The Role of Intestinal Cytochrome P450s in Vitamin D Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfection
2.2. Plasmid Constructs
2.3. Luciferin-IPA Assay
2.4. Dual Luciferase Reporter Assay
2.5. Animal Studies
2.6. RNA Isolation and Quantitative Reverse Transcription—PCR Analysis
2.7. Protein Preparation
2.8. Western Blotting
2.9. Measurement of Plasma 25OHD3 and 24,25(OH)2D3 Concentrations by LC/MS/MS Analysis
2.10. Statistics
3. Results
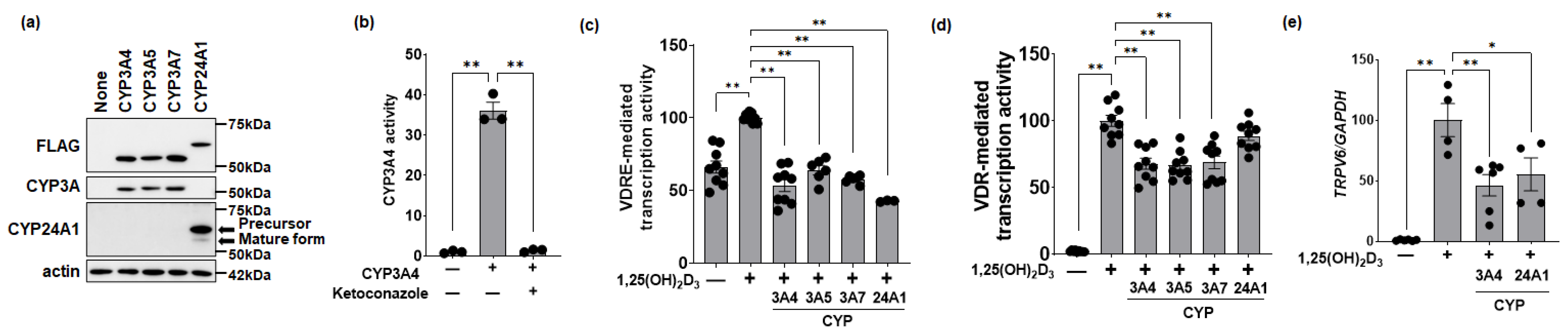
3.1. Effects of CYP3A or CYP24A1 on 1,25(OH)2D3 Activity in Human Colon Cells
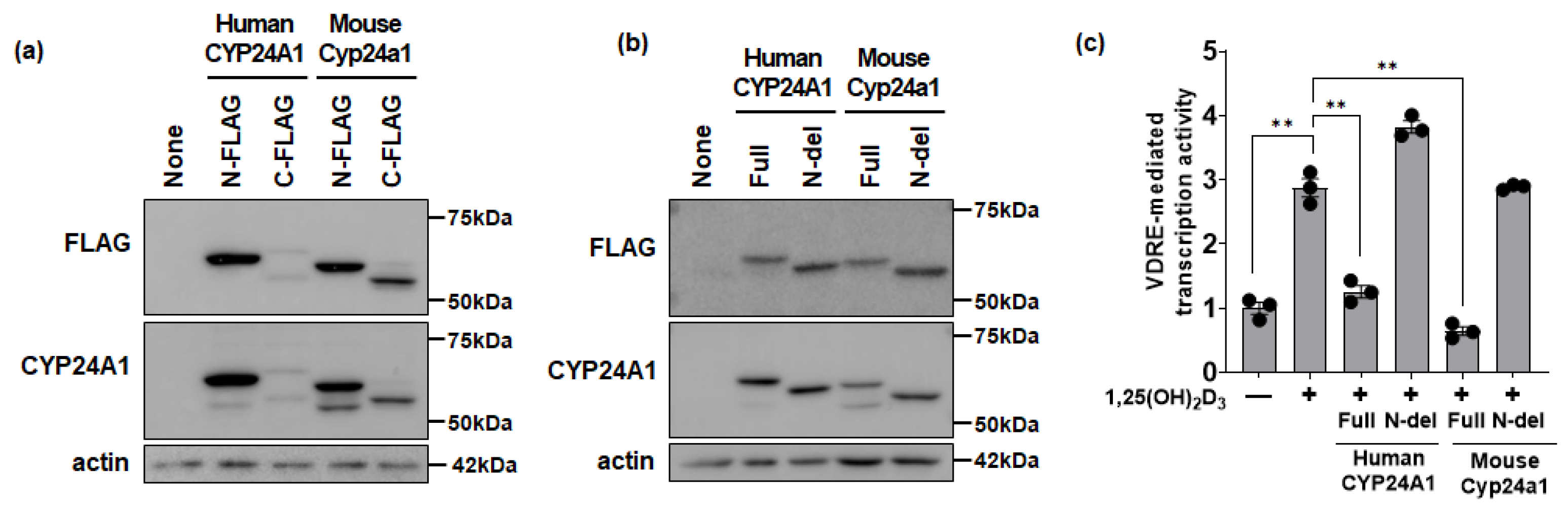
3.2. Functional Effects of CYP24A1 on 1,25(OH)2D3 Activity
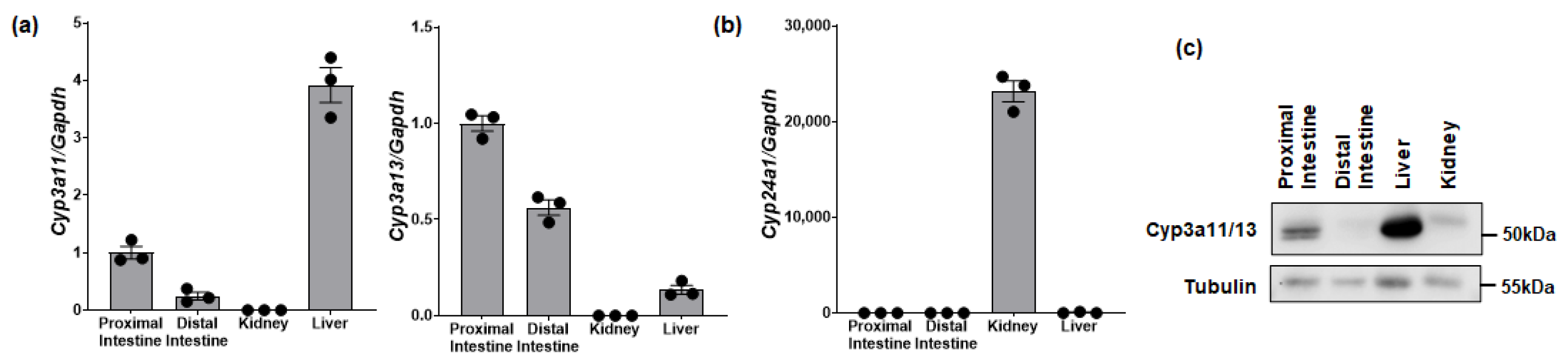
3.3. Tissue Expression of Cyp3a and Cyp24a1 in Mice
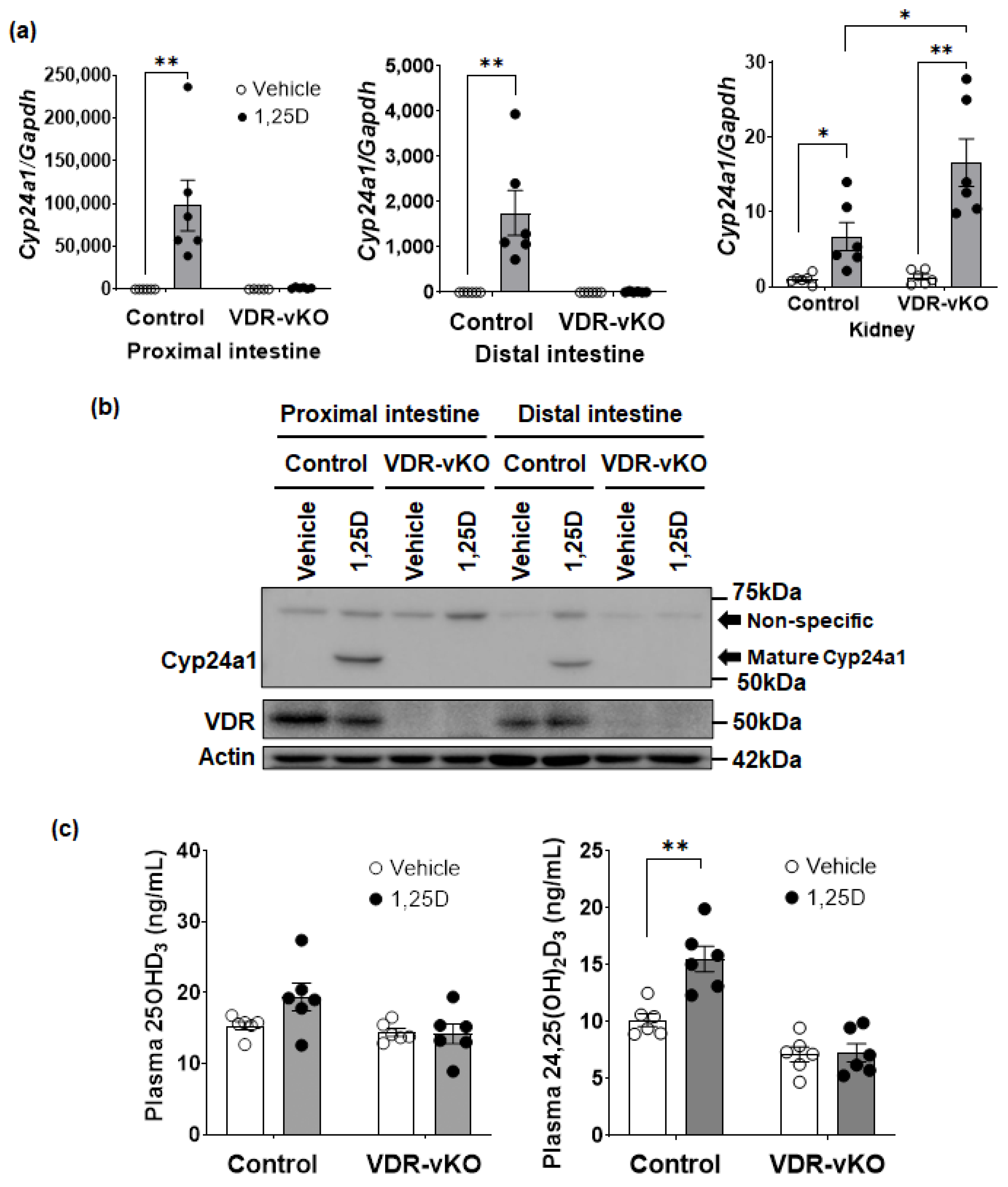
3.4. 1,25(OH)2D3-Dependent Regulation of Cyp3a or Cyp24a1 in the Mouse Intestine
3.5. 1,25(OH)2D3-Dependent Regulation of Cyp24a1 in Intestine-Specific VDR-KO Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jahnsen, J.; Falch, J.A.; Mowinckel, P.; Aadland, E. Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 2002, 37, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Nutrients in the Prevention of Osteoporosis in Patients with Inflammatory Bowel Diseases. Nutrients 2020, 12, 1702. [Google Scholar] [CrossRef] [PubMed]
- Grozić, A.; Coker, K.; Dussik, C.M.; Sabir, M.S.; Sabir, Z.; Bradley, A.; Zhang, L.; Park, J.; Yale, S.; Kaneko, I.; et al. Identification of putative transcriptomic biomarkers in irritable bowel syndrome (IBS): Differential gene expression and regulation of TPH1 and SERT by vitamin D. PLoS ONE 2022, 17, e0275683. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, T.; Grubler, M.R.; Smith, A.V.; Gudmundsson, E.F.; Keppel, M.; Cotch, M.F.; Harris, T.B.; Jorde, R.; Grimnes, G.; Joakimsen, R.; et al. Effect of Genetically Low 25-Hydroxyvitamin D on Mortality Risk: Mendelian Randomization Analysis in 3 Large European Cohorts. Nutrients 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Schottker, B.; Haug, U.; Schomburg, L.; Kohrle, J.; Perna, L.; Muller, H.; Holleczek, B.; Brenner, H. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am. J. Clin. Nutr. 2013, 97, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef]
- Meyer, M.B.; Benkusky, N.A.; Kaufmann, M.; Lee, S.M.; Onal, M.; Jones, G.; Pike, J.W. A kidney-specific genetic control module in mice governs endocrine regulation of the cytochrome P450 gene Cyp27b1 essential for vitamin D(3) activation. J. Biol. Chem. 2017, 292, 17541–17558. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Lee, S.M.; Carlson, A.H.; Benkusky, N.A.; Kaufmann, M.; Jones, G.; Pike, J.W. A chromatin-based mechanism controls differential regulation of the cytochrome P450 gene Cyp24a1 in renal and non-renal tissues. J. Biol. Chem. 2019, 294, 14467–14481. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.C.; Jeung, E.B. Molecular mechanism of regulation of the calcium-binding protein calbindin-D9k, and its physiological role(s) in mammals: A review of current research. J. Cell. Mol. Med. 2008, 12, 409–420. [Google Scholar] [CrossRef] [PubMed]
- van Abel, M.; Hoenderop, J.G.; Bindels, R.J. The epithelial calcium channels TRPV5 and TRPV6: Regulation and implications for disease. Naunyn Schmiedebergs Arch. Pharmacol. 2005, 371, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Inhibition of Cytochrome P450 Enzymes by Drugs-Molecular Basis and Practical Applications. Biomol. Ther. 2022, 30, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.S.; Prahl, J.M.; DeLuca, H.F. Isolation and expression of human 1,25-dihydroxyvitamin D3 24-hydroxylase cDNA. Proc. Natl. Acad. Sci. USA 1993, 90, 4543–4547. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.S.; DeLuca, H.F. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim. Biophys. Acta 1995, 1263, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Byford, V.; Arabian, A.; Sakai, Y.; Demay, M.B.; St-Arnaud, R.; Jones, G. Altered pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology 2005, 146, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, R.A.; Ismail, R.; DeLuca, H.F. Immunopurified 25-hydroxyvitamin D 1 alpha-hydroxylase and 1,25-dihydroxyvitamin D 24-hydroxylase are closely related but distinct enzymes. J. Biol. Chem. 1992, 267, 3498–3505. [Google Scholar]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Van Cromphaut, S.J.; Dewerchin, M.; Hoenderop, J.G.; Stockmans, I.; Van Herck, E.; Kato, S.; Bindels, R.J.; Collen, D.; Carmeliet, P.; Bouillon, R.; et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: Functional and molecular aspects. Proc. Natl. Acad. Sci. USA 2001, 98, 13324–13329. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P-450 3A4: Regulation and role in drug metabolism. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hashizume, T.; Shuhart, M.C.; Davis, C.L.; Nelson, W.L.; Sakaki, T.; Kalhorn, T.F.; Watkins, P.B.; Schuetz, E.G.; Thummel, K.E. Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1alpha,25-dihydroxyvitamin D(3): Implications for drug-induced osteomalacia. Mol. Pharmacol. 2006, 69, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, Y.S.; Zheng, X.E.; Senn, T.; Hashizume, T.; Scian, M.; Dickmann, L.J.; Nelson, S.D.; Baillie, T.A.; Hebert, M.F.; et al. An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Mol. Pharmacol. 2012, 81, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Nishikawa, M.; Okamoto, K.; Horibe, K.; Mano, H.; Yamaguchi, M.; Okon, R.; Nakagawa, K.; Tsugawa, N.; Okano, T.; et al. Elucidation of metabolic pathways of 25-hydroxyvitamin D3 mediated by CYP24A1 and CYP3A using Cyp24a1 knockout rats generated by CRISPR/Cas9 system. J. Biol. Chem. 2021, 296, 100668. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.C.; Hines, R.N.; Gu, C.; Koukouritaki, S.B.; Manro, J.R.; Tandler, P.J.; Zaya, M.J. Developmental expression of the major human hepatic CYP3A enzymes. J. Pharmacol. Exp. Ther. 2003, 307, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Roizen, J.D.; Li, D.; O’Lear, L.; Javaid, M.K.; Shaw, N.J.; Ebeling, P.R.; Nguyen, H.H.; Rodda, C.P.; Thummel, K.E.; Thacher, T.D.; et al. CYP3A4 mutation causes vitamin D-dependent rickets type 3. J. Clin. Investig. 2018, 128, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Mantoanelli, L.; de Almeida, C.M.; Coelho, M.C.A.; Coutinho, M.; Levine, M.A.; Collett-Solberg, P.F.; Bordallo, A.P. Vitamin D-Dependent Rickets Type 3: A Case Report and Systematic Review. Calcif. Tissue Int. 2023, 112, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Lee, S.M.; Meyer, M.B. Molecular insights into mineralotropic hormone inter-regulation. Front. Endocrinol. 2023, 14, 1213361. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Lee, S.M.; Benkusky, N.A.; Meyer, M.B. Genomic Mechanisms Governing Mineral Homeostasis and the Regulation and Maintenance of Vitamin D Metabolism. J. Bone Miner. Res. Plus 2021, 5, e10433. [Google Scholar] [CrossRef] [PubMed]
- Meisenheimer, P.L.; Uyeda, H.T.; Ma, D.; Sobol, M.; McDougall, M.G.; Corona, C.; Simpson, D.; Klaubert, D.H.; Cali, J.J. Proluciferin acetals as bioluminogenic substrates for cytochrome P450 activity and probes for CYP3A inhibition. Drug Metab. Dispos. 2011, 39, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Lieben, L.; Masuyama, R.; Torrekens, S.; Van Looveren, R.; Schrooten, J.; Baatsen, P.; Lafage-Proust, M.H.; Dresselaers, T.; Feng, J.Q.; Bonewald, L.F.; et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J. Clin. Investig. 2012, 122, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yoshizawa, T.; Fukuda, T.; Shirode-Fukuda, Y.; Yu, T.; Sekine, K.; Sato, T.; Kawano, H.; Aihara, K.; Nakamichi, Y.; et al. Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology 2013, 154, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Zella, L.A.; Nerenz, R.D.; Pike, J.W. Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J. Biol. Chem. 2007, 282, 22344–22352. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, N.; Suhara, Y.; Kamao, M.; Okano, T. Determination of 25-hydroxyvitamin D in human plasma using high-performance liquid chromatography--tandem mass spectrometry. Anal. Chem. 2005, 77, 3001–3007. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.P.; Hollis, B.W.; Patel, S.B.; Patrick, K.S.; Bell, N.H. CYP3A4 is a human microsomal vitamin D 25-hydroxylase. J. Bone Miner. Res. 2004, 19, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.P.; He, Y.A.; Patrick, K.S.; Halpert, J.R.; Bell, N.H. CYP3A4 is a vitamin D-24- and 25-hydroxylase: Analysis of structure function by site-directed mutagenesis. J. Clin. Endocrinol. Metab. 2005, 90, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, Y.S.; Dickmann, L.J.; Poulton, E.J.; Eaton, D.L.; Lampe, J.W.; Shen, D.D.; Davis, C.L.; Shuhart, M.C.; Thummel, K.E. Enhancement of hepatic 4-hydroxylation of 25-hydroxyvitamin D3 through CYP3A4 induction in vitro and in vivo: Implications for drug-induced osteomalacia. J. Bone Miner. Res. 2013, 28, 1101–1116. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Jurutka, P.W.; Whitfield, G.K.; Myskowski, S.M.; Eichhorst, K.R.; Dominguez, C.E.; Haussler, C.A.; Haussler, M.R. Liganded VDR induces CYP3A4 in small intestinal and colon cancer cells via DR3 and ER6 vitamin D responsive elements. Biochem. Biophys. Res. Commun. 2002, 299, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Riley, E.M.; Meyer, M.B.; Benkusky, N.A.; Plum, L.A.; DeLuca, H.F.; Pike, J.W. 1,25-Dihydroxyvitamin D3 Controls a Cohort of Vitamin D Receptor Target Genes in the Proximal Intestine That Is Enriched for Calcium-regulating Components. J. Biol. Chem. 2015, 290, 18199–18215. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Lu, T.T.; Xie, W.; Whitfield, G.K.; Domoto, H.; Evans, R.M.; Haussler, M.R.; Mangelsdorf, D.J. Vitamin D Receptor As an Intestinal Bile Acid Sensor. Science 2002, 296, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi-Shibata, M.; Sakaki, T.; Ohyama, Y.; Noshiro, M.; Okuda, K.; Yabusaki, Y. Further oxidation of hydroxycalcidiol by calcidiol 24-hydroxylase. A study with the mature enzyme expressed in Escherichia coli. Eur. J. Biochem. 1994, 224, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Paschen, S.A.; Neupert, W. Protein import into mitochondria. IUBMB Life 2001, 52, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Nguyen, L.; Wu, S.; Encinas, C.; Adams, J.S.; Hewison, M. Alternative splicing of vitamin D-24-hydroxylase: A novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J. Biol. Chem. 2005, 280, 20604–20611. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, M.; Nakahashi, O.; Kagawa, T.; Masuda, M.; Ohminami, H.; Iwano, M.; Takeda, E.; Taketani, Y.; Yamamoto, H. Association of increased renal Cyp24a1 gene expression with low plasma 1,25-dihydroxyvitamin D levels in rats with streptozotocin-induced diabetes. J. Clin. Biochem. Nutr. 2020, 66, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.M.; McKee, D.D.; Watson, M.A.; Willson, T.M.; Moore, J.T.; Kliewer, S.A. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Investig. 1998, 102, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.B.; Parks, D.J.; Jones, S.A.; Bledsoe, R.K.; Consler, T.G.; Stimmel, J.B.; Goodwin, B.; Liddle, C.; Blanchard, S.G.; Willson, T.M.; et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J. Biol. Chem. 2000, 275, 15122–15127. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.B.; Wrighton, S.A.; Schuetz, E.G.; Molowa, D.T.; Guzelian, P.S. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J. Clin. Investig. 1987, 80, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H. CYP induction-mediated drug interactions: In vitro assessment and clinical implications. Pharm. Res. 2006, 23, 1089–1116. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.P.; Li, D.; Hakonarson, H.; Meyers, K.E.; Thummel, K.E.; Levine, M.A. CYP3A4 Induction by Rifampin: An Alternative Pathway for Vitamin D Inactivation in Patients with CYP24A1 Mutations. J. Clin. Endocrinol. Metab. 2017, 102, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.W.; Lee, A.M.C.; Xu, X.; Hua, B.; Tapp, H.; Wen, X.S.; Xian, C.J. Methotrexate Chemotherapy Causes Growth Impairments, Vitamin D Deficiency, Bone Loss, and Altered Intestinal Metabolism-Effects of Calcitriol Supplementation. Cancers 2023, 15, 4367. [Google Scholar] [CrossRef]

| Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| Human CYP3A4 | GCGCAGATCTATGGCTCTCATCCCAGACTTG | GCGCCTCGAGTCAGGCTCCACTTACGGTGCC |
| Human CYP3A5 | GCGCAGATCTATGGACCTCATCCCAAATTTG | GCGCCTCGAGTCATTCTCCACTTAGGGTTCC |
| Human CYP3A7 | GCGCAGATCTATGGATCTCATCCCAAACTTG | GCGCCTCGAGTCAGGCTCCACTTACGGTCTC |
| Human full-length CYP24A1 N-FLAG | GCGCGAATTCATGAGCTCCCCCATCAGCAAGAG | GCGCGTCGACTTATCGCTGGCAAAACGCGATGG |
| Mouse full-length Cyp24a1 N-FLAG | GCGCGAATTCATGAGCTGCCCCATTGACAAAAG | GCGCGTCGACCTACCGTGGACAGAACGCAATGG |
| Human full-length CYP24A1 C-FLAG | GCGCGAATTCATGAGCTCCCCCATCAGCAAGAG | GCGCGTCGACTCGCTGGCAAAACGCGATGGGG |
| Mouse full-length Cyp24a1 C-FLAG | GCGCGAATTCATGAGCTGCCCCATTGACAAAAG | GCGCGTCGACCCGTGGACAGAACGCAATGGGC |
| Human N-del CYP24A1 | GCGCGAATTCCCTCAGCCGCGAGAGGTGCC | GCGCGTCGACTTATCGCTGGCAAAACGCGATGG |
| Mouse N-del Cyp24a1 | GCGCGAATTCCGTGCGCCAAAAGAGGTGCC | GCGCGTCGACCTACCGTGGACAGAACGCAATGG |
| Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| GAPDH | CTGCACCACCAACTGCTTAGC | CATCCACAGTCTTCTGGGTG |
| Human TRPV6 | AAGCCTACATGACCCCTAAG | CCCATTCTGAAGATGTCTGG |
| Mouse Cyp3a11 | TGGACAGAATGAAGGAAAGCC | GGCTTTATGAGAGACTTTGTC |
| Mouse Cyp3a13 | GATGAAATTGATGCGGCTCTG | TCTCAAGTCTTCCAGCGATTG |
| Mouse Cyp24a1 exon 6–7 | TGGGAAGATGATGGTGACCC | TCGATGCAGGGCTTGACTG |
| Mouse Cyp24a1 exon 1 | TACTGCTCCTCGAGTGTCAC | CTTGGATGTCACGGACCTTG |
| Mouse Cyp24a1 intron 2—exon 3 | TAAGCATACCCCTTCTCTGC | CAGCTTCATGATTTCCACGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uga, M.; Kaneko, I.; Shiozaki, Y.; Koike, M.; Tsugawa, N.; Jurutka, P.W.; Miyamoto, K.-I.; Segawa, H. The Role of Intestinal Cytochrome P450s in Vitamin D Metabolism. Biomolecules 2024, 14, 717. https://doi.org/10.3390/biom14060717
Uga M, Kaneko I, Shiozaki Y, Koike M, Tsugawa N, Jurutka PW, Miyamoto K-I, Segawa H. The Role of Intestinal Cytochrome P450s in Vitamin D Metabolism. Biomolecules. 2024; 14(6):717. https://doi.org/10.3390/biom14060717
Chicago/Turabian StyleUga, Minori, Ichiro Kaneko, Yuji Shiozaki, Megumi Koike, Naoko Tsugawa, Peter W. Jurutka, Ken-Ichi Miyamoto, and Hiroko Segawa. 2024. "The Role of Intestinal Cytochrome P450s in Vitamin D Metabolism" Biomolecules 14, no. 6: 717. https://doi.org/10.3390/biom14060717





