Comparative Analysis of Osteoblastic Responses to Titanium and Alumina-Toughened Zirconia Implants: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Osteoblast Cells
2.2. Implant Materials and Surface Treatments
2.3. Cell Surface Characterization
2.4. Isolation of Mononuclear Cells from Tissue
2.5. Cell Culture
2.6. Morphogenesis
2.7. Investigation of the Implant on the Proliferation of Osteoblast Cells
2.8. Cell Death Assay by Implant
2.9. Data Analysis
3. Results
3.1. Microscopic Evaluation of Osteoblast Cells
3.2. Cell Morphology of Osteoblasts
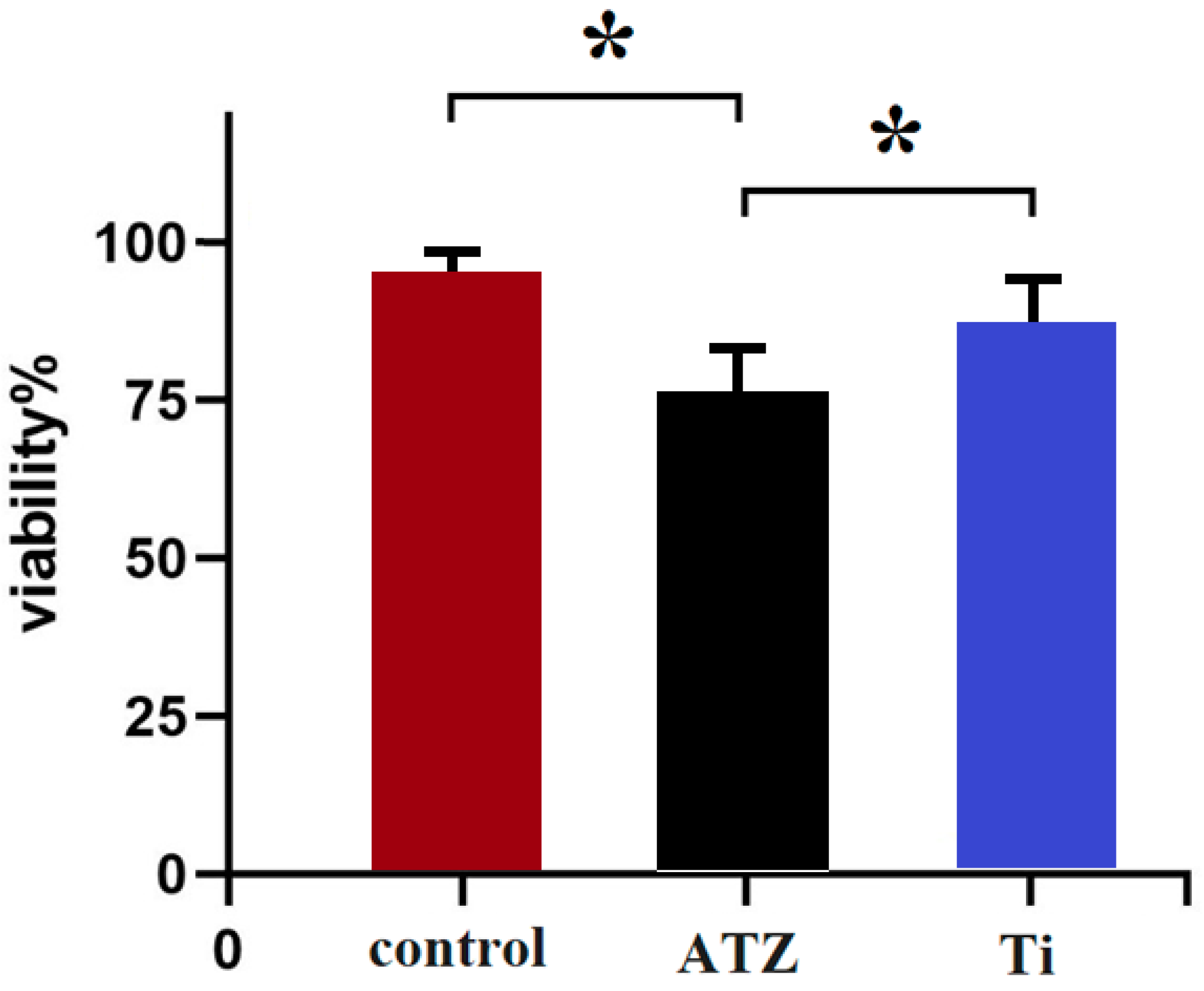
3.3. Effects of the Implants on Survival
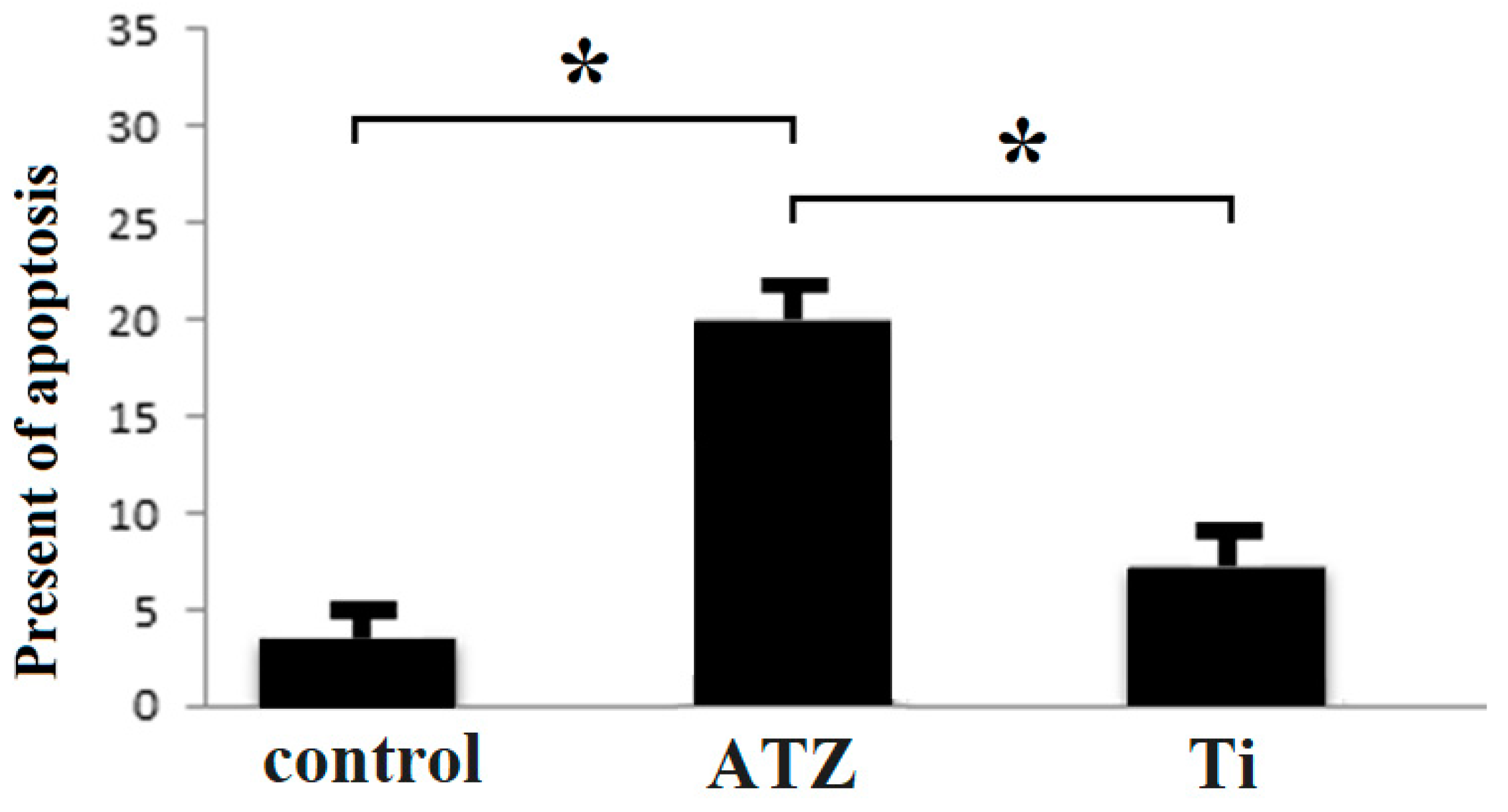
3.4. Investigation of Cell Proliferation and Cell Death
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anapu, M.P.; Atluri, K.R.; Tripuraneni, S.C.; Issrani, R.; Bader, A.K.; Alkhalaf, Z.A.; Sghaireen, M.G.; Prabhu, N.; Alshammari, R.R.D.; Khalid, G. Evaluation of effect on stability of implants with and without platelet rich fibrin using a resonance frequency analyzer-An in-vivo study. Heliyon 2024, 10, E27971. [Google Scholar] [CrossRef] [PubMed]
- Blanch-Martínez, N.; Arias-Herrera, S.; Martínez-González, A. Behavior of polyether-ether-ketone (PEEK) in prostheses on dental implants. A review. J. Clin. Exp. Dent. 2021, 13, e520. [Google Scholar] [CrossRef] [PubMed]
- Größner-Schreiber, B.; Herzog, M.; Hedderich, J.; Dück, A.; Hannig, M.; Griepentrog, M. Focal adhesion contact formation by fibroblasts cultured on surface-modified dental implants: An in vitro study. Clin. Oral Implant. Res. 2006, 17, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Ramenzoni, L.L.; Attin, T.; Schmidlin, P.R. In vitro effect of modified polyetheretherketone (PEEK) implant abutments on human gingival epithelial keratinocytes migration and proliferation. Materials 2019, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Wang, D.; Xu, L.; Wang, J.; Zeng, D.; Lin, S.; Huang, C.; Liu, X.; Jiang, X. The response of human osteoblasts, epithelial cells, fibroblasts, macrophages and oral bacteria to nanostructured titanium surfaces: A systematic study. Int. J. Nanomed. 2017, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y.-T.; Johansson, C.; Wennerberg, A.; Cho, L.-R.; Chang, B.-S.; Albrektsson, T. Optimum Surface Properties of Oxidized Implants for Reinforcement of Osseointeg ration: Surface Chemistry, Oxide Thickness, Porosity, Roughness, and Crystal Structure. Int. J. Oral Maxillofac. Implant. 2005, 20, 349. [Google Scholar]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Peri-implantitis–onset and pattern of progression. J. Clin. Periodontol. 2016, 43, 383–388. [Google Scholar] [CrossRef]
- Toffoli, A.; Parisi, L.; Bianchi, M.G.; Lumetti, S.; Bussolati, O.; Macaluso, G.M. Thermal treatment to increase titanium wettability induces selective proteins adsorption from blood serum thus affecting osteoblasts adhesion. Mater. Sci. Eng. C 2020, 107, 110250. [Google Scholar] [CrossRef]
- Lopes, M.A.; Monteiro, F.; Santos, J.; Serro, A.; Saramago, B. Hydrophobicity, surface tension, and zeta potential measurements of glass-reinforced hydroxyapatite composites. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1999, 45, 370–375. [Google Scholar] [CrossRef]
- Ng, K.; Fan, M.; Leung, M.; Fokas, G.; Mattheos, N. Peri-implant inflammation and marginal bone level changes around dental implants in relation to proximity with and bone level of adjacent teeth. Aust. Dent. J. 2018, 63, 467–477. [Google Scholar] [CrossRef]
- Insua, A.; Monje, A.; Wang, H.L.; Miron, R.J. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J. Biomed. Mater. Res. Part A 2017, 105, 2075–2089. [Google Scholar] [CrossRef]
- Ivanovski, S.; Bartold, P.M.; Huang, Y.S. The role of foreign body response in peri-implantitis: What is the evidence? Periodontology 2000 2022, 90, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.; Tay, J.R.H.; Mattheos, N.; Bostanci, N.; Belibasakis, G.N.; Seneviratne, C.J. A Mapping Review of the Pathogenesis of Peri-Implantitis: The Biofilm-Mediated Inflammation and Bone Dysregulation (BIND) Hypothesis. Cells 2024, 13, 315. [Google Scholar] [CrossRef] [PubMed]
- Homa, K.; Zakrzewski, W.; Dobrzyński, W.; Piszko, P.J.; Piszko, A.; Matys, J.; Wiglusz, R.J.; Dobrzyński, M. Surface Functionalization of Titanium-Based Implants with a Nanohydroxyapatite Layer and Its Impact on Osteoblasts: A Systematic Review. J. Funct. Biomater. 2024, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-C.; Han, J.-S.; Bächle, M.; Jang, J.-H.; Shin, S.-W.; Kim, D.-J. Initial osteoblast-like cell response to pure titanium and zirconia/alumina ceramics. Dent. Mater. 2007, 23, 1349–1355. [Google Scholar] [CrossRef]
- Kostina, D.; Lobov, A.; Klausen, P.; Karelkin, V.; Tikhilov, R.; Bozhkova, S.; Sereda, A.; Ryumina, N.; Enukashvily, N.; Malashicheva, A. Isolation of human osteoblast cells capable for mineralization and synthetizing bone-related proteins in vitro from adult bone. Cells 2022, 11, 3356. [Google Scholar] [CrossRef] [PubMed]
- Stoilov, M.; Stoilov, L.; Enkling, N.; Stark, H.; Winter, J.; Marder, M.; Kraus, D. Effects of Different Titanium Surface Treatments on Adhesion, Proliferation and Differentiation of Bone Cells: An In Vitro Study. J. Funct. Biomater. 2022, 13, 143. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Fos-Parra, I.; Cabanillas-Balsera, D.; Gil, J.; Ortiz-García, I.; Giner, M.; Bocio-Núñez, J.; Montoya-García, M.-J.; Jiménez-Guerra, Á. Osteoblastic cell behavior and gene expression related to bone metabolism on different titanium surfaces. Int. J. Mol. Sci. 2023, 24, 3523. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, A.; Torok-Storb, B.; Pillai, M.M. Primary marrow-derived stromal cells: Isolation and manipulation. Stem Cell Niche: Methods Protoc. 2013, 1035, 75–101. [Google Scholar]
- Remmers, S.J.; de Wildt, B.W.; Vis, M.A.; Spaander, E.S.; de Vries, R.B.; Ito, K.; Hofmann, S. Osteoblast-osteoclast co-cultures: A systematic review and map of available literature. PLoS ONE 2021, 16, e0257724. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Taheri-Kafrani, A.; Ordooei, M.; Amiri, A.; Karimi-Zarchi, M. Evaluation of toxicity of functionalized graphene oxide with ginsenoside Rh2, lysine and arginine on blood cancer cells (K562), red blood cells, blood coagulation and cardiovascular tissue: In vitro and in vivo studies. J. Taiwan Inst. Chem. Eng. 2018, 93, 70–78. [Google Scholar] [CrossRef]
- Jin, J.; Jaspers, R.T.; Wu, G.; Korfage, J.A.; Klein-Nulend, J.; Bakker, A.D. Shear stress modulates osteoblast cell and nucleus morphology and volume. Int. J. Mol. Sci. 2020, 21, 8361. [Google Scholar] [CrossRef]
- Iwasaki, M.; Kuroda, J.; Kawakami, K.; Wada, H. Epidermal regulation of bone morphogenesis through the development and regeneration of osteoblasts in the zebrafish scale. Dev. Biol. 2018, 437, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef]
- Levin, M.; Spiro, R.C.; Jain, H.; Falk, M.M. Effects of titanium implant surface topology on bone cell attachment and proliferation in vitro. Med. Devices Evid. Res. 2022, 103–119. [Google Scholar] [CrossRef]
- Haghgoo, J.M.; Khoshhal, M.; Sharifi, S.; Khodadadi, I.; Pour, H.G.; Rabie, M.A.S.; Rabienejad, N. Determination of Osteoblast Cell Viability and Histological Changes of Samples Obtained from Different Implant Drills during Osteotomy. J. Dent. 2023, 24, 220. [Google Scholar]
- Miralami, R.; Sharp, J.G.; Namavar, F.; Hartman, C.W.; Garvin, K.L.; Thiele, G.M. Effects of nano-engineered surfaces on osteoblast adhesion, growth, differentiation, and apoptosis. Proc. Inst. Mech. Eng. Part N J. Nanomater. Nanoeng. Nanosyst. 2020, 234, 59–66. [Google Scholar] [CrossRef]
- Campos-Bijit, V.; Inostroza, N.C.; Orellana, R.; Rivera, A.; Von Marttens, A.; Cortez, C.; Covarrubias, C. Influence of Topography and Composition of Commercial Titanium Dental Implants on Cell Adhesion of Human Gingiva-Derived Mesenchymal Stem Cells: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 16686. [Google Scholar] [CrossRef] [PubMed]
- Aung, L.M.; Lin, J.C.-Y.; Salamanca, E.; Wu, Y.-F.; Pan, Y.-H.; Teng, N.-C.; Huang, H.-M.; Sun, Y.-S.; Chang, W.-J. Functionalization of zirconia ceramic with fibronectin proteins enhanced bioactivity and osteogenic response of osteoblast-like cells. Front. Bioeng. Biotechnol. 2023, 11, 1159639. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The impact of dental implant surface modifications on osseointegration and biofilm formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. Effect of plasma treatment on the surface properties of polylactic acid films. Polym. Test. 2021, 96, 107097. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface modification techniques of titanium and titanium alloys for biomedical dental applications: A review. Mater. Today Proc. 2021, 39, 84–90. [Google Scholar] [CrossRef]
- Bays, J.L.; DeMali, K.A. Vinculin in cell–cell and cell–matrix adhesions. Cell. Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Egashira, K.; Kajiya, H.; Tsutsumi, T.; Taniguchi, Y.; Kakura, K.; Ohno, J.; Kido, H. AMPK activation enhances osteoblast differentiation on a titanium disc via autophagy. Int. J. Implant. Dent. 2024, 10, 2. [Google Scholar] [CrossRef]
- Weivoda, M.M.; Chew, C.K.; Monroe, D.G.; Farr, J.N.; Atkinson, E.J.; Geske, J.R.; Eckhardt, B.; Thicke, B.; Ruan, M.; Tweed, A.J. Identification of osteoclast-osteoblast coupling factors in humans reveals links between bone and energy metabolism. Nat. Commun. 2020, 11, 87. [Google Scholar] [CrossRef]
- Cochran, D.L.; Nummikoski, P.V.; Higginbottom, F.L.; Hermann, J.S.; Makins, S.R.; Buser, D. Evaluation of an endosseous titanium implant with a sandblasted and acid-etched surface in the canine mandible: Radiographic results. Clin. Oral Implants Res. 1996, 7, 240–252. [Google Scholar] [CrossRef]
- Zhu, Q.; Haglund, R.; Safavi, K.E.; Spangberg, L.S. Adhesion of human osteoblasts on root-end filling materials. J. Endod. 2000, 26, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Bidar, M.; Tavakkol Afshari, J.; Shahrami, F. Evaluation of Adhesion and Morphology of Human Osteoblasts to White MTA and Portland Cement. Iran. Endod. J. 2007, 2, 87–90. [Google Scholar]
- Torabinejad, M.; Hong, C.U.; Pitt Ford, T.R.; Kettering, J.D. Cytotoxicity of four root end filling materials. J. Endod. 1995, 21, 489–492. [Google Scholar] [CrossRef]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mater. 2020, 103, 103574. [Google Scholar] [CrossRef] [PubMed]
- Marticorena, M.; Corti, G.; Olmedo, D.; Guglielmotti, M.; Duhalde, S. Laser surface modification of Ti implants to improve osseointegration. J. Phys. Conf. Ser. 2007, 59, 662. [Google Scholar] [CrossRef]
- Plecko, M.; Sievert, C.; Andermatt, D.; Frigg, R.; Kronen, P.; Klein, K.; Stübinger, S.; Nuss, K.; Bürki, A.; Ferguson, S.; et al. Osseointegration and biocompatibility of different metal implants—A comparative experimental investigation in sheep. BMC Musculoskelet. Disord. 2012, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Rabel, K.; Kohal, R.J.; Steinberg, T.; Rolauffs, B.; Adolfsson, E.; Altmann, B. Human osteoblast and fibroblast response to oral implant biomaterials functionalized with non-thermal oxygen plasma. Sci. Rep. 2021, 11, 17302. [Google Scholar] [CrossRef] [PubMed]
- Robles, D.; Brizuela, A.; Fernández-Domínguez, M.; Gil, J. Osteoblastic and Bacterial Response of Hybrid Dental Implants. J. Funct. Biomater. 2023, 14, 321. [Google Scholar] [CrossRef] [PubMed]
- Lye, K.W.; Deatherage, J.R.; Waite, P.D. The use of demineralized bone matrix for grafting during Le Fort I and chin osteotomies: Techniques and complications. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2008, 66, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Friederici, V.; Bruinink, A.; Imgrund, P.; Seefried, S. Getting the powder mix right for design of bone implants. Met. Powder Rep. 2010, 65, 14–16. [Google Scholar] [CrossRef]
- AlJasser, R.N.; AlSarhan, M.A. Analysis of Prosthetic Factors Affecting Peri-Implant Health: An in vivo Retrospective Study. J. Multidiscip. Healthc. 2021, 14, 1183–1191. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saberian, E.; Jenča, A.; Seyfaddini, R.; Jenča, A.; Zare-Zardini, H.; Petrášová, A.; Jenčová, J. Comparative Analysis of Osteoblastic Responses to Titanium and Alumina-Toughened Zirconia Implants: An In Vitro Study. Biomolecules 2024, 14, 719. https://doi.org/10.3390/biom14060719
Saberian E, Jenča A, Seyfaddini R, Jenča A, Zare-Zardini H, Petrášová A, Jenčová J. Comparative Analysis of Osteoblastic Responses to Titanium and Alumina-Toughened Zirconia Implants: An In Vitro Study. Biomolecules. 2024; 14(6):719. https://doi.org/10.3390/biom14060719
Chicago/Turabian StyleSaberian, Elham, Andrej Jenča, Rahman Seyfaddini, Andrej Jenča, Hadi Zare-Zardini, Adriána Petrášová, and Janka Jenčová. 2024. "Comparative Analysis of Osteoblastic Responses to Titanium and Alumina-Toughened Zirconia Implants: An In Vitro Study" Biomolecules 14, no. 6: 719. https://doi.org/10.3390/biom14060719
APA StyleSaberian, E., Jenča, A., Seyfaddini, R., Jenča, A., Zare-Zardini, H., Petrášová, A., & Jenčová, J. (2024). Comparative Analysis of Osteoblastic Responses to Titanium and Alumina-Toughened Zirconia Implants: An In Vitro Study. Biomolecules, 14(6), 719. https://doi.org/10.3390/biom14060719




