Aromatic Characterisation of Moscato Giallo by GC-MS/MS and Validation of Stable Isotopic Ratio Analysis of the Major Volatile Compounds
Abstract
1. Introduction
2. Material and Methods
2.1. Samples Collection
2.2. Winemaking
2.3. Base Compositional Parameters of Must and Wine
2.4. Sample Preparation and Extraction
2.5. GC-MS/MS Analysis
2.6. GC-C/Py-IRMS Analysis
3. Results
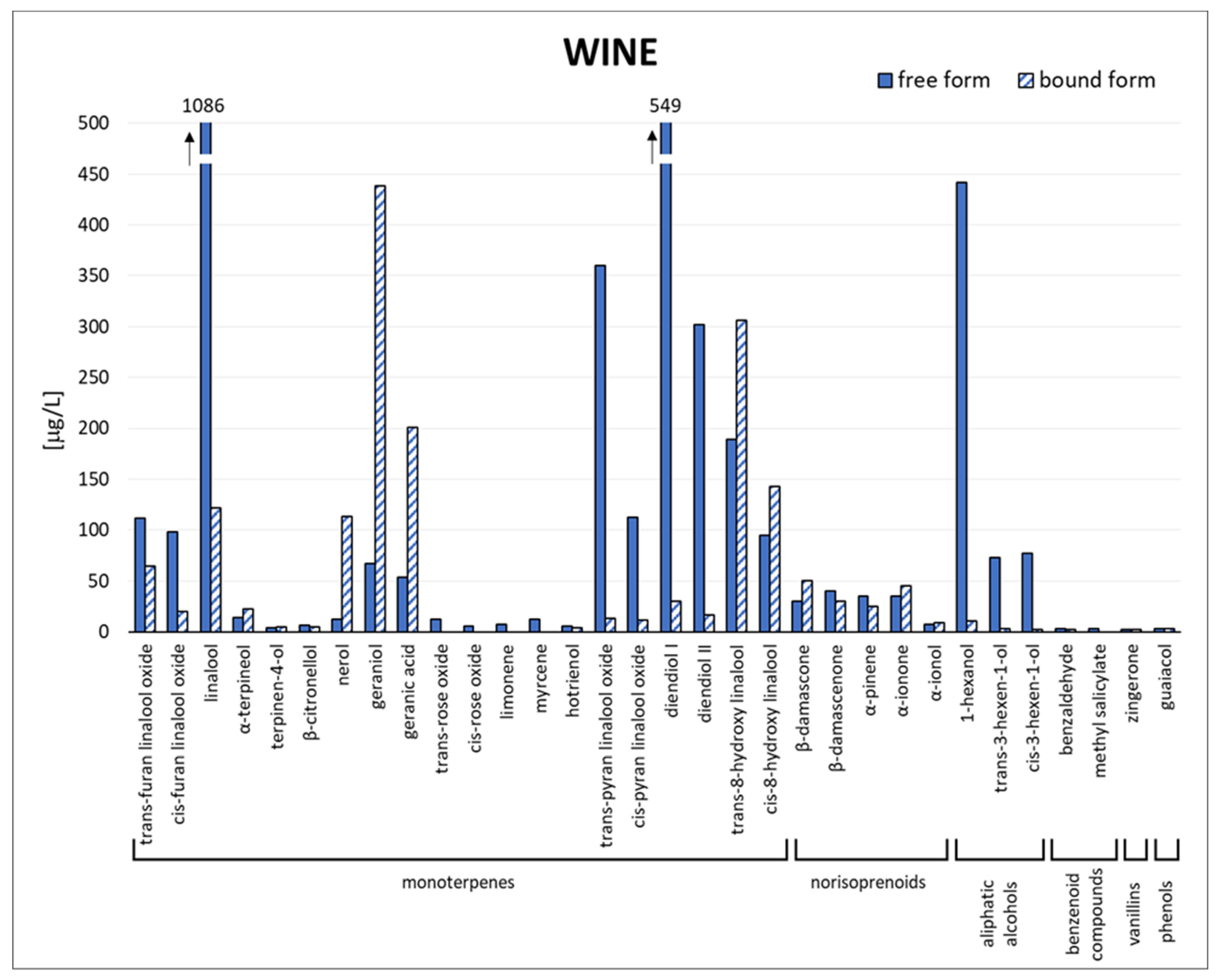
3.1. Grape and Wine Composition
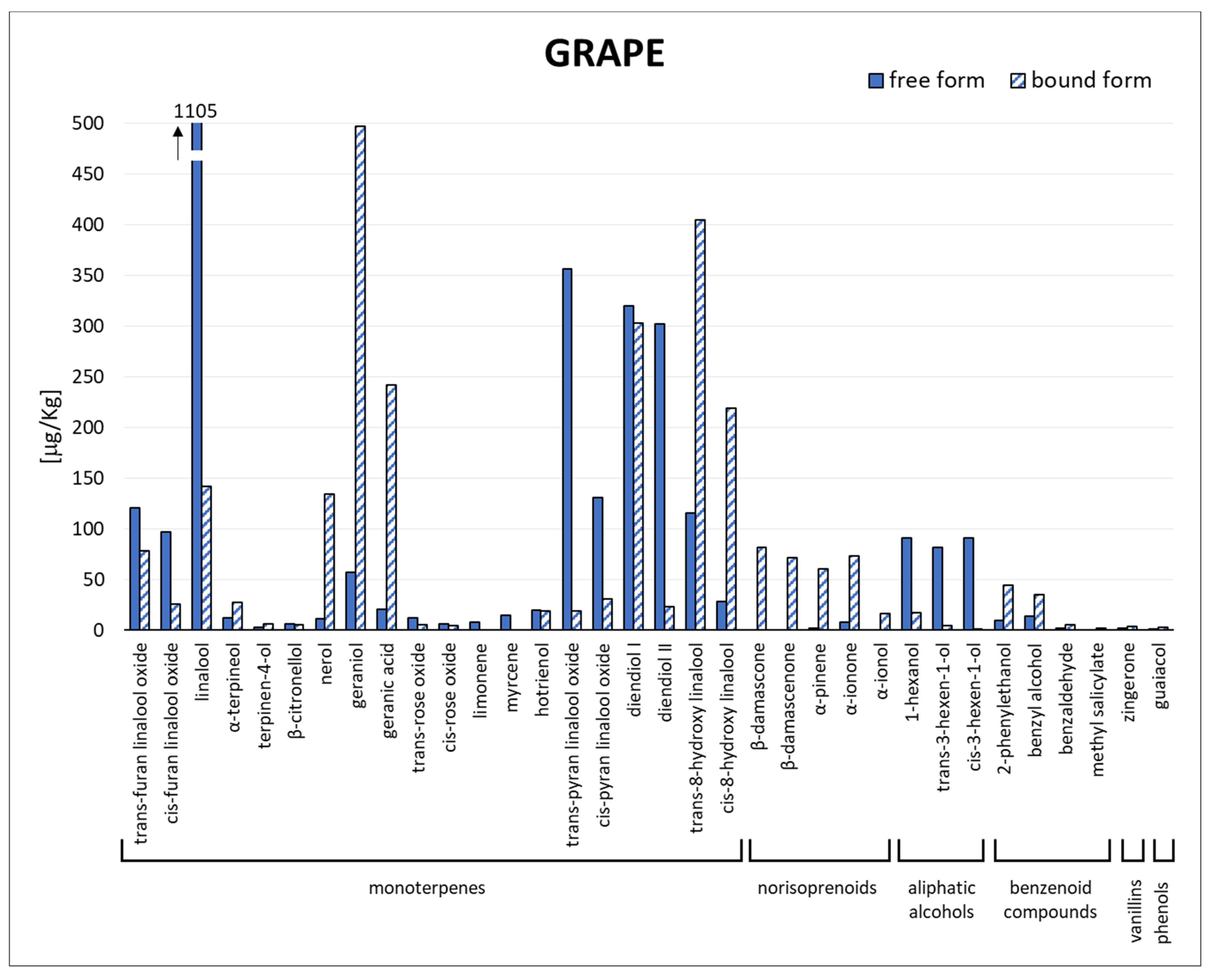
3.2. Quali-Quantitative Analysis by GC-MS/MS
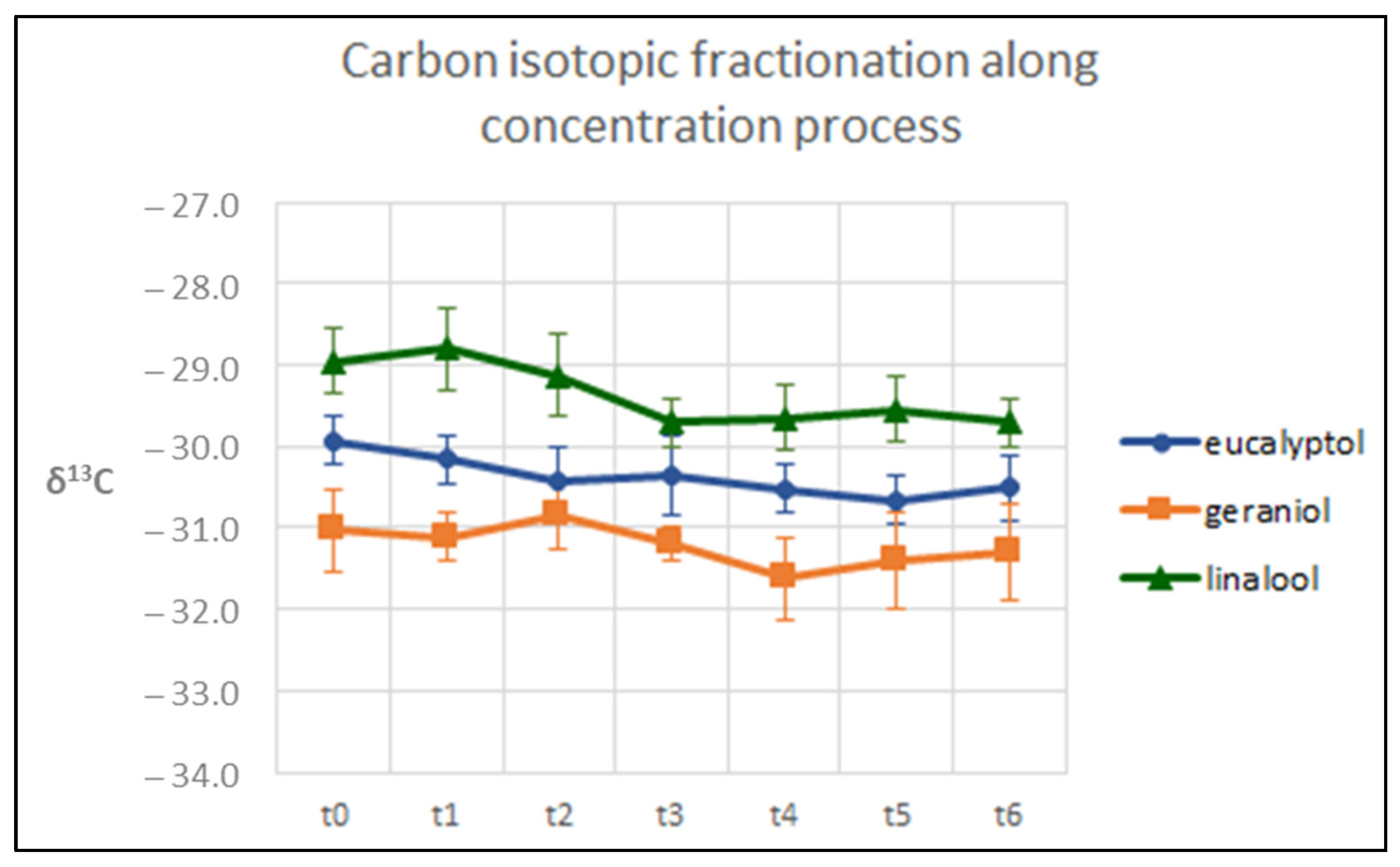
3.3. Evaluation of the Isotopic Fractionation along the Concentration Process
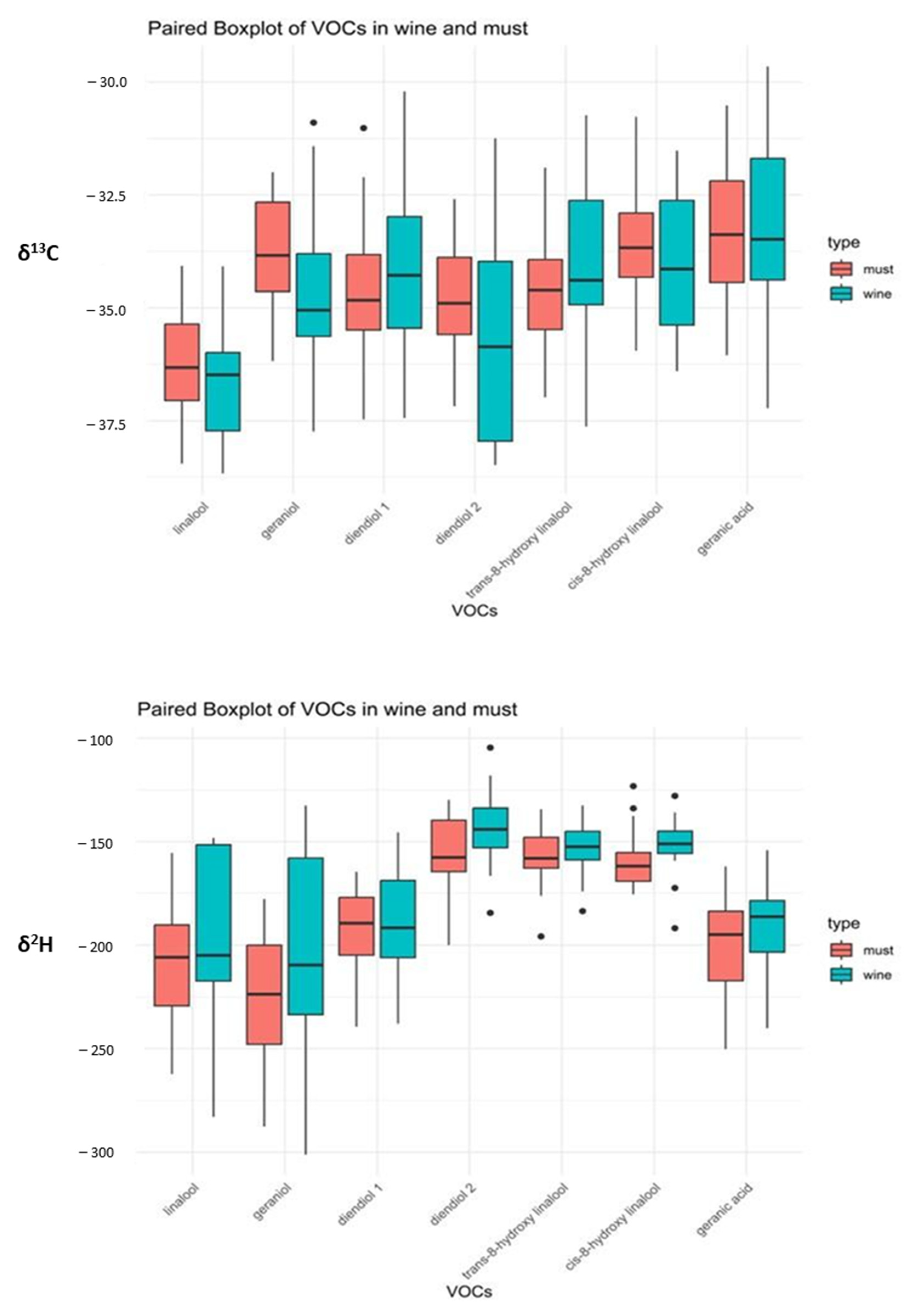
3.4. Isotopic Values of Grape Must and Wine from Moscato Giallo
3.5. Isotopic and VOCs Combination for Differentiation of Grape Must and Wine from Moscato Giallo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grand View Research. Moscato Wine Market Size, Share & Trends Analysis Report by Type (Moscato Bianco, Moscato Rosa, Moscato Giallo, Others), by Sales Channel, by Region, and Segment Forecasts, 2023–2030; Grand View Research: San Francisco, CA, USA, 2021. [Google Scholar]
- Berger, R.G. (Ed.) Flavours and Fragrances; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Villamor, R.R.; Ross, C.F. Wine matrix compounds affect perception of wine aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.H. 3—Biochemical Basis of Carbon Isotope Fractionation. In Stable Isotopes and Plant Carbon-Water Relations; Ehleringer, J.R., Hall, A.E., Farquhar, G.D., Eds.; Academic Press: San Diego, CA, USA, 1993; pp. 19–28. [Google Scholar]
- Bontempo, L.; Camin, F.; Paolini, M.; Micheloni, C.; Laursen, K.H. Multi-isotopic signatures of organic and conventional Italian pasta along the production chain. J. Mass Spectrom. 2016, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.K.; Casiello, G.; Kontakos, S.; Louppis, A.P.; Longobardi, F.; Kontominas, M.G. Investigating the impact of botanical origin and harvesting period on carbon stable isotope ratio values (13C/12C) and different parameter analysis of Greek unifloral honeys: A chemometric approach for correct botanical discrimination. Int. J. Food Sci. Technol. 2016, 51, 2460–2467. [Google Scholar] [CrossRef]
- Bontempo, L.; Perini, M.; Pianezze, S.; Horacek, M.; Roßmann, A.; Kelly, S.D.; Thomas, F.; Heinrich, K.; Schlicht, C.; Schellenberg, A.; et al. Characterization of Beef Coming from Different European Countries through Stable Isotope (H, C, N, and S) Ratio Analysis. Molecules 2023, 28, 2856. [Google Scholar] [CrossRef] [PubMed]
- Laursen, K.H.; Bontempo, L.; Camin, F.; Roßmann, A. 9—Advances in Isotopic Analysis for Food Authenticity Testing. In Advances in Food Authenticity Testing; Downey, G., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 227–252. [Google Scholar]
- Bowen, G.J.; West, J.B. Isotope Landscapes for Terrestrial Migration Research. Terr. Ecol. 2008, 2, 79–105. [Google Scholar]
- Roßmann, A.; Reniero, F.; Moussa, I.; Schmidt, H.-L.; Versini, G.; Merle, M.H. Stable oxygen isotope content of water of EU data-bank wines from Italy, France and Germany. Z. Leb. Forsch. A 1999, 208, 400–407. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV). Compendium of International Methods of Wine and Must Analysis Edition 2022; OIV: Dijon, France, 2021; Volume 1, Available online: https://www.oiv.int/sites/default/files/publication/2022-10/Compendium%20Methods%20of%20Analysis%20of%20Wine%20and%20Musts%20Vol1%20and%20Vol2.pdf (accessed on 10 February 2024).
- Martin, G.J.; Guillou, C.; Martin, M.L.; Cabanis, M.T.; Tep, Y.; Aerny, J. Natural factors of isotope fractionation and the characterization of wines. J. Agric. Food Chem. 1988, 36, 316–322. [Google Scholar] [CrossRef]
- Dordevic, N.; Camin, F.; Marianella, R.M.; Postma, G.J.; Buydens, L.M.C.; Wehrens, R. Detecting the addition of sugar and water to wine. Aust. J. Grape Wine Res. 2013, 19, 324–330. [Google Scholar] [CrossRef]
- European Commision. Regolamento di Esecuzione (Ue) 2018/274 della Commissione Dell’11 Dicembre 2017; European Commision: Maastricht, The Netherlands, 2018; Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=CELEX:32018R0274&from=HR (accessed on 10 February 2024).
- Dordevic, N. Chemometrics and Stable Isotope Ratios of Wine. 2015. Available online: https://repository.ubn.ru.nl/bitstream/handle/2066/147420/147420.pdf (accessed on 16 February 2024).
- Manitto, P.; Sammes, P.G. Biosynthesis of Natural Products; Ellis Horwood: Chichester, UK; Wiley: New York, NY, USA, 1981. [Google Scholar]
- Selli, S.; Cabaroglu, T.; Canbas, A.; Erten, H.; Nurgel, C. Effect of skin contact on the aroma composition of the musts of Vitis vinifera L. cv. Muscat of Bornova and Narince grown in Turkey. Food Chem. 2003, 81, 341–347. [Google Scholar]
- Voirin, S.G.; Baumes, R.L.; Bitteur, S.M.; Gunata, Z.Y.; Bayonove, C.L. Novel monoterpene disaccharide glycosides of Vitis vinifera grapes. J. Agric. Food Chem. 1990, 38, 1373–1378. [Google Scholar] [CrossRef]
- Diéguez, S.C.; Lois, L.C.; Gómez, E.F.; de la Peña, M.L.G. Aromatic composition of the Vitis vinifera grape Albariño. LWT—Food Sci. Technol. 2003, 36, 585–590. [Google Scholar] [CrossRef]
- Fenoll, J.; Manso, A.; Hellín, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Bayonove, C.L.; Baumes, R.L.; Cordonnier, R.E. Stability of Free and Bound Fractions of Some Aroma Components of Grapes cv. Muscat During the Wine Processing: Preliminary Results. Am. J. Enol. Vitic. 1986, 37, 112–114. [Google Scholar] [CrossRef]
- Spangenberg, J.E.; Zufferey, V. Carbon isotope compositions of whole wine, wine solid residue, and wine ethanol, determined by EA/IRMS and GC/C/IRMS, can record the vine water status—A comparative reappraisal. Anal. Bioanal. Chem. 2019, 411, 2031–2043. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, L.; Wu, S.; Huang, M.; Yu, W.; Zhang, S. Developing an authentication approach using SPME-GC-IRMS based on compound-specific δ 13C analysis of six typical volatiles in wine. Food Qual. Saf. 2021, 5, fyaa031. [Google Scholar] [CrossRef]
- Paolini, M.; Tonidandel, L.; Moser, S.; Larcher, R. Development of a fast gas chromatography-tandem mass spectrometry method for volatile aromatic compound analysis in oenological products. J. Mass Spectrom. 2018, 53, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Khatri, P.K.; Paolini, M.; Larcher, R.; Ziller, L.; Magdas, D.A.; Marincas, O.; Roncone, A.; Bontempo, L. Validation of gas chromatographic methods for lavender essential oil authentication based on volatile organic compounds and stable isotope ratios. Microchem. J. 2023, 186, 108343. [Google Scholar] [CrossRef]
- Verzera, A.; Merlino, M.; Cincotta, F.; Prestia, O.; Sparacio, A.; Sparla, S. Varietal Aromas of Fortified Wines from Different Moscato Var. (Vitis vinifera L.) under the Same Pedoclimatic Conditions. Foods 2021, 10, 2549. [Google Scholar] [CrossRef]
- Potisek, M.; Krebelj, A.; Šuklje, K.; Škvarč, A.; Čuš, F. Viticultural and oenological characterization of Muscat a Petits Grains Blancs and Muscat giallo clones. J. Cent. Eur. Agric. 2023, 24, 422–433. [Google Scholar] [CrossRef]
- Mipaaf. Disciplinare di Produzione dei vini a Denominazione di Origine Controllata «Trentino»; Mipaaf: Roma, Italy, 1971; Available online: https://vinideltrentino.com/wp-content/uploads/2020/09/DOC_Trentino.pdf (accessed on 15 April 2024).
- Mipaaf. Disciplinare di Produzione dei Vini a Denominazione di Origine Controllata dei Vini “Colli Euganei”; Mipaaf: Roma, Italy, 1969; Available online: https://www.collieuganeidoc.com/wp-content/uploads/2014/10/DOC-Colli-Euganei.pdf (accessed on 15 April 2024).
- Versini, G.; Dalla Serra, A.; Monetti, A.; De Micheli, L.; Mattivi, F. Free and bound grape aroma profiles variability within the family of Muscat-called varieties. In Proceedings of the Symposium International Connaissance Aromatiques des Cépages et Qualité des Vins, Montpellier, France, 9–10 February 1993; Available online: http://hdl.handle.net/10449/16575 (accessed on 27 March 2024).
- Flamini, R. Some advances in the knowledge of grape, wine and distillates chemistry as achieved by mass spectrometry. J. Mass Spectrom. 2005, 40, 705–713. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, C.; Matarese, F.; Cuzzola, A. Study of the terpene profile at harvest and during berry development of Vitis vinifera L. aromatic varieties Aleatico, Brachetto, Malvasia di Candia aromatica and Moscato bianco. J. Sci. Food Agric. 2017, 97, 2898–2907. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Morrison, J.C.; Adams, D.O.; Noble, A.C. Distribution of free and glycosidically bound monoterpenes in the skin and mesocarp of Muscat of Alexandria grapes during development. J. Agric. Food Chem. 1991, 39, 514–518. [Google Scholar] [CrossRef]
- Ugliano, M.; Moio, L. Free and hydrolytically released volatile compounds of Vitis vinifera L. cv. Fiano grapes as odour-active constituents of Fiano wine. Anal. Chim. Acta 2008, 621, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Y.; Liang, Z.; Fan, P.; Wu, B.; Yang, L.; Wang, Y.; Li, S. Volatiles of grape berries evaluated at the germplasm level by headspace-SPME with, GC–MS. Food Chem. 2009, 114, 1106–1114. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical changes throughout Grape Berry development and fruit and wine quality. Food 2007, 1, 1–22. Available online: https://www.researchgate.net/publication/228555202_Biochemical_changes_throughout_Grape_Berry_development_and_fruit_and_wine_quality (accessed on 15 April 2024).
- Molina, A.M.; Guadalupe, V.; Varela, C.; Swiegers, J.H.; Pretorius, I.S.; Agosin, E. Differential synthesis of fermentative aroma compounds of two related commercial wine yeast strains. Food Chem. 2009, 117, 189–195. [Google Scholar] [CrossRef]
- Herraiz, T.; Martin-Alvarez, P.J.; Reglero, G.; Herraiz, M.; Cabezudo, M.D. Effects of the presence of skins during alcoholic fermentation on the composition of wine volatiles. Vitis 1990, 29, 239–249. [Google Scholar]
- Nicolini, G.; Moser, S.; Borin, G.; Tonidandel, L.; Roman, T.; Larcher, R. Gli aromi del Moscato giallo nelle sue interpretazioni nel Trentino e nei colli Euganei. L’Enologo 2013, 49, 65–72. Available online: https://www.researchgate.net/publication/265846875_Gli_aromi_del_Moscato_giallo_nelle_sue_interpretazioni_in_Trentino_e_nei_Colli_Euganei (accessed on 20 April 2024).
- Williams, P.J.; Strauss, C.R.; Wilson, B. Hydroxylated linalool derivatives as precursors of volatile monoterpenes of muscat grapes. J. Agric. Food Chem. 1980, 28, 766–771. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef]
- Slaghenaufi, D.; Boscaini, A.; Prandi, A.; Dal Cin, A.; Zandonà, V.; Luzzini, G.; Ugliano, M. Influence of Different Modalities of Grape Withering on Volatile Compounds of Young and Aged Corvina Wines. Molecules 2020, 25, 2141. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Shuai, M.; Song, M.; Qiao, D.; Peng, W.; Chen, L. Characterization of Evodia rutaecarpa (Juss) Benth honey: Volatile profile, odor-active compounds and odor properties. J. Sci. Food Agric. 2024, 104, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Coelho, E.; Vilanova, M.; Genisheva, Z.; Oliveira, J.M.; Teixeira, J.A.; Domingues, L. Systematic approach for the development of fruit wines from industrially processed fruit concentrates, including optimization of fermentation parameters, chemical characterization and sensory evaluation. LWT—Food Sci. Technol. 2015, 62, 1043–1052. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Aroma potential of Brancellao grapes from different cluster positions. Food Chem. 2012, 132, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Chigo-Hernandez, M.M.; Tomasino, E. Aroma Perception of Limonene, Linalool and α-Terpineol Combinations in Pinot Gris Wine. Foods 2023, 12, 2389. [Google Scholar] [CrossRef] [PubMed]
- Deterre, S.; Rega, B.; Delarue, J.; Decloux, M.; Lebrun, M.; Giampaoli, P. Identification of key aroma compounds from bitter orange (Citrus aurantium L.) products: Essential oil and macerate–distillate extract. Flavour Fragr. J. 2012, 27, 77–88. [Google Scholar] [CrossRef]
- Slegers, A.; Angers, P.; Pedneault, K. Volatile compounds from must and wines from five white grape varieties. J. Food Chem. Nanotechnol. 2017, 3, 8–18. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the aroma of grapes and wines: A review. S. Afr. J. Enol. Vitic. 2017, 4, 49–58. [Google Scholar] [CrossRef]
- Shi, J.; Tong, G.; Yang, Q.; Huang, M.; Ye, H.; Liu, Y.; Wu, J.; Zhang, J.; Sun, X.; Zhao, D. Characterization of Key Aroma Compounds in Tartary Buckwheat (Fagopyrum tataricum Gaertn.) by Means of Sensory-Directed Flavor Analysis. J. Agric. Food Chem. 2021, 69, 11361–11371. [Google Scholar] [CrossRef]
- Schreiner, L.; Bauer, P.; Buettner, A. Resolving the smell of wood—Identification of odour-active compounds in Scots pine (Pinus sylvestris L.). Sci. Rep. 2018, 8, 8294. [Google Scholar] [CrossRef] [PubMed]
- Slaghenaufi, D.; Luzzini, G.; Samaniego Solis, J.; Forte, F.; Ugliano, M. Two Sides to One Story-Aroma Chemical and Sensory Signature of Lugana and Verdicchio Wines. Molecules 2021, 26, 2127. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; de-la-Fuente-Blanco, A.; Sáenz-Navajas, M.-P. A New Classification of Perceptual Interactions between Odorants to Interpret Complex Aroma Systems. Application to Model Wine Aroma. Foods 2021, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, J.; Chatterjee, S.; Gamre, S.; Chattopadhyay, S.; Variyar, P.S.; Sharma, A. Analysis of free and bound aroma compounds of pomegranate (Punica granatum L.). LWT—Food Sci. Technol. 2014, 59, 461–466. [Google Scholar] [CrossRef]
- Budić-Leto, I.; Humar, I.; Gajdoš Kljusurić, J.; Zdunić, G.; Zlatić, E. Free and bound volatile aroma compounds of ‘Maraština’ grapes as influenced by dehydration techniques. Appl. Sci. 2020, 10, 8928. [Google Scholar] [CrossRef]
- (PDF) Aromatic Complexity in Verdicchio Wines: A Case Study. Available online: https://www.researchgate.net/publication/336468331_Aromatic_complexity_in_Verdicchio_wines_a_case_study (accessed on 29 March 2024).
- Mateo, J.J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Linskens, H.F.; Jackson, J.F.; Conte, L.S. Wine Analysis; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Khatri, P.K.; Paolini, M.; Larcher, R.; Ziller, L.; Magdas, D.A.; Marincas, O.; Roncone, A.; Bontempo, L. Botanical characterization and authentication of lavender essential oil using its volatile organic compounds and compound-specific carbon and hydrogen isotope ratio analysis. Food Control 2023, 154, 110002. [Google Scholar] [CrossRef]
- Cuchet, A.; Anchisi, A.; Schiets, F.; Clément, Y.; Lantéri, P.; Bonnefoy, C.; Jame, P.; Carénini, E.; Casabianca, H. Determination of enantiomeric and stable isotope ratio fingerprints of active secondary metabolites in neroli (Citrus aurantium L.) essential oils for authentication by multidimensional gas chromatography and GC-C/P-IRMS. J. Chromatogr. B 2021, 1185, 123003. [Google Scholar] [CrossRef]
- Camin, F.; Dordevic, N.; Wehrens, R.; Neteler, M.; Delucchi, L.; Postma, G.; Buydens, L. Climatic and geographical dependence of the H, C and O stable isotope ratios of Italian wine. Anal. Chim. Acta 2015, 853, 384–390. [Google Scholar] [CrossRef]
- Luan, F.; Wüst, M. Differential incorporation of 1-deoxy-D-xylulose into (3S)-linalool and geraniol in grape berry exocarp and mesocarp. Phytochemistry 2002, 60, 451–459. [Google Scholar] [CrossRef]
- Hayes, J.M. Fractionation of Carbon and Hydrogen Isotopes in Biosynthetic Processes. In Stable Isotope Geochemistry; Walter de Gruyter: Berlin, Germany, 2001; pp. 225–278. [Google Scholar]
- Strojnik, L.; Stopar, M.; Zlatič, E.; Kokalj, D.; Gril, M.N.; Ženko, B.; Žnidaršič, M.; Bohanec, M.; Boshkovska, B.M.; Luštrek, M.; et al. Authentication of key aroma compounds in apple using stable isotope approach. Food Chem. 2019, 277, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Hanneguelle, S.; Thibault, J.N.; Naulet, N.; Martin, G.J. Authentication of essential oils containing linalool and linalyl acetate by isotopic methods. J. Agric. Food Chem. 1992, 40, 81–87. [Google Scholar] [CrossRef]
- Beale, D.J.; Morrison, P.D.; Karpe, A.V.; Dunn, M.S. Chemometric Analysis of Lavender Essential Oils Using Targeted and Untargeted GC-MS Acquired Data for the Rapid Identification and Characterization of Oil Quality. Molecules 2017, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
- Lafhal, S.; Vanloot, P.; Bombarda, I.; Kister, J.; Dupuy, N. Chemometric analysis of French lavender and lavandin essential oils by near infrared spectroscopy. Ind. Crops Prod. 2016, 80, 156–164. [Google Scholar] [CrossRef]
- Nhu-Trang, T.-T.; Casabianca, H.; Grenier-Loustalot, M.-F. Authenticity control of essential oils containing citronellal and citral by chiral and stable-isotope gas-chromatographic analysis. Anal. Bioanal. Chem. 2006, 386, 2141–2152. [Google Scholar] [CrossRef]


| BRIX (°) | Reducing Sugars (g/L) | pH | Titratable Acidity (g/L) | Density (g/mL) | Tartaric Acid (g/L) | Malic Acid (g/L) | K (g/L) | YAN (mg/L) | NH4+ (mg/L) | α-aa (mg/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | 20.03 | 181 | 3.22 | 7.62 | 1.07718 | 4.76 | 5.44 | 1.36 | 155 | 28 | 127 |
| Sample 2 | 22.83 | 172 | 3.12 | 7.14 | 1.07208 | 4.98 | 4.48 | 1.17 | <20 | <20 | <20 |
| Sample 3 | 21.19 | 188 | 3.23 | 4.81 | 1.07795 | 5.01 | 2.65 | 1.32 | 25 | <20 | 23 |
| Sample 4 | 21.16 | 152 | 3.08 | 7.74 | 1.06447 | 4.86 | 5.10 | 1.00 | 83 | 27 | 56 |
| Sample 5 | 20.84 | 182 | 3.18 | 6.76 | 1.07677 | 4.76 | 4.19 | 1.27 | 54 | <20 | 46 |
| Sample 6 | 22.00 | 185 | 3.19 | 6.23 | 1.07743 | 4.78 | 4.20 | 1.29 | 58 | <20 | 48 |
| Sample 7 | 21.76 | 225 | 3.35 | 5.01 | 1.09410 | 4.71 | 2.46 | 1.53 | <20 | <20 | <20 |
| Sample 8 | 22.47 | 189 | 3.28 | 4.68 | 1.07872 | 4.79 | 2.46 | 1.33 | 69 | <20 | 62 |
| Sample 9 | 23.55 | 189 | 3.29 | 5.76 | 1.07925 | 4.40 | 3.52 | 1.37 | 57 | <20 | 52 |
| Sample 10 | 21.64 | 217 | 3.55 | 6.39 | 1.09341 | 5.47 | 4.93 | 2.06 | 152 | <20 | 135 |
| Sample 11 | 22.81 | 201 | 3.40 | 5.67 | 1.08498 | 5.13 | 3.29 | 1.63 | 81 | <20 | 76 |
| Sample 12 | 21.93 | 202 | 3.32 | 6.54 | 1.08573 | 4.79 | 4.13 | 1.56 | 109 | <20 | 92 |
| Sample 13 | 20.09 | 192 | 3.26 | 7.02 | 1.08180 | 5.42 | 4.30 | 1.48 | 120 | 23 | 97 |
| Sample 14 | 21.02 | 169 | 3.26 | 7.30 | 1.07228 | 5.03 | 4.95 | 1.39 | 163 | 42 | 120 |
| Sample 15 | 20.40 | 193 | 3.41 | 6.40 | 1.08209 | 3.65 | 5.45 | 1.58 | 137 | 17 | 120 |
| Sample 16 | 20.42 | 208 | 3.41 | 5.15 | 1.08787 | 5.39 | 2.64 | 1.60 | 69 | <20 | 69 |
| Sample 17 | 20.58 | 182 | 3.30 | 7.28 | 1.07741 | 4.24 | 5.51 | 1.42 | 140 | 23 | 118 |
| Ethanol (% v/v) | Reducing Sugars (g/L) | Non-Reducing Extract (g/L) | pH | Total Acidity (g/L) | Volatile Acidity (g/L) | Density (g/mL) | Tartaric Acid (g/L) | Malic Acid (g/L) | Lactic Acid (g/L) | K (g/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | 11.13 | <1.00 | 24.39 | 3.35 | 7.57 | <0.10 | 0.99599 | 1.63 | 5.30 | <0.50 | 1.30 |
| Sample 2 | 11.60 | <1.00 | 25.07 | 3.08 | 7.96 | <0.10 | 0.99716 | 2.16 | 4.19 | <0.50 | 1.03 |
| Sample 3 | 11.64 | <1.00 | 23.55 | 3.36 | 5.97 | <0.10 | 0.99612 | 1.11 | 3.68 | <0.50 | 1.08 |
| Sample 4 | 11.84 | <1.00 | 25.52 | 3.19 | 8.08 | <0.10 | 0.99722 | 2.04 | 4.87 | <0.50 | 1.16 |
| Sample 5 | 12.07 | <1.00 | 24.81 | 3.25 | 7.35 | <0.10 | 0.99553 | 1.33 | 4.41 | <0.50 | 1.10 |
| Sample 6 | 12.28 | <1.00 | 24.07 | 3.17 | 8.01 | <0.10 | 0.99738 | 1.88 | 4.79 | <0.50 | 1.28 |
| Sample 7 | 11.06 | <1.00 | 23.71 | 3.30 | 7.28 | <0.10 | 0.99582 | 1.66 | 4.40 | <0.50 | 1.28 |
| Sample 8 | 11.08 | <1.00 | 24.28 | 3.21 | 6.40 | <0.10 | 0.99527 | 1.84 | 3.07 | <0.50 | 1.00 |
| Sample 9 | 11.16 | <1.00 | 24.73 | 3.26 | 7.70 | <0.10 | 0.99623 | 1.45 | 4.87 | <0.50 | 1.23 |
| Sample 10 | 11.03 | <1.00 | 24.03 | 3.46 | 7.24 | <0.10 | 0.99528 | 0.94 | 5.09 | <0.50 | 1.39 |
| Sample 11 | 11.97 | <1.00 | 23.44 | 3.28 | 6.85 | <0.10 | 0.99731 | 1.87 | 4.30 | <0.50 | 1.41 |
| Sample 12 | 11.62 | <1.00 | 24.62 | 3.20 | 8.07 | <0.10 | 0.99686 | 1.95 | 4.74 | <0.50 | 1.24 |
| Sample 13 | 11.06 | <1.00 | 22.10 | 3.31 | 7.50 | <0.10 | 0.99580 | 1.35 | 4.91 | <0.50 | 1.13 |
| Sample 14 | 11.88 | <1.00 | 22.81 | 3.21 | 7.05 | <0.10 | 0.99666 | 1.62 | 4.35 | <0.50 | 1.11 |
| Sample 15 | 11.81 | <1.00 | 22.75 | 3.33 | 7.41 | <0.10 | 0.99676 | 1.69 | 4.77 | <0.50 | 1.43 |
| Sample 16 | 11.80 | <1.00 | 23.13 | 3.37 | 6.32 | <0.10 | 0.99629 | 1.12 | 3.46 | <0.50 | 1.18 |
| Sample 17 | 11.84 | <1.00 | 24.94 | 3.19 | 8.56 | <0.10 | 0.99755 | 1.78 | 5.86 | <0.50 | 1.22 |
| Chemical Class | Compound | Odour Threshold [μg/L] | Sensory Descriptors |
|---|---|---|---|
| Monoterpenes | trans-furan linalool oxide | 3000 [42] | Sweet, floral [43] |
| cis-furan linalool oxide | 6000 [42] | Floral, sweet, woody [43] | |
| linalool | 25 [44] | Citrus, floral, sweet [45] | |
| α-terpineol | 250 [42] | Floral, sweet [45] | |
| terpinen-4-ol | 250 [45] | Sweet, herbaceous [45] | |
| β-citronellol | 100 [46] | Lemongrass [46] | |
| nerol | 400 [46] | Lime, floral-hyacinth, roses [46] | |
| geraniol | 30 [44] | Rose, geranium [44] | |
| geranic acid | 40 [47] | Green [47] | |
| trans-rose oxide | 100 [44] | Rose-like, floral, sweet [43] | |
| cis-rose oxide | 100 [44] | Floral, lychee-like, rose [44] | |
| limonene | 200 [48] | Orange, mint, lemon, floral, citrus [49] | |
| myrcene | 14 [50] | Green, floral, grass, citrus [49] | |
| hotrienol | 110 [43] | Floral, green, woody [43] | |
| trans-pyran linalool oxide | 3000–5000 [51] | Sweet, floral, earthy [43] | |
| cis-pyran linalool oxide | 3000–5000 [51] | Sweet, floral, earthy [43] | |
| diendiol I | n.d. | n.d. | |
| diendiol II | n.d. | n.d. | |
| trans-8-hydroxy linalool | n.d. | Floral [43] | |
| cis-8-hydroxy linalool | n.d. | Floral [43] | |
| Norisoprenoids | β-damascone | 0.09 [44] | Fruity-flowery, exotic-spicy [52] |
| β-damascenone | 0.05 [44] | Apple, rose, honey [44] | |
| α-pinene | n.d. | Woody, resinous [53] | |
| α-ionone | 2.6 [54] | Violets, berry [55] | |
| α-ionol | n.d. | Floral and woody [55] | |
| Aliphatic alcohols | 1-hexanol | 8000 [44] | Resin, green (cut grass) [44] |
| trans-3-hexen-1-ol | 1000 [54] | Grassy green, earthy [56] | |
| cis-3-hexen-1-ol | 400 [44] | Lettuce-like, green, grass [44] | |
| Benzenoid | benzaldehyde | 2000 [57] | Bitter almond [57] |
| methyl salicylate | 50 [58] | Mint-like [43] | |
| Vanillins | zingerone | n.d. | Toasty, dry fruit |
| Phenols | guaiacol | 10 [44] | Smoke, sweet, medicine [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolini, M.; Roncone, A.; Cucinotta, L.; Sciarrone, D.; Mondello, L.; Camin, F.; Moser, S.; Larcher, R.; Bontempo, L. Aromatic Characterisation of Moscato Giallo by GC-MS/MS and Validation of Stable Isotopic Ratio Analysis of the Major Volatile Compounds. Biomolecules 2024, 14, 710. https://doi.org/10.3390/biom14060710
Paolini M, Roncone A, Cucinotta L, Sciarrone D, Mondello L, Camin F, Moser S, Larcher R, Bontempo L. Aromatic Characterisation of Moscato Giallo by GC-MS/MS and Validation of Stable Isotopic Ratio Analysis of the Major Volatile Compounds. Biomolecules. 2024; 14(6):710. https://doi.org/10.3390/biom14060710
Chicago/Turabian StylePaolini, Mauro, Alberto Roncone, Lorenzo Cucinotta, Danilo Sciarrone, Luigi Mondello, Federica Camin, Sergio Moser, Roberto Larcher, and Luana Bontempo. 2024. "Aromatic Characterisation of Moscato Giallo by GC-MS/MS and Validation of Stable Isotopic Ratio Analysis of the Major Volatile Compounds" Biomolecules 14, no. 6: 710. https://doi.org/10.3390/biom14060710
APA StylePaolini, M., Roncone, A., Cucinotta, L., Sciarrone, D., Mondello, L., Camin, F., Moser, S., Larcher, R., & Bontempo, L. (2024). Aromatic Characterisation of Moscato Giallo by GC-MS/MS and Validation of Stable Isotopic Ratio Analysis of the Major Volatile Compounds. Biomolecules, 14(6), 710. https://doi.org/10.3390/biom14060710









