Correlation between Melatonin and Colostral Regulatory T Cells in Giardia lamblia Infection
Abstract
1. Introduction
2. Materials and Methods
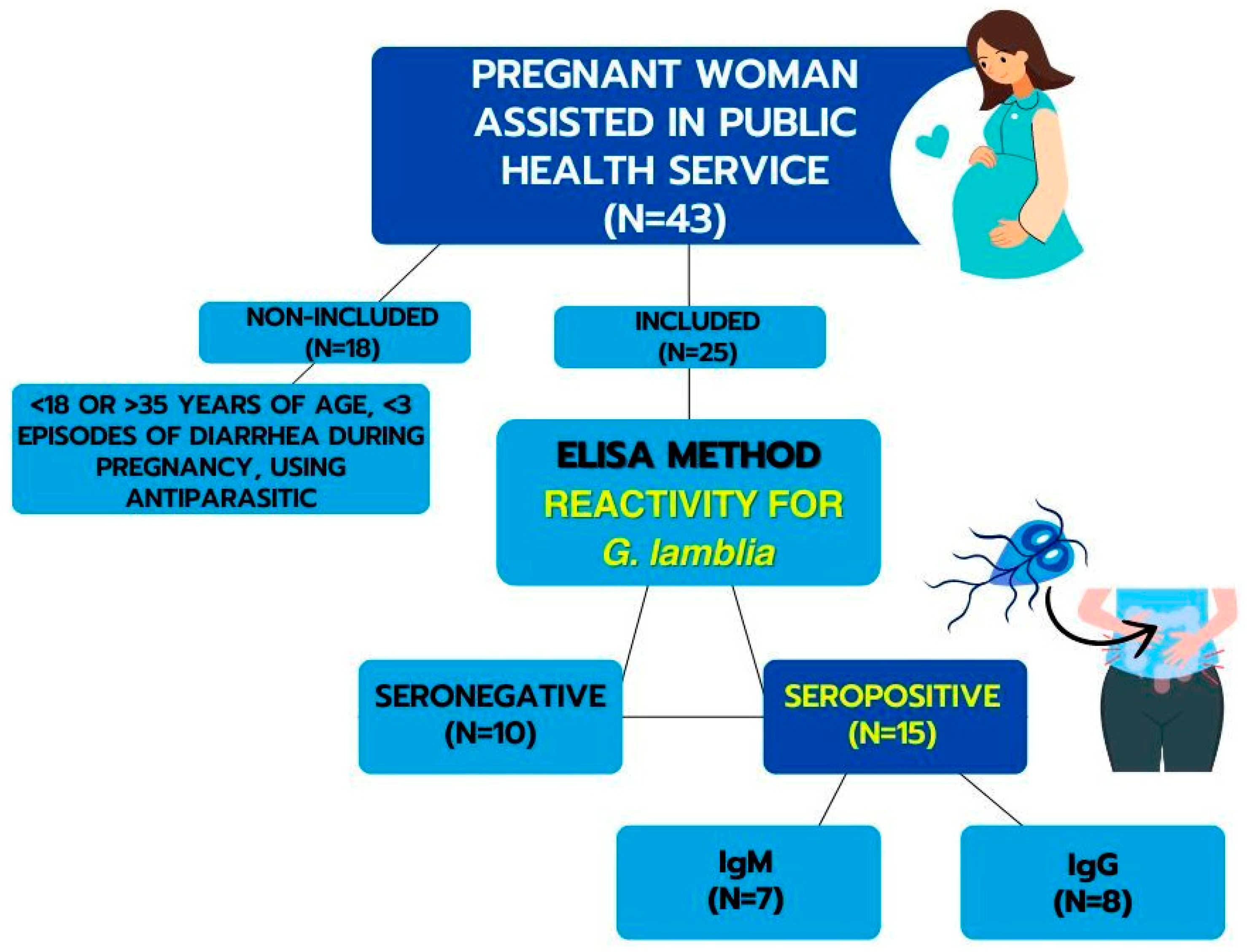
2.1. Subjects
2.2. Obtaining Serum and Determination of Serology for G. lamblia
2.3. Obtaining Supernatant and Colostrum Cells
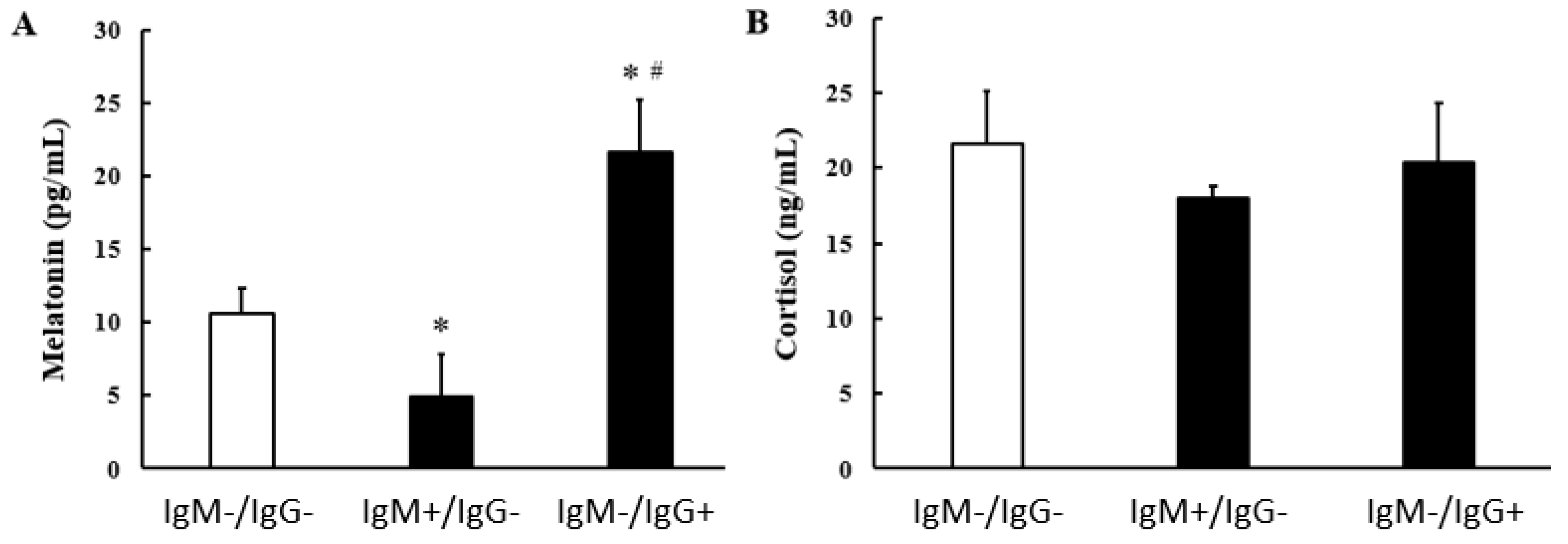
2.4. Melatonin Concentration Dosage
2.5. Cortisol Concentration Measurement
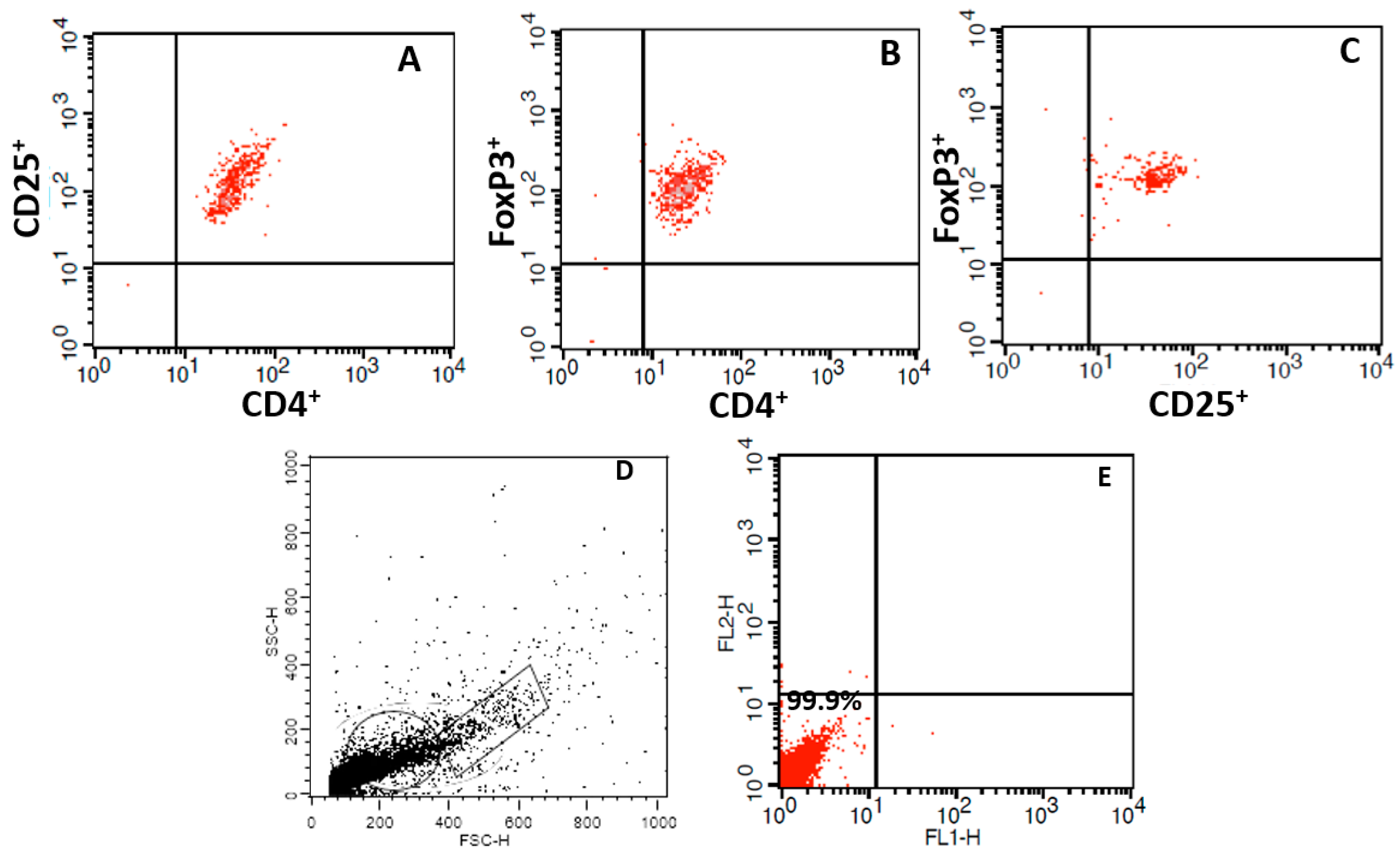
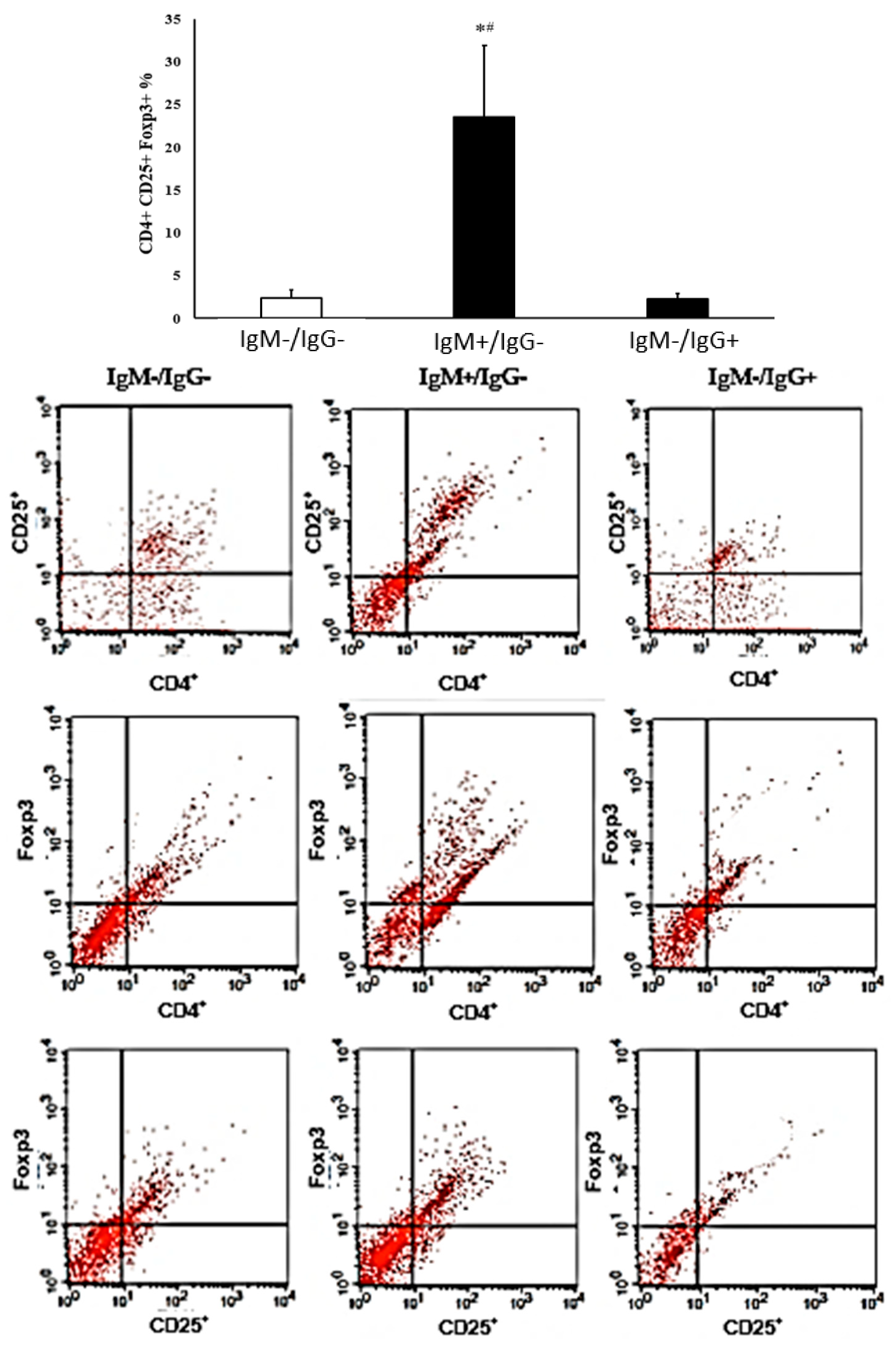
2.6. Immunophenotyping
2.7. Quantification of IL-10 and TGF-β Cytokines
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adam, R.D. Biology of Giardia lamblia. Clin. Microbiol. Rev. 2001, 14, 447–475. [Google Scholar] [CrossRef] [PubMed]
- Savioli, L.; Smith, H.; Thompson, A. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol. 2006, 22, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, E.; Ma’ayeh, S.; Svärd, S.G. An update on Giardia and giardiasis. Curr. Opin. Microbiol. 2016, 34, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Buret, A.G. Mechanisms of epithelial dysfunction in giardiasis. Gut 2007, 56, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Berkman, D.S.; Lescano, A.G.; Gilman, R.H.; Lopez, S.L.; Black, M.M. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: A follow-up study. Lancet 2002, 359, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Mondal, D.; Karim, A.; Molla, I.H.; Rahim, A.; Faruque, A.S.; Ahmad, N.; Kirkpatrick, B.D.; Houpt, E.; Snider, C.; et al. Prospective case-control study of the association between common enteric protozoal parasites and diarrhea in Bangladesh. Clin. Infect. Dis. 2009, 48, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.D. Intestinal protozoal parasites and diarrheal disease in Bangladesh. Clin. Infect. Dis. 2009, 48, 1198–1200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gottstein, B.; Harriman, G.R.; Conrad, J.T.; Nash, T.E. Antigenic variation in Giardia lamblia: Cellular and humoral immune response in a mouse model. Parasite Immunol. 1990, 12, 659–673. [Google Scholar] [CrossRef] [PubMed]
- Hanevik, K.; Hausken, T.; Morken, M.H.; Strand, E.A.; Mørch, K.; Coll, P.; Helgeland, L.; Langeland, N. Persisting symptoms and duodenal inflammation related to Giardia duodenalis infection. J. Infect. 2007, 55, 524–530. [Google Scholar] [CrossRef]
- Pereira, Q.L.C.; Hara, C.C.P.; Fernandes, R.T.S.; Fagundes, D.L.G.; França-Botelho, A.D.C.; Gomes, M.A.; França, E.L.; Honorio-França, A.C. Human colostrum action against Giardia lamblia infection influenced by hormones and advanced maternal age. Parasitol. Res. 2018, 117, 1783–1791. [Google Scholar] [CrossRef]
- Rópolo, A.S.; Touz, M.C. A lesson in survival, by Giardia lamblia. Sci. World J. 2010, 10, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.J.; Hanevik, K.; Escobedo, A.A.; Mørch, K.; Langeland, N. Giardiasis—Why do the symptoms sometimes never stop? Trends Parasitol. 2010, 26, 75–82. [Google Scholar] [CrossRef]
- França-Botelho, A.C.; Honório-França, A.C.; França, E.L.; Gomes, M.A.; Costa-Cruz, J.M. Phagocytosis of Giardia lamblia trophozoites by human colostral leukocytes. Acta Paediatr. 2006, 95, 438–443. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Chappell, C.L.; Hossain, M.M.; Huang, D.B.; Habib, M.; DuPont, H.L. Impact of breastfeeding on Giardia lamblia infections in Bilbeis, Egypt. Am. J. Trop. Med. Hyg. 2001, 65, 257–260. [Google Scholar] [CrossRef]
- Cérbulo-Vázquez, A.; Hernández-Peláez, G.; Arriaga-Pizano, L.A.; Bautista-Pérez, P.; Romero-Venado, J.; Flores-González, J.C.; Figueroa-Damian, R.; Soriano-Becerril, D.; Mancilla-Herrera, I. Characterization of CD127− CD25++ Treg from human colostrum. Am. J. Reprod. Immunol. 2018, 79, e12806. [Google Scholar] [CrossRef]
- Palmeira, P.; Carneiro-Sampaio, M. Immunology of breast milk. Rev. Assoc. Med. Bras. 2016, 62, 584–593. [Google Scholar] [CrossRef]
- Verhasselt, V. Neonatal tolerance under breastfeeding influence: The presence of allergen and transforming growth factor-beta in breast milk protects the progeny from allergic asthma. J. Pediatr. 2010, 156, S16–S20. [Google Scholar] [CrossRef] [PubMed]
- Grit, G.H.; Devriendt, B.; Van Coppernolle, S.; Geurden, T.; Hope, J.; Vercruysse, J.; Cox, E.; Geldhof, P.; Claerebout, E. Giardia duodenalis stimulates partial maturation of bovine dendritic cells associated with altered cytokine secretion and induction of T-cell proliferation. Parasite Immunol. 2014, 36, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, D.L.G.; França, E.L.; Hara, C.C.P.; Honorio-França, A.C. Immunomodulatory Effects of Poly (Ethylene Glycol) Microspheres Adsorbed with Cortisol on Activity of Colostrum Phagocytes. Int. J. Pharmacol. 2012, 8, 510–518. [Google Scholar] [CrossRef]
- Hara, C.C.P.; Honorio-França, A.C.; Fagundes, D.L.G.; Guimarães, P.C.L.; França, E.L. Melatonin Nanoparticles Adsorbed to Polyethylene Glycol Microspheres as Activators of Human Colostrum Macrophages. J. Nanomater. 2013, 2013, 973179. [Google Scholar] [CrossRef]
- Honorio-França, A.C.; Hara, C.C.P.; Ormonde, J.V.S.; Nunes, G.T.; França, E.L. Human colostrum melatonin exhibits a day-night variation and modulates the activity of colostral phagocytes. J. Appl. Biomed. 2013, 11, 153–162. [Google Scholar] [CrossRef]
- França, E.L.; Calderon, I.M.P.; Vieira, E.L.; Morceli, G.; Honorio-França, A.C. Transfer of maternal immunity to newborns of diabetic mothers. J. Immuol. Res. 2012, 2012, 928187. [Google Scholar] [CrossRef] [PubMed]
- Diamond, L.S.; Harlow, D.R.; Cunnick, C.C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 431–432. [Google Scholar] [CrossRef]
- Pacheco, F.T.F.; de Carvalho, S.S.; Santos, S.A.; das Chagas, G.M.T.; Santos, M.C.; Santos, J.G.S.; da Costa-Ribeiro, H., Jr.; Ribeiro, T.C.M.; de Mattos, Â.P.; Gomes, M.A.; et al. Specific IgG and IgA Antibody Reactivities in Sera of Children by Enzyme-Linked Immunoassay and Comparison with Giardia duodenalis Diagnosis in Feces. Ann. Lab. Med. 2020, 40, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for quantitating microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.R.; Thompson, R.C. The zoonotic transmission of Giardia and Cryptosporidium. Int. J. Parasitol. 2005, 35, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.E.P.; Barbosa, M.C.R.; Ferreira, B.R. High prevalence of enteroparasites in children from Ribeirão Preto, São Paulo, Brazil. Rev. Bras. Enferm. 2017, 70, 566–571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, Y.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef]
- Liu, L.; Chu, Y.; Oza, S.; Hogan, D.; Perin, J.; Bassani, D.G.; Ram, U.; Fadel, S.A.; Pandey, A.; Dhingra, N.; et al. National, Regional, and State-Level All-Cause and Cause-Specific Under-5 Mortality in India in 2000–15: A Systematic Analysis with Implications for the Sustainable Development Goals. Lancet Glob. Health 2019, 7, e721–e734. [Google Scholar] [CrossRef] [PubMed]
- Reiner, D.S.; Wang, C.S.; Gillin, F.D. Human milk kills Giardia lamblia by generating toxic lipolytic products. J. Infect. Dis. 1986, 154, 825–832. [Google Scholar] [CrossRef]
- França-Botelho, A.C.; França, J.L.; Oliveira, F.M.; Franca, E.L.; Honório-França, A.C.; Caliari, M.V.; Gomes, M.A. Melatonin reduces the severity of experimental amoebiasis. Parasit. Vectors 2011, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Daryani, A.; Montazeri, M.; Pagheh, A.S.; Sharif, M.; Sarvi, S.; Hosseinzadeh, A.; Reiter, R.J.; Hadighi, R.; Joghataei, M.T.; Ghaznavi, H.; et al. The potential use of melatonin to treat protozoan parasitic infections: A review. Biomed. Pharmacother. 2018, 97, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.I.R.; Neto, J.R.C.; Guerra, R.O.; Silva, P.E.F.; Braga, Y.L.L.; Celes, M.R.N.; Menezes, L.B.; Miguel, M.P.; Machado, J.R. Melatonin: A Look at Protozoal and Helminths. Biochimie 2024, 219, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Santello, F.H.; Frare, E.O.; dos Santos, C.D.; Toldo, M.P.; Kawasse, L.M.; Zucoloto, S.; do Prado, J.C., Jr. Melatonin Treatment Reduces the Severity of Experimental Trypanosoma cruzi Infection. J. Pineal Res. 2007, 42, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadraawya, S.K.; Al-Turabian, M.E.; Al-musawib, M.M.; Alzeyadia, M. Ghrelin and melatonin as biomarkers in patients with giardiasis. Biotechnol. Biotechnol. Equip. 2016, 30, 553–557. [Google Scholar] [CrossRef][Green Version]
- Amaral, R.S.; Freitas, J.F.; Ribeiro, M.R.S.; Machado, D.C.C.; Rocha, F.F.; Teixeira, M.C.A.; Cardoso, V.N.; Andrade, M.E.R.; Silva, C.A.V.; Caliari, M.V.; et al. Effect of dexamethasone on experimental enteritis produced by Giardia lamblia in a Meriones unguiculatus model. Exp. Parasitol. 2021, 230, 108158. [Google Scholar] [CrossRef] [PubMed]
- Josefowicz, S.Z.; Lu, L.F.; Rudensky, A.Y. Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 2012, 30, 531–564. [Google Scholar] [CrossRef] [PubMed]
- Majlessi, L.; Lo-Man, R.; Leclerc, C. Regulatory B and T cells in infections. Microbes Infect. 2008, 10, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Kizilbash, N.; Suhail, N.; Alzahrani, A.K.; Basha, W.J.; Soliman, M. Natural regulatory T cells increase significantly in pediatric patients with parasitic infections: Flow cytometry study. Indian. J. Pathol. Microbiol. 2023, 66, 556–559. [Google Scholar] [CrossRef]
- Fernández-Lainez, C.; de la Mora-de la Mora, I.; Enríquez-Flores, S.; García-Torres, I.; Flores-López, L.A.; Gutiér-rez-Castrellón, P.; de Vos, P.; López-Velázquez, G. The Giardial Arginine Deiminase Participates in Giardia-Host Immunomodulation in a Structure-Dependent Fashion via Toll-like Receptors. Int. J. Mol. Sci. 2022, 23, 11552. [Google Scholar] [CrossRef]
- Grit, G.H.; Van Coppernolle, S.; Devriendt, B.; Geurden, T.; Dreesen, L.; Hope, J.; Vercruysse, J.; Cox, E.; Geldhof, P.; Claerebout, E. Evaluation of cellular and humoral systemic immune response against Giardia duodenalis infection in cattle. Vet. Parasitol. 2014, 202, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, I.A.; Cortés, A.; Klotz, C.; Kühl, A.A.; Heimesaat, M.M.; Cantacessi, C.; Hartmann, S.; Rausch, S. RORγt+ Treg to Th17 ratios correlate with susceptibility to Giardia infection. Sci. Rep. 2019, 9, 20328. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Niu, C.; Sun, C.; Ma, Y.; Guo, R.; Ruan, Z.; Gao, Y.; Lu, X.; Li, H.; Lin, Y.; et al. Melatonin exerts immunoregulatory effects by balancing peripheral effector and regulatory T helper cells in myasthenia gravis. Aging 2020, 12, 21147–21160. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, L.; Wei, J.E.; Xie, M.R.; Wang, S.E.; Zhou, R.X. Role of CD4+ CD25+ regulatory T cells in melatonin-mediated inhibition of murine gastric cancer cell growth in vivo and in vitro. Anat. Rec. 2011, 294, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Li, T.; et al. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62, e12394. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Campillo, P.; Sarmiento-Soto, H.; Álvarez-Sánchez, N.; Álvarez-Ríos, A.I.; Guerrero, J.M.; Rodríguez-Prieto, I.; Castillo-Palma, M.J.; Lardone, P.J.; Carrillo-Vico, A. Evaluation of the immunomodulatory effect of melatonin on the T-cell response in peripheral blood from systemic lupus erythematosus patients. J. Pineal Res. 2015, 58, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Bocian, K.; Kiernozek, E.; Domagała-Kulawik, J.; Korczak-Kowalska, G.; Stelmaszczyk-Emmel, A.; Drela, N. Expanding Diversity and Common Goal of Regulatory T and B Cells. I: Origin, Phenotype, Mechanisms. Arch. Immunol. Ther. Exp. 2017, 65, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Romero, G.; Quintero, J.; Astiazarán-García, H.; Velazquez, C. Host defenses against Giardia lamblia. Parasite Immunol. 2015, 37, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, M.R.; Mehmet, N.; Durmaz, R. Role of IL-2, IL-4, and IL-10 in patients infected with Giardia lamblia. Turk. Parazitol. Derg. 2005, 29, 160–162. [Google Scholar]
- Moraes, L.C.; França, E.L.; Pessoa, R.S.; Fagundes, D.L.; Hernandes, M.G.; Ribeiro, V.P.; Gomes, M.A.; Honorio-França, A.C. The effect of IFN-γ and TGF-β in the functional activity of mononuclear cells in the presence of Entamoeba histolytica. Parasit. Vectors 2015, 8, 413. [Google Scholar] [CrossRef]
- Brune, M.W.; França, E.L.; Moraes, L.C.A.; Ribeiro, V.P.; Gomes, M.A.; Honorio-França, A.C. Effects of Cytokines IFN-γ and TGF-β on the Functional Activity of Blood Mononuclear Cells against Giardia lamblia. Iran. J. Parasitol. 2021, 16, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Masoori, L.; Khalaf, A.K.; Ezatkhah, F.; Balaña-Fouce, R.; Mahmoudvand, H. Promising Effects of 1,8 Cineole to Control Giardia lamblia Infection: Targeting the Inflammation, Oxidative Stress, and Infectivity. Acta Trop. 2024, 255, 107201. [Google Scholar] [CrossRef] [PubMed]
- Fontenele, A.L.A.; Nóbrega, C.G.O.; Nascimento, W.R.C.; Santos, P.D.A.; Lorena, V.M.B.; Peixoto, D.M.; Alburquerque, M.C.P.A.; Solé, D.; Costa, V.M.A.; Sarinho, E.S.C.; et al. Respiratory Allergy Symptoms and Cytokine Profiles in the Presence of Anti-Ascaris Antibody in Giardia lamblia–Infected Children. Parasitol. Res. 2023, 122, 3147–3158. [Google Scholar] [CrossRef] [PubMed]
- Saghaug, C.S.; Sørnes, S.; Peirasmaki, D.; Svärd, S.; Langeland, N.; Hanevik, K. Human Memory CD4+ T Cell Immune Responses against Giardia lamblia. Clin. Vaccine Immunol. 2015, 23, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Weatherhead, J.; Cortés, A.A.; Sandoval, C.; Vaca, M.; Chico, M.; Loor, S.; Cooper, P.J.; Mejia, R. Comparison of Cytokine Responses in Ecuadorian Children Infected with Giardia, Ascaris, or Both Parasites. Am. J. Trop. Med. Hyg. 2017, 96, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, M.M.; Hussein, M.H.; Hafedh, A.A. Evaluation of IL-2, IL-4 and IL-10 levels in patients with giardiasis. Ann. Parasitol. 2021, 67, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Serradell, M.C.; Gargantini, P.R.; Saura, A.; Oms, S.R.; Rupil, L.L.; Berod, L.; Sparwasser, T.; Luján, H.D. Cytokines, Antibodies, and Histopathological Profiles during Giardia Infection and Variant-Specific Surface Protein-Based Vaccination. Infect. Immun. 2018, 86, e00773-17. [Google Scholar] [CrossRef] [PubMed]
- Bansal, D.; Bhatti, H.S.; Sehgal, R. Role of cholesterol in parasitic infections. Lipids Health Dis. 2005, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Dann, S.M.; Le, C.H.Y.; Hanson, E.M.; Ross, M.C.; Eckmann, L. Giardia Infection of the Small Intestine Induces Chronic Colitis in Genetically Susceptible Hosts. J. Immunol. 2018, 201, 548–559. [Google Scholar] [CrossRef]
- França, E.L.; Nicomedes, T.R.; Calderon, I.M.P.; Honorio-França, A.C. Time-dependent Alterations of Soluble and Cellular Components in Human Milk. Biol. Rhythm. Res. 2011, 41, 333–347. [Google Scholar] [CrossRef]
- Morceli, G.; França, E.L.; Magalhães, V.B.; Damasceno, D.C.; Calderon, I.M.P.; Honorio França, A.C. Diabetes induced immunological and biochemical changes in human colostrum. Acta Paediatr. 2011, 100, 100550–100556. [Google Scholar] [CrossRef] [PubMed]
- Bilenko, N.; Ghosh, R.; Levy, A.; Deckelbaum, R.J.; Fraser, D. Partial breastfeeding protects Bedouin infants from infection and morbidity: Prospective cohort study. Asia Pac. J. Clin. Nutr. 2008, 17, 243–249. [Google Scholar] [PubMed]
- Morais, T.C.; Honorio-França, A.C.; Fujimori, M.; de Quental, O.B.; Pessoa, R.S.; França, E.L.; de Abreu, L.C. Melatonin Action on the Activity of Phagocytes from the Colostrum of Obese Women. Medicina 2019, 55, 625. [Google Scholar] [CrossRef] [PubMed]
| Cytokines | Antibody Anti-G. lamblia | ||
|---|---|---|---|
| Non-Reagent | IgM+/IgG− | IgM−/IgG+ | |
| IL-10 (pg/mL) | 25.53 ± 11.40 | 38.59 ± 7.17 | 32.47 ± 13.45 |
| TGF-β (pg/mL) | 95.73 ± 18.91 | 124.14 ± 2.14 * | 103.05 ± 9.63 |
| Antibody Anti-G. lamblia | Non-Reagent | IgM+/IgG− | IgM−/IgG+ | |||
|---|---|---|---|---|---|---|
| Rs | p | Rs | p | Rs | p | |
| Melatonin/Cortisol | −0.2985 | 0.5655 | 0.6455 | 0.2394 | −0.7537 | 0.0835 |
| Melatonin/Treg | −0.8317 | 0.0401 * | 0.8660 | 0.0476 * | −0.3339 | 0.5177 |
| Melatonin/IL-10 | −0.5643 | 0.3217 | 0.2962 | 0.6285 | −0.2571 | 0.6228 |
| Melatonin/TGF-β | 0.2899 | 0.5773 | 0.5774 | 0.3080 | 0.4857 | 0.3287 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Queiroz, A.A.; França, E.L.; Gadenz, G.R.B.; Dalcin, L.D.L.; Fujimori, M.; França, D.C.H.; Gomes, M.A.; Honorio-França, A.C. Correlation between Melatonin and Colostral Regulatory T Cells in Giardia lamblia Infection. Biomolecules 2024, 14, 744. https://doi.org/10.3390/biom14070744
de Queiroz AA, França EL, Gadenz GRB, Dalcin LDL, Fujimori M, França DCH, Gomes MA, Honorio-França AC. Correlation between Melatonin and Colostral Regulatory T Cells in Giardia lamblia Infection. Biomolecules. 2024; 14(7):744. https://doi.org/10.3390/biom14070744
Chicago/Turabian Stylede Queiroz, Adriele Ataides, Eduardo Luzía França, Gabriella Regina Borges Gadenz, Letícia Damas Leão Dalcin, Mahmi Fujimori, Danielle Cristina Honorio França, Maria Aparecida Gomes, and Adenilda Cristina Honorio-França. 2024. "Correlation between Melatonin and Colostral Regulatory T Cells in Giardia lamblia Infection" Biomolecules 14, no. 7: 744. https://doi.org/10.3390/biom14070744
APA Stylede Queiroz, A. A., França, E. L., Gadenz, G. R. B., Dalcin, L. D. L., Fujimori, M., França, D. C. H., Gomes, M. A., & Honorio-França, A. C. (2024). Correlation between Melatonin and Colostral Regulatory T Cells in Giardia lamblia Infection. Biomolecules, 14(7), 744. https://doi.org/10.3390/biom14070744






