pH Homeodynamics and Male Fertility: A Coordinated Regulation of Acid-Based Balance during Sperm Journey to Fertilization
Abstract
:1. Introduction
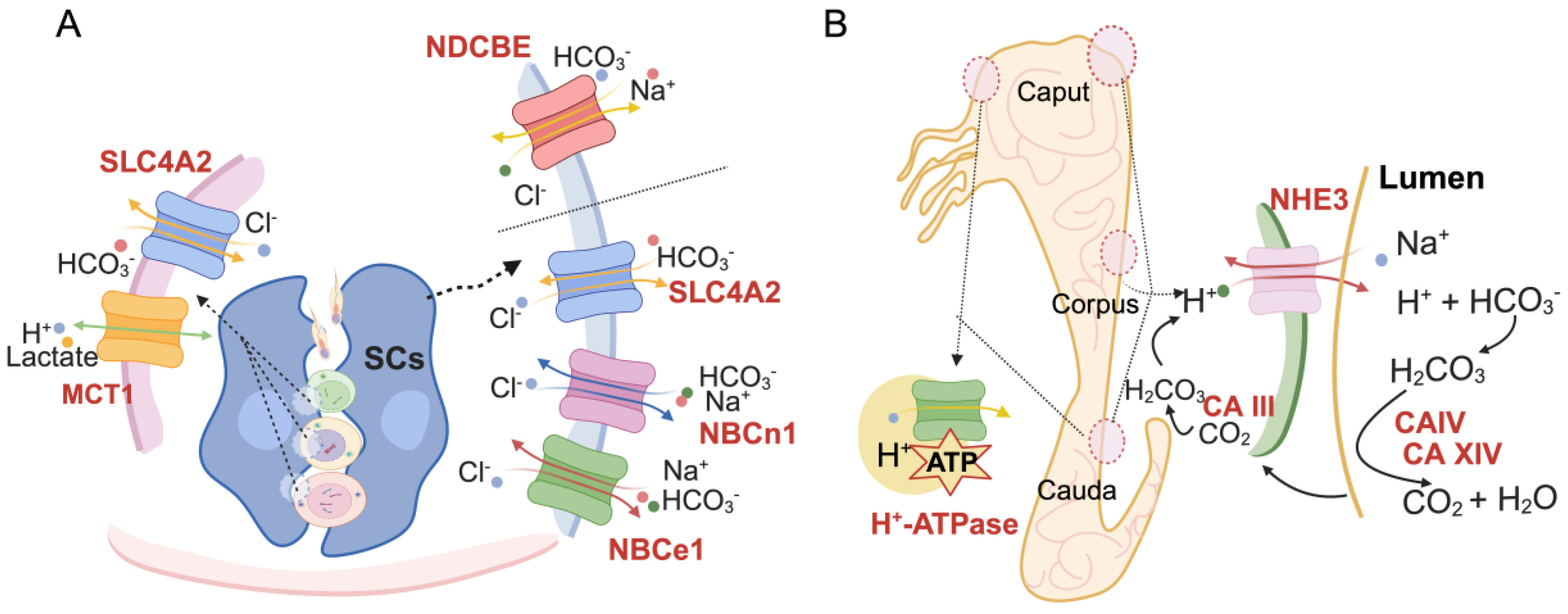
2. pH Homeostasis on Spermatogenesis
3. pH Involvement in Sperm Maturation
4. pH and Regulation of Sperm Function
4.1. pH-Regulated Ion Channels in Spermatozoa
4.2. pH and Sperm Motility
4.3. pH Promotion in Sperm Capacitation and Hyperactivation
4.3.1. HCO3− Transport
4.3.2. H+ Transport
4.4. Acrosome Reaction
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Macaluso, M.; Wright-Schnapp, T.J.; Chandra, A.; Johnson, R.; Satterwhite, C.L.; Pulver, A.; Berman, S.M.; Wang, R.Y.; Farr, S.L.; Pollack, L.A. A public health focus on infertility prevention, detection, and management. Fertil. Steril. 2010, 93, 16.e1–16.e11. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Palmer, M.J.; Tanton, C.; Gibson, L.J.; Jones, K.G.; Macdowall, W.; Glasier, A.; Sonnenberg, P.; Field, N.; Mercer, C.H.; et al. Prevalence of infertility and help seeking among 15,000 women and men. Hum. Reprod. 2016, 31, 2108–2118. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; La Vignera, S.; Calogero, A.E. Molecular Biology of Spermatogenesis: Novel Targets of Apparently Idiopathic Male Infertility. Int. J. Mol. Sci. 2020, 21, 1728. [Google Scholar] [CrossRef] [PubMed]
- Lotti, F.; Maggi, M. Sexual dysfunction and male infertility. Nat. Rev. Urol. 2018, 15, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.G.; Publicover, S.J.; Barratt, C.L.R.; Martins da Silva, S.J. Human sperm ion channel (dys)function: Implications for fertilization. Hum. Reprod. Update 2019, 25, 758–776. [Google Scholar] [CrossRef]
- Brugh, V.M.; Lipshultz, L.I. Male factor infertility: Evaluation and management. Med. Clin. N. Am. 2004, 88, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Lutz, W. Fertility rates and future population trends: Will Europe’s birth rate recover or continue to decline? Int. J. Androl. 2006, 29, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M. Spermatogenesis-physiology and pathophysiology. Der Urologe. Ausg. A 2005, 44, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Socorro, S.; Cavaco, J.E.B.; Oliveira, P.F. Tubular fluid secretion in the seminiferous epithelium: Ion transporters and aquaporins in Sertoli cells. J. Membr. Biol. 2010, 236, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Gatti, J.L. Role of the ionic environment and internal pH on sperm activity. Hum. Reprod. 1998, 13 (Suppl. 4), 20–30. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-J.; Maeng, J.-H.; Hwang, S.Y.; Ahn, Y. Design, fabrication, and testing of a microfluidic device for thermotaxis and chemotaxis assays of sperm. Slas Technol. Transl. Life Sci. Innov. 2018, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.Y.B.; Mingels, R.; Morgan, H.; Macklon, N.; Cheong, Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: A systematic review. Hum. Reprod. Update 2018, 24, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.A.; Meldrum, S.J.; Watson, B.W. Continuous measurement by radio-telemetry of vaginal pH during human coitus. J. Reprod. Fertil. 1973, 33, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.W.; Acott, T.S. Intracellular pH regulates bovine sperm motility and protein phosphorylation. Biol. Reprod. 1989, 41, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S. Control of hyperactivation in sperm. Hum. Reprod. Update 2008, 14, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Bermúdez, A.; Yeste, M.; Bonet, S.; Pinart, E. A Review on the Role of Bicarbonate and Proton Transporters during Sperm Capacitation in Mammals. Int. J. Mol. Sci. 2022, 23, 6333. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, T.; José, O.; González-Cota, A.L.; Romero, F.; Treviño, C.L.; Darszon, A. Intracellular pH in sperm physiology. Biochem. Biophys. Res. Commun. 2014, 450, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-H.; Yang, C.; Kim, S.T.; Lingle, C.J.; Xia, X.-M. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc. Natl. Acad. Sci. USA 2011, 108, 5879–5884. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Krapf, D.; de la Vega-Beltrán, J.L.; Acevedo, J.J.; Darszon, A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl. 2011, 13, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Hess, R.A.; Schaeffer, D.J.; Ko, C.; Hudgin-Spivey, S.; Chambon, P.; Shur, B.D. Absence of Estrogen Receptor Alpha Leads to Physiological Alterations in the Mouse Epididymis and Consequent Defects in Sperm Function. Biol. Reprod. 2010, 82, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Boussouar, F.; Mauduit, C.; Tabone, E.; Pellerin, L.; Magistretti, P.J.; Benahmed, M. Developmental and hormonal regulation of the monocarboxylate transporter 2 (MCT2) expression in the mouse germ cells. Biol. Reprod. 2003, 69, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, R.L.; Martins, A.D.; Jesus, T.T.; Sá, R.; Sousa, M.; Alves, M.G.; Oliveira, P.F. Estrogenic regulation of bicarbonate transporters from SLC4 family in rat Sertoli cells. Mol. Cell. Biochem. 2015, 408, 47–54. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.; Smith, L.B.; Rebourcet, D. Sertoli cells as key drivers of testis function. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2022; pp. 2–9. [Google Scholar]
- Oliveira, P.F.; Alves, M.G.; Rato, L.; Laurentino, S.; Silva, J.; Sá, R.; Barros, A.; Sousa, M.; Carvalho, R.A.; Cavaco, J.E.; et al. Effect of insulin deprivation on metabolism and metabolism-associated gene transcript levels of in vitro cultured human Sertoli cells. Biochim. Biophys. Acta 2012, 1820, 84–89. [Google Scholar] [CrossRef]
- Holappa, K. Primary structure of a sperm cell anion exchanger and its messenger ribonucleic acid expression during spermatogenesis. Biol. Reprod. 1999, 61, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.F.; Recalde, S.; Prieto, J.; Lecanda, J.; Saez, E.; Funk, C.D.; Vecino, P.; van Roon, M.A.; Ottenhoff, R.; Bosma, P.J.; et al. Anion exchanger 2 is essential for spermiogenesis in mice. Proc. Natl. Acad. Sci. USA 2003, 100, 15847–15852. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Chen, L.; Nowak, R.A. Tissue distribution of basigin and monocarboxylate transporter 1 in the adult male mouse: A study using the wild-type and basigin gene knockout mice. Anat. Record. Part A Discov. Mol. Cell. Evol. Biol. 2006, 288, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, A.; Ishiguro-Oonuma, T.; Takahashi, R.; Maekawa, M.; Toshimori, K.; Watanabe, M.; Iwanaga, T. Immunohistochemical localization of GLUT3, MCT1, and MCT2 in the testes of mice and rats: The use of different energy sources in spermatogenesis. Biomed. Res. 2015, 36, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, R.L.; D’Souza, W.N.; Rato, L.; Rothstein, J.L.; Dias, T.R.; Chui, D.; Wannberg, S.; Alves, M.G.; Oliveira, P.F. Knockout of MCT1 results in total absence of spermatozoa, sex hormones dysregulation, and morphological alterations in the testicular tissue. Cell Tissue Res. 2019, 378, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Chávez, J.C.; Hernández-González, E.O.; Wertheimer, E.; Visconti, P.E.; Darszon, A.; Treviño, C.L. Participation of the Cl−/HCO3− exchangers SLC26A3 and SLC26A6, the Cl− channel CFTR, and the regulatory factor SLC9A3R1 in mouse sperm capacitation. Biol Reprod 2012, 86, 1–14. [Google Scholar] [CrossRef] [PubMed]
- El Khouri, E.; Whitfield, M.; Stouvenel, L.; Kini, A.; Riederer, B.; Lores, P.; Roemermann, D.; di Stefano, G.; Drevet, J.R.; Saez, F.; et al. Slc26a3 deficiency is associated with epididymis dysplasia and impaired sperm fertilization potential in the mouse. Mol. Reprod. Dev. 2018, 85, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Wedenoja, S.; Khamaysi, A.; Shimshilashvili, L.; Anbtawe-Jomaa, S.; Elomaa, O.; Toppari, J.; Höglund, P.; Aittomäki, K.; Holmberg, C.; Hovatta, O.; et al. A missense mutation in Slc26a3 is associated with human male subfertility and impaired activation of CFTR. Sci. Rep. 2017, 7, 14208. [Google Scholar] [CrossRef] [PubMed]
- Touré, A.; Lhuillier, P.; Gossen, J.A.; Kuil, C.W.; Lhôte, D.; Jégou, B.; Escalier, D.; Gacon, G. The testis anion transporter 1 (Slc26a8) is required for sperm terminal differentiation and male fertility in the mouse. Hum. Mol. Genet. 2007, 16, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Lhuillier, P.; Rode, B.; Escalier, D.; Lorès, P.; Dirami, T.; Bienvenu, T.; Gacon, G.; Dulioust, E.; Touré, A. Absence of annulus in human asthenozoospermia: Case report. Hum. Reprod. 2009, 24, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.P.; Tse, C.M.; Chew, S.B.; Wong, P.Y. Expression of multiple Na+/H+ exchanger isoforms in cultured epithelial cells from rat efferent duct and cauda epididymidis. Biol. Reprod. 2001, 64, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.C.; James, P.F. Na+/H+ Exchangers (NHEs) in Mammalian Sperm: Essential Contributors to Male Fertility. Int. J. Mol. Sci. 2023, 24, 14981. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Clarke, L.; Nie, R.; Carnes, K.; Lai, L.-W.; Lien, Y.-H.H.; Verkman, A.; Lubahn, D.; Fisher, J.S.; Katzenellenbogen, B.S. Estrogen action and male fertility: Roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc. Natl. Acad. Sci. USA 2001, 98, 14132–14137. [Google Scholar] [CrossRef] [PubMed]
- Bagnis, C.; Marsolais, M.; Biemesderfer, D.; Laprade, R.; Breton, S. Na+/H+-exchange activity and immunolocalization of NHE3 in rat epididymis. Am. J. Physiology. Ren. Physiol. 2001, 280, F426–F436. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-R.; Chen, M.; Deng, S.L.; Hao, X.X.; Wang, X.X.; Liu, Y.X. Sodium-hydrogen exchanger NHA1 and NHA2 control sperm motility and male fertility. Cell Death Dis. 2016, 7, e2152. [Google Scholar] [CrossRef]
- Kumar, P.L.; James, P.F. Identification and characterization of methylation-dependent/independent DNA regulatory elements in the human Slc9b1 gene. Gene 2015, 561, 235–248. [Google Scholar] [CrossRef]
- Wang, D.; King, S.M.; Quill, T.A.; Doolittle, L.K.; Garbers, D.L. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat. Cell Biol. 2003, 5, 1117–1122. [Google Scholar] [CrossRef]
- Windler, F.; Bönigk, W.; Körschen, H.G.; Grahn, E.; Strünker, T.; Seifert, R.; Kaupp, U.B. The solute carrier SLC9C1 is a Na+/H+-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding. Nat. Commun. 2018, 9, 2809. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Wu, H.; Zhang, H.; Zhang, H.; Mao, J.; Liu, D.; Zhao, L.; Lin, H.; Tang, W.; et al. Sodium-Hydrogen-Exchanger expression in human sperm and its relationship with semen parameters. J. Assist. Reprod. Genet. 2017, 34, 795–801. [Google Scholar] [CrossRef]
- Xu, W.M.; Shi, Q.X.; Chen, W.Y.; Zhou, C.X.; Ni, Y.; Rowlands, D.K.; Yi Liu, G.; Zhu, H.; Ma, Z.G.; Wang, X.F.; et al. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc. Natl. Acad. Sci. USA 2007, 104, 9816–9821. [Google Scholar] [CrossRef]
- Grubb, B.R.; Gabriel, S.E. Intestinal physiology and pathology in gene-targeted mouse models of cystic fibrosis. Am. J. Physiol. 1997, 273, G258–G266. [Google Scholar] [CrossRef]
- Ahmed, N.; Corey, M.; Forstner, G.; Zielenski, J.; Tsui, L.C.; Ellis, L.; Tullis, E.; Durie, P. Molecular consequences of cystic fibrosis transmembrane regulator (Cftr) gene mutations in the exocrine pancreas. Gut 2003, 52, 1159–1164. [Google Scholar] [CrossRef]
- Grasemann, H.; Ratjen, F. Cystic Fibrosis. N. Engl. J. Med. 2023, 389, 1693–1707. [Google Scholar] [CrossRef]
- Brown, D.; Lui, B.; Gluck, S.; Sabolić, I. A plasma membrane proton ATPase in specialized cells of rat epididymis. Am. J. Physiol. 1992, 263, C913–C916. [Google Scholar] [CrossRef]
- Breton, S.; Smith, P.J.; Lui, B.; Brown, D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat. Med. 1996, 2, 470–472. [Google Scholar] [CrossRef]
- Smith, A.N.; Finberg, K.E.; Wagner, C.A.; Lifton, R.P.; Devonald, M.A.; Su, Y.; Karet, F.E. Molecular cloning and characterization of Atp6n1b: A novel fourth murine vacuolar H+-ATPase a-subunit gene. J. Biol. Chem. 2001, 276, 42382–42388. [Google Scholar] [CrossRef]
- Sun-Wada, G.-H.; Imai-Senga, Y.; Yamamoto, A.; Murata, Y.; Hirata, T.; Wada, Y.; Futai, M. A proton pump ATPase with testis-specific E1-subunit isoform required for acrosome acidification. J. Biol. Chem. 2002, 277, 18098–18105. [Google Scholar] [CrossRef]
- Codelia, V.A.; Cortes, C.J.; Moreno, R.D. Inhibition of the vacuolar H(+)-pump with bafilomycin A1 does not induce acrosome reaction or activate proacrosin in mouse spermatozoa. Biochem. Biophys. Res. Commun. 2005, 337, 1337–1344. [Google Scholar] [CrossRef]
- Blomqvist, S.R.; Vidarsson, H.; Söder, O.; Enerbäck, S. Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. EMBO J. 2006, 25, 4131–4141. [Google Scholar] [CrossRef]
- Dai, P.; Qiao, F.; Chen, Y.; Chan, D.; Yim, H.; Fok, K.; Chen, H. SARS-CoV-2 and male infertility: From short-to long-term impacts. J. Endocrinol. Investig. 2023, 46, 1491–1507. [Google Scholar] [CrossRef]
- Bernardino, R.L.; Martins, A.D.; Socorro, S.; Alves, M.G.; Oliveira, P.F. Effect of prediabetes on membrane bicarbonate transporters in testis and epididymis. J. Membr. Biol. 2013, 246, 877–883. [Google Scholar] [CrossRef]
- Romero, M.F.; Chen, A.-P.; Parker, M.D.; Boron, W.F. The SLC4 family of bicarbonate (HCO3−) transporters. Mol. Asp. Med. 2013, 34, 159–182. [Google Scholar] [CrossRef]
- Oliveira, P.F.; Sousa, M.; Barros, A.; Moura, T.; Rebelo da Costa, A. Membrane transporters and cytoplasmatic pH regulation on bovine Sertoli cells. J. Membr. Biol. 2009, 227, 49–55. [Google Scholar] [CrossRef]
- Xu, H.; Chen, H.; Li, J.; Zhao, Y.; Ghishan, F.K. Disruption of NHE8 expression impairs Leydig cell function in the testes. Am. J. Physiol. Cell Physiol. 2015, 308, C330–C338. [Google Scholar] [CrossRef]
- Bernardino, R.L.; Costa, A.R.; Martins, A.D.; Silva, J.; Barros, A.; Sousa, M.; Sá, R.; Alves, M.G.; Oliveira, P.F. Estradiol modulates Na+-dependent HCO3− transporters altering intracellular pH and ion transport in human Sertoli cells: A role on male fertility? Biol. Cell 2016, 108, 179–188. [Google Scholar] [CrossRef]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.J.N.b. Towards a knowledge-based human protein atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef]
- Felmlee, M.A.; Jones, R.S.; Rodriguez-Cruz, V.; Follman, K.E.; Morris, M.E. Monocarboxylate transporters (SLC16): Function, regulation, and role in health and disease. Pharmacol. Rev. 2020, 72, 466–485. [Google Scholar] [CrossRef]
- Mannowetz, N.; Wandernoth, P.; Wennemuth, G. Basigin interacts with both MCT1 and MCT2 in murine spermatozoa. J. Cell. Physiol. 2012, 227, 2154–2162. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef]
- Halestrap, A.P.; PRICE, N.T. The proton-linked monocarboxylate transporter (MCT) family: Structure, function and regulation. Biochem. J. 1999, 343, 281–299. [Google Scholar] [CrossRef]
- Soudmand, P.; Tofighi, A.; Azar, J.T.; Razi, M.; Pakdel, F.G. Different continuous exercise training intensities induced effect on sertoli-germ cells metabolic interaction; implication on GLUT-1, GLUT-3 and MCT-4 transporting proteins expression level. Gene 2021, 783, 145553. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Socorro, S.; Carvalho, R.A.; Cavaco, J.E.; Oliveira, P.F. Metabolic modulation induced by oestradiol and DHT in immature rat Sertoli cells cultured in vitro. Biosci. Rep. 2012, 32, 61–69. [Google Scholar] [CrossRef]
- Pereira-Vieira, J.; Azevedo-Silva, J.; Preto, A.; Casal, M.; Queirós, O. MCT1, MCT4 and CD147 expression and 3-bromopyruvate toxicity in colorectal cancer cells are modulated by the extracellular conditions. Biol. Chem. 2019, 400, 787–799. [Google Scholar] [CrossRef]
- Okamura, N.; Tajima, Y.; Ishikawa, H.; Yoshii, S.; Koiso, K.; Sugita, Y. Lowered levels of bicarbonate in seminal plasma cause the poor sperm motility in human infertile patients. Fertil. Steril. 1986, 45, 265–272. [Google Scholar] [CrossRef]
- Acott, T.S.; Carr, D.W. Inhibition of bovine spermatozoa by caudal epididymal fluid: II. Interaction of pH and a quiescence factor. Biol. Reprod. 1984, 30, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.J.; Stuart-Tilley, A.K.; Peters, L.L.; Lux, S.E.; Alper, S.L.; Breton, S. Immunolocalization of AE2 anion exchanger in rat and mouse epididymis. Biol. Reprod. 1999, 61, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, J.-Y.; Wang, D.-K.; Wang, L.; Chen, L.-M. Cloning and identification of two novel Nbce1 splice variants from mouse reproductive tract tissues: A comparative study of NCBT genes. Genomics 2011, 98, 112–119. [Google Scholar] [CrossRef]
- PUSHKIN, A.; CLARK, I.; KWON, T.H.; Nielsen, S.; KURTZ, I. Immunolocalization of NBC3 and NHE3 in the rat epididymis: Colocalization of NBC3 and the vacuolar H+-ATPase. J. Androl. 2000, 21, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, E.; Ridderstråle, Y. Histochemical localization of carbonic anhydrase in the testis and epididymis of the rabbit. Acta Anat. 1992, 143, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.J.; Schmitt, B.M.; Berger, U.V.; Nsumu, N.N.; Boron, W.F.; Hediger, M.A.; Brown, D.; Breton, S. Localization of sodium bicarbonate cotransporter (NBC) protein and messenger ribonucleic acid in rat epididymis. Biol. Reprod. 1999, 60, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases and metabolism. Metabolites 2018, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Caflisch, C.R.; DuBose Jr, T. Direct evaluation of acidification by rat testis and epididymis: Role of carbonic anhydrase. Am. J. Physiol.-Endocrinol. Metab. 1990, 258, E143–E150. [Google Scholar] [CrossRef]
- Hermo, L.; Chong, D.L.; Moffatt, P.; Sly, W.S.; Waheed, A.; Smith, C.E. Region-and cell-specific differences in the distribution of carbonic anhydrases II, III, XII, and XIV in the adult rat epididymis. J. Histochem. Cytochem. 2005, 53, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Kaunisto, K.; Parkkila, S.; Parkkila, A.-K.; Waheed, A.; Sly, W.S.; Rajaniemi, H. Expression of carbonic anhydrase isoenzymes IV and II in rat epididymal duct. Biol. Reprod. 1995, 52, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Karhumaa, P.; Kaunisto, K.; Parkkila, S.; Waheed, A.; Pastoreková, S.; Pastorek, J.; Sly, W.S.; Rajaniemi, H. Expression of the transmembrane carbonic anhydrases, CA IX and CA XII, in the human male excurrent ducts. Mol. Hum. Reprod. 2001, 7, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Levine, N.; Marsh, D.J. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J. Physiol. 1971, 213, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Finberg, K.E.; Wagner, C.A.; Bailey, M.A.; Paunescu, T.G.; Breton, S.; Brown, D.; Giebisch, G.; Geibel, J.P.; Lifton, R.P. The B1-subunit of the H+ATPase is required for maximal urinary acidification. Proc. Natl. Acad. Sci. USA 2005, 102, 13616–13621. [Google Scholar] [CrossRef] [PubMed]
- Oldereid, N.B.; Thomassen, Y.; Attramadal, A.; Olaisen, B.; Purvis, K. Concentrations of lead, cadmium and zinc in the tissues of reproductive organs of men. J. Reprod. Fertil. 1993, 99, 421–425. [Google Scholar] [CrossRef]
- Caflisch, C.R.; DuBose, T.D. Cadmium-induced changes in luminal fluid pH in testis and epididymis of the rat in vivo. J. Toxicol. Environ. Health 1991, 32, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Herak-Kramberger, C.M.; Sabolić, I.; Blanusa, M.; Smith, P.J.; Brown, D.; Breton, S. Cadmium inhibits vacuolar H+ATPase-mediated acidification in the rat epididymis. Biol. Reprod. 2000, 63, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.; Shum, W.W.; Breton, S. Regulation of vacuolar proton pumping ATPase-dependent luminal acidification in the epididymis. Asian J. Androl. 2007, 9, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Silva, J.V.; Howl, J.; Santos, M.A.; Fardilha, M. All you need to know about sperm RNAs. Hum. Reprod. Update 2022, 28, 67–91. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Szymczak-Cendlak, M. Structure and Function of Ion Channels Regulating Sperm Motility-An Overview. Int. J. Mol. Sci. 2021, 22, 3259. [Google Scholar] [CrossRef]
- Kirichok, Y.; Navarro, B.; Clapham, D.E. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 2006, 439, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Navarro, B.; Kirichok, Y.; Clapham, D.E. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc. Natl. Acad. Sci. USA 2007, 104, 7688–7692. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-H.; Zhu, Y.-Y.; Wang, L.; Liu, H.-L.; Ling, Y.; Li, Z.-L.; Sun, L.-B. The Catsper channel and its roles in male fertility: A systematic review. Reprod. Biol. Endocrinol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Brenker, C.; Goodwin, N.; Weyand, I.; Kashikar, N.D.; Naruse, M.; Krähling, M.; Müller, A.; Kaupp, U.B.; Strünker, T. The CatSper channel: A polymodal chemosensor in human sperm. EMBO J. 2012, 31, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.; Nishigaki, T. Comparative genomic analysis suggests that the sperm-specific sodium/proton exchanger and soluble adenylyl cyclase are key regulators of CatSper among the Metazoa. Zool. Lett. 2019, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.E.; Westenbroek, R.E.; Quill, T.; Ren, D.; Clapham, D.E.; Hille, B.; Garbers, D.L.; Babcock, D.F. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl. Acad. Sci. USA 2003, 100, 14864–14868. [Google Scholar] [CrossRef] [PubMed]
- Quill, T.A.; Sugden, S.A.; Rossi, K.L.; Doolittle, L.K.; Hammer, R.E.; Garbers, D.L. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc. Natl. Acad. Sci. USA 2003, 100, 14869–14874. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Jin, N.; Zheng, H.; Ro, S.; Tafolla, D.; Sanders, K.M.; Yan, W. Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol. Reprod. 2007, 77, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Navarro, B.; Perez, G.; Jackson, A.C.; Hsu, S.; Shi, Q.; Tilly, J.L.; Clapham, D.E. A sperm ion channel required for sperm motility and male fertility. Nature 2001, 413, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Mannowetz, N.; Zhang, Y.; Everley, R.A.; Gygi, S.P.; Bewersdorf, J.; Lishko, P.V.; Chung, J.-J. Dual Sensing of Physiologic pH and Calcium by EFCAB9 Regulates Sperm Motility. Cell 2019, 177, 1480–1494. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Kirichok, Y. The role of Hv1 and CatSper channels in sperm activation. J. Physiol. 2010, 588, 4667–4672. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, X.; Lingle, C.J. SLO3 K+ channels: Voltage and pH dependence of macroscopic currents. J. Gen. Physiol. 2006, 128, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zeng, X.-H.; Zhou, Y.; Xia, X.-M.; Lingle, C.J. LRRC52 (leucine-rich-repeat-containing protein 52), a testis-specific auxiliary subunit of the alkalization-activated SLO3 channel. Proc. Natl. Acad. Sci. USA 2011, 108, 19419–19424. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, M.D.; Yuan, P.; Hsiung, Y.; Mackinnon, R. Functional and structural analysis of the human SLO3 pH- and voltage-gated K+ channel. Proc. Natl. Acad. Sci. USA 2012, 109, 19274–19279. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-H.; Yang, C.; Xia, X.-M.; Liu, M.; Lingle, C.J. SLO3 auxiliary subunit LRRC52 controls gating of sperm KSPER currents and is critical for normal fertility. Proc. Natl. Acad. Sci. USA 2015, 112, 2599–2604. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Liu, M.; Zhang, W.; Huang, R.-Z.; Zhao, N.; Chen, C.; Zeng, X.-H. Na+/H+ Exchangers Involve in Regulating the pH-Sensitive Ion Channels in Mouse Sperm. Int. J. Mol. Sci. 2021, 22, 1612. [Google Scholar] [CrossRef]
- Chávez, J.C.; Ferreira, J.J.; Butler, A.; De La Vega Beltrán, J.L.; Treviño, C.L.; Darszon, A.; Salkoff, L.; Santi, C.M. SLO3 K+ channels control calcium entry through CATSPER channels in sperm. J. Biol. Chem. 2014, 289, 32266–32275. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.M.; Martínez-López, P.; de la Vega-Beltrán, J.L.; Butler, A.; Alisio, A.; Darszon, A.; Salkoff, L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010, 584, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Mannowetz, N.; Naidoo, N.M.; Choo, S.-A.S.; Smith, J.F.; Lishko, P.V. SLO1 is the principal potassium channel of human spermatozoa. eLife 2013, 2, e01009. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, U.B.; Strunker, T. Signaling in Sperm: More Different than Similar. Trends Cell Biol. 2017, 27, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.G.; Publicover, S.J.; Mansell, S.A.; Lishko, P.V.; Williams, H.L.; Ramalingam, M.; Wilson, S.M.; Barratt, C.L.R.; Sutton, K.A.; Da Silva, S.M. Depolarization of sperm membrane potential is a common feature of men with subfertility and is associated with low fertilization rate at IVF. Hum. Reprod. 2016, 31, 1147–1157. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kumar, A.; Swain, D.K.; Yadav, S.; Nigam, R. Insights into pH regulatory mechanisms in mediating spermatozoa functions. Vet. World 2018, 11, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Giroux-Widemann, V.; Jouannet, P.; Pignot-Paintrand, I.; Feneux, D. Effects of pH on the reactivation of human spermatozoa demembranated with Triton X-100. Mol. Reprod. Dev. 1991, 29, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.M.; Matsumura, N.; Shiba, K.; Itoh, N.; Takahashi, K.G.; Inaba, K.; Osada, M. Roles of extracellular ions and pH in 5-HT-induced sperm motility in marine bivalve. Reproduction 2014, 147, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, M.; Selvaraju, S. Microenvironment of the male and female reproductive tracts regulate sperm fertility: Impact of viscosity, pH, and osmolality. Andrology 2022, 10, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Speer, K.F.; Allen-Waller, L. Molecular mechanisms of sperm motility are conserved in an early-branching metazoan. Proc. Natl. Acad. Sci. USA 2021, 118, e2109993118. [Google Scholar] [CrossRef] [PubMed]
- Vacquier, V.D.; Loza-Huerta, A.; García-Rincón, J.; Darszon, A.; Beltrán, C. Soluble adenylyl cyclase of sea urchin spermatozoa. Biochim. Biophys. Acta 2014, 1842, 2621–2628. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Garcia, M.A.; Carlson, A.E.; Schuh, S.M.; Babcock, D.F.; Jaiswal, B.S.; Gossen, J.A.; Esposito, G.; van Duin, M.; Conti, M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev. Biol. 2006, 296, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Boulais, M.; Suquet, M.; Arsenault-Pernet, E.J. pH controls spermatozoa motility in the Pacific oyster (Crassostrea gigas). Biol. Open 2018, 7, bio031427. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.; Gallego, V.; Asturiano, J.F. Intracellular pH regulation and sperm motility in the European eel. Theriogenology 2020, 145, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Boitano, S.; Omoto, C.K. Membrane hyperpolarization activates trout sperm without an increase in intracellular pH. J. Cell Sci. 1991, 98 Pt 3, 343–349. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, L.; Li, J.; Li, H.; Hong, Z.; Xie, M.; Chen, S.; Yao, B. The Semen pH Affects Sperm Motility and Capacitation. PLoS ONE 2015, 10, e0132974. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, S.S.; Naik, P.; Dakshinamurthy, S.; Sullia, K. Semen pH and its correlation with motility and count—A study in subfertile men. JBRA Assist. Reprod. 2021, 25, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Mannowetz, N.; Wandernoth, P.M.; Wennemuth, G. Glucose is a pH-dependent motor for sperm beat frequency during early activation. PLoS ONE 2012, 7, e41030. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Dong, H.B.; Ma, D.L.; Li, Y.W.; Han, D.; Luo, M.J.; Chang, Z.L.; Tan, J.H. Effects of pH during liquid storage of goat semen on sperm viability and fertilizing potential. Anim. Reprod. Sci. 2016, 164, 47–56. [Google Scholar] [CrossRef]
- de Mercado, E.; Tomás-Almenar, C.; Gómez-Izquierdo, E. Improvement of the motility of boar sperm after cryopreservation. Anim. Reprod. Sci. 2020, 222, 106610. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wu, S.; Huang, M.; Wang, Y.; Zhang, K.; Kang, J.; Zhang, Y.; Quan, F. Effects of Diluent pH on Enrichment and Performance of Dairy Goat X/Y Sperm. Front. Cell Dev. Biol. 2021, 9, 747722. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Cao, X.Y.; He, Q.F.; Yang, H.W.; Chen, Y.Z.; Zhao, J.L.; Ma, H.W.; Kang, J.; Liu, J.; Quang, F.S. Alkaline semen diluent combined with R848 for separation and enrichment of dairy goat X-sperm. J. Dairy Sci. 2022, 105, 10020–10032. [Google Scholar] [CrossRef]
- He, Q.; Wu, S.; Gao, F.; Xu, X.; Wang, S.; Xu, Z.; Huang, M.; Zhang, K.; Zhang, Y.; Quan, F. Diluent pH affects sperm motility via GSK3 α/β-hexokinase pathway for the efficient enrichment of X-sperm to increase the female kids rate of dairy goats. Theriogenology 2023, 201, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Shin, D.H.; Pang, W.K.; Ryu, D.Y.; Rahman, M.S.; Adegoke, E.O.; Pang, M.G. Short-term storage of semen samples in acidic extender increases the proportion of females in pigs. BMC Vet. Res. 2021, 17, 362. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kwon, K.J.; Song, W.H.; Pang, W.K.; Ryu, D.Y.; Saidur Rahman, M.; Pang, M.G. New technique of sex preselection for increasing female ratio in boar sperm model. Reprod. Domest. Anim. 2021, 56, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Leemans, B.; Stout, T.A.E.; De Schauwer, C.; Heras, S.; Nelis, H.; Hoogewijs, M.; Van Soom, A.; Gadella, B.M. Update on mammalian sperm capacitation: How much does the horse differ from other species? Reproduction 2019, 157, R181–R197. [Google Scholar] [CrossRef] [PubMed]
- Krasznai, Z.; Krasznai, Z.T.; Morisawa, M.; Bazsáné, Z.K.; Hernádi, Z.; Fazekas, Z.; Trón, L.; Goda, K.; Márián, T. Role of the Na+/Ca2+ exchanger in calcium homeostasis and human sperm motility regulation. Cell Motil. Cytoskelet. 2006, 63, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Oberdorf, J.A.; Florman, H.M. pH regulation in mouse sperm: Identification of Na+-, Cl−, and HCO3−-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev. Biol. 1996, 173, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B.; Suarez, S.S. Bovine sperm hyperactivation is promoted by alkaline-stimulated Ca2+ influx. Biol. Reprod. 2007, 76, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.C.; Suarez, S.S. Hyperactivation of mammalian spermatozoa: Function and regulation. Reproduction 2001, 122, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-C.; Granish, K.A.; Suarez, S.S. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev. Biol. 2002, 250, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Llavanera, M.; Mateo-Otero, Y.; Catalán, J.; Bonet, S.; Pinart, E. HVCN1 Channels Are Relevant for the Maintenance of Sperm Motility During In Vitro Capacitation of Pig Spermatozoa. Int. J. Mol. Sci. 2020, 21, 3255. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cann, M.J.; Litvin, T.N.; Iourgenko, V.; Sinclair, M.L.; Levin, L.R.; Buck, J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 2000, 289, 625–628. [Google Scholar] [CrossRef] [PubMed]
- De La Vega-Beltran, J.L.; Sánchez-Cárdenas, C.; Krapf, D.; Hernandez-González, E.O.; Wertheimer, E.; Treviño, C.L.; Visconti, P.E.; Darszon, A. Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. J. Biol. Chem. 2012, 287, 44384–44393. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Jaiswal, B.S.; Xie, F.; Krajnc-Franken, M.A.M.; Robben, T.J.A.A.; Strik, A.M.; Kuil, C.; Philipsen, R.L.A.; van Duin, M.; Conti, M.; et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc. Natl. Acad. Sci. USA 2004, 101, 2993–2998. [Google Scholar] [CrossRef] [PubMed]
- Hess, K.C.; Jones, B.H.; Marquez, B.; Chen, Y.; Ord, T.S.; Kamenetsky, M.; Miyamoto, C.; Zippin, J.H.; Kopf, G.S.; Suarez, S.S.; et al. The "soluble" adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell. 2005, 9, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Balbach, M.; Ghanem, L.; Rossetti, T.; Kaur, N.; Ritagliati, C.; Ferreira, J.; Krapf, D.; Puga Molina, L.C.; Santi, C.M.; Hansen, J.N.; et al. Soluble adenylyl cyclase inhibition prevents human sperm functions essential for fertilization. Mol. Hum. Reprod. 2021, 27, gaab054. [Google Scholar] [CrossRef] [PubMed]
- Pierucci-Alves, F.; Akoyev, V.; Schultz, B.D. Bicarbonate exchangers SLC26A3 and SLC26A6 are localized at the apical membrane of porcine vas deferens epithelium. Physiol. Rep. 2015, 3, e12380. [Google Scholar] [CrossRef]
- Seidler, U.; Nikolovska, K. SLC26 Family of Anion Transporters in the Gastrointestinal Tract: Expression, Function, Regulation, and Role in Disease. Compr. Physiol. 2019, 9, 839–872. [Google Scholar] [CrossRef] [PubMed]
- Schweinfest, C.W.; Spyropoulos, D.D.; Henderson, K.W.; Kim, J.-H.; Chapman, J.M.; Barone, S.; Worrell, R.T.; Wang, Z.; Soleimani, M. Slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J. Biol. Chem. 2006, 281, 37962–37971. [Google Scholar] [CrossRef] [PubMed]
- Rode, B.; Dirami, T.; Bakouh, N.; Rizk-Rabin, M.; Norez, C.; Lhuillier, P.; Lorès, P.; Jollivet, M.; Melin, P.; Zvetkova, I.; et al. The testis anion transporter TAT1 (SLC26A8) physically and functionally interacts with the cystic fibrosis transmembrane conductance regulator channel: A potential role during sperm capacitation. Hum. Mol. Genet. 2012, 21, 1287–1298. [Google Scholar] [CrossRef]
- Quinton, P.M. Too much salt, too little soda: Cystic fibrosis. Sheng Li Xue Bao 2007, 59, 397–415. [Google Scholar] [PubMed]
- Li, C.-Y.; Jiang, L.-Y.; Chen, W.-Y.; Li, K.; Sheng, H.-Q.; Ni, Y.; Lu, J.-X.; Xu, W.-X.; Zhang, S.-Y.; Shi, Q.-X. CFTR is essential for sperm fertilizing capacity and is correlated with sperm quality in humans. Hum. Reprod. 2010, 25, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Hernández-González, E.O.; Treviño, C.L.; Castellano, L.E.; de la Vega-Beltrán, J.L.; Ocampo, A.Y.; Wertheimer, E.; Visconti, P.E.; Darszon, A. Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. J. Biol. Chem. 2007, 282, 24397–24406. [Google Scholar] [CrossRef]
- Puga Molina, L.C.; Pinto, N.A.; Torres, N.I.; González-Cota, A.L.; Luque, G.M.; Balestrini, P.A.; Romarowski, A.; Krapf, D.; Santi, C.M.; Treviño, C.L.; et al. CFTR/ENaC-dependent regulation of membrane potential during human sperm capacitation is initiated by bicarbonate uptake through NBC. J. Biol. Chem. 2018, 293, 9924–9936. [Google Scholar] [CrossRef] [PubMed]
- Matamoros-Volante, A.; Treviño, C.L. Capacitation-associated alkalization in human sperm is differentially controlled at the subcellular level. J. Cell Sci. 2020, 133, jcs238816. [Google Scholar] [CrossRef]
- Liang, M.; Ji, N.; Song, J.; Kang, H. Flagellar pH homeostasis mediated by Na+/H+ exchangers regulates human sperm functions through coupling with CatSper and KSper activation. Hum. Reprod. 2024, 39, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Donowitz, M.; Tse, C.M.; Fuster, D. SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol. Asp. Med. 2013, 34, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.L.; James, P.F.; Lingrel, J.B. Roles of the Na,K-ATPase alpha4 isoform and the Na+/H+ exchanger in sperm motility. Mol. Reprod. Dev. 2002, 62, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Muzzachi, S.; Guerra, L.; Martino, N.A.; Favia, M.; Punzi, G.; Silvestre, F.; Guaricci, A.C.; Roscino, M.T.; Pierri, C.L.; Dell’Aquila, M.E.; et al. Effect of cariporide on ram sperm pH regulation and motility: Possible role of NHE1. Reproduction 2018, 155, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.M.; Schreiner, C.M.; Schultheis, P.J.; Miller, M.L.; Evans, R.L.; Vorhees, C.V.; Shull, G.E.; Scott, W.J. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am. J. Physiol. Cell Physiol. 1999, 276, C788–C795. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; Tang, L.; Wang, J.; Shen, C.; Liu, J.; Lu, S.; Zhang, H.; Kuang, Y.; Fei, J. Nhe5 deficiency enhances learning and memory via upregulating BDNF/TRKB signaling in mice. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.B.; Leung, G.P.; Leung, P.Y.; Tse, C.M.; Wong, P.Y. Polarized distribution of NHE1 and NHE2 in the rat epididymis. Biol. Reprod. 2000, 62, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Malakooti, J.; Dahdal, R.Y.; Schmidt, L.; Layden, T.J.; Dudeja, P.K.; Ramaswamy, K. Molecular cloning, tissue distribution, and functional expression of the human Na+/H+ exchanger NHE2. Am. J. Physiol. 1999, 277, G383–G390. [Google Scholar] [CrossRef] [PubMed]
- Oberheide, K.; Puchkov, D.; Jentsch, T.J. Loss of the Na+/H+ exchanger NHE8 causes male infertility in mice by disrupting acrosome formation. J. Biol. Chem. 2017, 292, 10845–10854. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Mansell, S.A.; Meyers, S.A.; Lishko, P.V. Flagellar ion channels of sperm: Similarities and differences between species. Cell Calcium. 2015, 58, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.C.; James, P.F. The Slc9c2 Gene Product (Na+/H+ Exchanger Isoform 11; NHE11) Is a Testis-Specific Protein Localized to the Head of Mature Mammalian Sperm. Int. J. Mol. Sci. 2023, 24, 5329. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.K.; Fußhöller, D.M.; Goodwin, N.; Bönigk, W.; Müller, A.; Dokani Khesroshahi, N.; Brenker, C.; Wachten, D.; Krause, E.; Kaupp, U.B.; et al. Post-translational cleavage of Hv1 in human sperm tunes pH- and voltage-dependent gating. J. Physiol. 2017, 595, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Seredenina, T.; Demaurex, N.; Krause, K.-H. Voltage-Gated Proton Channels as Novel Drug Targets: From NADPH Oxidase Regulation to Sperm Biology. Antioxid. Redox. Signal 2015, 23, 490–513. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.R.; Kang, S.J.; Lee, K.P.; Choi, B.R.; Kim, H.K.; Park, J.K.; Kim, C.Y.; Lee, S.W. Onion (Allium cepa L.) peel extract (OPE) regulates human sperm motility via protein kinase C-mediated activation of the human voltage-gated proton channel. Andrology 2017, 5, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Musset, B.; Capasso, M.; Cherny, V.V.; Morgan, D.; Bhamrah, M.; Dyer, M.J.S.; DeCoursey, T.E. Identification of Thr29 as a critical phosphorylation site that activates the human proton channel HVCN1 in leukocytes. J. Biol. Chem. 2010, 285, 5117–5121. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Kumar, A.; Yadav, S.; Anand, M.; Yadav, B.; Nigam, R.; Garg, S.K.; Swain, D.K. Functional insights into voltage gated proton channel (Hv1) in bull spermatozoa. Theriogenology 2019, 136, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Keshtgar, S.; Ghanbari, H.; Ghani, E.; Shid Moosavi, S.M. Effect of CatSper and Hv1 Channel Inhibition on Progesterone Stimulated Human Sperm. J. Reprod. Infertil. 2018, 19, 133–139. [Google Scholar] [PubMed]
- Zhao, R.; Dai, H.; Arias, R.J.; De Blas, G.A.; Orta, G.; Pavarotti, M.A.; Shen, R.; Perozo, E.; Mayorga, L.S.; Darszon, A.; et al. Direct activation of the proton channel by albumin leads to human sperm capacitation and sustained release of inflammatory mediators by neutrophils. Nat. Commun. 2021, 12, 3855. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Chu, X.-P.; Goodson, R.; Gamel, P.; Peng, S.; Vance, J.; Wang, S. Cholesterol inhibits human voltage-gated proton channel hHv1. Proc. Natl. Acad. Sci. USA 2022, 119, e2205420119. [Google Scholar] [CrossRef] [PubMed]
- Casslén, B.; Nilsson, B. Human uterine fluid, examined in undiluted samples for osmolarity and the concentrations of inorganic ions, albumin, glucose, and urea. Am. J. Obs. Gynecol. 1984, 150, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Allouche-Fitoussi, D.; Breitbart, H. The Role of Zinc in Male Fertility. Int. J. Mol. Sci. 2020, 21, 7796. [Google Scholar] [CrossRef] [PubMed]
- Vigil, P.; Orellana, R.F.; Cortés, M.E. Modulation of spermatozoon acrosome reaction. Biol. Res. 2011, 44, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Honda, A.; Fulka, H.; Tamura-Nakano, M.; Matoba, S.; Tomishima, T.; Mochida, K.; Hasegawa, A.; Nagashima, K.; Inoue, K.; et al. Acrosin is essential for sperm penetration through the zona pellucida in hamsters. Proc. Natl. Acad. Sci. USA 2020, 117, 2513–2518. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Ikawa, M.; Yamada, S.; Toshimori, K.; Okabe, M. Alkalinization of acrosome measured by GFP as a pH indicator and its relation to sperm capacitation. Dev. Biol. 2001, 237, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Working, P.K.; Meizel, S. Correlation of increased intraacrosomal pH with the hamster sperm acrosome reaction. J. Exp. Zool. 1983, 227, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Panneerdoss, S.; Siva, A.B.; Kameshwari, D.B.; Rangaraj, N.; Shivaji, S. Association of lactate, intracellular pH, and intracellular calcium during capacitation and acrosome reaction: Contribution of hamster sperm dihydrolipoamide dehydrogenase, the E3 subunit of pyruvate dehydrogenase complex. J. Androl. 2012, 33, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, B.; Egge, N.; Cornwall, G.A. Functional amyloids in the mouse sperm acrosome. Mol. Cell Biol. 2014, 34, 2624–2634. [Google Scholar] [CrossRef] [PubMed]
- Mata-Martínez, E.; Darszon, A.; Treviño, C.L. pH-dependent Ca2+ oscillations prevent untimely acrosome reaction in human sperm. Biochem. Biophys. Res. Commun. 2018, 497, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.L. Effect of pH on the development of acrosomal responsiveness of human sperm. Andrologia 2007, 39, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Carrasquel Martínez, G.; Aldana, A.; Carneiro, J.; Treviño, C.L.; Darszon, A. Acrosomal alkalinization occurs during human sperm capacitation. Mol. Hum. Reprod. 2022, 28, gaac005. [Google Scholar] [CrossRef] [PubMed]
- Chávez, J.C.; De la Vega-Beltrán, J.L.; José, O.; Torres, P.; Nishigaki, T.; Treviño, C.L.; Darszon, A. Acrosomal alkalization triggers Ca2+ release and acrosome reaction in mammalian spermatozoa. J. Cell. Physiol. 2018, 233, 4735–4747. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, P.; Kane, S. Elevated and Sustained Intracellular Calcium Signalling Is Necessary for Efficacious Induction of the Human Sperm Acrosome Reaction. Int. J. Mol. Sci. 2022, 23, 11253. [Google Scholar] [CrossRef] [PubMed]
- Donà, G.; Tibaldi, E.; Andrisani, A.; Ambrosini, G.; Sabbadin, C.; Pagano, M.A.; Brunati, A.M.; Armanini, D.; Ragazzi, E.; Bordin, L. Human Sperm Capacitation Involves the Regulation of the Tyr-Phosphorylation Level of the Anion Exchanger 1 (AE1). Int. J. Mol. Sci. 2020, 21, 4063. [Google Scholar] [CrossRef] [PubMed]
- El Khouri, E.; Touré, A. Functional interaction of the cystic fibrosis transmembrane conductance regulator with members of the SLC26 family of anion transporters (SLC26A8 and SLC26A9): Physiological and pathophysiological relevance. Int. J. Biochem. Cell Biol. 2014, 52, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Recuero, S.; Maside, C.; Salas-Huetos, A.; Bonet, S.; Pinart, E. Blocking NHE Channels Reduces the Ability of In Vitro Capacitated Mammalian Sperm to Respond to Progesterone Stimulus. Int. J. Mol. Sci. 2021, 22, 12646. [Google Scholar] [CrossRef] [PubMed]
- Genetet, S.; Desrames, A.; Chouali, Y.; Ripoche, P.; Lopez, C.; Mouro-Chanteloup, I. Stomatin modulates the activity of the Anion Exchanger 1 (AE1, SLC4A1). Sci. Rep. 2017, 7, 46170. [Google Scholar] [CrossRef]
- Yannoukakos, D.; Stuart-Tilley, A.; Fernandez, H.A.; Fey, P.; Duyk, G.; Alper, S.L. Molecular cloning, expression, and chromosomal localization of two isoforms of the AE3 anion exchanger from human heart. Circ. Res. 1994, 75, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, K.; Dam, V.S.; Kjaer-Sorensen, K.; Pedersen, L.N.; Skeberdis, V.A.; Jurevičius, J.; Treinys, R.; Petersen, I.; Nielsen, M.S.; Oxvig, C.; et al. Loss-of-activity-mutation in the cardiac chloride-bicarbonate exchanger AE3 causes short QT syndrome. Nat. Commun. 2017, 8, 1696. [Google Scholar] [CrossRef] [PubMed]
- Felder, R.A.; Jose, P.A.; Xu, P.; Gildea, J.J. The renal sodium bicarbonate cotransporter NBCe2: Is it a major contributor to sodium and pH homeostasis? Curr. Hypertens. Rep. 2016, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- L Bernardino, R.; T Jesus, T.; D Martins, A.; Sousa, M.; Barros, A.; E Cavaco, J.; Socorro, S.; G Alves, M.; F Oliveira, P. Molecular basis of bicarbonate membrane transport in the male reproductive tract. Curr. Med. Chem. 2013, 20, 4037–4049. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Carrillo, L.; Aragón-Herrera, A.; Giménez-Escamilla, I.; Delgado-Arija, M.; García-Manzanares, M.; Anido-Varela, L.; Lago, F.; Martínez-Dolz, L.; Portolés, M.; Tarazón, E.J.P. Cardiac sodium/hydrogen exchanger (NHE11) as a novel potential target for SGLT2i in heart failure: A preliminary study. Pharmaceutics 2022, 14, 1996. [Google Scholar] [CrossRef] [PubMed]
- Lindskog, S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Haapasalo, J.; Nordfors, K.; Haapasalo, H.; Parkkila, S. The Expression of Carbonic Anhydrases II, IX and XII in Brain Tumors. Cancers 2020, 12, 1723. [Google Scholar] [CrossRef] [PubMed]
- Sly, W.S.; Hewett-Emmett, D.; Whyte, M.P.; Yu, Y.S.; Tashian, R.E. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc. Natl. Acad. Sci. USA 1983, 80, 2752–2756. [Google Scholar] [CrossRef]
- Lönnerholm, G.; Knutson, L.; Wistrand, P.J.; Flemström, G. Carbonic anhydrase in the normal rat stomach and duodenum and after treatment with omeprazole and ranitidine. Acta Physiol. Scand. 1989, 136, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cheng, Z.; Liu, F.; Zhang, H.; Li, J.; Li, F. CREB is a key negative regulator of carbonic anhydrase IX (CA9) in gastric cancer. Cell Signal. 2015, 27, 1369–1379. [Google Scholar] [CrossRef]
- Nortunen, M.; Parkkila, S.; Saarnio, J.; Huhta, H.; Karttunen, T.J. Carbonic Anhydrases II and IX in Non-ampullary Duodenal Adenomas and Adenocarcinoma. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2021, 69, 677–690. [Google Scholar] [CrossRef]
- Liao, S.Y.; Ivanov, S.; Ivanova, A.; Ghosh, S.; Cote, M.A.; Keefe, K.; Coca-Prados, M.; Stanbridge, E.J.; Lerman, M.I. Expression of cell surface transmembrane carbonic anhydrase genes Ca9 and Ca12 in the human eye: Overexpression of CA12 (CAXII) in glaucoma. J. Med. Genet. 2003, 40, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Beasley, N.; Watson, P.H.; Campo, L.; Chia, S.K.; English, R.; Pastorek, J.; Sly, W.S.; Ratcliffe, P.; Harris, A.L. Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am. J. Pathol. 2001, 158, 1011–1019. [Google Scholar] [CrossRef]
- Song, X.; Zhu, S.; Xie, Y.; Liu, J.; Sun, L.; Zeng, D.; Wang, P.; Ma, X.; Kroemer, G.; Bartlett, D.L.; et al. JTC801 Induces pH-dependent Death Specifically in Cancer Cells and Slows Growth of Tumors in Mice. Gastroenterology 2018, 154, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Vecchio-Pagán, B.; Sharma, N.; Waheed, A.; Li, X.; Raraigh, K.S.; Robbins, S.; Han, S.T.; Franca, A.L.; Pellicore, M.J.; et al. Loss of carbonic anhydrase XII function in individuals with elevated sweat chloride concentration and pulmonary airway disease. Hum. Mol. Genet. 2016, 25, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Silagi, E.S.; Schoepflin, Z.R.; Seifert, E.L.; Merceron, C.; Schipani, E.; Shapiro, I.M.; Risbud, M.V. Bicarbonate Recycling by HIF-1-Dependent Carbonic Anhydrase Isoforms 9 and 12 Is Critical in Maintaining Intracellular pH and Viability of Nucleus Pulposus Cells. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2018, 33, 338–355. [Google Scholar] [CrossRef] [PubMed]
- Groeneweg, S.; Kersseboom, S.; van den Berge, A.; Dolcetta-Capuzzo, A.; van Geest, F.S.; van Heerebeek, R.E.A.; Arjona, F.J.; Meima, M.E.; Peeters, R.P.; Visser, W.E.; et al. In Vitro Characterization of Human, Mouse, and Zebrafish MCT8 Orthologues. Thyroid 2019, 29, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yuan, H.; Li, W.; Xiong, Z.; Dong, W.; Xiao, W.; Zhang, X. Solute carrier family 16 member 5 downregulation and its methylation might serve as a prognostic indicator of prostate cancer. IUBMB Life 2021, 73, 1363–1377. [Google Scholar] [CrossRef] [PubMed]

| Protein Symbol, Gene ID | Location | Functions | Phenotype (Knockout in Mouse) | Clinical Symptoms/Disease |
|---|---|---|---|---|
| SLC4A2 (AE2), 6522 | Sertoli cells (SCs), spermatozoa [25,28] | Sodium-independent anion exchanger, transports Cl−/HCO3− in sperm. | Spermatogenesis interrupted, male infertility [29] | NR |
| SLC16A1 (MCT1), 6566 | Elongated spermatids, spermatozoa tail [30,31] | Bidirectional proton-coupled monocarboxylate transporter, transports nutrients, and regulates pH. | Seminiferous tubules and SCs morphologically changed, and spermatogenesis failed [32] | NR |
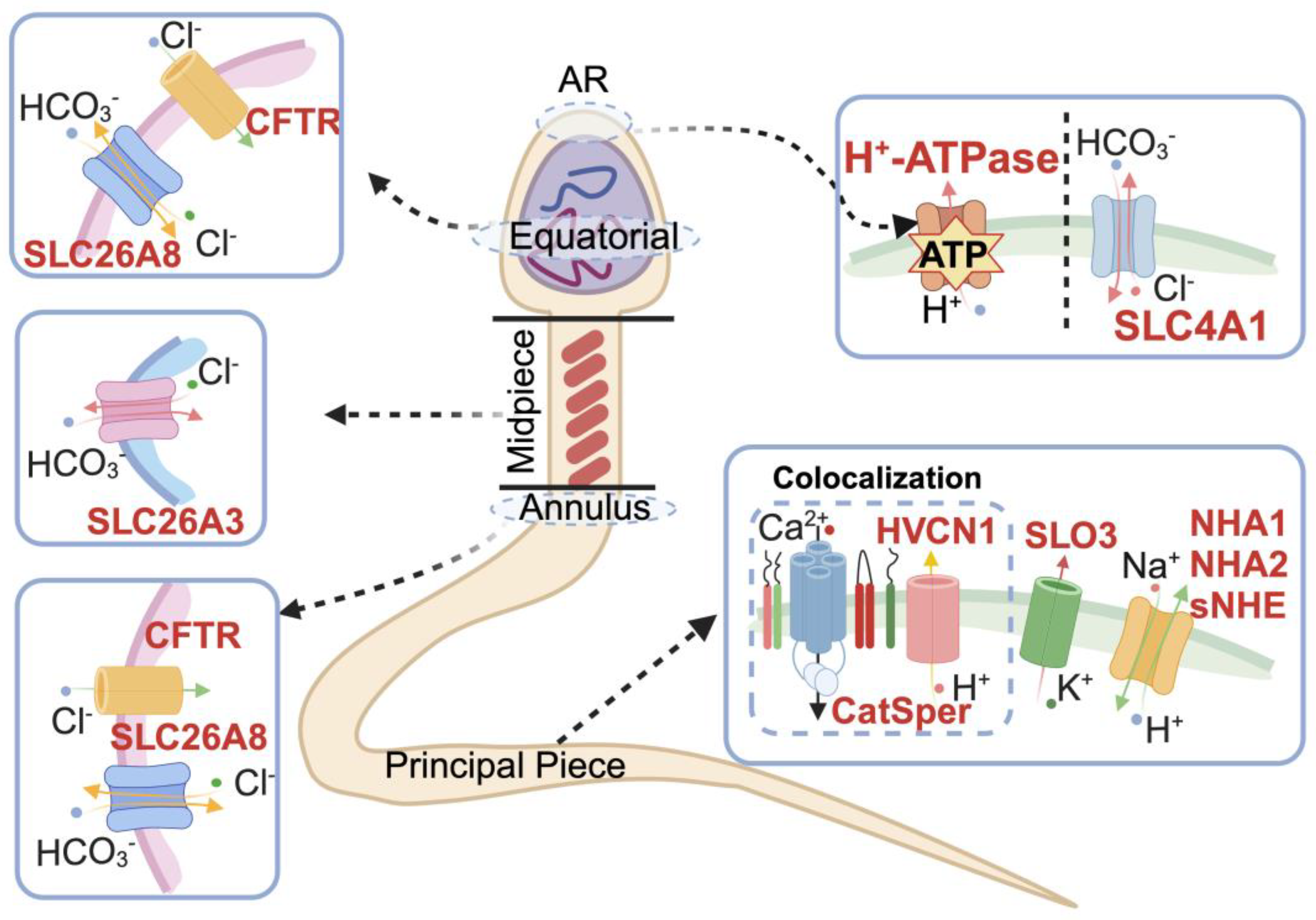
| SLC26A3, 1811 | Midpiece [33] | The chloride/bicarbonate transporter, participates in energy acquisition, induces sperm tyrosine phosphorylation and is hyperactivated. | Epididymal morphology altered, and mature sperm decreased [34] | Male infertility [35] |
| SLC26A8 (TAT1), 116369 | Annulus of spermatozoa [36,37] | Co-localization with CFTR in the equatorial segment of the sperm head, involves sperm capacitation and hyperactivation. | Sperm flagellar differentiation is defective, and sperm capacitation fails [36] | Asthenozoospermia [37] |
| SLC9A3 (NHE3), 6550 | Efferent duct [38,39], developing acrosomal granule [40], epididymis [41] | Reabsorb Na+ in the efferent duct, and regulate lumen fluid pH. | The lumen of the rete testis and efferent duct dilated, causing obstructive azoospermia [39] | NR |
| SLC9B1 (NHA1), 150159 SLC9B2 (NHA2), 133308 | Principal piece [42] | Na+/H+ exchanger, regulates the intracellular pH of spermatozoa, and maintains sperm motility and fertility. | Sperm count decreased, causing low fertility (NHA1 or NHA2 cKO mice) and male infertility (double KO mice) [42] | Teratospermia, caused by NHA1 deficiency [43] |
| SLC9C1 (NHE10/sNHE), 285335 | Principal piece [44] | Sperm-specific sodium/hydrogen exchanger, regulates the intracellular pH of spermatozoa, and maintains sperm hyperactivation. | Male infertility and asthenozoospermia [45]. | Asthenospermia [46] |
| CFTR, 1080 | The equatorial region of the sperm head and middle piece [47] | The epithelial ion channel, maintains chloride and bicarbonate homeostasis during sperm epididymal maturation and capacitation. | Homozygotes for targeted null mutations cause death around puberty. Cftr+/− male mice exhibited abnormal capacitation [48] | Cystic fibrosis: progressive lung disorder, pancreatic insufficiency, male infertility [49,50] |
| B1 subunit of the H+-ATPase (ATP6V1B1), 110935 | The initial segment of the epididymis, narrow cells in the caput epididymis and bright cells in the cauda epididymis [51,52,53], acrosome [54,55] | Participate in the secretion of the H+ in epididymal lumen and maintain the acidic pH of epididymal lumen fluid, and acrosome alkalization. | FOXI1 regulates the synthesis of ATP6V1B1. Foxi1−/− mice sperm maturation was blocked, and fertilization failed [56] | NR |
| Protein Symbol, Gene ID | Location in Other Tissues | Functions | Location in the Male Reproductive System |
|---|---|---|---|
| SLC4A1 (AE1), 6521 | Erythroid, kidney [188] | Electroneutral anion transporter and structural protein [188] | Leydig cells [63] |
| SLC4A3 (AE3), 6508 | brain, heart, and adrenal gland [189,190] | Chloride ion exchangers, regulate intracellular pH and cardiac action potential [189,190] | Spermatogenic cells and seminal vesicle cells [63] |
| SLC4A5 (NBCe2), 57835 | Liver, spleen, kidney, etc. [191] | Sodium-bicarbonate cotransporter [191] | Testis, epididymis, prostate, and seminal vesicles [192] |
| SLC9A2 (NHE2, ID:6549) | Kidney, stomach [160] | Plasma membrane Na+-H+ exchanger, maintains intracellular pH [160] | Epididymis, vas deferens, testis [159,160] |
| SLC9C2 (NHE11), 284525 | Heart [193] | Sodium/hydrogen exchanger, correlates with human cardiac dysfunction, and regulates intracellular pH [193]. | Spermatozoa head [163] |
| CA II, 760 | Stomach, brain, duodenum, etc. [194,195,196,197] | Catalyzes reversible hydration, creates and maintains the pH differential in tumor cells, and regulates the pH of duodenal villous epithelial cells [194,195,196,197] | Epididymis (narrow cells of the initial segment and principal cells of all regions) [81] |
| CA IX, 768 | Brain, duodenum, stomach, eye [195,198,199,200] | Catalyzes reversible hydration, participates in necrosis, calcification, acid-base balance, and the formation of aqueous humor, and gastric acid, and maintains pH homeostasis [198,200,201,202] | Epididymis (efferent duct) [82] |
| CA XII, 771 | Expresses widely in normal tissues [203,204] | Reversible hydration of carbon dioxide [203], and maintains intracellular pH [204] | Epididymis [81,82] |
| SLC16A2 (MCT8), 6567 | Brain, thyroid, kidney [205] | Specific thyroid hormone transmembrane transporter, mediating efflux of thyroid hormone across cell membranes [205] | Mouse testis, epididymis, epididymal spermatozoa [65] |
| SLC16A5 (MCT5), ID: 9121 | Liver, kidney, brain, prostate, etc. [206] | Proton-linked monocarboxylate transporter [206] | Mouse testis, epididymis, epididymal spermatozoa [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, P.; Zou, M.; Cai, Z.; Zeng, X.; Zhang, X.; Liang, M. pH Homeodynamics and Male Fertility: A Coordinated Regulation of Acid-Based Balance during Sperm Journey to Fertilization. Biomolecules 2024, 14, 685. https://doi.org/10.3390/biom14060685
Dai P, Zou M, Cai Z, Zeng X, Zhang X, Liang M. pH Homeodynamics and Male Fertility: A Coordinated Regulation of Acid-Based Balance during Sperm Journey to Fertilization. Biomolecules. 2024; 14(6):685. https://doi.org/10.3390/biom14060685
Chicago/Turabian StyleDai, Pengyuan, Meng Zou, Ziyi Cai, Xuhui Zeng, Xiaoning Zhang, and Min Liang. 2024. "pH Homeodynamics and Male Fertility: A Coordinated Regulation of Acid-Based Balance during Sperm Journey to Fertilization" Biomolecules 14, no. 6: 685. https://doi.org/10.3390/biom14060685





