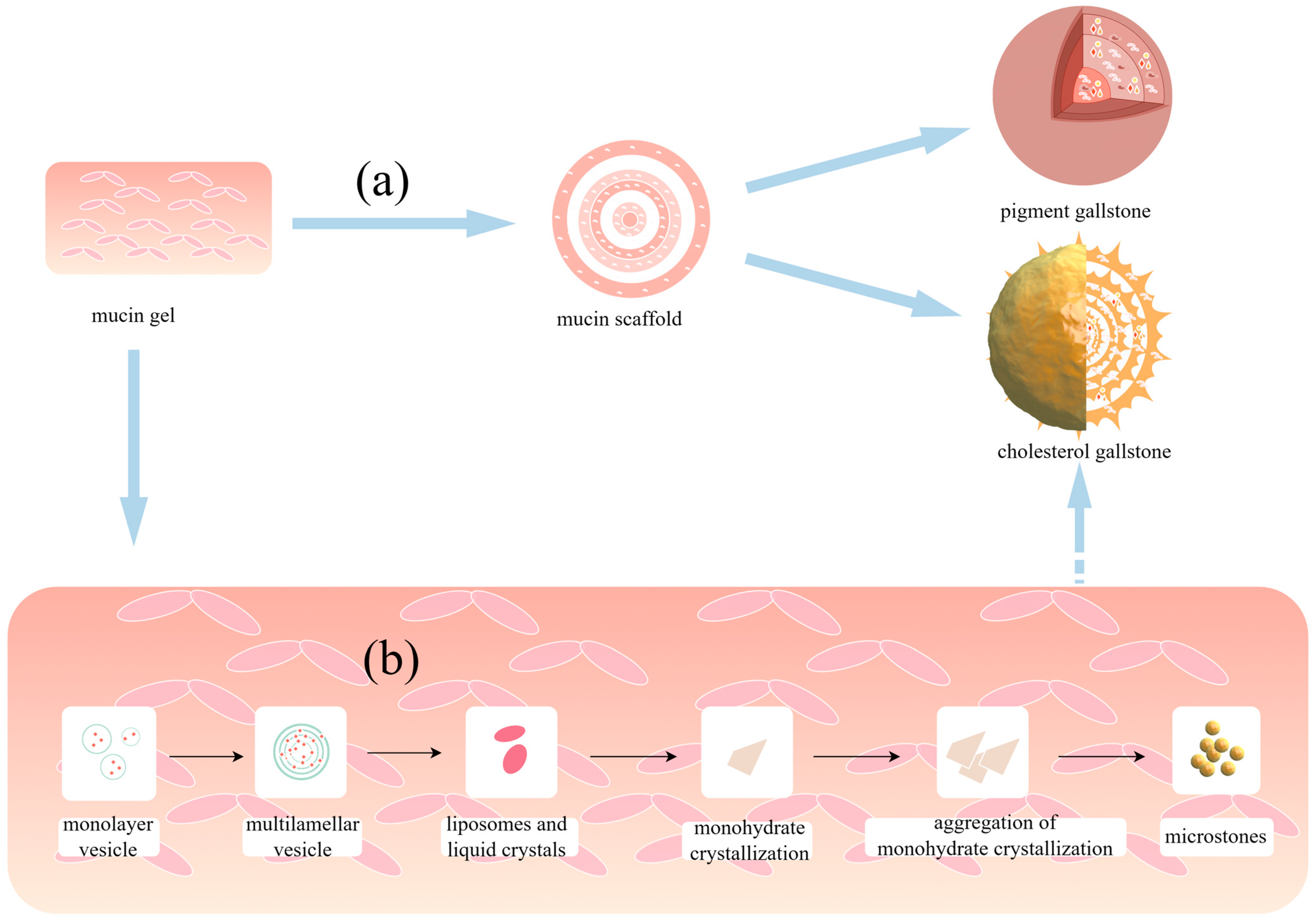
4. Mucin Gel Promotes Stone Formation
5. The Interactions between Mucin and Vesicles Affect Cholesterol Stone Formation
6. Bacterial Infections and Inflammation
7. Mucin Hypersecretion Causes Cholestasis and Reduces Gallbladder Activity
| First Author’s Name | Publication Year | Study Design | Study Results | Conclusions |
|---|---|---|---|---|
| Nezam H Afdhal [24] | 2004 | Fluorescence recovery after photobleaching (FRAP) and fluorescence correlation spectroscopy (FCS) was utilised to examine the role of gallbladder mucin (GBM) in promoting the aggregation and/or fusion of cholesterol-enriched vesicles. | GBM had a profound effect on inducing vesicles to aggregate/fuse. | Both glycosylated and nonglycosylated domains of GBM are involved in early aggregation of cholesterol-enriched vesicles. |
| P C Sheen [34] | 1998 | Periodic acid–Schiff–alcian blue double staining was performed for gallbladder specimens to compare the mucin area to the total epithelial area ratio. | Gallbladders with pigment gallstones contained more mucin than gallbladders with or without cholesterol gallstones. | Mucin might play a more important role in the formation of brown pigment stones than that of cholesterol stones. |
| N H Afdhal [60] | 1995 | Three kinds of physical and chemical technology were used to quantify the gallbladder mucins affecting vesicle aggregation and fusion. | Without mucin, fusion was slow and took up to 24 h; however, with a physiological concentration of mucin, complete fusion of the vesicles occurred within 6 h. | The mucin hydrophobic domain promotes nucleation of cholesterol monohydrate crystals. |
| P F Levy [61] | 1984 | Mucin was added to model bile to observe cholesterol crystal nucleation time. | The number of cholesterol crystals in the 4 mg/mL group was significantly more compared to the other group | Nucleation of hydrated cholesterol crystals in gallbladder bile promotes the formation of cholesterol stones. |
| Zhitang Lyu [77] | 2021 | 16s rRNA sequencing analysis was performed on patients with gallstones and biliary tract disease and on patients with biliary and duodenal microbial communities. | There were similarities in the abundance of major taxa between the two groups. | The reflux of bacteria in the duodenum into the common bile duct through the sphincter of Oddi should be an important factor in bacterial infection and stone formation in the common bile duct. |
| Xiaodong, Wu [86] | 2021 | Different concentrations of LPS stimulation in HIBEpiC and the detection of MUC5AC transcription and secretion level were studied. | LPS upregulated mucin expression in bile duct epithelial cells in a dose-dependent manner. | LPS promotes the secretion of MUC5AC to promote stone formation. |
| Sven Fischer [110] | 2004 | The effects of ursodeoxycholic acid (UDCA) on gallbladder bile composition, viscosity, and precipitable fractions were studied in 25 patients with cholesterol gallstones. | The concentrations of protein and mucin in gallbladder bile tended to be lower in UDCA-treated groups. | UDCA treatment reduces total and vesicular cholesterol, the formation of cholesterol crystals, viscosity, and the total amount of sedimentable fractions in gallbladder bile. |
| Sung Ill Jang [111] | 2019 | The effect of n-3 polyunsaturated fatty acids (PUFA) in combination with UDCA was evaluated in a mouse model of cholesterol gallstones. | Expression levels of mucin genes were significantly lower in the UDCA, PUFA, and combination groups. | Combination treatment with PUFA and UDCA dissolves cholesterol gallstones in mice by decreasing mucin production, increasing levels of phospholipids and bile acids in bile, and decreasing cholesterol saturation. |
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weerakoon, H.; Vithanage, I.; Alahakoon, O.; Weerakoon, K. Clinico-Epidemiology and Aetiopathogenesis of Gallstone Disease in the South Asian Region: A Scoping Review Protocol. BMJ Open 2022, 12, e057808. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, T.; Agabin, E.; Varghese, J.; Talukder, A. Gallbladder Dysfunction: Cholecystitis, Choledocholithiasis, Cholangitis, and Biliary Dyskinesia. Prim. Care 2017, 44, 575–597. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, J.R.; Charles, A. Acute Cholecystitis: A Review. JAMA 2022, 327, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Cafasso, D.E.; Smith, R.R. Symptomatic Cholelithiasis and Functional Disorders of the Biliary Tract. Surg. Clin. N. Am. 2014, 94, 233–256. [Google Scholar] [CrossRef] [PubMed]
- Hsing, A.W.; Gao, Y.-T.; Han, T.-Q.; Rashid, A.; Sakoda, L.C.; Wang, B.-S.; Shen, M.-C.; Zhang, B.-H.; Niwa, S.; Chen, J.; et al. Gallstones and the Risk of Biliary Tract Cancer: A Population-Based Study in China. Br. J. Cancer 2007, 97, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Assakran, B.S.; Khalid, R.; Albadrani, H.; Alsuhaibani, A.; Almutairi, A.; Alhomidan, R.; Alfarhan, G.; Alshaya, R. Incidence of Asymptomatic Gallstones in Obese Patients Who Underwent Bariatric Surgery in Qassim Region at King Fahad Specialist Hospital. Cureus 2023, 15, e44154. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Cho, S.K.; Kim, C.S.; Park, J.S. Big Data and Analysis of Risk Factors for Gallbladder Disease in the Young Generation of Korea. PLoS ONE 2019, 14, e0211480. [Google Scholar] [CrossRef] [PubMed]
- Marschall, H.-U.; Einarsson, C. Gallstone Disease. J. Intern. Med. 2007, 261, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Sebghatollahi, V.; Parsa, M.; Minakari, M.; Azadbakht, S. A Clinician’s Guide to Gallstones and Common Bile Duct (CBD): A Study Protocol for a Systematic Review and Evidence-based Recommendations. Health Sci. Rep. 2023, 6, e1555. [Google Scholar] [CrossRef]
- Shikano, R.; Ohno, K.; Nagahara, T.; Nagao, I.; Toyoda, H.; Nakagawa, T.; Goto-Koshino, Y.; Chambers, J.K.; Tomiyasu, H.; Tsujimoto, H. Effects of Proportions of Carbohydrates and Fats in Diets on Mucin Concentration and Bile Composition in Gallbladder of Dogs. J. Vet. Med. Sci. 2022, 84, 1465–1468. [Google Scholar] [CrossRef]
- Bar Dayan, Y.; Vilkin, A.; Niv, Y. Gallbladder Mucin Plays a Role in Gallstone Formation. Eur. J. Intern. Med. 2004, 15, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Thornton, D.J. From Mucins to Mucus: Toward a More Coherent Understanding of This Essential Barrier. Proc. Am. Thorac. Soc. 2004, 1, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, R.; Fujitani, N.; Takegawa, Y.; Kurogochi, M.; Matsushita, T.; Naruchi, K.; Ohyabu, N.; Hinou, H.; Gao, X.D.; Manri, N.; et al. An Efficient Approach for the Characterization of Mucin-Type Glycopeptides: The Effect of O-Glycosylation on the Conformation of Synthetic Mucin Peptides. Chemistry 2011, 17, 2393–2404. [Google Scholar] [CrossRef] [PubMed]
- Afdhal, N.H.; Offner, G.D.; Smith, B.F. Characterization of Bovine Gallbladder Mucin. Amino Acid Sequences of Tryptic Peptides from the Glycosylated Domain of the Protein Core. Gastroenterology 1990, 99, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, N.; Skrypek, N.; Frénois, F.; Van Seuningen, I. Membrane-Bound Mucin Modular Domains: From Structure to Function. Biochimie 2013, 95, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C. Mucins and the Microbiome. Annu. Rev. Biochem. 2020, 89, 769–793. [Google Scholar] [CrossRef] [PubMed]
- Jüngst, C.; Sreejayan, N.; Eder, M.I.; von Stillfried, N.; Zündt, B.; Spelsberg, F.W.; Kullak-Ublick, G.A.; Jüngst, D.; von Ritter, C. Lipid Peroxidation and Mucin Secretagogue Activity in Bile of Gallstone Patients. Eur. J. Clin. Invest. 2007, 37, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, X.; He, Y.; Sun, B.; Zhu, C.; Zhao, R.; Zhang, S.; Huang, X.; Liu, Y. Effect of P38 Mitogen-Activate Protein Kinase on MUC5AC Protein Expression of Bile Duct Epithelial Cells in Hepatolithiasis Patients. Int. J. Clin. Exp. Pathol. 2015, 8, 13753–13758. [Google Scholar] [PubMed]
- Portincasa, P.; Di Ciaula, A.; Bonfrate, L.; Stella, A.; Garruti, G.; Lamont, J.T. Metabolic Dysfunction-Associated Gallstone Disease: Expecting More from Critical Care Manifestations. Intern. Emerg. Med. 2023, 18, 1897–1918. [Google Scholar] [CrossRef]
- Stinton, L.M.; Shaffer, E.A. Epidemiology of Gallbladder Disease: Cholelithiasis and Cancer. Gut Liver 2012, 6, 172–187. [Google Scholar] [CrossRef]
- Ceci, L.; Han, Y.; Krutsinger, K.; Baiocchi, L.; Wu, N.; Kundu, D.; Kyritsi, K.; Zhou, T.; Gaudio, E.; Francis, H.; et al. Gallstone and Gallbladder Disease: Biliary Tract and Cholangiopathies. Compr. Physiol. 2023, 13, 4909–4943. [Google Scholar] [CrossRef]
- Van Erpecum, K.J. Pathogenesis of Cholesterol and Pigment Gallstones: An Update. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-C.; Hsi, E.; Lee, K.-T. Genetics of Gallstone Disease. Adv. Clin. Chem. 2013, 60, 143–185. [Google Scholar] [CrossRef] [PubMed]
- Afdhal, N.H.; Cao, X.; Bansil, R.; Hong, Z.; Thompson, C.; Brown, B.; Wolf, D. Interaction of Mucin with Cholesterol Enriched Vesicles: Role of Mucin Structural Domains. Biomacromolecules 2004, 5, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Lammert, F.; Gurusamy, K.; Ko, C.W.; Miquel, J.-F.; Méndez-Sánchez, N.; Portincasa, P.; van Erpecum, K.J.; van Laarhoven, C.J.; Wang, D.Q.-H. Gallstones. Nat. Rev. Dis. Primers 2016, 2, 16024. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tian, F.; Feng, X.; He, Y.; Jiang, P.; Li, J.; Guo, F.; Zhao, X.; Chang, H.; Wang, S. LPS Increases MUC5AC by TACE/TGF-α/EGFR Pathway in Human Intrahepatic Biliary Epithelial Cell. Biomed. Res. Int. 2013, 2013, 165715. [Google Scholar] [CrossRef] [PubMed]
- Venneman, N.G.; van Erpecum, K.J. Pathogenesis of Gallstones. Gastroenterol. Clin. N. Am. 2010, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhou, Y.; Cheng, N.; Mao, H.; Jiang, L.; Li, N.; Li, Q.; de Jong, M.C.; Pawlik, T.M. Epidermal Growth Factor Receptor as a Target for Anti-Proliferative Treatment of Proliferative Cholangitis in Hepatolithiasis. J. Surg. Res. 2011, 166, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Portincasa, P.; Wang, D.Q.-H. Molecular Pathophysiology and Physical Chemistry of Cholesterol Gallstones. Front. Biosci. 2008, 13, 401–423. [Google Scholar] [CrossRef]
- Portincasa, P.; Di Ciaula, A.; de Bari, O.; Garruti, G.; Palmieri, V.O.; Wang, D.Q.-H. Management of Gallstones and Its Related Complications. Expert. Rev. Gastroenterol. Hepatol. 2016, 10, 93–112. [Google Scholar] [CrossRef]
- Ma, H.; Li, W.; Bi, P.; Wang, Q.; Li, J.; Yang, B. Hsa-miR-93 Regulates MUCIN Family Gene Expression via WNT/β-Catenin Pathway in Intrahepatic Stone Disease. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Shabanzadeh, D.M. Incidence of Gallstone Disease and Complications. Curr. Opin. Gastroenterol. 2018, 34, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Portincasa, P.; Liu, M.; Tso, P.; Samuelson, L.C.; Wang, D.Q.-H. Effect of Gallbladder Hypomotility on Cholesterol Crystallization and Growth in CCK-Deficient Mice. Biochim. Biophys. Acta 2010, 1801, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Sheen, P.C.; Lee, K.T.; Liu, Y.E. Mucin Content in Gallbladders with Brown Pigment Stones or Combination Stones with a Brown Periphery. Digestion 1998, 59, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tu, Z.; Liu, J.; Wu, T.; Li, D.; Zhang, N.; Cui, Y. Therapeutic Effect of Yinchenhao Decoction on Cholelithiasis via Mucin in the Gallbladder and Intestine. Fitoterapia 2023, 172, 105746. [Google Scholar] [CrossRef]
- Yao, D.; Dai, W.; Dong, M.; Dai, C.; Wu, S. MUC2 and Related Bacterial Factors: Therapeutic Targets for Ulcerative Colitis. EBioMedicine 2021, 74, 103751. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Stanley, E.; LaMont, J.T. Mucin Biophysics. Annu. Rev. Physiol. 1995, 57, 635–657. [Google Scholar] [CrossRef] [PubMed]
- Lidell, M.E.; Johansson, M.E.V.; Hansson, G.C. An Autocatalytic Cleavage in the C Terminus of the Human MUC2 Mucin Occurs at the Low pH of the Late Secretory Pathway. J. Biol. Chem. 2003, 278, 13944–13951. [Google Scholar] [CrossRef] [PubMed]
- Lidell, M.E.; Johansson, M.E.V.; Mörgelin, M.; Asker, N.; Gum, J.R.; Kim, Y.S.; Hansson, G.C. The Recombinant C-Terminus of the Human MUC2 Mucin Forms Dimers in Chinese-Hamster Ovary Cells and Heterodimers with Full-Length MUC2 in LS 174T Cells. Biochem. J. 2003, 372, 335–345. [Google Scholar] [CrossRef]
- Terao, J.; Ingemansson, T.; Ioku, K.; Yuki, H.; Ito, Y. Effects of Rat Bile-Pancreatic Juice on Fe2+ Induced Peroxidation of Phospholipids. Biosci. Biotechnol. Biochem. 1995, 59, 55–58. [Google Scholar] [CrossRef]
- Lesuffleur, T.; Roche, F.; Hill, A.S.; Lacasa, M.; Fox, M.; Swallow, D.M.; Zweibaum, A.; Real, F.X. Characterization of a Mucin cDNA Clone Isolated from HT-29 Mucus-Secreting Cells. The 3′ End of MUC5AC? J. Biol. Chem. 1995, 270, 13665–13673. [Google Scholar] [CrossRef] [PubMed]
- Jirsa, M.; Groen, A.K. Role of Biliary Proteins and Non-Protein Factors in Kinetics of Cholesterol Crystallisation and Gallstone Growth. Front. Biosci. 2001, 6, E154–E167. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Hansson, G.C.; Samuelsson, T. Gel-Forming Mucins Appeared Early in Metazoan Evolution. Proc. Natl. Acad. Sci. USA 2007, 104, 16209–16214. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.K.; Kirkham, S.; Howard, M.; Woodman, P.; Kutay, S.; Brazeau, C.; Buckley, J.; Thornton, D.J. Identification of Molecular Intermediates in the Assembly Pathway of the MUC5AC Mucin. J. Biol. Chem. 2004, 279, 15698–15705. [Google Scholar] [CrossRef] [PubMed]
- Dhanisha, S.S.; Guruvayoorappan, C.; Drishya, S.; Abeesh, P. Mucins: Structural Diversity, Biosynthesis, Its Role in Pathogenesis and as Possible Therapeutic Targets. Crit. Rev. Oncol. Hematol. 2018, 122, 98–122. [Google Scholar] [CrossRef]
- Carey, M.C. Pathogenesis of Gallstones. Am. J. Surg. 1993, 165, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Finzi, L.; Barbu, V.; Burgel, P.-R.; Mergey, M.; Kirkwood, K.S.; Wick, E.C.; Scoazec, J.-Y.; Peschaud, F.; Paye, F.; Nadel, J.A.; et al. MUC5AC, a Gel-Forming Mucin Accumulating in Gallstone Disease, Is Overproduced via an Epidermal Growth Factor Receptor Pathway in the Human Gallbladder. Am. J. Pathol. 2006, 169, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Attri, M.R.; Kumar, I.A.; Din, F.M.U.; Raina, A.H.; Attri, A.; Attri, M.R.; Kumar, I.A.; Din, F.M.U.; Raina, A.H.; Attri, A. Pathophysiology of Gallstones. In Gallstones—Review and Recent Progress; IntechOpen: London, UK, 2021; ISBN 978-1-83880-676-7. [Google Scholar]
- Chen, Y.; Kong, J.; Wu, S. Cholesterol Gallstone Disease: Focusing on the Role of Gallbladder. Lab. Invest. 2015, 95, 124–131. [Google Scholar] [CrossRef]
- Wittenburg, H. Hereditary Liver Disease: Gallstones. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 747–756. [Google Scholar] [CrossRef]
- Smith, B.F. Human Gallbladder Mucin Binds Biliary Lipids and Promotes Cholesterol Crystal Nucleation in Model Bile. J. Lipid. Res. 1987, 28, 1088–1097. [Google Scholar] [CrossRef]
- Jones, M.W.; Small, K.; Kashyap, S.; Deppen, J.G. Physiology, Gallbladder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Konikoff, F.M.; Danino, D.; Weihs, D.; Rubin, M.; Talmon, Y. Microstructural Evolution of Lipid Aggregates in Nucleating Model and Human Biles Visualized by Cryogenic Transmission Electron Microscopy. Hepatology 2000, 31, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.J.; Carey, M.C.; Fox, J.G. Roles of Infection, Inflammation, and the Immune System in Cholesterol Gallstone Formation. Gastroenterology 2009, 136, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Portincasa, P.; Liu, M.; Wang, D.Q.-H. Effects of Biliary Phospholipids on Cholesterol Crystallization and Growth in Gallstone Formation. Adv. Ther. 2023, 40, 743–768. [Google Scholar] [CrossRef]
- Jüngst, C.; Kullak-Ublick, G.A.; Jüngst, D. Gallstone Disease: Microlithiasis and Sludge. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.-M.; Yan, R.; Gao, Y.-D.; Yang, H.-J.; Bi, H.-Y.; Duan, Y.-Q. Cholesterol Crystals Activate NLRP3 Inflammasomes and Promote Gallstone Formation by Increasing Mucin Secretion. Biotech. Histochem. 2022, 97, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wiedmann, T.S. Formation of Cholesterol Crystals at a Mucin Coated Substrate. Pharm. Res. 2006, 23, 2413–2416. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Moschetta, A.; van Erpecum, K.J.; Calamita, G.; Margari, A.; vanBerge-Henegouwen, G.P.; Palasciano, G. Pathways of Cholesterol Crystallization in Model Bile and Native Bile. Dig. Liver Dis. 2003, 35, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Afdhal, N.H.; Niu, N.; Nunes, D.P.; Bansil, R.; Cao, X.X.; Gantz, D.; Small, D.M.; Offner, G.D. Mucin-Vesicle Interactions in Model Bile: Evidence for Vesicle Aggregation and Fusion before Cholesterol Crystal Formation. Hepatology 1995, 22, 856–865. [Google Scholar] [PubMed]
- Levy, P.F.; Smith, B.F.; LaMont, J.T. Human Gallbladder Mucin Accelerates Nucleation of Cholesterol in Artificial Bile. Gastroenterology 1984, 87, 270–275. [Google Scholar] [CrossRef]
- Afdhal, N.H.; Niu, N.; Gantz, D.; Small, D.M.; Smith, B.F. Bovine Gallbladder Mucin Accelerates Cholesterol Monohydrate Crystal Growth in Model Bile. Gastroenterology 1993, 104, 1515–1523. [Google Scholar] [CrossRef]
- Lee, T.J.; Smith, B.F. Bovine Gallbladder Mucin Promotes Cholesterol Crystal Nucleation from Cholesterol-Transporting Vesicles in Supersaturated Model Bile. J. Lipid Res. 1989, 30, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmi, M.; Jüngst, C.; Mock, M.; Meyer, G.; Zündt, B.; Del Pozo, R.; Jüngst, D. Effect of Gallbladder Mucin on the Crystallization of Cholesterol in Bile. Eur. J. Gastroenterol. Hepatol. 2004, 16, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-K.; Pan, H.; Huang, S.-M.; Huang, N.-L.; Yao, C.-C.; Hsiao, K.-M.; Wu, C.-W. Calcium Content of Different Compositions of Gallstones and Pathogenesis of Calcium Carbonate Gallstones. Asian J. Surg. 2013, 36, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sachan, S.G.; Sharma, S.R. In Vitro Analysis of Gallstone Formation in the Presence of Bacteria. Indian J. Gastroenterol. 2020, 39, 473–480. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, A.A.; van Buul, J.D.; Tytgat, G.N.; Groen, A.K.; Ostrow, J.D. Mucins and Calcium Phosphate Precipitates Additively Stimulate Cholesterol Crystallization. J. Lipid Res. 1998, 39, 1744–1751. [Google Scholar] [CrossRef]
- Smith, B.F.; LaMont, J.T. Hydrophobic Binding Properties of Bovine Gallbladder Mucin. J. Biol. Chem. 1984, 259, 12170–12177. [Google Scholar] [CrossRef] [PubMed]
- Çelebioğlu, H.Y.; Lee, S.; Chronakis, I.S. Interactions of Salivary Mucins and Saliva with Food Proteins: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Turner, B.S. The Biology of Mucus: Composition, Synthesis and Organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.; Grifiss, J.M.; Jarvis, G.A.; Way, L.W. Biliary Bacterial Factors Determine the Path of Gallstone Formation. Am. J. Surg. 2006, 192, 598–603. [Google Scholar] [CrossRef]
- Grigor’eva, I.N.; Romanova, T.I. Gallstone Disease and Microbiome. Microorganisms 2020, 8, 835. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, M.; Qin, C.; Hong, J. Role of the Biliary Microbiome in Gallstone Disease. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, M.G.; Guo, L.; Birchall, J.P.; Pearson, J.P. LPS Up-Regulates Mucin and Cytokine mRNA Expression and Stimulates Mucin and Cytokine Secretion in Goblet Cells. Cell Immunol. 2003, 221, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Karpel, E.; Madej, A.; Bułdak, Ł.; Duława-Bułdak, A.; Nowakowska-Duława, E.; Łabuzek, K.; Haberka, M.; Stojko, R.; Okopień, B. Bile Bacterial Flora and Its in Vitro Resistance Pattern in Patients with Acute Cholangitis Resulting from Choledocholithiasis. Scand. J. Gastroenterol. 2011, 46, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Gibiino, G.; Coluccio, C.; Sbrancia, M.; Dajti, E.; Sinagra, E.; Capurso, G.; Sambri, V.; Cucchetti, A.; Ercolani, G.; et al. Biliary Diseases from the Microbiome Perspective: How Microorganisms Could Change the Approach to Benign and Malignant Diseases. Microorganisms 2022, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Yu, T.; Zhang, L.; Xu, X.; Zhang, Y.; Li, J.; Li, Z.; Zhang, W.; Hou, S. Analysis of the Relationship between Bile Duct and Duodenal Microbiota Reveals That Potential Dysbacteriosis Is the Main Cause of Primary Common Bile Duct Stones. Synth. Syst. Biotechnol. 2021, 6, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wu, S.; Fan, Y.; Jin, J.; Zhang, Z. The Preliminary Experimental and Clinical Study of the Relationship between the Pigment Gallstone and Intestinal Mucosal Barrier. J. Gastroenterol. Hepatol. 2009, 24, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, J.-S.; Bae, J.; Hwang, N. Bile Microbiota in Patients with Pigment Common Bile Duct Stones. J. Korean Med. Sci. 2021, 36, e94. [Google Scholar] [CrossRef]
- Kawai, M.; Iwahashi, M.; Uchiyama, K.; Ochiai, M.; Tanimura, H.; Yamaue, H. Gram-Positive Cocci Are Associated with the Formation of Completely Pure Cholesterol Stones. Am. J. Gastroenterol. 2002, 97, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, S.; Fan, Y.; Tian, Y.; Kong, J. Biliary Microbiota in Choledocholithiasis and Correlation with Duodenal Microbiota. Front. Cell Infect. Microbiol. 2021, 11, 625589. [Google Scholar] [CrossRef]
- Stewart, L. Pigment Gallstone Pathogenesis: Slime Production by Biliary Bacteria Is More Important than Beta-Glucuronidase Production. J. Gastrointest. Surg. 2000, 4, 547–553. [Google Scholar] [CrossRef]
- Li, M.; Tian, Y.; Wu, S.; Yu, H.; Li, Y. LPS Stimulates MUC5AC Expression in Human Biliary Epithelial Cells: Whether There Exists a Possible Pathway of PKC/NADPH/ROS? Mol. Cell Biochem. 2014, 385, 87–93. [Google Scholar] [CrossRef]
- Ma, W.-J.; Wu, Z.-R.; Yang, Q.; Hu, H.-J.; Wang, J.-K.; Shi, Y.-J.; Li, F.-Y.; Cheng, N.-S. Biliary Antibiotics Irrigation for E. Coli-Induced Chronic Proliferative Cholangitis and Hepatolithiasis: A Pathophysiological Study in Rabbits. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Sasaki, M.; Ishikawa, A.; Sato, Y.; Harada, K.; Zen, Y.; Kazumori, H.; Nakanuma, Y. Interaction of Toll-like Receptors with Bacterial Components Induces Expression of CDX2 and MUC2 in Rat Biliary Epithelium in Vivo and in Culture. Lab. Invest. 2007, 87, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yao, C.; Kong, J.; Tian, Y.; Fan, Y.; Zhang, Z.; Han, J.; Wu, S. Molecular Mechanism Underlying miR-130b-Sp1 Transcriptional Regulation in LPS-induced Upregulation of MUC5AC in the Bile Duct Epithelium. Mol. Med. Rep. 2021, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Vítek, L.; Carey, M.C. New Pathophysiological Concepts Underlying Pathogenesis of Pigment Gallstones. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 122–129. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, N.; Li, D. Effect of clearing heat and removing dampness method on formation of pigment gallstones in rabbits. Zhongguo Zhong Xi Yi Jie He Za Zhi 2007, 27, 241–243. [Google Scholar] [PubMed]
- Yang, L.; Junmin, S.; Hong, Y.; Shuodong, W. PGE(2) Induces MUC2 and MUC5AC Expression in Human Intrahepatic Biliary Epithelial Cells via EP4/p38MAPK Activation. Ann. Hepatol. 2013, 12, 479–486. [Google Scholar] [PubMed]
- Vilkin, A.; Nudelman, I.; Morgenstern, S.; Geller, A.; Bar Dayan, Y.; Levi, Z.; Rodionov, G.; Hardy, B.; Konikoff, F.; Gobbic, D.; et al. Gallbladder inflammation is associated with increase in mucin expression and pigmented stone formation. Dig. Dis. Sci. 2007, 52, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Swarne, E.; Srikanth, M.S.; Shreyas, A.; Desai, S.; Mehdi, S.; Gangadharappa, H.V.; Suman; Krishna, K.L. Recent Advances, Novel Targets and Treatments for Cholelithiasis; a Narrative Review. Eur. J. Pharmacol. 2021, 908, 174376. [Google Scholar] [CrossRef]
- Ikeda, H.; Sasaki, M.; Ohira, S.; Ishikawa, A.; Sato, Y.; Harada, K.; Zen, Y.; Nakanuma, Y. Tumor Necrosis Factor-α Induces the Aberrant Expression of Mucus Core Protein-2 in Non-Neoplastic Biliary Epithelial Cells via the Upregulation of CDX2 in Chronic Cholangitis. Hepatol. Res. 2008, 38, 1006–1017. [Google Scholar] [CrossRef]
- Dey, B.; Kaushal, G.; Jacob, S.E.; Barwad, A.; Pottakkat, B. Pathogenesis and Management of Hepatolithiasis: A Report of Two Cases. J. Clin. Diagn. Res. 2016, 10, PD11-13. [Google Scholar] [CrossRef] [PubMed]
- Zen, Y.; Harada, K.; Sasaki, M.; Tsuneyama, K.; Katayanagi, K.; Yamamoto, Y.; Nakanuma, Y. Lipopolysaccharide Induces Overexpression of MUC2 and MUC5AC in Cultured Biliary Epithelial Cells: Possible Key Phenomenon of Hepatolithiasis. Am. J. Pathol. 2002, 161, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Motta, R.V.; Saffioti, F.; Mavroeidis, V.K. Hepatolithiasis: Epidemiology, presentation, classification and management of a complex disease. World J. Gastroenterol. 2024, 30, 1836–1850. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-H.; Yoon, Y.B.; Kim, Y.-T.; Cheon, J.H.; Jeong, J.B. Risk Factors for Recurrent Cholangitis after Initial Hepatolithiasis Treatment. J. Clin. Gastroenterol. 2004, 38, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, M.; Cao, J.; Zhang, X.; Mi, N.; Yang, X.; Wang, H.; Gao, L.; Bai, M.; Fu, W.; et al. Saline Irrigation for Reducing the Recurrence of Common Bile Duct Stones after Lithotripsy: A Randomized Controlled Trial. EClinicalMedicine 2023, 59, 101978. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Meng, F.; Venter, J.; Giang, T.; Glaser, S.; Alpini, G. The Role of the Secretin/Secretin Receptor Axis in Inflammatory Cholangiocyte Communication via Extracellular Vesicles. Sci. Rep. 2017, 7, 11183. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Komori, A.; Nakamura, M.; Takii, Y.; Kamihira, T.; Shimoda, S.; Mori, T.; Fujiwara, S.; Koyabu, M.; Taniguchi, K.; et al. Human Intrahepatic Biliary Epithelial Cells Function in Innate Immunity by Producing IL-6 and IL-8 via the TLR4-NF-kappaB and -MAPK Signaling Pathways. Liver Int. 2006, 26, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.F.; Peetermans, J.A.; Tanaka, T.; LaMont, J.T. Subunit Interactions and Physical Properties of Bovine Gallbladder Mucin. Gastroenterology 1989, 97, 179–187. [Google Scholar] [CrossRef]
- Jüngst, D.; Niemeyer, A.; Müller, I.; Zündt, B.; Meyer, G.; Wilhelmi, M.; del Pozo, R. Mucin and Phospholipids Determine Viscosity of Gallbladder Bile in Patients with Gallstones. World J. Gastroenterol. 2001, 7, 203–207. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Wang, D.Q.-H.; Bonfrate, L.; Portincasa, P. Current Views on Genetics and Epigenetics of Cholesterol Gallstone Disease. Cholesterol 2013, 2013, 298421. [Google Scholar] [CrossRef]
- Gründel, D.; Jüngst, C.; Straub, G.; Althaus, R.; Schneider, B.; Spelsberg, F.W.; Hüttl, T.P.; del Pozo, R.; Jüngst, D.; Neubrand, M. Relation of Gallbladder Motility to Viscosity and Composition of Gallbladder Bile in Patients with Cholesterol Gallstones. Digestion 2009, 79, 229–234. [Google Scholar] [CrossRef]
- Ko, C.W.; Lee, S.P. Gallstone Formation: Local Factors. Gastroenterol. Clin. N. Am. 1999, 28, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-Y.; Cheng, N.-S.; Cheng, J.-Q.; Mao, H.; Jiang, L.-S.; Li, Q.-S.; Zhou, Y. Practical Value of Applying Cdc2 Kinase shRNA to Chronic Proliferative Cholangitis in Treatment of Hepatolithiasis. Hepatogastroenterology 2009, 56, 1477–1482. [Google Scholar]
- Kim, H.J.; Kim, J.S.; Joo, M.K.; Lee, B.J.; Kim, J.H.; Yeon, J.E.; Park, J.-J.; Byun, K.S.; Bak, Y.-T. Hepatolithiasis and Intrahepatic Cholangiocarcinoma: A Review. World J. Gastroenterol. 2015, 21, 13418–13431. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Choi, J.-W.; Kim, S.-T.; Cho, M.-C.; Sung, R.H.; Jang, L.-C.; Park, J.-W.; Lee, S.P.; Park, Y.-H. Suppression of Proliferative Cholangitis in a Rat Model by Local Delivery of Paclitaxel. J. Hepatobiliary Pancreat. Surg. 2003, 10, 176–182. [Google Scholar] [CrossRef]
- Stokes, C.S.; Gluud, L.L.; Casper, M.; Lammert, F. Ursodeoxycholic Acid and Diets Higher in Fat Prevent Gallbladder Stones during Weight Loss: A Meta-Analysis of Randomized Controlled Trials. Clin. Gastroenterol. Hepatol. 2014, 12, 1090–1100.e2; quiz e61. [Google Scholar] [CrossRef]
- Portincasa, P.; Moschetta, A.; Palasciano, G. Cholesterol Gallstone Disease. Lancet 2006, 368, 230–239. [Google Scholar] [CrossRef]
- Fischer, S.; Müller, I.; Zündt, B.Z.; Jüngst, C.; Meyer, G.; Jüngst, D. Ursodeoxycholic Acid Decreases Viscosity and Sedimentable Fractions of Gallbladder Bile in Patients with Cholesterol Gallstones. Eur. J. Gastroenterol. Hepatol. 2004, 16, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Fang, S.; Kim, K.P.; Ko, Y.; Kim, H.; Oh, J.; Hong, G.Y.; Lee, S.Y.; Kim, J.M.; Noh, I.; et al. Combination Treatment with N-3 Polyunsaturated Fatty Acids and Ursodeoxycholic Acid Dissolves Cholesterol Gallstones in Mice. Sci. Rep. 2019, 9, 12740. [Google Scholar] [CrossRef]
- Kim, J.K.; Cho, S.M.; Kang, S.H.; Kim, E.; Yi, H.; Yun, E.S.; Lee, D.G.; Cho, H.J.; Paik, Y.H.; Choi, Y.K.; et al. N-3 Polyunsaturated Fatty Acid Attenuates Cholesterol Gallstones by Suppressing Mucin Production with a High Cholesterol Diet in Mice. J. Gastroenterol. Hepatol. 2012, 27, 1745–1751. [Google Scholar] [CrossRef]
- Yamamoto, R.; Tazuma, S.; Kanno, K.; Igarashi, Y.; Inui, K.; Ohara, H.; Tsuyuguchi, T.; Ryozawa, S. Ursodeoxycholic Acid after Bile Duct Stone Removal and Risk Factors for Recurrence: A Randomized Trial. J. Hepatobiliary Pancreat. Sci. 2016, 23, 132–136. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Yang, Y.; Wu, S.; Yao, D. Role of Secretory Mucins in the Occurrence and Development of Cholelithiasis. Biomolecules 2024, 14, 676. https://doi.org/10.3390/biom14060676
Zhao Z, Yang Y, Wu S, Yao D. Role of Secretory Mucins in the Occurrence and Development of Cholelithiasis. Biomolecules. 2024; 14(6):676. https://doi.org/10.3390/biom14060676
Chicago/Turabian StyleZhao, Zeying, Ye Yang, Shuodong Wu, and Dianbo Yao. 2024. "Role of Secretory Mucins in the Occurrence and Development of Cholelithiasis" Biomolecules 14, no. 6: 676. https://doi.org/10.3390/biom14060676
APA StyleZhao, Z., Yang, Y., Wu, S., & Yao, D. (2024). Role of Secretory Mucins in the Occurrence and Development of Cholelithiasis. Biomolecules, 14(6), 676. https://doi.org/10.3390/biom14060676







