Functional Validation of the Cytochrome P450 Family PgCYP309 Gene in Panax ginseng
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Data and Materials
2.2. Identification and Analysis of Candidate Genes
2.3. Bioinformatics Analysis of Candidate Genes
2.4. Determination of Adventitious Roots and Saponin Content and Gene Expression in Ginseng under MeJA Treatment
2.5. Cloning of PgCYP309 Gene
2.6. Construction of PgCYP309 Gene Overexpression Vector and Interference Vector
2.7. Genetic Transformation of Ginseng Adventitious Roots
2.8. Positive Material Detection and its Gene Expression Change and Saponin Content Change Analysis
3. Results
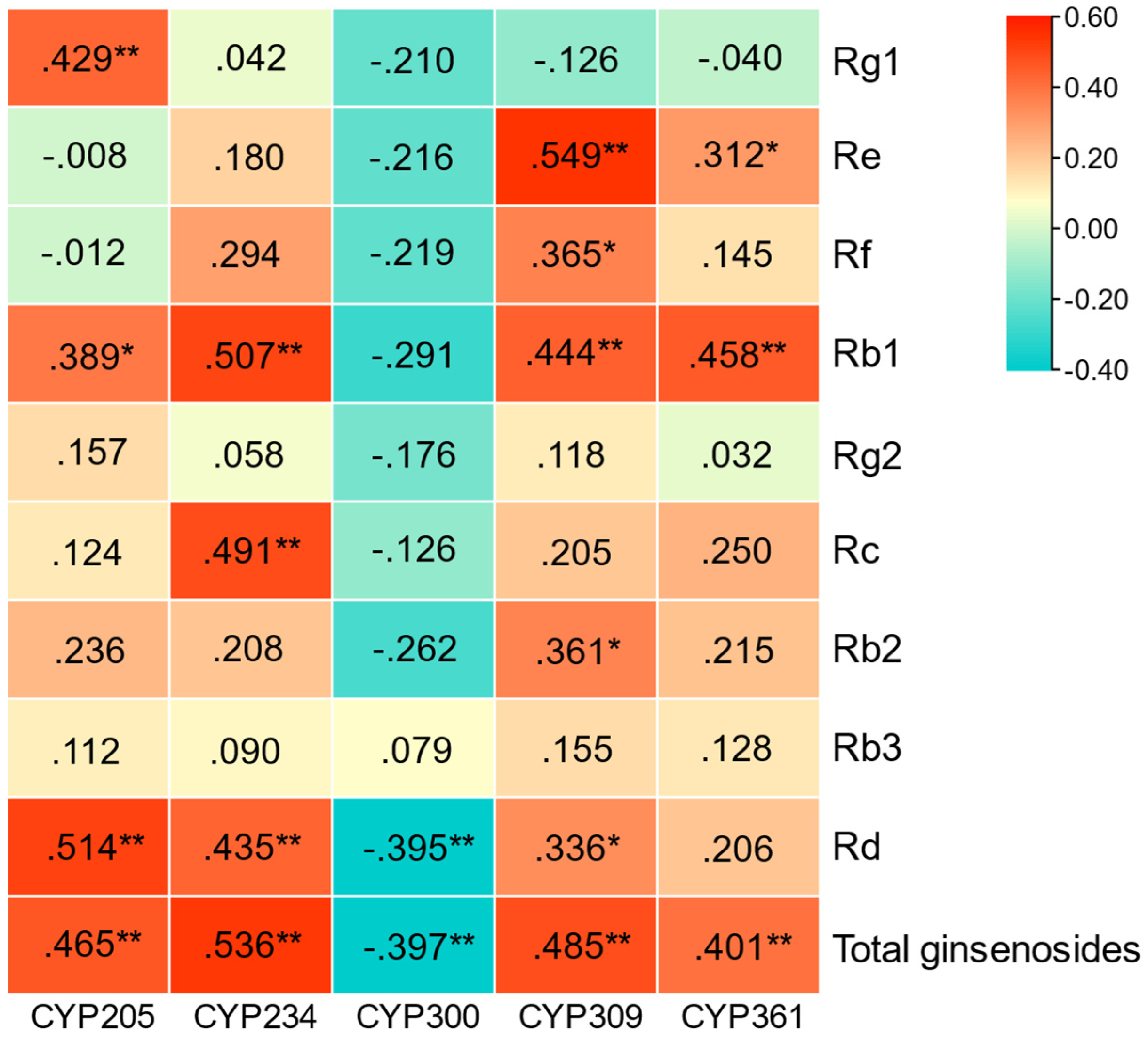
3.1. Correlation Analysis between PgCYP450 Gene and Ginseng Saponin Content
3.2. Interaction Analysis of PgCYP450 Gene with Key Enzyme Genes
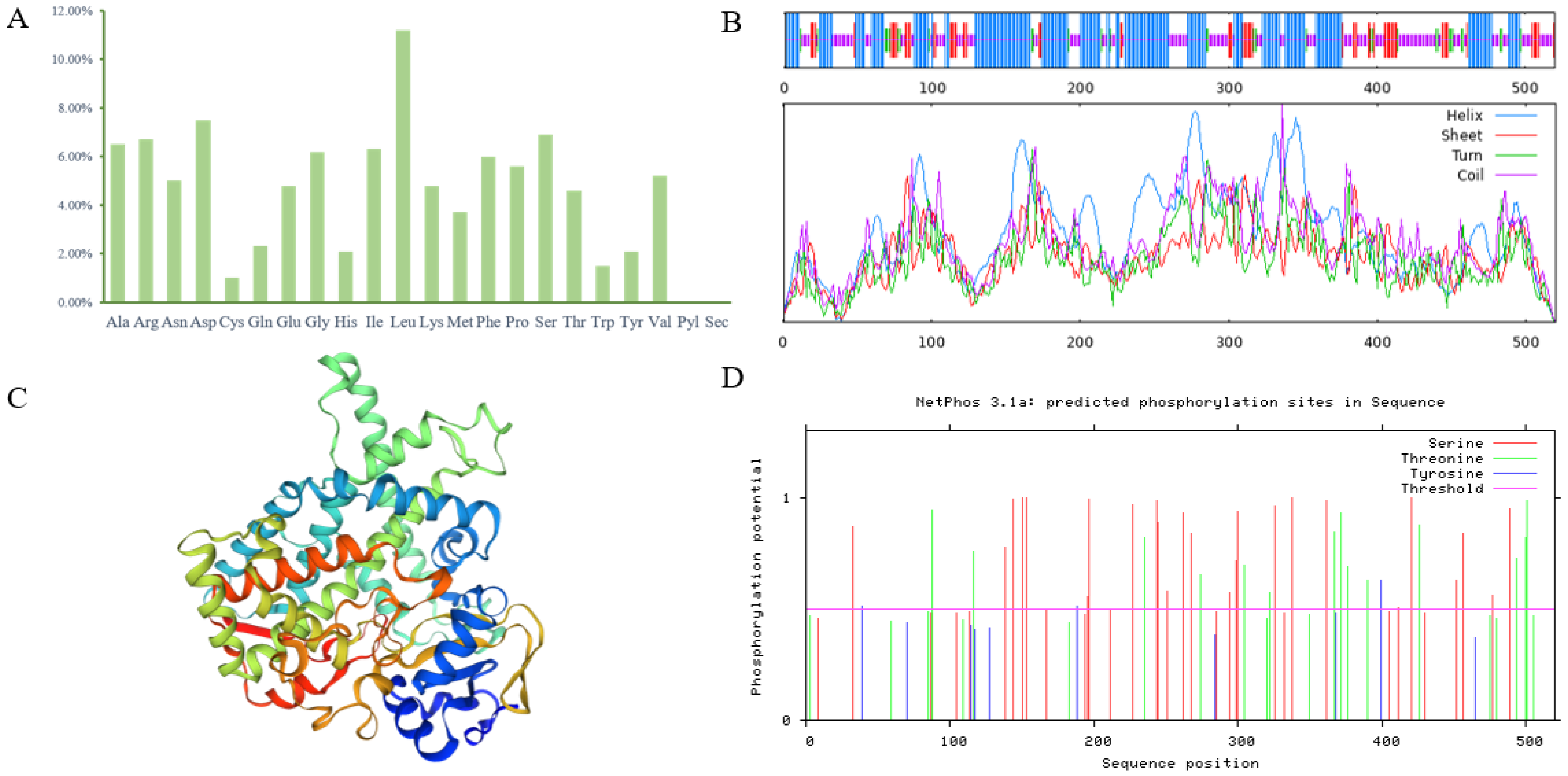
3.3. Bioinformatics Analysis of the PgCYP309 Gene
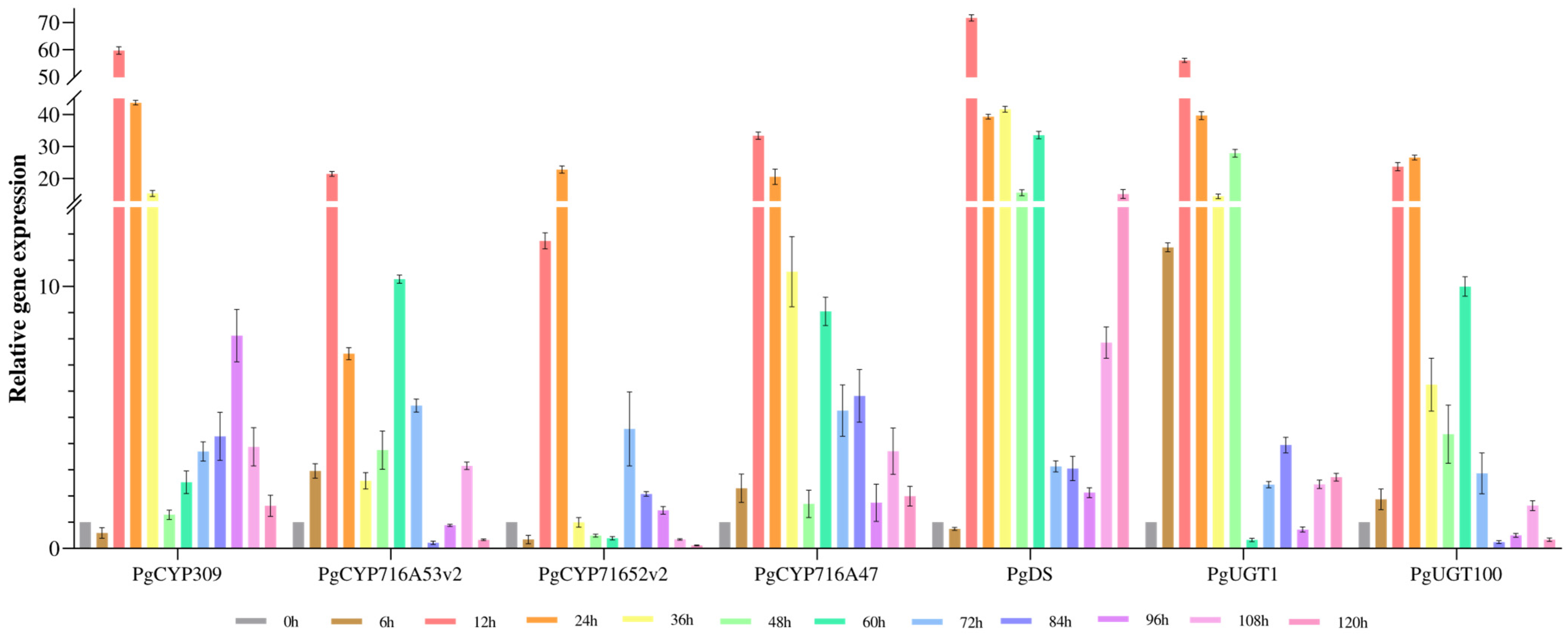
3.4. Analysis of Saponin Content and Gene Expression after MeJA-induced Treatment of Ginseng Adventitious Roots
3.5. Cloning of PgCYP309 Gene and Construction of Vector
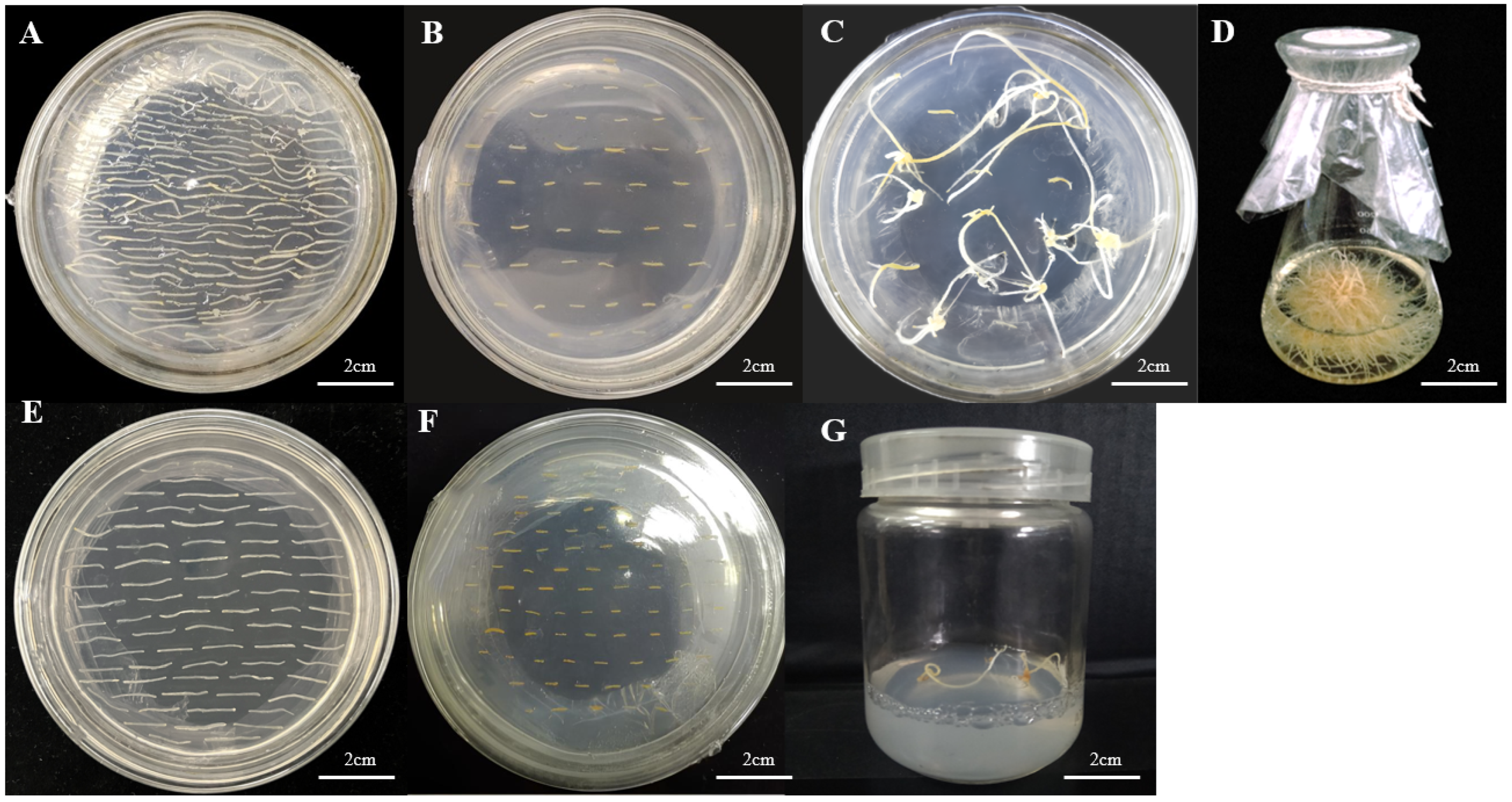
3.6. Genetic Transformation of Ginseng Explants and Detection of Positive Material
3.7. Detection of Gene Expression and Saponin Content in Positive Hair Roots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPD | protopanaxadiol |
| PPT | protopanaxatriol |
| MeJA | Methyl jasmonate |
| RT-qPCR | Quantitative real-time PCR |
| ddH2O | Double distilled water |
| nm | Nanometer |
| TPS | Extraction solution (Tris-HCl, EDTA, KCl, ddH2O) |
| B5 | Gamborg B5 |
| MS | Murashige and skoog |
| OD | Optical density |
| AS | Acetosyringone |
References
- Choi, K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Gao, L.L.; Zhu, P. Advances in the biosynthesis research of ginsenosides. Ao Xue Xue Bao Acta Pharm. Sin. 2013, 48, 170–178. [Google Scholar]
- Zhang, T.; Zhong, S.; Hou, L.; Wang, Y.; Xing, X.; Guan, T.; Zhang, J.; Li, T. Computational and experimental characterization of estrogenic activities of 20(S, R)-protopanaxadiol and 20(S, R)-protopanaxatriol. J. Ginseng Res. 2020, 44, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv. Food Nutr. Res. 2009, 55, 1–99. [Google Scholar] [PubMed]
- Kim, S.; Na, J.Y.; Song, K.B.; Choi, D.S.; Kim, J.H.; Kwon, Y.B.; Kwon, J. Protective Effect of Ginsenoside Rb1 on Hydrogen Peroxide-induced Oxidative Stress in Rat Articular Chondrocytes. J. Ginseng Res. 2012, 36, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, J.; Wang, J.; Li, X.; Li, J.; Chu, S.; Li, L.; Chen, N.; Zhang, L. Ginsenoside Rg1 prevent and treat inflammatory diseases: A review. Int. Immunopharmacol. 2020, 87, 106805. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chu, S.; Lin, M.; Gao, Y.; Liu, Y.; Yang, S.; Zhou, X.; Zhang, Y.; Hu, Y.; Wang, H.; et al. Anticancer property of ginsenoside Rh2 from ginseng. Eur. J. Med. Chem. 2020, 203, 112627. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Sureda, A.; Habtemariam, S.; Nabavi, S.M. Ginsenoside Rd and ischemic stroke; a short review of literatures. J. Ginseng Res. 2015, 39, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Popovich, D.; Yeo, C.R.; Zhang, W. Ginsenosides derived from Asian (Panax ginseng), American ginseng (Panax quinquefolius) and potential cytoactivity. Int. J. Biomed. Pharm. Sci. 2012, 6, 56–62. [Google Scholar]
- Kim, C.; Choo, G.C.; Cho, H.S.; Lim, J.T. Soil properties of cultivation sites for mountain-cultivated ginseng at local level. J. Ginseng Res. 2015, 39, 76–80. [Google Scholar] [CrossRef]
- Baeg, I.H.; So, S.H. The world ginseng market and the ginseng (Korea). J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.; Wolf, F.; Proulx, J.; Cuellar, R.; Saunders, C. Is the Reaction Catalyzed by 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase a Rate-Limiting Step for Isoprenoid Biosynthesis in Plants? Plant Physiol. 1995, 109, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Seemann, M.; Tse, S.B.B.; Wolff, M.; Miginiac-Maslow, M.; Rohmer, M. Isoprenoid biosynthesis in plant chloroplasts via the MEP pathway: Direct thylakoid/ferredoxin-dependent photoreduction of GcpE/IspG. FEBS Lett. 2006, 580, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M. Impacts of diversification of cytochrome P450 on plant metabolism. Biol. Pharm. Bull. 2012, 35, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Denisov, I.G.; Makris, T.M.; Sligar, S.G. Schlichting I: Structure and chemistry of cytochrome P450. Chem. Rev. 2005, 105, 2253–2277. [Google Scholar] [CrossRef]
- Mizutani, M.; Ohta, D. Diversification of P450 genes during land plant evolution. Annu. Rev. Plant. Biol. 2010, 61, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Werck-Reichhart, D. A P450-centric view of plant evolution. Plant J. 2011, 66, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C. Molecular-Genetic Analysis of Plant Cytochrome p450-Dependent Monooxygenases. Annu. Rev. Plant Biol. 1998, 49, 311–343. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R.; Ming, R.; Alam, M.; Schuler, M.A. Comparison of Cytochrome P450 Genes from Six Plant Genomes. Trop. Plant Biol. 2008, 1, 216–235. [Google Scholar] [CrossRef]
- Schuler, M.A.; Werck-Reichhart, D. Functional genomics of P450s. Annu. Rev. Plant. Biol. 2003, 54, 629–667. [Google Scholar] [CrossRef]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant. Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Pollier, J.; Almagro, L.; Buyst, D.; Van Montagu, M.; Pedreño, M.A.; Martins, J.C.; Thevelein, J.M.; Goossens, A. Combinatorial biosynthesis of sapogenins and saponins in Saccharomyces cerevisiae using a C-16α hydroxylase from Bupleurum falcatum. Proc. Natl. Acad. Sci. USA 2014, 111, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Kim, J.; Mijakovic, I.; Jung, K.H.; Choi, G.; Kim, S.C.; Kim, Y.J. Triterpenoid-biosynthetic UDP-glycosyltransferases from plants. Biotechnol. Adv. 2019, 37, 107394. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.T.; Bang, K.H.; Jung, S.J.; Kim, Y.C.; Hyun, D.Y.; Kim, S.H.; Cha, S.W. Molecular characterization of ginseng farnesyl diphosphate synthase gene and its up-regulation by methyl jasmonate. Biol. Plant. 2010, 54, 47–53. [Google Scholar] [CrossRef]
- Han, J.Y.; In, J.G.; Kwon, Y.S.; Choi, Y.E. Regulation of ginsenoside and phytosterol biosynthesis by RNA interferences of squalene epoxidase gene in Panax ginseng. Phytochemistry 2010, 71, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Kwon, Y.S.; Yang, D.C.; Jung, Y.R.; Choi, Y.E. Expression and RNA interference-induced silencing of the dammarenediol synthase gene in Panax ginseng. Plant Cell Physiol. 2006, 47, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Fiallos-Jurado, J.; Pollier, J.; Moses, T.; Arendt, P.; Barriga-Medina, N.; Morillo, E.; Arahana, V.; de Lourdes, T.M.; Goossens, A.; Leon-Reyes, A. Saponin determination, expression analysis and functional characterization of saponin biosynthetic genes in Chenopodium quinoa leaves. Plant Sci. 2016, 250, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Hwang, H.S.; Choi, S.W.; Kim, H.J.; Choi, Y.E. Cytochrome P450 CYP716A53v2 catalyzes the formation of protopanaxatriol from protopanaxadiol during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol. 2012, 53, 1535–1545. [Google Scholar] [CrossRef]
- Han, J.Y.; Kim, H.J.; Kwon, Y.S.; Choi, Y.E. The Cyt P450 enzyme CYP716A47 catalyzes the formation of protopanaxadiol from dammarenediol-II during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol. 2011, 52, 2062–2073. [Google Scholar] [CrossRef]
- Lu, C.; Zhao, S.; Wang, X. Functional regulation of a UDP-glucosyltransferase gene (Pq3-O-UGT1) by RNA interference and overexpression in Panax quinquefolius. Plant Cell Tissue Organ Cult. (PCTOC) 2017, 129, 445–456. [Google Scholar] [CrossRef]
- Niu, Y.; Luo, H.; Sun, C.; Yang, T.J.; Dong, L.; Huang, L.; Chen, S. Expression profiling of the triterpene saponin biosynthesis genes FPS, SS, SE, and DS in the medicinal plant Panax notoginseng. Gene 2014, 533, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, Z.J.; Xu, Y.; Qian, X.; Zhong, J.J. Efficient induction of ginsenoside biosynthesis and alteration of ginsenoside heterogeneity in cell cultures of Panax notoginseng by using chemically synthesized 2-hydroxyethyl jasmonate. Appl. Microbiol. Biotechnol. 2006, 70, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lin, Y.; Wang, Y.; Li, X.; Han, Y.; Wang, K.; Sun, C.; Wang, Y.; Zhang, M. Transcriptome analysis identifies strong candidate genes for ginsenoside biosynthesis reveals its underlying molecular mechanism in Panax ginseng, C.A. Meyer. Sci. Rep. 2019, 9, 615. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, S.; Sun, C.; Lin, Y.; Yin, R.; Wang, Y.; Zhang, M. The Spatial and Temporal Transcriptomic Landscapes of Ginseng, Panax ginseng C. A. Meyer. Sci. Rep. 2015, 5, 18283. [Google Scholar] [CrossRef]
- China Pharmacopoeia Committee. China Pharmacopoeia; China Medical Science Press: Beijing, China, 2010; Volume 1, pp. 367–368. (In Chinese)
- Zhang, Y.; Feng, C.; Bie, S.; Wang, X.; Yi, X.; Zhang, C.; Qin, H. An Improved TPS Method for Rapid DNA Extraction from Cotton Leaves. Cotton Sci. 2016, 28, 413–417. (In Chinese) [Google Scholar]
- Suzuki, H.; Achnine, L.; Xu, R.; Matsuda, S.P.; Dixon, R.A. A genomics approach to the early stages of triterpene saponin biosynthesis in Medicago truncatula. Plant J. 2002, 32, 1033–1048. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hahn, E.J.; Murthy, H.N.; Paek, K.Y. Adventitious root growth and ginsenoside accumulation in Panax ginseng cultures as affected by methyl jasmonate. Biotechnol. Lett. 2004, 26, 1619–1622. [Google Scholar] [CrossRef]
- Ren, A.; Li, M.J.; Shi, L.; Mu, D.S.; Jiang, A.L.; Han, Q.; Zhao, M.W. Profiling and quantifying differential gene transcription provide insights into ganoderic acid biosynthesis in Ganoderma lucidum in response to methyl jasmonate. PLoS ONE 2013, 8, e65027. [Google Scholar] [CrossRef]
- Xiang, L.; Zhu, S.; Zhao, T.; Zhang, M.; Liu, W.; Chen, M.; Lan, X.; Liao, Z. Enhancement of artemisinin content and relative expression of genes of artemisinin biosynthesis in Artemisia annua by exogenous MeJA treatment. Plant Growth Regul. 2015, 75, 435–441. [Google Scholar] [CrossRef]
- Han, J.Y.; Kim, M.J.; Ban, Y.W.; Hwang, H.S.; Choi, Y.E. The involvement of β-amyrin 28-oxidase (CYP716A52v2) in oleanane-type ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol. 2013, 54, 2034–2046. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; He, G.; Li, R.; Wang, K.; Wang, Y.; Zhao, M.; Zhang, M. Functional Validation of the Cytochrome P450 Family PgCYP309 Gene in Panax ginseng. Biomolecules 2024, 14, 715. https://doi.org/10.3390/biom14060715
Jiang Y, He G, Li R, Wang K, Wang Y, Zhao M, Zhang M. Functional Validation of the Cytochrome P450 Family PgCYP309 Gene in Panax ginseng. Biomolecules. 2024; 14(6):715. https://doi.org/10.3390/biom14060715
Chicago/Turabian StyleJiang, Yang, Gaohui He, Ruiqi Li, Kangyu Wang, Yi Wang, Mingzhu Zhao, and Meiping Zhang. 2024. "Functional Validation of the Cytochrome P450 Family PgCYP309 Gene in Panax ginseng" Biomolecules 14, no. 6: 715. https://doi.org/10.3390/biom14060715
APA StyleJiang, Y., He, G., Li, R., Wang, K., Wang, Y., Zhao, M., & Zhang, M. (2024). Functional Validation of the Cytochrome P450 Family PgCYP309 Gene in Panax ginseng. Biomolecules, 14(6), 715. https://doi.org/10.3390/biom14060715






