PPARs in Clinical Experimental Medicine after 35 Years of Worldwide Scientific Investigations and Medical Experiments
Abstract
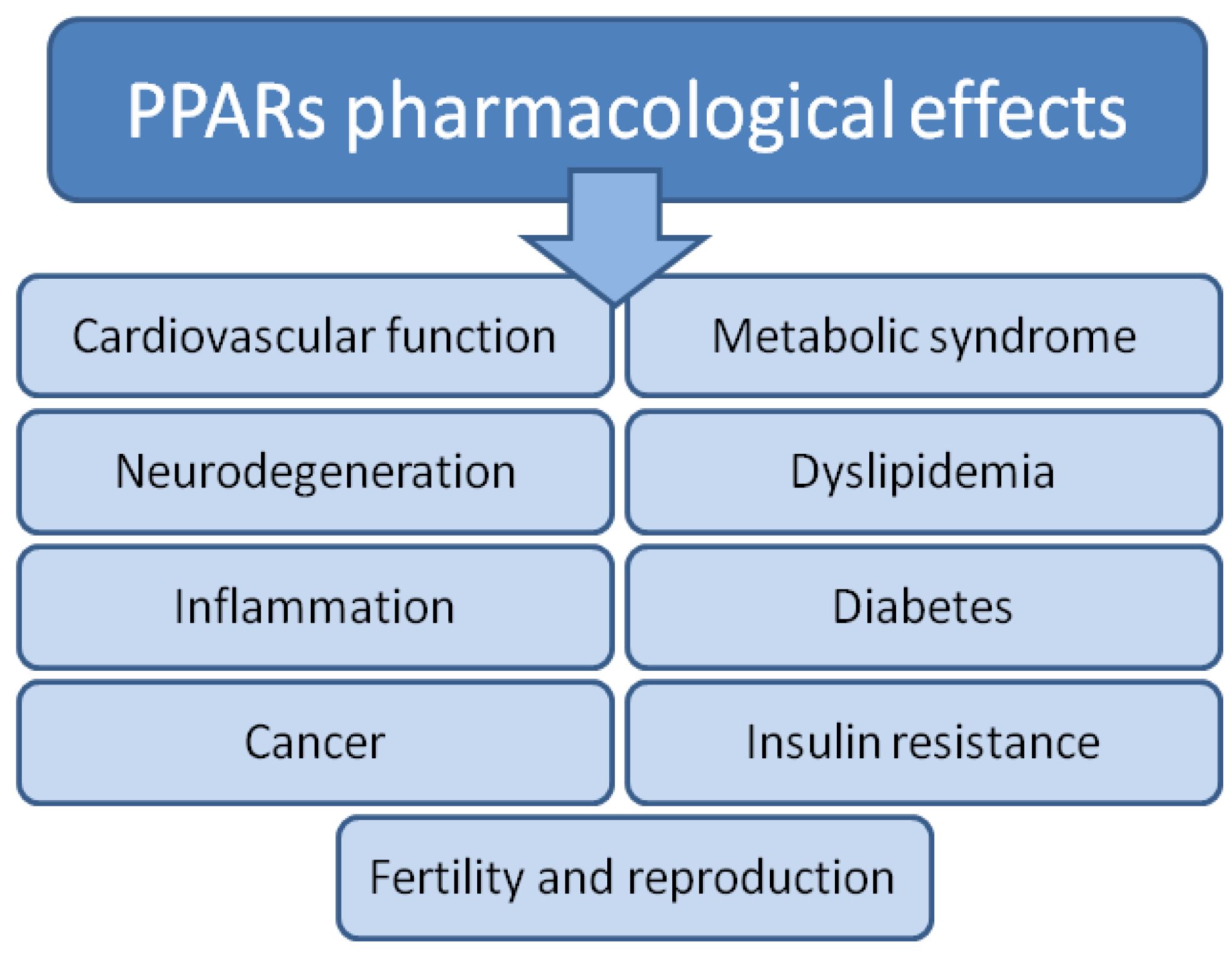
1. Introduction
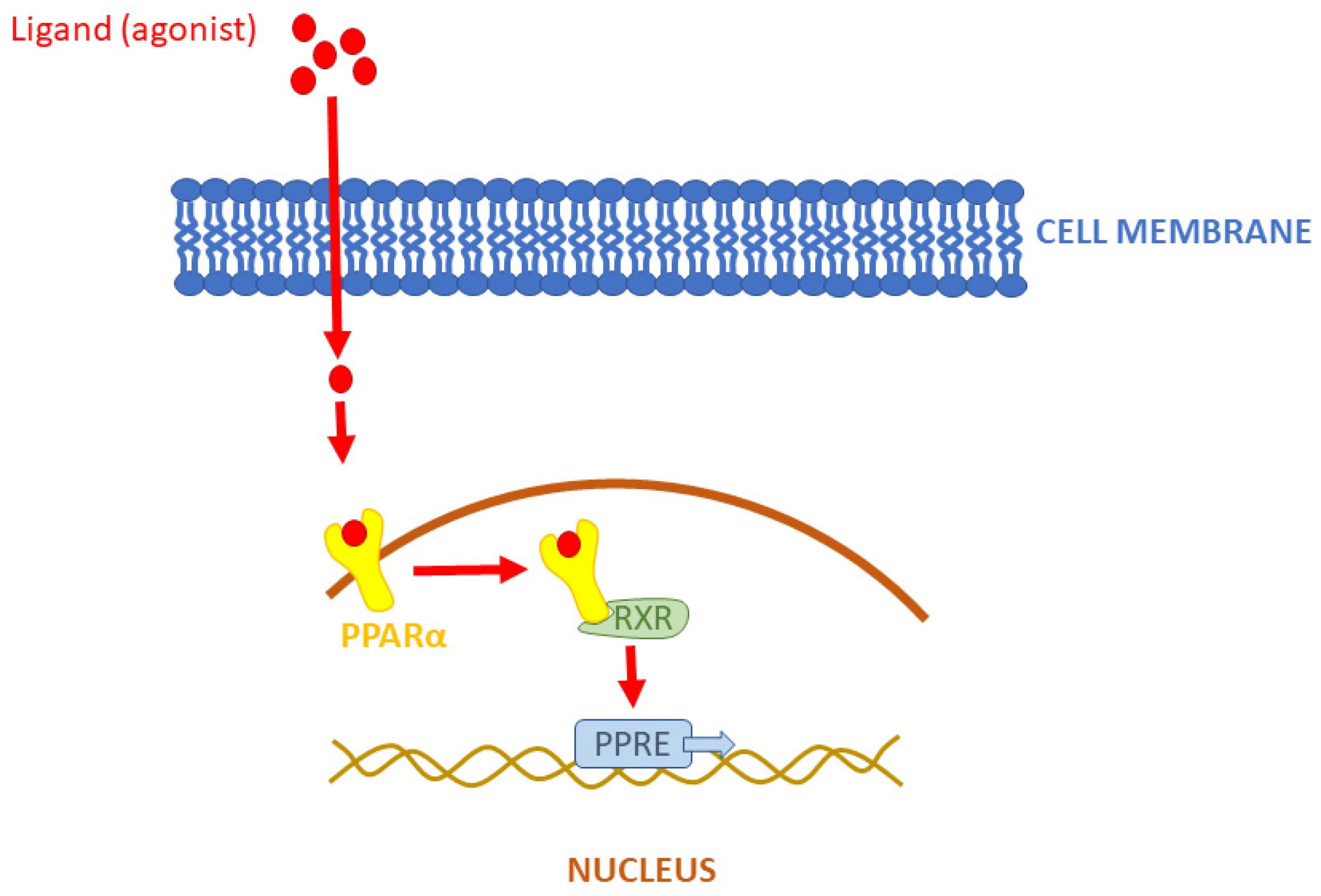
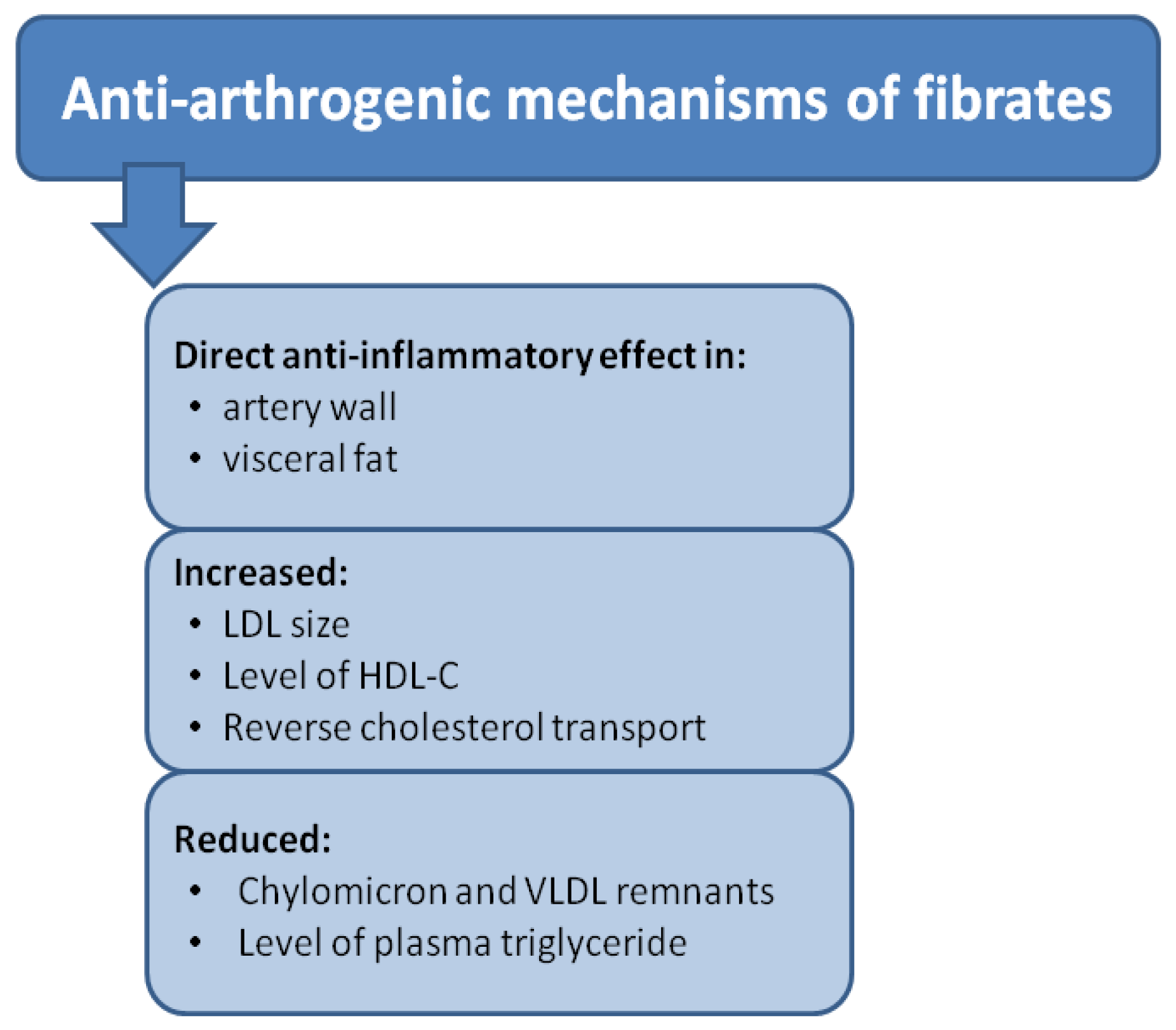
1.1. Peroxisome Proliferator-Activated Receptor Alpha (PPARα)
1.2. Peroxisome Proliferator-Activated Receptor Beta/Delta (PPARβ/δ)
1.3. Peroxisome Proliferator-Activated Receptor Gamma (PPARγ)
1.4. Pan-PPAR (Alpha, Beta/Delta, Gamma)
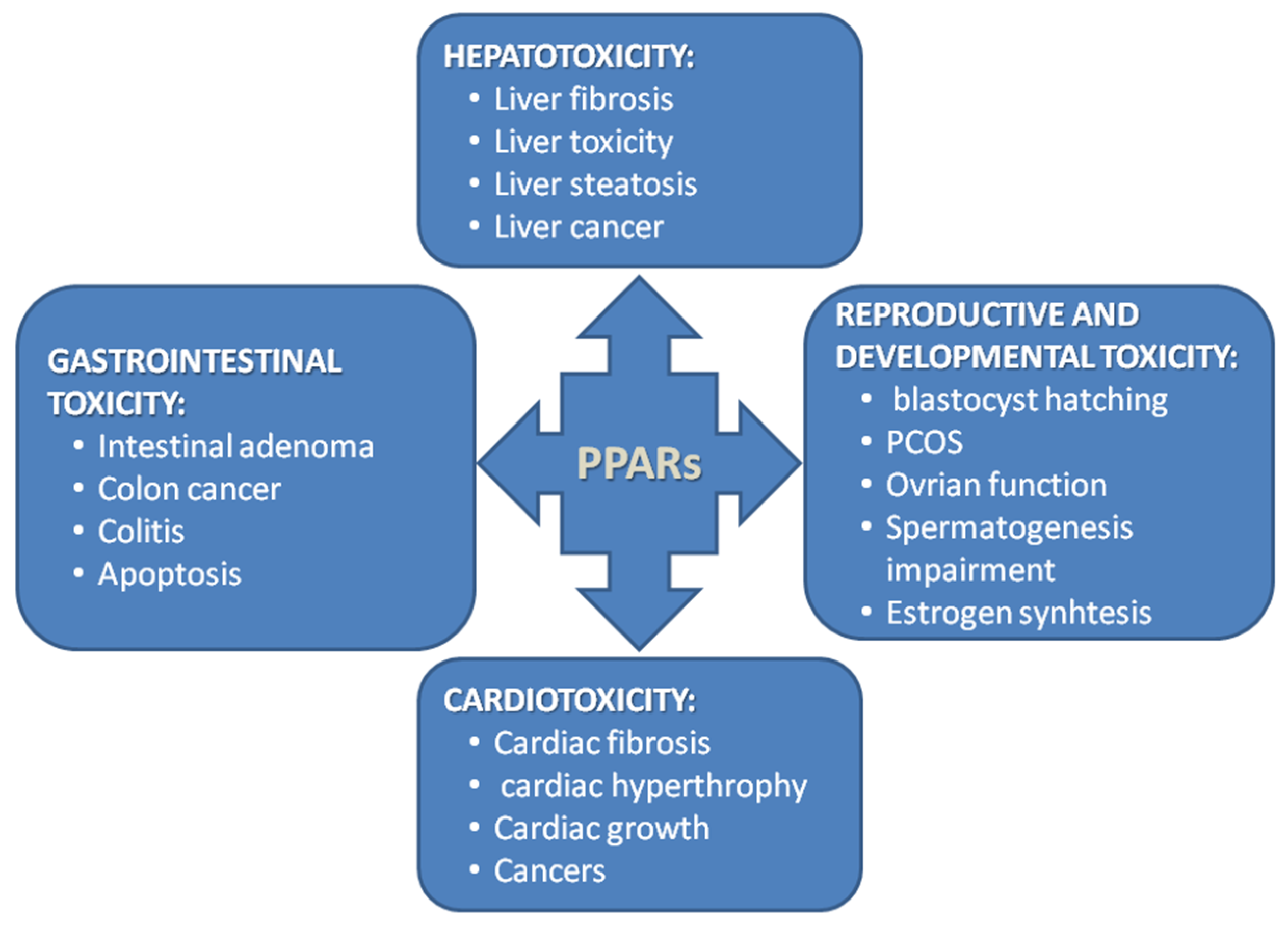
2. Aim of the Work
3. Results
3.1. Introduction
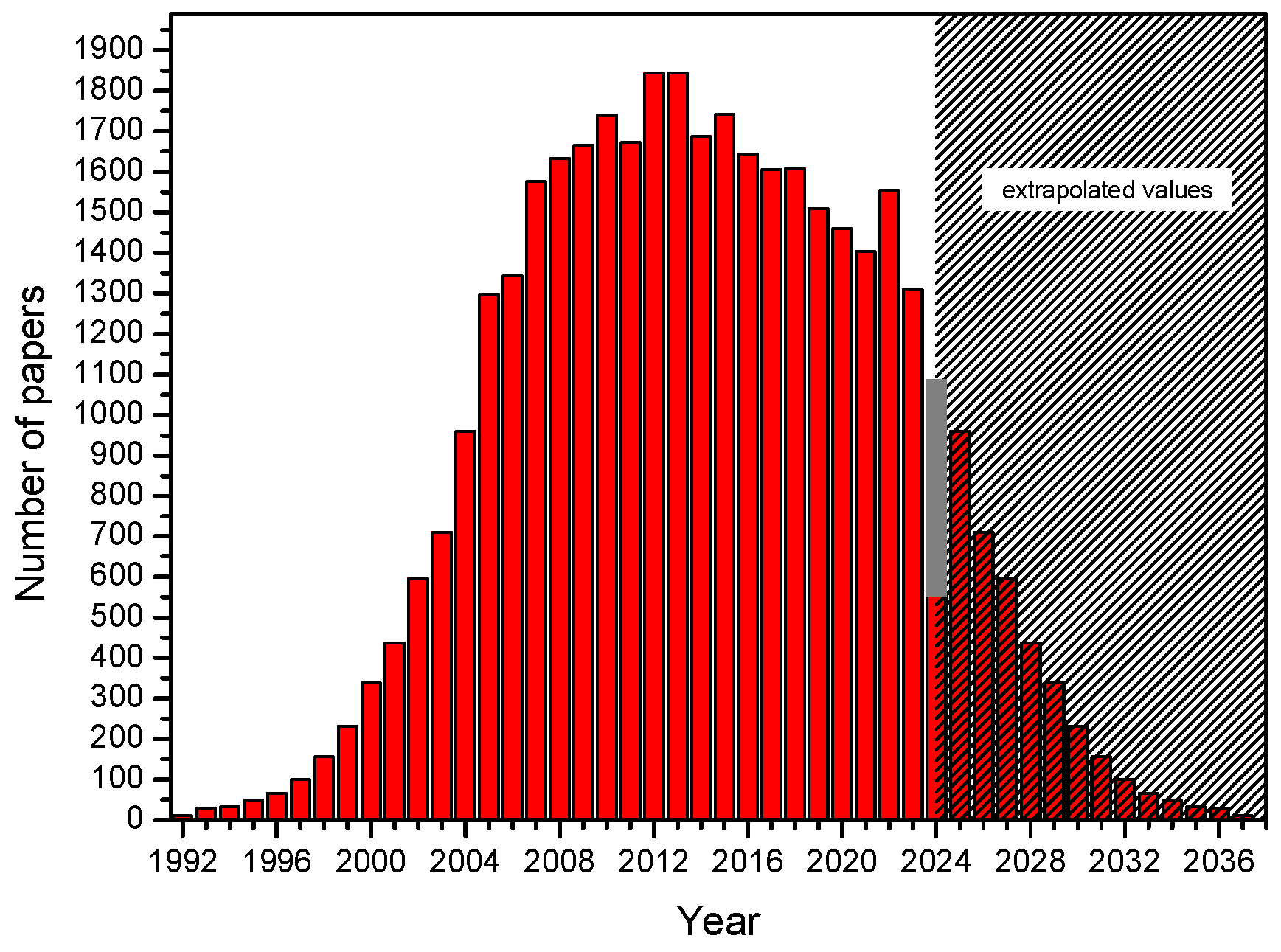
3.2. Electronic Search Strategy
3.3. Calendar with Commentary
4. Discussion
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vamecq, J.; Latruffe, N. Medical significance of peroxisome proliferator-activated receptors. Lancet 1999, 354, 141–148. [Google Scholar] [CrossRef]
- Issemann, I.; Green, S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990, 347, 645–650. [Google Scholar] [CrossRef]
- Dreyer, C.; Krey, G.; Keller, H.; Givel, F.; Helftenbein, G.; Wahli, W. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell 1992, 68, 879–887. [Google Scholar] [CrossRef]
- Reilly, M.M.; Rossor, A.M. Humans: The Ultimate Animal Models. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1132–1136. [Google Scholar] [CrossRef]
- Tahri-Joutey, M.; Andreoletti, P.; Surapureddi, S.; Nasser, B.; Cherkaoui-Malki, M.; Latruffe, N. Mechanisms Mediating the Regulation of Peroxisomal Fatty Acid Beta-Oxidation by PPARα. Int. J. Mol. Sci. 2021, 22, 8969. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yan, X.; Wang, G.; Liu, H.; Gan, X.; Zhang, T.; Wang, J.; Li, L. Evolutionary Pattern and Regulation Analysis to Support Why Diversity Functions Existed within PPAR Gene Family Members. Biomed. Res. Int. 2015, 2015, 613910. [Google Scholar] [CrossRef] [PubMed]
- Lathion, C.; Michalik, L.; Wahli, W. Physiological Ligands of PPARs in Inflammation and Lipid Homeostasis. Future Lipidol. 2006, 1, 191–201. [Google Scholar] [CrossRef]
- Willson, T.M.; Brown, P.J.; Sternbach, D.D.; Henke, B.R. The PPARs: From orphan receptors to drug discovery. J. Med. Chem. 2000, 43, 527–550. [Google Scholar] [CrossRef]
- Chinetti-Gbaguidi, G.; Fruchart, J.C.; Staels, B. Role of the PPAR family of nuclear receptors in the regulation of metabolic and cardiovascular homeostasis: New approaches to therapy. Curr. Opin. Pharmacol. 2005, 5, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.; Cha, B.S. Refocusing Peroxisome Proliferator Activated Receptor-α: A New Insight for Therapeutic Roles in Diabetes. Diabetes Metab. J. 2013, 37, 326–332. [Google Scholar] [CrossRef]
- Waterham, H.R.; Ferdinandusse, S.; Wanders, R.J. Human disorders of peroxisome metabolism and biogenesis. Biochim. Biophys. Acta. 2016, 1863, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Fruchart, J.C. Therapeutic Roles of Peroxisome Proliferator–Activated Receptor Agonists. Diabetes 2005, 54, 2460–2470. [Google Scholar] [CrossRef]
- Pyper, S.R.; Viswakarma, N.; Jia, Y.; Zhu, Y.J.; Fondell, J.D.; Reddy, J.K. PRIC295, a Nuclear Receptor Coactivator, Identified from PPARα-Interacting Cofactor Complex. PPAR Res. 2010, 2010, 173907. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, P.; Chinetti, G.; Fruchart, J.-C.; Staels, B. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. J. Clin. Investig. 2006, 116, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Gacka, M.; Adamiec, R. Mutacje genu receptora aktywowanego przez proliferatory peroksysomów γ (PPARγ)—Implikacje kliniczne. Postępy Hig. I Med. Doświadczalnej 2004, 58, 483–489. [Google Scholar]
- Cullingford, T.E.; Dolphin, C.T.; Sato, H. The peroxisome proliferator-activated receptor alpha-selective activator ciprofibrate upregulates expression of genes encoding fatty acid oxidation and ketogenesis enzymes in rat brain. Neuropharmacology 2002, 42, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.; Chen, L.; Majumdar, D.; Bullock, W.H.; Burns, M.; Claus, T.; Dela Cruz, F.E.; Daly, M.; Ehrgott, F.J.; Johnson, J.S.; et al. Indanylacetic acid derivatives carrying 4-thiazolyl-phenoxy tail groups, a new class of potent PPAR alpha/gamma/delta pan agonists: Synthesis, structure-activity relationship, and in vivo efficacy. J. Med. Chem. 2007, 50, 984–1000. [Google Scholar] [CrossRef] [PubMed]
- Huang-Jin, H.; Kuei-Jen, L.; Hsin, W.Y.; Hsin-Yi, C.; Fuu-Jen, T.; Calvin, Y.-C.C. A novel strategy for designing the selective PPAR agonist by the “sum of activity” model. J. Biomol. Struct. Dyn. 2010, 28, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Vamecq, J.; Cherkaoui-Malki, M.; Andreoletti, P.; Latruffe, N. The human peroxisome in health and disease: The story of an oddity becoming a vital organelle. Biochimie 2014, 98, 4–15. [Google Scholar] [CrossRef]
- Reddy, J.K.; Lalvvai, N.D.; Farber, E. Carcinogenesis by peroxisome proliferators: Evaluation of the risk of hypolipidemic drugs and industrial plasticizers to humans. Crit. Rev. Toxicol. 1988, 18, 1–58. [Google Scholar] [CrossRef]
- Reddy, J.K.; Rao, M.S. Peroxisome proliferators and cancer: Mechanisms and implications. Adv. Exp. Med. Biol. 1988, 283, 131–148. [Google Scholar]
- Martinez, E.; Givel, F.; Wahli, W.; Keller, H. The peroxisome proliferator-activated receptor (PPAR) is a transcriptional regulator of the peroxisomal beta-oxidation pathway. J. Cell Sci. 1991, 100, 711–717. [Google Scholar]
- Liu, J.; Ormö, M.; Nyström, A.C.; Claesson, J.; Giordanetto, F. Transient expression, purification and characterisation of human full-length PPARγ2 in HEK293 cells. Protein Expr. Purif. 2013, 89, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Alvares, K.; Carrillo, A.; Yuan, P.M.; Kawano, H.; Morimoto, R.I.; Reddy, J.K. Identification of cytosolic peroxisome proliferator binding protein as a member of the heat shock protein HSP70 family. Proc. Natl. Acad. Sci. USA 1990, 87, 5293–5297. [Google Scholar] [CrossRef] [PubMed]
- Maksymowych, A.B.; Hsu, T.C.; Litwack, G. A novel, highly conserved structural motif is present in all members of the steroid receptor superfamily. Receptor 1992, 2, 225–240. [Google Scholar] [PubMed]
- Göttlicher, M.; Demoz, A.; Svensson, D.; Tollet, P.; Berge, R.K.; Gustafsson, J.A. Structural and metabolic requirements for activators of the peroxisome proliferator-activated receptor. Biochem. Pharmacol. 1993, 46, 2177–2184. [Google Scholar] [CrossRef]
- Keller, H.; Wahli, W. Peroxisome proliferator-activated receptors A link between endocrinology and nutrition? Trends Endocrinol. Metab. 1993, 4, 291–296. [Google Scholar] [CrossRef]
- Keller, H.; Mahfoudi, A.; Dreyer, C.; Hihi, A.K.; Medin, J.; Ozato, K.; Wahli, W. Peroxisome proliferator-activated receptors and lipid metabolism. Ann. N. Y. Acad. Sci. 1993, 684, 157–173. [Google Scholar] [CrossRef]
- Issemann, I.; Prince, R.A.; Tugwood, J.D.; Green, S. The retinoid X receptor enhances the function of the peroxisome proliferator activated receptor. Biochimie 1993, 75, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Bardot, O.; Aldridge, T.C.; Latruffe, N.; Green, S. PPAR-RXR heterodimer activates a peroxisome proliferator response element upstream of the bifunctional enzyme gene. Biochem. Biophys. Res. Commun. 1993, 192, 37–45. [Google Scholar] [CrossRef]
- Boie, Y.; Adam, M.; Rushmore, T.H.; Kennedy, B.P. Enantioselective activation of the peroxisome proliferator-activated receptor. J. Biol. Chem. 1993, 268, 5530–5534. [Google Scholar] [CrossRef] [PubMed]
- Motojima, K. Proliferator-Activated Receptor (PPAR): Structure, Mechanisms of Activation and Diverse Functions. Cell Struct. Funct. 1993, 18, 267–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sher, T.; Yi, H.F.; McBride, O.W.; Gonzalez, F.J. cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry 1993, 32, 5598–5604. [Google Scholar] [CrossRef] [PubMed]
- Bass, N.M. Cellular binding proteins for fatty acids and retinoids: Similar or specialized functions? Mol. Cell Biochem. 1993, 123, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.G. Peroxisome proliferators: Paradigms and prospects. In Proceedings of the 1992 Conference on Toxicology: Application of Advances in Toxicology to Risk Assessment; Dodd, D.E., Clewell, H.J., III, Mattie, D.R., Eds.; ManTech Environmental Technology, Inc.: Dayton, OH, USA, 1993; pp. 194–202. [Google Scholar]
- Chen, F.; Law, S.W.; O’Malley, B.W. Identification of two mPPAR related receptors and evidence for the existence of five subfamily members. Biochem. Biophys. Res. Commun. 1993, 196, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Kainu, T.; Wikström, A.C.; Gustafsson, J.A.; Pelto-Huikko, M. Localization of the peroxisome proliferator-activated receptor in the brain. Neuroreport 1994, 5, 2481–2485. [Google Scholar] [CrossRef] [PubMed]
- Aleshin, S.; Grabeklis, S.; Hanck, T.; Sergeeva, M.; Reiser, G. Peroxisome proliferator-activated receptor (PPAR)-gamma positively controls and PPARalpha negatively controls cyclooxygenase-2 expression in rat brain astrocytes through a convergence on PPARbeta/delta via mutual control of PPAR expression levels. Mol Pharmacol. 2009, 76, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Lemberger, T.; Saladin, R.; Vázquez, M.; Assimacopoulos, F.; Staels, B.; Desvergne, B.; Wahli, W.; Auwerx, J. Expression of the peroxisome proliferator-activated receptor alpha gene is stimulated by stress and follows a diurnal rhythm. J. Biol. Chem. 1996, 271, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Wahli, W.; Braissant, O.; Desvergne, B. Peroxisome proliferator activated receptors: Transcriptional regulators of adipogenesis, lipid metabolism and more…. Chem. Biol. 1995, 2, 261–266. [Google Scholar] [CrossRef]
- Gustafsson, J.A. Receptor-mediated toxicity. Toxicol. Lett. 1995, 82–83, 465–470. [Google Scholar] [CrossRef]
- Bocos, C.; Göttlicher, M.; Gearing, K.; Banner, C.; Enmark, E.; Teboul, M.; Crickmore, A.; Gustafsson, J.A. Fatty acid activation of peroxisome proliferator-activated receptor (PPAR). J. Steroid. Biochem. Mol. Biol. 1995, 53, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Lemberger, T.; Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: A nuclear receptor signaling pathway in lipid physiology. Annu. Rev. Cell Dev. Biol. 1996, 12, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Bayona, W.; Kallen, C.B.; Harding, H.P.; Ravera, C.P.; McMahon, G.; Brown, M.; Lazar, M.A. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J. Biol. Chem. 1995, 270, 23975–23983. [Google Scholar] [CrossRef]
- Keller, H.; Givel, F.; Perroud, M.; Wahli, W. Signaling cross-talk between peroxisome proliferator-activated receptor/retinoid X receptor and estrogen receptor through estrogen response elements. Mol. Endocrinol. 1995, 9, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Juge-Aubry, C.E.; Gorla-Bajszczak, A.; Pernin, A.; Lemberger, T.; Wahli, W.; Burger, A.G.; Meier, C.A. Peroxisome proliferator-activated receptor mediates cross-talk with thyroid hormone receptor by competition for retinoid X receptor. Possible role of a leucine zipper-like heptad repeat. J. Biol. Chem. 1995, 270, 18117–18122. [Google Scholar] [CrossRef]
- Baes, M.; Castelein, H.; Desmet, L.; Declercq, P.E. Antagonism of COUP-TF and PPAR alpha/RXR alpha on the activation of the malic enzyme gene promoter: Modulation by 9-cis RA. Biochem. Biophys. Res. Commun. 1995, 215, 338–345. [Google Scholar] [CrossRef]
- Forman, B.M.; Chen, J.; Evans, R.M. The peroxisome proliferator-activated receptors: Ligands and activators. Ann. N. Y. Acad. Sci. 1996, 804, 266–275. [Google Scholar] [CrossRef]
- Willson, T.M.; Cobb, J.E.; Cowan, D.J.; Wiethe, R.W.; Correa, I.D.; Prakash, S.R.; Beck, K.D.; Moore, L.B.; Kliewer, S.A.; Lehmann, J.M. The structure-activity relationship between peroxisome proliferator- activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J. Med. Chem. 1996, 39, 665–668. [Google Scholar] [CrossRef]
- Aubert, J.; Ailhaud, G.; Negrel, R. Evidence for a novel regulatory pathway activated by (carba)prostacyclin in preadipose and adipose cells. FEBS Lett. 1996, 397, 117–121. [Google Scholar] [CrossRef]
- Patel, C.B.; De Lemos, J.A.; Wyne, K.L.; McGuire, D.K. Thiazolidinediones and risk for atherosclerosis: Pleiotropic effects of PPar gamma agonism. Diab. Vasc. Dis. Res. 2006, 3, 65–71. [Google Scholar] [CrossRef]
- Brun, R.P.; Tontonoz, P.; Forman, B.M.; Ellis, R.; Chen, J.; Evans, R.M.; Spiegelman, B.M. Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev. 1996, 10, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhang, Y.; Davis, L.; Breyer, M.D. Expression of peroxisome proliferator-activated receptors in urinary tract of rabbits and humans. Am. J. Physiol. 1997, 273, F1013–F1022. [Google Scholar] [CrossRef] [PubMed]
- Bernlohr, D.A.; Simpson, M.A.; Hertzel, A.V.; Banaszak, L.J. Intracellular lipid-binding proteins and their genes. Annu. Rev. Nutr. 1997, 17, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.D.; Jump, D. Polyunsaturated fatty acids regulate lipogenic and peroxisomal gene expression by independent mechanisms. Prostaglandins Leukot Essent Fat. Acids. 1997, 57, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A. Retinoid X receptors. Int. J. Biochem. Cell Biol. 1997, 29, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Schönbeck, U.; Lazar, M.A.; Libby, P.; Plutzky, J. Peroxisome Proliferator-Activated Receptor Gamma Activators Inhibit Gene Expression and Migra-tion in Human Vascular Smooth Muscle Cells. Circ Res. 1998, 83, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Kruszynska, Y.T.; Mukherjee, R.; Jow, L.; Dana, S.; Paterniti, J.R.; Olefsky, J.M. Skeletal muscle peroxisome proliferator- activated receptor-gamma expression in obesity and non- insulin-dependent diabetes mellitus. J. Clin. Investig. 1998, 101, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Schulman, I.G.; Shao, G.; Heyman, R.A. Transactivation by Retinoid X Receptor–Peroxisome Proliferator-Activated Receptor gamma (PPARgamma) Heterodimers: Intermolecular Synergy Requires Only the PPAR? Hormone-Dependent Activation Function. Mol. Cell Biol. 1998, 18, 3483–3494. [Google Scholar] [CrossRef]
- Ricote, M.; Huang, J.; Fajas, L.; Li, A.; Welch, J.; Najib, J.; Witztum, J.L.; Auwerx, J.; Palinski, W.; Glass, C.K. Expression of the peroxisome proliferator-activated receptor gamma (PPARgamma) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA 1998, 95, 7614–7619. [Google Scholar] [CrossRef]
- Zhou, Y.-T.; Shimabukuro, M.; Wang, M.-Y.; Lee, Y.; Higa, M.; Milburn, J.L.; Newgard, C.B.; Unger, R.H. Role of peroxisome proliferator-activated receptor alpha in disease of pancreatic ß cells. Proc. Natl. Acad. Sci. USA 1998, 95, 8898–8903. [Google Scholar] [CrossRef]
- Ribon, V.; Johnson, J.H.; Camp, H.S.; Saltiel, A.R. Thiazolidinediones and insulin resistance: Peroxisome proliferator activated receptor gamma activation stimulates expression of the CAP gene. Proc. Natl. Acad. Sci. USA 1998, 95, 14751–14756. [Google Scholar] [CrossRef]
- Shimomura, I.; Hammer, R.E.; Richardson, J.A.; Ikemoto, S.; Bashmakov, Y.; Goldstein, J.L.; Brown, M.S. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adi-pose tissue: Model for congenital generalized lipodystrophy. Genes Dev. 1998, 12, 3182–3194. [Google Scholar] [CrossRef] [PubMed]
- Elstner, E.; Müller, C.; Koshizuka, K.; Williamson, E.A.; Park, D.; Asou, H.; Shintaku, P.; Said, J.W.; Heber, D.; Koeffler, H.P. Ligands for peroxisome proliferator-activated receptor gamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc. Natl. Acad. Sci. USA 1998, 95, 8806–8811. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.N.; Treuter, E.; Gustafsson, J.A. Regulation of peroxisome proliferator-activated receptors. Vitam. Horm. 1998, 54, 121–166. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.B. Three independent lines of evidence suggest retinoids as causal to schizophrenia. Proc. Natl. Acad. Sci. USA 1998, 95, 7240–7244. [Google Scholar] [CrossRef]
- Guan, Y.-F.; Zhang, Y.-H.; Breyer, R.M.; Davis, L.; Breyer, M.D. Expression of Peroxisome Proliferator-Activated Receptor Gamma (PPARgamma) in Human Transition-al Bladder Cancer and its Role in Inducing Cell Death. Neoplasia 1999, 1, 330–339. [Google Scholar] [CrossRef]
- Demetri, G.D.; Fletcher, C.D.M.; Mueller, E.; Sarraf, P.; Naujoks, R.; Campbell, N.; Spiegelman, B.M.; Singer, S. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc. Natl. Acad. Sci. USA 1999, 96, 3951–3956. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Miyazaki, M.; Shinomura, Y.; Kondo, S.; Kanayama, S.; Matsuzawa, Y. Peroxisome Proliferator-activated Receptor Gamma Induces Growth Arrest and Differentiation Markers of Human Colon Cancer Cells. Jpn J. Cancer Res. 1999, 90, 75–80. [Google Scholar] [CrossRef]
- Sarraf, P.; Mueller, E.; Smith, W.M.; Wright, H.M.; Kum, J.B.; Aaltonen, L.A.; de la Chapelle, A.; Spiegelman, B.M.; Eng, C. Loss-of-function mutations in PPAR gamma associated with human colon cancer. Mol. Cell. 1999, 3, 799–804. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, C.; Jain, S.; Le Beau, M.M.; Espinosa, R., 3rd; Atkins, G.B.; Lazar, M.A.; Yeldandi, A.V.; Rao, M.S.; Reddy, J.K. Amplification and overexpression of peroxisome proliferator activated receptor binding protein (PBP/PPARBP) gene in breast cancer. Proc. Natl. Acad. Sci. USA 1999, 96, 10848–10853. [Google Scholar] [CrossRef]
- Doucas, V.; Evans, R.M. The human T-cell leukemia virus type 1 tax oncoprotein represses nuclear receptor signaling. Proc. Natl. Acad. Sci. USA 1999, 96, 2633–2638. [Google Scholar] [CrossRef] [PubMed]
- Shappell, S.B.; Boeglin, W.E.; Olson, S.J.; Kasper, S.; Brash, A.R. 15-Lipoxygenase-2 (15-LOX-2) Is Expressed in Benign Prostatic Epithelium and Reduced in Prostate Adenocarcinoma. Am. J. Pathol. 1999, 155, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G.; Canaple, L.; Saugy, D.; Wahli, W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Mol. Endocrinol. 2000, 14, 1962–1975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawahito, Y.; Kondo, M.; Tsubouchi, Y.; Hashiramoto, A.; Bishop-Bailey, D.; Inoue, K.; Kohno, M.; Yamada, R.; Hla, T.; Sano, H. 15-deoxy-delta(12,14)-PGJ(2) induces synoviocyte apoptosis and sup-presses adjuvant-induced arthritis in rats. J. Clin. Investig. 2000, 106, 189–197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tong, B.J.; Tan, J.; Tajeda, T.; Das, S.K.; Chapman, J.A.; DuBoist, R.N.; Dey, S.K. Heightened Expression of Cyclooxygenase-2 and Peroxisome Proliferator-Activated Recep-tor-δ in Human Endometrial Adenocarcinoma. Neoplasia 2000, 2, 483–490. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, R.A.; Tan, J.; Krause, W.F.; Geraci, M.W.; Willson, T.M.; Dey, S.K.; DuBois, R.N. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor δ in colorectal cancer. Proc. Natl. Acad. Sci. USA 2000, 97, 13275–13280. [Google Scholar] [CrossRef] [PubMed]
- Jehl-Pietri, C.; Bastie, C.; Gillot, I.; Luquet, S.; Grimaldi, P.A. Peroxisome-proliferator-activated recep-tor delta mediates the effects of long-chain fatty acids on post-confluent cell proliferation. Biochem. J. 2000, 350, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Ishihara, S.; Kawashima, K.; Moriyama, N.; Suetsugu, H.; Kazumori, H.; Okuyama, T.; Rumi, M.A.; Fukuda, R.; Nagasue, N.; et al. Expression of peroxisome proliferator-activated receptor (PPAR)gamma in gastric cancer and inhibitory effects of PPARgamma agonists. Br. J. Cancer. 2000, 83, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.; Smith, M.; Sarraf, P.; Kroll, T.; Aiyer, A.; Kaufman, D.S.; Oh, W.; Demetri, G.; Figg, W.D.; Zhou, X.P.; et al. Effects of ligand activation of peroxisome proliferator-activated receptor gamma in human prostate cancer. Proc. Natl. Acad. Sci. USA 2000, 97, 10990–10995. [Google Scholar] [CrossRef]
- Combs, C.K.; Johnson, D.E.; Karlo, J.C.; Cannady, S.B.; Landreth, G.E. Inflammatory mechanisms in Alz-heimer’s disease: Inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxi-city by PPARgamma agonists. J. Neurosci. 2000, 20, 558–567. [Google Scholar] [CrossRef]
- Zhou, X.P.; Smith, W.M.; Gimm, O.; Mueller, E.; Gao, X.; Sarraf, P.; Prior, T.W.; Plass, C.; von Deimling, A.; Black, P.M.; et al. Over-representation of PPARgamma sequence variants in sporadic cases of glioblastoma multiforme: Preliminary evidence for common low penetrance modifiers for brain tumour risk in the general population. J. Med. Genet. 2000, 37, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Debril, M.B.; Renaud, J.P.; Fajas, L.; Auwerx, J.; Girard, J. Transcription factors and nuclear receptors interact with the SWI/SNF complex through the BAF60c subunit. J. Biol. Chem. 1999, 274, 8945–8951. [Google Scholar] [CrossRef]
- Chinetti, G.; Fruchart, J.C.; Staels, B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors with functions in the vascular wall. Z Kardiol. 2001, 90 (Suppl. S3), 125–132. [Google Scholar] [CrossRef]
- Delerive, P.; Fruchart, J.C.; Staels, B. Peroxisome Proliferator-Activated Receptors in Inflammation Control. J. Endocrinol. 2001, 169, 453–459. [Google Scholar] [CrossRef]
- Duez, H.; Fruchart, J.C.; Staels, B. PPARS in Inflammation, Atherosclerosis and Thrombosis. J. Cardiovasc. Risk 2001, 8, 187–194. [Google Scholar] [CrossRef]
- Elangbam, C.S.; Tyler, R.D.; Lightfoot, R.M. Peroxisome Proliferator-Activated Receptors in Atherosclerosis and Inflammation--an Update. Toxicol. Pathol. 2001, 29, 224–231. [Google Scholar] [CrossRef]
- Stumvoll, M.; Häring, H. The Peroxisome Proliferator-Activated Receptor-Gamma2 Pro12Ala Polymorphism. Diabetes 2002, 51, 2341–2347. [Google Scholar] [CrossRef]
- Roberts, R.A.; Chevalier, S.; Hasmall, S.C.; James, N.H.; Cosulich, S.C.; Macdonald, N. PPARα and the Regulation of Cell Division and Apoptosis. Toxicology 2002, 181–182, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Moller, D.E. The Mechanisms of Action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Fauconnet, S.; Lascombe, I.; Chabannes, E.; Adessi, G.-L.; Desvergne, B.; Wahli, W.; Bittard, H. Differential Regulation of Vascular Endothelial Growth Factor Expression by Peroxisome Proliferator-Activated Receptors in Bladder Cancer Cells. J. Biol. Chem. 2002, 277, 23534–23543. [Google Scholar] [CrossRef]
- Haydon, R.C.; Zhou, L.; Feng, T.; Breyer, B.; Cheng, H.; Jiang, W.; Ishikawa, A.; Peabody, T.; Montag, A.; Simon, M.A.; et al. Nuclear receptor agonists as potential differentiation therapy agents for human osteosarcoma. Clin. Cancer Res. 2002, 8, 1288–1294. [Google Scholar] [PubMed]
- Koumanis, D.J.; Christou, N.V.; Wang, X.L.; Gilfix, B.M. Pilot study examining the frequency of several gene polymorphisms in a morbidly obese population. Obes. Surg. 2002, 12, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Wagner, J.A. Physiological and therapeutic roles of peroxisome proliferator-activated receptors. Diabetes Technol. Ther. 2002, 4, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.A.; Annicotte, J.-S.; Auwerx, J. PPAR Agonists in the Treatment of Atherosclerosis. Curr. Opin. Pharmacol. 2003, 3, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Margeli, A.; Kouraklis, G.; Theocharis, S. Peroxisome Proliferator Activated Receptor-γ (PPAR-γ) Ligands and Angiogenesis. Angiogenesis 2003, 6, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.M.; Toubro, S.; Astrup, A. PPARgamma Agonists in the Treatment of Type II Diabetes: Is Increased Fatness Commensurate with Long-Term Efficacy? Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2003, 27, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Shappell, S.B.; Hayward, S.W. Approaches to Understanding the Importance and Clinical Implications of Peroxisome Proliferator-Activated Receptor Gamma (PPARgamma) Signaling in Prostate Cancer. J. Cell. Biochem. 2004, 91, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Bedu, E.; Wahli, W.; Desvergne, B. Peroxisome proliferator-activated receptor beta/delta as a therapeutic target for metabolic diseases. Expert Opin. Ther. Targets. 2005, 9, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, A.; Motro, M.; Fisman, E.Z. Dual and pan-peroxisome proliferator-activated receptors (PPAR) co-agonism: The bezafibrate lessons. Cardiovasc. Diabetol. 2005, 4, 14. [Google Scholar] [CrossRef]
- Buzzetti, R.; Petrone, A.; Caiazzo, A.M.; Alemanno, I.; Zavarella, S.; Capizzi, M.; Mein, C.A.; Osborn, J.A.; Vania, A.; di Mario, U. PPAR-Gamma2 Pro12Ala Variant Is Associated with Greater Insulin Sensitivity in Childhood Obesity. Pediatr. Res. 2005, 57, 138–140. [Google Scholar] [CrossRef][Green Version]
- Tyagi, S.C.; Rodriguez, W.; Patel, A.M.; Roberts, A.M.; Falcone, J.C.; Passmore, J.C.; Fleming, J.T.; Joshua, I.G. Hyperhomocysteinemic diabetic cardiomyopathy: Oxidative stress, remodeling, and endothelial-myocyte uncoupling. J. Cardiovasc. Pharmacol. Ther. 2005, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.; Ghazallah, R.A.; Nascene, D.; Wuertz, B.; Ondrey, F.G. PPAR activation and decreased proliferation in oral carcinoma cells with 4-HPR. Otolaryngol. Head Neck Surg. 2005, 133, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.B.; Bifulco, N.; Bullock, W.H.; Claus, T.; Coish, P.; Dai, M.; Dela Cruz, F.E.; Dickson, D.; Fan, D.; Hoover-Litty, H.; et al. Substituted indanylacetic acids as PPAR-alpha-gamma activators. Bioorg. Med. Chem. Lett. 2006, 16, 297–301. [Google Scholar] [CrossRef] [PubMed]
- LoVerme, J.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The search for the palmitoylethanolamide receptor. Life Sci. 2005, 77, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Michalik, L.; Auwerx, J.; Berger, J.P.; Chatterjee, V.K.; Glass, C.K.; Gonzalez, F.J.; Grimaldi, P.A.; Kadowaki, T.; Lazar, M.A.; O’Rahilly, S.; et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006, 58, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Aung, C.S.; Faddy, H.M.; Lister, E.J.; Monteith, G.R.; Roberts-Thomson, S.J. Isoform Specific Changes in PPAR Alpha and Beta in Colon and Breast Cancer with Differentiation. Biochem. Biophys. Res. Commun. 2006, 340, 656–660. [Google Scholar] [CrossRef]
- Trivedi, N.R.; Cong, Z.; Nelson, A.M.; Albert, A.J.; Rosamilia, L.L.; Sivarajah, S.; Gilliland, K.L.; Liu, W.; Mauger, D.T.; Gabbay, R.A.; et al. Peroxisome Proliferator-Activated Receptors Increase Human Sebum Production. J. Investig. Dermatol. 2006, 126, 2002–2009. [Google Scholar] [CrossRef]
- Burdick, A.D.; Kim, D.J.; Peraza, M.A.; Gonzalez, F.J.; Peters, J.M. The Role of Peroxisome Proliferator-Activated Receptor-Beta/Delta in Epithelial Cell Growth and Differentiation. Cell. Signal. 2006, 18, 9–20. [Google Scholar] [CrossRef]
- Michalik, L.; Wahli, W. Peroxisome proliferator-activated receptors (PPARs) in skin health, repair and disease. Biochim. Biophys. Acta. 2007, 1771, 991–998. [Google Scholar] [CrossRef]
- Akhmetov, I.I.; Astranenkova, I.V.; Rogozkin, V.A. Association of PPARD gene polymorphism with human physical performance. Mol. Biol. 2007, 41, 852–857. (In Russian) [Google Scholar]
- Piqueras, L.; Reynolds, A.R.; Hodivala-Dilke, K.M.; Alfranca, A.; Redondo, J.M.; Hatae, T.; Tanabe, T.; Warner, T.D.; Bishop-Bailey, D. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Grarup, N.; Albrechtsen, A.; Ek, J.; Borch-Johnsen, K.; Jørgensen, T.; Schmitz, O.; Hansen, T.; Pedersen, O. Variation in the peroxisome proliferator-activated receptor delta gene in relation to common metabolic traits in 7495 middle-aged white people. Diabetologia 2007, 50, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Hollingshead, H.E.; Killins, R.L.; Borland, M.G.; Girroir, E.E.; Billin, A.N.; Willson, T.M.; Sharma, A.K.; Amin, S.; Gonzalez, F.J.; Peters, J.M. Peroxisome Proliferator-Activated Receptor-Beta/Delta (PPARbeta/Delta) Ligands Do Not Potentiate Growth of Human Cancer Cell Lines. Carcinogenesis 2007, 28, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Pedchenko, T.V.; Gonzalez, A.L.; Wang, D.; DuBois, R.N.; Massion, P.P. Peroxisome Proliferator-Activated Receptor Beta/Delta Expression and Activation in Lung Cancer. Am. J. Respir. Cell Mol. Biol. 2008, 39, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Borland, M.G.; Foreman, J.E.; Girroir, E.E.; Zolfaghari, R.; Sharma, A.K.; Amin, S.; Gonzalez, F.J.; Ross, A.C.; Peters, J.M. Ligand Activation of Peroxisome Proliferator-Activated Receptor-Beta/Delta Inhibits Cell Proliferation in Human HaCaT Keratinocytes. Mol. Pharmacol. 2008, 74, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Riley, C.; Quinn, M.A. An Immunohistochemical Perspective of PPAR Beta and One of Its Putative Targets PDK1 in Normal Ovaries, Benign and Malignant Ovarian Tumours. Br. J. Cancer 2008, 98, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Billin, A.N. PPAR-Beta/Delta Agonists for Type 2 Diabetes and Dyslipidemia: An Adopted Orphan Still Looking for a Home. Expert Opin. Investig. Drugs 2008, 17, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Soler, A.; Gómez-Nieto, C.; Campo, M.L.; Marathe, C.; Tontonoz, P.; Castrillo, A.; Corraliza, I. Arginase I induction by modified lipoproteins in macrophages: A peroxisome proliferator-activated receptor-gamma/delta-mediated effect that links lipid metabolism and immunity. Mol. Endocrinol. 2008, 22, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.Z. Peroxisome proliferator-activated receptors in the modulation of the im-mune/inflammatory response in atherosclerosis. PPAR Res. 2008, 2008, 285842. [Google Scholar] [CrossRef]
- Takata, Y.; Liu, J.; Yin, F.; Collins, A.R.; Lyon, C.J.; Lee, C.H.; Atkins, A.R.; Downes, M.; Barish, G.D.; Evans, R.M.; et al. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc. Natl. Acad. Sci. USA 2008, 105, 4277–4282. [Google Scholar] [CrossRef]
- Cavalieri, D.; Calura, E.; Romualdi, C.; Marchi, E.; Radonjic, M.; Van Ommen, B.; Müller, M. Filling gaps in PPAR-alpha signaling through comparative nutrigenomics analysis. BMC Genom. 2009, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Porcuna, J.; Mínguez-Martínez, J.; Ricote, M. The PPARα and PPARγ Epigenetic Landscape in Cancer and Immune and Metabolic Disorders. Int. J. Mol. Sci. 2021, 22, 10573. [Google Scholar] [CrossRef] [PubMed]
- Małodobra-Mazur, M.; Cierzniak, A.; Ryba, M.; Sozański, T.; Piórecki, N.; Kucharska, A.Z. Cornus mas L. Increases Glucose Uptake and the Expression of PPARG in Insulin-Resistant Adipocytes. Nutrients 2022, 14, 2307. [Google Scholar] [CrossRef] [PubMed]
- Danesi, F.; Di Nunzio, M.; Boschetti, E.; Bordoni, A. Green Tea Extract Selectively Activates Peroxisome Proliferator-Activated Receptor Beta/Delta in Cultured Cardiomyocytes. Br. J. Nutr. 2009, 101, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liang, P.; Zhang, B.; Song, J.; Huang, X.; Xiao, X. Role of PPAR-Beta in Hydrogen Peroxide-Induced Apoptosis in Human Umbilical Vein Endothelial Cells. Atherosclerosis 2009, 204, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liang, P.; Zhang, B.; Huang, X.; Xiao, X. Enhancement of PPAR-β Activity by Repetitive Low-Grade H2O2 Stress Protects Human Umbilical Vein Endothelial Cells from Subsequent Oxidative Stress-Induced Apoptosis. Free Radic. Biol. Med. 2009, 46, 555–563. [Google Scholar] [CrossRef]
- Bishop-Bailey, D.; Bystrom, J. Emerging Roles of Peroxisome Proliferator-Activated Receptor-Beta/Delta in Inflammation. Pharmacol. Ther. 2009, 124, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, S.; Zhao, W.; Riddle, D.R.; Robbins, M.E. Role of PPARs in Radiation-Induced Brain Injury. PPAR Res. 2010, 2010, 234975. [Google Scholar] [CrossRef] [PubMed]
- Genovese, S.; Foreman, J.E.; Borland, M.G.; Epifano, F.; Gonzalez, F.J.; Curini, M.; Peters, J.M. A natural propenoic acid derivative activates peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta). Life Sci. 2010, 86, 493–498. [Google Scholar] [CrossRef]
- Grimaldi, P.A. Metabolic and Nonmetabolic Regulatory Functions of Peroxisome Proliferator-Activated Receptor Beta. Curr. Opin. Lipidol. 2010, 21, 186–191. [Google Scholar] [CrossRef]
- McKinnon, B.; Bersinger, N.A.; Huber, A.W.; Kuhn, A.; Mueller, M.D. PPAR-γ Expression in Peritoneal Endometriotic Lesions Correlates with Pain Experienced by Patients. Fertil. Steril. 2010, 93, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Mistry, N.F.; Cresci, S. PPAR transcriptional activator complex polymorphisms and the promise of individualized therapy for heart failure. Heart Fail Rev. 2010, 15, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Bassaganya-Riera, J.; Song, R.; Roberts, P.C.; Hontecillas, R. PPAR-gamma activation as an anti-inflammatory therapy for respiratory virus infections. Viral Immunol. 2010, 23, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Housley, W.J.; Adams, C.O.; Vang, A.G.; Brocke, S.; Nichols, F.C.; LaCombe, M.; Rajan, T.V.; Clark, R.B. Peroxisome proliferator-activated receptor gamma is required for CD4+ T cell-mediated lymphopenia-associated autoimmunity. J. Immunol. 2011, 187, 4161–4169. [Google Scholar] [CrossRef]
- Shahin, D.; Toraby, E.E.; Abdel-Malek, H.; Boshra, V.; Elsamanoudy, A.Z.; Shaheen, D. Effect of peroxisome proliferator-activated receptor gamma agonist (pioglitazone) and methotrexate on disease activity in rheumatoid arthritis (experimental and clinical study). Clin. Med. Insights Arthritis Musculoskelet Disord. 2011, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sertznig, P.; Reichrath, J. Peroxisome proliferator-activated receptors (PPARs) in dermatology: Challenge and promise. Dermatoendocrinol 2011, 3, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Menger, M.D. Anti-angiogenic treatment strategies for the therapy of endometriosis. Hum. Reprod. Update. 2012, 18, 682–702. [Google Scholar] [CrossRef] [PubMed]
- Nickkho-Amiry, M.; McVey, R.; Holland, C. Peroxisome proliferator-activated receptors modulate proliferation and angiogenesis in human endometrial carcinoma. Mol. Cancer Res. 2012, 10, 441–453. [Google Scholar] [CrossRef]
- Knapp, P.; Chabowski, A.; Błachnio-Zabielska, A.; Jarząbek, K.; Wołczyński, S. Altered peroxisome-proliferator activated receptors expression in human endometrial cancer. PPAR Res. 2011, 2012, 471524. [Google Scholar] [CrossRef]
- Montero, T.D.; Racordon, D.; Bravo, L.; Owen, G.I.; Bronfman, M.L.; Leisewitz, A.V. PPARα and PPARγ regulate the nucleoside transporter hENT1. Biochem. Biophys. Res. Commun. 2012, 419, 405–411. [Google Scholar] [CrossRef]
- Abbas, A.; Blandon, J.; Rude, J.; Elfar, A.; Mukherjee, D. PPAR- γ agonist in treatment of diabetes: Cardiovascular safety considerations. Cardiovasc. Hematol. Agents Med. Chem. 2012, 10, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Wu, J.S.; Tsai, H.D.; Huang, C.Y.; Chen, J.J.; Sun, G.Y.; Lin, T.N. Peroxisome proliferator-activated receptor gamma (PPAR-γ) and neurodegenerative disorders. Mol. Neurobiol. 2012, 46, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Greene, N.P.; Fluckey, J.D.; Lambert, B.S.; Greene, E.S.; Riechman, S.E.; Crouse, S.F. Regulators of blood lipids and lipoproteins? PPARδ and AMPK, induced by exercise, are correlated with lipids and lipoproteins in overweight/obese men and women. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1212–E1221. [Google Scholar] [CrossRef] [PubMed]
- Wadosky, K.M.; Willis, M.S. The story so far: Post-translational regulation of peroxisome proliferator-activated receptors by ubiquitination and SUMOylation. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H515–H526. [Google Scholar] [CrossRef] [PubMed]
- Peyrou, M.; Ramadori, P.; Bourgoin, L.; Foti, M. PPARs in Liver Diseases and Cancer: Epigenetic Regulation by MicroRNAs. PPAR Res. 2012, 2012, 757803. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Ciccodicola, A. Is PPARG the key gene in diabetic retinopathy? Br. J. Pharmacol. 2012, 165, 1–3. [Google Scholar] [CrossRef]
- Balakumar, P.; Mahadevan, N. Interplay between statins and PPARs in improving cardiovascular outcomes: A double-edged sword? Br. J. Pharmacol. 2012, 165, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Wahli, W.; Michalik, L. PPARs at the crossroads of lipid signaling and inflammation. Trends. Endocrinol. Metab. 2012, 23, 351–363. [Google Scholar] [CrossRef]
- Reichenbach, G.; Starzinski-Powitz, A.; Sloane, B.F.; Doll, M.; Kippenberger, S.; Bernd, A.; Kaufmann, R.; Meissner, M. PPARα agonist Wy14643 suppresses cathepsin B in human endothelial cells via transcriptional, post-transcriptional and post-translational mechanisms. Angiogenesis 2013, 16, 223–233. [Google Scholar] [CrossRef]
- Tailleux, A.; Wouters, K.; Staels, B. Roles of PPARs in NAFLD: Potential therapeutic targets. Biochim. Biophys. Acta 2012, 1821, 809–818. [Google Scholar] [CrossRef]
- Videla, L.A.; Pettinelli, P. Misregulation of PPAR Functioning and Its Pathogenic Consequences Associated with Nonalcoholic Fatty Liver Disease in Human Obesity. PPAR Res. 2012, 2012, 107434. [Google Scholar] [CrossRef] [PubMed]
- Benedusi, V.; Martorana, F.; Brambilla, L.; Maggi, A.; Rossi, D. The Peroxisome Proliferator-activated Receptor γ (PPARγ) Controls Natural Protective Mechanisms against Lipid Peroxidation in Amyotrophic Lateral Sclerosis*. J. Biol. Chem. 2012, 287, 35899–35911. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, X.; Ding, X.; Li, Y.; Zhang, X.; Xie, P.; Yang, J.; Wang, S. MicroRNA-141 represses HBV replication by targeting PPARA. PLoS ONE 2012, 7, e34165. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.; Liang, P.; Huang, X. Role of PPARbeta in fibroblast response to heat injury. Indian J. Biochem. Biophys. 2012, 49, 219–227. [Google Scholar] [PubMed]
- Le Foll, B.; Di Ciano, P.; Panlilio, L.V.; Goldberg, S.R.; Ciccocioppo, R. Peroxisome proliferator-activated receptor (PPAR) agonists as promising new medications for drug addiction: Preclinical evidence. Curr. Drug Targets 2013, 14, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Aleshin, S.; Reiser, G. Role of the peroxisome proliferator-activated receptors (PPAR)-α, β/δ and γ triad in regulation of reactive oxygen species signaling in brain. Biol. Chem. 2013, 394, 1553–1570. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.V.; Torres, N.; Tovar, A.R. PPAR-α as a key nutritional and environmental sensor for metabolic adaptation. Adv. Nutr. 2013, 4, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Akyürek, N.; Aycan, Z.; Çetinkaya, S.; Akyürek, Ö.; Yilmaz Ağladioğlu, S.; Ertan, Ü. Peroxisome proliferator activated receptor (PPAR)-gamma concentrations in childhood obesity. Scand J. Clin. Lab. Investig. 2013, 73, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.L.; Morales, J.L.; Zhu, B.; Kang, B.H.; Gonzalez, F.J.; Peters, J.M. Activation of Peroxisome Proliferator-Activated Receptor-β/δ (PPAR-β/δ) Inhibits Human Breast Cancer Cell Line Tumorigenicity. Mol. Cancer Ther. 2014, 13, 1008–1017. [Google Scholar] [CrossRef]
- Young, K.C.; Bai, C.H.; Su, H.C.; Tsai, P.J.; Pu, C.Y.; Liao, C.S.; Tsao, C.W. Fluoxetine a novel anti-hepatitis C virus agent via ROS-, JNK-, and PPARβ/γ-dependent pathways. Antivir. Res. 2014, 110, 158–167. [Google Scholar] [CrossRef]
- Montagner, A.; Wahli, W.; Tan, N.S. Nuclear receptor peroxisome proliferator activated receptor (PPAR) β/δ in skin wound healing and cancer. Eur. J. Dermatol. 2015, Suppl. 1, 4–11. [Google Scholar] [CrossRef]
- de Melo, N.F.; de Macedo, C.G.; Bonfante, R.; Abdalla, H.B.; da Silva, C.M.; Pasquoto, T.; de Lima, R.; Fraceto, L.F.; Clemente-Napimoga, J.T.; Napimoga, M.H. 15d-PGJ2-Loaded Solid Lipid Nanopar-ticles: Physicochemical Characterization and Evaluation of Pharmacological Effects on In-flammation. PLoS ONE 2016, 11, e0161796. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xu, X.; Zhang, X.Q.; Farokhzad, O.C.; Langer, R. Preventing diet-induced obesity in mice by adipose tissue transformation and angiogenesis using targeted nanoparticles. Proc. Natl. Acad. Sci. USA. 2016, 113, 5552–5557. [Google Scholar] [CrossRef]
- Strojny, B.; Grodzik, M.; Sawosz, E.; Winnicka, A.; Kurantowicz, N.; Jaworski, S.; Kutwin, M.; Urbańska, K.; Hotowy, A.; Wierzbicki, M.; et al. Diamond Nanoparticles Modify Curcumin Activity: In Vitro Studies on Cancer and Normal Cells and In Ovo Studies on Chicken Embryo Model. PLoS ONE 2016, 11, e0164637. [Google Scholar] [CrossRef] [PubMed]
- Fidoamore, A.; Cristiano, L.; Laezza, C.; Galzio, R.; Benedetti, E.; Cinque, B.; d’Angelo, M.; Castelli, V.; Cifone, M.G.; Ippoliti, R.; et al. Annamaria Cimini Energy metabolism in glioblastoma stem cells: PPARα a metabolic adaptor to intratumoral microenvironment. Oncotarget 2017, 8, 108430–108450. [Google Scholar] [CrossRef] [PubMed]
- Rolver, M.G.; Holland, L.K.K.; Ponniah, M.; Prasad, N.S.; Yao, J.; Schnipper, J.; Kramer, S.; Elingaard-Larsen, L.; Pedraz-Cuesta, E.; Liu, B.; et al. Chronic acidosis rewires cancer cell metabolism through PPARα signaling. Int. J. Cancer. 2023, 152, 1668–1684. [Google Scholar] [CrossRef] [PubMed]
- Kado, T.; Kusakari, N.; Tamaki, T.; Murota, K.; Tsujiuchi, T.; Fukushima, N. Oleic acid stimulates cell proliferation and BRD4-L-MYC-dependent glucose transporter transcription through PPARα activation in ovarian cancer cells. Biochem. Biophys. Res. Commun. 2023, 657, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.K.; Wuertz, B.R.; Harris, G.; Abu Ghazallah, R.; Miller, W.A.; Gaffney, P.M.; Ondrey, F.G. Functional Activation of PPARγ in Human Upper Aerodigestive Cancer Cell Lines. Mol. Carcinog. 2017, 56, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Orekhov, A.N. Peroxisome Proliferator-Activated Receptor (PPAR) Gamma Agonists as Therapeutic Agents for Cardiovascular Disorders: Focus on Atherosclerosis. Curr. Pharm. Des. 2017, 23, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in Obesity-Induced T2DM, Dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49. [Google Scholar] [CrossRef]
- Borland, M.G.; Kehres, E.M.; Lee, C.; Wagner, A.L.; Shannon, B.E.; Albrecht, P.P.; Zhu, B.; Gonzalez, F.J.; Peters, J.M. Inhibition of tumorigenesis by peroxisome proliferator-activated receptor (PPAR)-dependent cell cycle blocks in human skin carcinoma cells. Toxicology 2018, 404–405, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Leiguez, E.; Giannotti, K.C.; Viana, M.D.N.; Matsubara, M.H.; Fernandes, C.M.; Gutiérrez, J.M.; Lomonte, B.; Teixeira, C. A Snake Venom-Secreted Phospholipase A2 Induces Foam Cell Formation Depending on the Activation of Factors Involved in Lipid Homeostasis. Mediat. Inflamm. 2018, 2018, 2547918. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Shao, W.; Liu, H.; Jiang, Z. Exposure to 2,4-dichlorophenoxyacetic acid induced PPARβ-dependent disruption of glucose metabolism in HepG2 cells. Environ. Sci. Pollut. Res. 2018, 25, 17050–17057. [Google Scholar] [CrossRef] [PubMed]
- Quintão, N.L.M.; Santin, J.R.; Stoeberl, L.C.; Corrêa, T.P.; Melato, J.; Costa, R. Pharmacological Treatment of Chemotherapy-Induced Neuropathic Pain: PPAR Agonists as a Promising Tool. Front. Neurosci. 2019, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Lecarpentier, Y.; Vallée, J.N. Targeting the Canonical WNT/β-Catenin Pathway in Cancer Treatment Using Non-Steroidal Anti-Inflammatory Drugs. Cells 2019, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Toraih, E.A.; Fawzy, M.S.; Abushouk, A.I.; Shaheen, S.; Hobani, Y.H.; Alruwetei, A.M.; Badran, D.I. Prognostic Value of the miRNA-27a and PPAR/RXRα Signaling Axis in Patients with Thyroid Carcinoma. Epigenomics 2020, 12, 1825–1843. [Google Scholar] [CrossRef] [PubMed]
- Hirao-Suzuki, M.; Takeda, S.; Koga, T.; Takiguchi, M.; Toda, A. Cannabidiolic acid dampens the expression of cyclooxygenase-2 in MDA-MB-231 breast cancer cells: Possible implication of the peroxisome proliferator-activated receptor β/δ abrogation. J. Toxicol. Sci. 2020, 45, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Elie-Caille, C.; Lascombe, I.; Péchery, A.; Bittard, H.; Fauconnet, S. Molecular and nanoscale evaluation of N-cadherin expression in invasive bladder cancer cells under control conditions or GW501516 exposure. Mol. Cell Biochem. 2020, 471, 113–127. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Akhavan Sepahi, A.; Mousavi, S.F.; Vaziri, F.; Siadat, S.D. The effect of Faecalibacterium prausnitzii and its extracellular vesicles on the permeability of intestinal epithelial cells and expression of PPARs and ANGPTL4 in the Caco-2 cell culture model. J. Diabetes Metab. Disord. 2020, 19, 1061–1069. [Google Scholar] [CrossRef]
- Liu, X.; Qian, D.; Liu, H.; Abbruzzese, J.L.; Luo, S.; Walsh, K.M.; Wei, Q. Genetic variants of the peroxisome proliferator-activated receptor (PPAR) signaling pathway genes and risk of pancreatic cancer. Mol Carcinog. 2020, 59, 930–939. [Google Scholar] [CrossRef]
- Wouters, E.; Grajchen, E.; Jorissen, W.; Dierckx, T.; Wetzels, S.; Loix, M.; Tulleners, M.P.; Staels, B.; Stinissen, P.; Haidar, M.; et al. Altered PPARγ Expression Promotes Myelin-Induced Foam Cell Formation in Macrophages in Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 9329. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, J.; Chen, K.; Feng, J.; Guo, C. Bergenin Attenuates Hepatic Fibrosis by Regulating Autophagy Mediated by the PPAR-γ/TGF-β Pathway. PPAR Res. 2020, 2020, e6694214. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sun, B.; Yu, C.; Cao, Y.; Cai, C.; Yao, J. Choline and methionine regulate lipid metabolism via the AMPK signaling pathway in hepatocytes exposed to high concentrations of nonesterified fatty acids. J. Cell Biochem. 2020, 121, 3667–3678. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Berga, S.L.; Zou, W.; Rajakumar, A.; Man, M.; Sidell, N.; Taylor, R.N. Human Endometrial Stromal Cell Differentiation is Stimulated by PPARβ/δ Activation: New Targets for Infertility? J. Clin. Endocrinol. Metab. 2020, 105, 2983–2995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Y.W.; Chi, Z.; Chen, B. Ligand-Activated Peroxisome Proliferator-Activated Receptor β/δ Facilitates Cell Proliferation in Human Cholesteatoma Keratinocytes. PPAR Res. 2020, 2020, e8864813. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, S.; Strosznajder, A.K.; Jeżyna, M.; Strosznajder, J.B. The Novel Role of PPAR Alpha in the Brain: Promising Target in Therapy of Alzheimer’s Disease and Other Neurodegenerative Disorders. Neurochem. Res. 2020, 45, 972–988. [Google Scholar] [CrossRef] [PubMed]
- Manickam, R.; Duszka, K.; Wahli, W. PPARs and Microbiota in Skeletal Muscle Health and Wasting. Int. J. Mol. Sci. 2020, 21, 8056. [Google Scholar] [CrossRef]
- da Cruz, B.O.; Cardozo, L.F.M.F.; Magliano, D.C.; Stockler-Pinto, M.B. Nutritional strategies to modulate inflammation pathways via regulation of peroxisome proliferator-activated receptor β/δ. Nutr. Rev. 2020, 78, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.D.; Wagner, N. PPARs and Myocardial Infarction. Int. J. Mol. Sci. 2020, 21, 9436. [Google Scholar] [CrossRef]
- Phua, W.W.T.; Tan, W.R.; Yip, Y.S.; Hew, I.D.; Wee, J.W.K.; Cheng, H.S.; Leow, M.K.S.; Wahli, W.; Tan, N.S. PPARβ/δ Agonism Upregulates Forkhead Box A2 to Reduce Inflammation in C2C12 Myoblasts and in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 1747. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, H.; Ahmed, R.Z.; Zheng, Y.; Jin, X. Critical biomarkers for myocardial damage by fine particulate matter: Focused on PPARα-regulated energy metabolism. Environ. Pollut. 2020, 264, 114659. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Souza, V.A.; Ghiarone, T.; Sansonio, A.; Santos Silva, K.A.; Tomazini, F.; Arcoverde, L.; Fyfe, J.; Perri, E.; Saner, N.; Kuang, J.; et al. Exercise twice-a-day potentiates markers of mitochondrial biogenesis in men. FASEB J. 2020, 34, 1602–1619. [Google Scholar] [CrossRef]
- Faulkner, A.; Lynam, E.; Purcell, R.; Jones, C.; Lopez, C.; Board, M.; Wagner, K.-D.; Wagner, N.; Carr, C.; Wheeler-Jones, C. Context-dependent regulation of endothelial cell metabolism: Differential effects of the PPARβ/δ agonist GW0742 and VEGF-A. Sci. Rep. 2020, 10, 7849. [Google Scholar] [CrossRef]
- Chai, C.Y.; Tai, I.C.; Zhou, R.; Song, J.; Zhang, C.; Sun, S. MicroRNA-9-5p inhibits proliferation and induces apoptosis of human hypertrophic scar fibroblasts through targeting peroxisome proliferator-activated receptor β. Biol. Open. 2020, 9, bio051904. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, M.E.; Savage, S.R.; McCollum, G.W.; Hammer, S.S.; Ramos, C.J.; Yang, R.; Bretz, C.A.; Penn, J.S. The peroxisome proliferator-activated receptor-β/δ antagonist GSK0660 mitigates retinal cell inflammation and leukostasis. Exp. Eye Res. 2020, 190, 107885. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Lopez, R.A.; Elizondo-Vega, R.; Torres, M.J.; Vega-Letter, A.M.; Luque-Campos, N.; Paredes-Martinez, M.J.; Pradenas, C.; Tejedor, G.; Oyarce, K.; Salgado, M.; et al. PPARβ/δ-dependent MSC metabolism determines their immunoregulatory properties. Sci. Rep. 2020, 10, 11423. [Google Scholar] [CrossRef]
- Petr, M.; Maciejewska-Skrendo, A.; Zajac, A.; Chycki, J.; Stastny, P. Association of Elite Sports Status with Gene Variants of Peroxisome Proliferator Activated Receptors and Their Transcriptional Coactivator. Int. J. Mol. Sci. 2019, 21, 162. [Google Scholar] [CrossRef]
- Tutunchi, H.; Ostadrahimi, A.; Saghafi-Asl, M.; Hosseinzadeh-Attar, M.J.; Shakeri, A.; Asghari-Jafarabadi, M.; Roshanravan, N.; Farrin, N.; Naemi, M.; Hasankhani, M. Oleoylethanolamide supplementation in obese patients newly diagnosed with non-alcoholic fatty liver disease: Effects on metabolic parameters, anthropometric indices, and expression of PPAR-α, UCP1, and UCP2 genes. Pharmacol. Res. 2020, 156, 104770. [Google Scholar] [CrossRef]
- Grabacka, M.; Pierzchalska, M.; Płonka, P.M.; Pierzchalski, P. The Role of PPAR Alpha in the Modulation of Innate Immunity. Int. J. Mol. Sci. 2021, 22, 10545. [Google Scholar] [CrossRef]
- Willems, S.; Morstein, J.; Hinnah, K.; Trauner, D.; Merk, D. A Photohormone for Light-Dependent Control of PPARα in Live Cells. J. Med. Chem. 2021, 64, 10393–10402. [Google Scholar] [CrossRef]
- Kharbanda, C.; Alam, M.S.; Hamid, H.; Ali, Y.; Nazreen, S.; Dhulap, A.; Alam, P.; Pasha, M.A.Q. In Silico Designing, in Vitro and in Vivo Evaluation of Potential PPAR-γ Agonists Derived from Aryl Propionic Acid Scaffold. Bioorganic Chem. 2021, 106, 104458. [Google Scholar] [CrossRef] [PubMed]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef] [PubMed]
- Rayner, M.L.D.; Healy, J.; Phillips, J.B. Repurposing Small Molecules to Target PPAR-γ as New Therapies for Peripheral Nerve Injuries. Biomolecules 2021, 11, 1301. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.M.; Coquet, J.M.; Tibbitt, C.A. The Role of PPAR-γ in Allergic Disease. Curr. Allergy Asthma Rep. 2021, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Yoneda, M.; Kurita, Y.; Nogami, A.; Honda, Y.; Hosono, K.; Nakajima, A. Cholestatic Liver Disease: Current Treatment Strategies and New Therapeutic Agents. Drugs 2021, 81, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.G.; Wanjari, U.R.; Gopalakrishnan, A.V.; Katturajan, R.; Kannampuzha, S.; Murali, R.; Namachivayamm, A.; Ganesan, R.; Renu, K.; Dey, A.; et al. Exploring the Regulatory Role of ncRNA in NAFLD: A Particular Focus on PPARs. Cells 2022, 24, 3959. [Google Scholar] [CrossRef] [PubMed]
- Dandare, A.; Khan, M.J.; Naeem, A.; Liaquat, A. Clinical relevance of circulating non-coding RNAs in metabolic diseases: Emphasis on obesity, diabetes, cardiovascular diseases and metabolic syndrome. Genes Dis. 2022, 10, 2393–2413. [Google Scholar] [CrossRef] [PubMed]
- Chatzopoulou, F.; Kyritsis, K.A.; Papagiannopoulos, C.I.; Galatou, E.; Mittas, N.; Theodoroula, N.F.; Papazoglou, A.S.; Karagiannidis, E.; Chatzidimitriou, M.; Papa, A.; et al. Dissecting miRNA-Gene Networks to Map Clinical Utility Roads of Pharmacogenomics-Guided Therapeutic Decisions in Cardiovascular Precision Medicine. Cells 2022, 11, 607. [Google Scholar] [CrossRef]
- Kaltdorf, M.; Breitenbach, T.; Karl, S.; Fuchs, M.; Kessie, D.K.; Psota, E.; Prelog, M.; Sarukhanyan, E.; Ebert, R.; Jakob, F.; et al. Software JimenaE allows efficient dynamic simulations of Boolean networks, centrality and system state analysis. Sci. Rep. 2023, 13, 1855. [Google Scholar] [CrossRef]
- Tanaka, Y.; Minami, Y.; Endo, M. Ror1 promotes PPARα-mediated fatty acid metabolism in astrocytes. Genes Cells. 2023, 28, 307–318. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Abdeljawaad, K.A.A.; Roshdy, E.; Mohamed, D.E.M.; Ali, T.F.S.; Gabr, G.A.; Jaragh-Alhadad, L.A.; Mekhemer, G.A.H.; Shawky, A.M.; Sidhom, P.A.; et al. In Silico Drug Discovery of SIRT2 Inhibitors from Natural Source as Anticancer Agents. Sci. Rep. 2023, 13, 2146. [Google Scholar] [CrossRef]
- Yang, W.; Ling, X.; He, S.; Cui, H.; Yang, Z.; An, H.; Wang, L.; Zou, P.; Chen, Q.; Liu, J.; et al. PPARα/ACOX1 as a novel target for hepatic lipid metabolism disorders induced by per- and polyfluoroalkyl substances: An integrated approach. Environ. Int. 2023, 178, 108138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhao, G.; Li, H.; Zhao, Q.; Liu, D. FNDC5/PPARa Pathway Alleviates THP-1-derived Macrophage Pyroptosis and Its Mechanism. Altern. Ther. Health Med. 2023, 29, 32–42. [Google Scholar] [PubMed]
- Páscoa, I.; Biltes, R.; Sousa, J.; Preto, M.A.C.; Vasconcelos, V.; Castro, L.F.; Ruivo, R.; Cunha, I. A Multiplex Molecular Cell-Based Sensor to Detect Ligands of PPARs: An Optimized Tool for Drug Discovery in Cyanobacteria. Sensors 2023, 23, 1338. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Chen, Z.; Li, J.; Luo, J.; Castro-Martinez, F.; Wisniewski, J.; Cui, K.; Wang, Y.; Sun, J.; Ren, X.; et al. Gut-liver axis calibrates intestinal stem cell fitness. Cell. 2024, 187, 914–930.e20. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, Q.; Zhu, G.; Zhang, T.; Tu, D.; Zhang, F.; Finel, M.; He, Y.; Ge, G. Characterization of the Glucuronidating Pathway of Pectolinarigenin, the Major Active Constituent of the Chinese Medicine Daji, in Humans and Its Influence on Biological Activities. J. Ethnopharmacol. 2024, 319, 117280. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wei, H.-Z.; Chen, H.-W.; Wang, Z.-Q.; Mao, P.; Zhang, H.-H. DNMT3a-Mediated Methylation of PPARγ Promote Intervertebral Disc Degeneration by Regulating the NF-κB Pathway. J. Cell. Mol. Med. 2024, 28, e18048. [Google Scholar] [CrossRef] [PubMed]
- Shyni, G.L.; Kavitha, S.; Indu, S.; Arya, A.D.; Anusree, S.S.; Vineetha, V.P.; Vandana, S.; Sundaresan, A.; Raghu, K.G. Chebulagic acid from Terminalia chebula enhances insulin mediated glucose uptake in 3T3-L1 adipocytes via PPARγ signaling pathway. Biofactors 2014, 40, 646–657. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, G. Green tea and the prevention of breast cancer: A case-control study in Southeast China. Carcinogenesis 2009, 30, 1351–1356. [Google Scholar] [CrossRef]
- Tang, J.; Zheng, J.S.; Fang, L.; Jin, Y. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules 2009, 14, 5004–5015. [Google Scholar]
- Chen, Z.P.; Schell, J.B.; Ho, C.T.; Chen, K.Y.; Li, S. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 2020, 278, 101–112. [Google Scholar] [CrossRef]
- Shao, M.; Guo, D.; Lu, W.; Chen, X.; Ma, L.; Wu, Y.; Zhang, X.; Wang, Q.; Wang, X.; Li, W.; et al. Identification of the active compounds and drug targets of Chinese medicine in heart failure based on the PPARs-RXRα pathway. J. Ethnopharmacol. 2020, 257, 112859. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Li, S.; Zhang, C.; Chen, H.; Wang, N.; Feng, Y. Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management. Acta Pharm. Sin B. 2021, 11, 2749–2767. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, J.; Hu, X.; Xu, P.; Huang, S.; Cui, S.; Guo, Y.; Yang, H.; Chen, X.; Jiang, C. Yi-shen-hua-shi granules modulate immune and inflammatory damage via the ALG3/PPARγ/NF-κB pathway in the treatment of immunoglobulin a nephropathy. J. Ethnopharmacol. 2024, 319, 117204. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Qiao, H.; Wang, Z.; Wang, H.; Han, M.; Zhang, W.; Zhou, Y.; Hassan, H.M.; Zhao, W.; Qin, T. Taohe Chengqi decoction alleviated metabolic-associated fatty liver disease by boosting branched chain amino acids catabolism in the skeletal muscles of type 2 diabetes mellitus. Phytomedicine 2024, 126, 155315. [Google Scholar] [CrossRef]
- Kuttan, R.; Bhanumathy, P.; Nirmala, K.; George, M.C.; Maliakel, B. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett. 2009, 136, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Anandharajan, R.; Jaiganesh, S.; Shankernarayanan, N.P.; Viswakarma, R.A.; Balakrishnan, A. In vitro glucose uptake activity of Aegles marmelos and Syzygium cumini by activation of Glut-4, PI3 kinase and PPARgamma in L6 myotubes. Phytomedicine 2006, 13, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Khosropoor, S.; Alavi, M.S.; Etemad, L.; Roohbakhsh, A. Cannabidiol goes nuclear: The role of PPARγ. Phytomedicine 2023, 114, 154771. [Google Scholar] [CrossRef]
- Burstein, S. PPAR-gamma: A nuclear receptor with affinity for cannabinoids. Life Sci. 2005, 77, 1674–1684. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An overview of some pharmacological aspects. J. Clin. Pharmacol. 2002, 42 (Suppl. S11), 11S–19S. [Google Scholar] [CrossRef] [PubMed]
- Robson, P. Human studies of cannabinoids and medicinal cannabis. Handb. Exp. Pharmacol. 2005, 168, 719–756. [Google Scholar] [CrossRef]
- Sun, Y.; Alexander, S.P.; Kendall, D.A.; Bennett, A.J. Cannabinoids and PPARalpha signalling. Biochem. Soc. Trans. 2006, 34, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.; Cascio, M.G.; Rotondo, D.; Pertwee, R.G.; Heys, S.D.; Wahle, K.W. Cannabinoids and omega-3/6 endocannabinoids as cell death and anticancer modulators. Prog. Lipid Res. 2013, 52, 80–109. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M.; Lübben, G.; Paschke, R. Analysis of the relationship between the Pro12Ala variant in the PPAR-gamma2 gene and the response rate to therapy with pioglitazone in patients with type 2 diabetes. Diabetes Care 2003, 26, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M.; Klemm, T.; Gerike, T.; Krankenberg, H.; Schuler, G.; Paschke, R. Lack of association between peroxisome proliferator-activated receptor-gamma-2 gene variants and the occurrence of coronary heart disease in patients with diabetes mellitus. Eur. J. Endocrinol. 2002, 146, 545–551. [Google Scholar] [CrossRef]
- Usuda, D.; Kanda, T. Peroxisome proliferator-activated receptors for hypertension. World J. Cardiol. 2014, 6, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Leibowitz, M.D.; Doebber, T.W.; Elbrecht, A.; Zhang, B.; Zhou, G.; Biswas, C.; Cullinan, C.A.; Hayes, N.S.; Li, Y.; et al. Novel peroxisome proliferator-activated receptor (PPAR) gamma and PPARdelta ligands produce distinct biological effects. J. Biol. Chem. 1999, 274, 6718–6725. [Google Scholar] [CrossRef] [PubMed]
- Bidzińska, B.; Demissie, M.; Tworowska, U.; Milewicz, A. Receptory aktywowane przez proliferatory peroksysomów a gospodarka lipidowa i węglowodanowa—rola fizjologiczna i znaczenie kliniczne. Diabetol. Pol. 2000, 7, 258–264. (In Polish) [Google Scholar]
- Houseknecht, K.L.; Cole, B.M.; Steele, P.J. Peroxisome proliferator-activated receptor gamma (PPARgamma) and its ligands: A review. Domest. Anim. Endocrinol. 2002, 22, 1–23. [Google Scholar] [CrossRef]
- Lindi, V.I.; Uusitupa, M.I.; Lindström, J.; Louheranta, A.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; et al. Finnish Diabetes Prevention Study. Association of the Pro12Ala polymorphism in the PPAR-gamma2 gene with 3-year incidence of type 2 diabetes and body weight change in the Finnish Diabetes Prevention Study. Diabetes 2002, 51, 2581–2586. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.L.G.; Rios, M.S.; Perez, C.F.; Laakso, M.; Larrad, M.T.M. Effect of the Pro12Ala polymorphism of the peroxisome proliferator-activated receptor g -2 gene on adiposity, insulin sensitivity and lipid profile in the Spanish population. Eur. J. Endocrinol. 2000, 147, 495–501. [Google Scholar]
- Demissie, M. Związek Polimorfizmu Genu Receptora Aktywowanego Proliferatorami Peroksysomów g 2 z Zaburzeniami Gospodarki Węglowodanowej i Lipidowej Oraz Profilem Hormonalnym u Osób z Należną Masą Ciała i Otyłych [Association of Peroxisome Proliferator-Activated Receptor Gene g 2 Polymorphism with Carbohydrate and Lipid Metabolism Disorders and Hormonal Profile in Normal-Weight and Obese Subjects]. Ph.D. Disertation, Wroclaw Medical Univerity, Wrocław, Poland, 2003. [Google Scholar]
- Douglas, J.A.; Erdos, M.R.; Watanabe, R.M.; Braun, A.; Johnston, C.L.; Oeth, P.; Mohlke, K.L.; Valle, T.T.; Ehnholm, C.; Buchanan, T.A.; et al. The peroxisome proliferator-activated receptor-gamma2 Pro12A1a variant: Association with type 2 diabetes and trait differences. Diabetes 2001, 50, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Huang, Q.; Sun, J.; Zhao, X.; Guo, X.; Jin, Y.; Ma, W.; Wang, W. Correlation between PPAR-α methylation level in peripheral blood and atherosclerosis of NAFLD patients with DM. Exp. Ther. Med. 2018, 15, 2727–2730. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Zhuo, Q.; Tseng, Y.; Wang, J.; Ma, Y.; Zhang, J.; Liu, J. TET1 promotes fatty acid oxidation and inhibits NAFLD progression by hydroxymethylation of PPARα promoter. Nutr. Metab. 2020, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Theys, C.; Lauwers, D.; Perez-Novo, C.; Vanden Berghe, W. PPARα in the Epigenetic Driver Seat of NAFLD: New Therapeutic Opportunities for Epigenetic Drugs? Biomedicines 2022, 10, 3041. [Google Scholar] [CrossRef] [PubMed]
- Yideng, J.; Zhihong, L.; Jiantuan, X.; Jun, C.; Guizhong, L.; Shuren, W. Homocysteine-Mediated PPARα,γ DNA Methylation and Its Potential Pathogenic Mechanism in Monocytes. DNA Cell Biol. 2008, 27, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Castillo, D.; Moreno-Indias, I.; Sanchez-Alcoholado, L.; Ramos-Molina, B.; Alcaide-Torres, J.; Morcillo, S.; Ocaña-Wilhelmi, L.; Tinahones, F.; Queipo-Ortuño, M.I.; Cardona, F. Altered Adipose Tissue DNA Methylation Status in Metabolic Syndrome: Relationships Between Global DNA Methylation and Specific Methylation at Adipogenic, Lipid Metabolism and Inflammatory Candidate Genes and Metabolic Variables. J. Clin. Med. 2019, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Xu, P.; Zhai, Y. The Opportunities and Challenges of Peroxisome Proliferator-Activated Receptors Ligands in Clinical Drug Discovery and Development. Int. J. Mol. Sci. 2018, 19, 2189. [Google Scholar] [CrossRef]
- Kahn, B.B.; McGraw, T.E. Rosiglitazone, PPARγ, and Type 2 Diabetes. N. Engl. J. Med. 2010, 363, 2667–2669. [Google Scholar] [CrossRef]
- Mitka, M. Panel Recommends Easing Restrictions on Rosiglitazone Despite Concerns About Cardiovascular Safety. JAMA 2013, 310, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.; Bell, S.; Sund, R.; Hartikainen, S.A.; Tuomilehto, J.; Pukkala, E.; Keskimäki, I.; Badrick, E.; Renehan, A.G.; Buchan, I.E.; et al. Pioglitazone and Bladder Cancer Risk: A Multipopulation Pooled, Cumulative Exposure Analysis. Diabetologia 2015, 58, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.G.; Kim, D.M.; Woo, J.-T.; Jang, H.C.; Chung, C.H.; Ko, K.S.; Park, J.H.; Park, Y.S.; Kim, S.J.; et al. Safety and Efficacy of Lobeglitazone Monotherapy in Patients with Type 2 Diabetes Mellitus over 52 Weeks: An Open-Label Extension Study. Diabetes Res. Clin. Pract. 2015, 110, e27–e30. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Kim, T.-E.; Yoon, S.H.; Cho, J.-Y.; Shin, S.-G.; Jang, I.-J.; Yu, K.-S. Assessment of the Pharmacokinetics of Co-Administered Metformin and Lobeglitazone, a Thiazolidinedione Antihyperglycemic Agent, in Healthy Subjects. Curr. Med. Res. Opin. 2012, 28, 1213–1220. [Google Scholar] [CrossRef]
- Priya, S.S.; Sankaran, R.; Ramalingam, S.; Sairam, T.; Somasundaram, L.S. Genotype Phenotype Correlation of Genetic Polymorphism of PPAR Gamma Gene and Therapeutic Response to Pioglitazone in Type 2 Diabetes Mellitus- A Pilot Study. J. Clin. Diagn. Res. JCDR 2016, 10, FC11–FC14. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Häring, H. Reduced Lipolysis as Possible Cause for Greater Weight Gain in Subjects with the Pro12Ala Polymorphism in PPARgamma2? Diabetologia 2002, 45, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Wahl, H.G.; Löblein, K.; Becker, R.; Machicao, F.; Jacob, S.; Häring, H. Pro12Ala Polymorphism in the Peroxisome Proliferator-Activated Receptor-Gamma2 Gene Is Associated with Increased Antilipolytic Insulin Sensitivity. Diabetes 2001, 50, 876–881. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jang, E.J.; Lee, D.H.; Im, S.-S.; Yee, J.; Gwak, H.S. Correlation between PPARG Pro12Ala Polymorphism and Therapeutic Responses to Thiazolidinediones in Patients with Type 2 Diabetes: A Meta-Analysis. Pharmaceutics 2023, 15, 1778. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.D.; Cheon, H.; Jun, J.B.; Choi, S.J.; Kim, Y.R.; Lee, Y.H.; Kim, T.H.; Chae, I.J.; Song, G.G.; Yoo, G.H.; et al. Effects of Peroxisome Proliferator-activated Receptor-γ (PPAR-γ) on the Expression of Inflammatory Cytokines and Apoptosis Induction in Rheumatoid Synovial Fibroblasts and Monocytes. J. Autoimmun. 2001, 17, 215–221. [Google Scholar] [CrossRef]
- Ganeb, S.S.; El-Brashy, A.E.-W.S.; Baraka, E.A.; Aboelazm, A.A.; Shaza, A.; Abdul Basset, S.A.A. Peroxisome proliferator-activated receptor gamma expression in peripheral monocytes from rheumatoid arthritis patients. Egypt. Rheumatol. 2016, 38, 141–146. [Google Scholar] [CrossRef][Green Version]
- Li, X.F.; Sun, Y.Y.; Bao, J.; Chen, X.; Li, Y.H.; Yang, Y.; Zhang, L.; Huang, C.; Wu, B.-M.; Meng, X.-M.; et al. Functional role of PPAR-γ on the proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.; Lefebvre, B.; Dubuquoy, L.; Lefebvre, P.; Romano, O.; Auwerx, J.; Metzger, D.; Wahli, W.; Desvergne, B.; Naccari, G.C.; et al. Intestinal anti-inflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-γ. J. Exp. Med. 2005, 201, 1205–1215. [Google Scholar] [CrossRef]
- Dubuquoy, L.; Rousseaux, C.; Thuru, X.; Peyrin-Biroulet, L.; Romano, O.; Chavatte, P.; Chamaillard, M.; Desreumaux, P. PPARgamma as a new therapeutic target in inflammatory bowel diseases. Gut 2006, 55, 1341–1349. [Google Scholar] [CrossRef]
- Annese, V.; Rogai, F.; Settesoldi, A.; Bagnoli, S. PPARγ in Inflammatory Bowel Disease. PPAR Res. 2011, 2012, 620839. [Google Scholar] [CrossRef]
- da Rocha, G.H.O.; de Paula-Silva, M.; Broering, M.F.; Scharf, P.R.D.S.; Matsuyama, L.S.A.S.; Maria-Engler, S.S.; Farsky, S.H.P. Pioglitazone-Mediated Attenuation of Experimental Colitis Relies on Cleaving of Annexin A1 Released by Macrophages. Front. Pharmacol. 2020, 11, 591561. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Luo, S.; Zhan, Y.; Lu, Q. The roles of PPARγ and its agonists in autoimmune diseases: A comprehensive review. J. Autoimmun. 2020, 113, 102510. [Google Scholar] [CrossRef]
- Li, X.F.; Yin, S.Q.; Li, H.; Yang, Y.L.; Chen, X.; Song, B.; Wu, S.; Wu, Y.Y.; Wang, H.; Li, J. PPAR-γ alleviates the inflammatory response in TNF-α-induced fibroblast-like synoviocytes by binding to p53 in rheumatoid arthritis. Acta Pharmacol Sin. 2023, 44, 454–464. [Google Scholar] [CrossRef]
- Lewis, J.D.; Lichtenstein, G.R.; Stein, R.B.; Deren, J.J.; Judge, T.A.; Fogt, F.; Furth, E.E.; Demissie, E.J.; Hurd, L.B.; Su, C.G.; et al. An open-label trial of the PPAR-gamma ligand rosiglitazone for active ulcerative colitis. Am. J. Gastroenterol. 2001, 96, 3323–3328. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.L.; Ouyang, Q. A clinical trial of rosiglitazone and 5-aminosalicylate combination for ulcerative colitis. Zhonghua Nei ke Za Zhi 2006, 45, 548–551. [Google Scholar]
- Bassaganya-Riera, J.; Rynolds, K.; Martino-Catt, S.; Cui, Y.; Hennighausen, L.; Gonzalez, F.; Rohrer, J.; Benninghoff, A.U.; Hontecillas, R. Activation of PPAR γ and δ by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology 2004, 127, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Li, P.; Zhang, J.; Shi, Y.; Chen, K.; Yang, J.; Wu, Y.; Ye, X. Association between peroxisome proliferator-activated receptor-alpha, delta, and gamma polymorphisms and risk of coronary heart disease: A case-control study and meta-analysis. Medicine 2016, 95, e4299. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz-Aydogan, H.; Kurnaz, O.; Kucukhuseyin, O.; Akadam-Teker, B.; Kurt, O.; Eronat, A.P.; Tekeli, A.; Bugra, Z.; Ozturk, O. Different effects of PPARA, PPARG and ApoE SNPs on serum lipids in patients with coronary heart disease based on the presence of diabetes. Gene 2013, 523, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Skoczynska, A.; Dobosz, T.; Poreba, R.; Turczyn, B.; Derkacz, A.; Zoledziewska, M.; Jonkisz, A.; Lebioda, A. The dependence of serum interleukin-6 level on PPAR-alpha polymorphism in men with coronary atherosclerosis. Eur. J. Intern. Med. 2005, 16, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Gouni-Berthold, I.; Giannakidou, E.; Müller-Wieland, D.; Faust, M.; Kotzka, J.; Berthold, H.K.; Krone, W. Association between the PPARalpha L162V polymorphism, plasma lipoprotein levels, and atherosclerotic disease in patients with diabetes mellitus type 2 and in nondiabetic controls. Am. Heart J. 2004, 147, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zhou, H.; Shen, C.; Yu, L.G.; Ding, Y.; Zhang, Y.H.; Guo, Z.R. Role of peroxisome proliferator-activated receptors gene polymorphisms in type 2 diabetes and metabolic syndrome. World J. Diabetes 2015, 6, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. ESC National Cardiac Societies, ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3237. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, P.; Prejbisz, A.; Kuryłowicz, A.; Baska, A.; Burchardt, P.; Chlebus, K.; Dzida, G.; Jankowski, P.; Jaroszewicz, J.; Jaworski, P.; et al. Metabolic syndrome—A new definition and management guidelines: A joint position paper by the Polish Society of Hypertension, Polish Society for the Treatment of Obesity, Polish Lipid Association, Polish Association for Study of Liver, Polish Society of Family Medicine, Polish Society of Lifestyle Medicine, Division of Prevention and Epidemiology Polish Cardiac Society, “Club 30” Polish Cardiac Society, and Division of Metabolic and Bariatric Surgery Society of Polish Surgeons. Arch. Med. Sci. 2022, 18, 1133–1156. [Google Scholar] [CrossRef]
- Cheng, H.S.; Tan, W.R.; Low, Z.S.; Marvalim, C.; Lee, J.Y.H.; Tan, N.S. Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence. Int. J. Mol. Sci. 2019, 20, 5055. [Google Scholar] [CrossRef]
- Colapietro, F.; Gershwin, M.E.; Lleo, A. PPAR agonists for the treatment of primary biliary cholangitis: Old and new tales. J. Transl. Autoimmun. 2023, 6, 100188. [Google Scholar] [CrossRef]
- Xi, Y.; Zhang, Y.; Zhu, S.; Luo, Y.; Xu, P.; Huang, Z. PPAR-Mediated Toxicology and Applied Pharmacology. Cells 2020, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Okopień, B.; Bułdak, Ł.; Bołdys, A. Benefits and risks of the treatment with fibrates––A comprehensive summary. Expert Rev. Clin. Pharmacol. 2018, 11, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S.; Wahli, W. Peroxisome proliferator-activated receptor agonists. Exp.-Suppl. Only 2000, 89, 141–152. [Google Scholar]

| Possibilities | PPARα | PPARβ/δ | PPARγ |
|---|---|---|---|
| 1 | ↑ activity | ↓ activity | ↓ activity |
| 2 | ↓ activity | ↓ activity | ↑ activity |
| 3 | ↓ activity | ↑ activity | ↓ activity |
| Category | Description |
|---|---|
| Study ID | Authors Year Journal |
| Model | Human |
| Intervention | PPAR isotypes:
|
| Other | The term “PPAR” reported in the title or abstract—yes/no Criteria for inclusion and/or exclusion of data provided—yes/no |
| Search term type | Free-text |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoczyńska, A.; Ołdakowska, M.; Dobosz, A.; Adamiec, R.; Gritskevich, S.; Jonkisz, A.; Lebioda, A.; Adamiec-Mroczek, J.; Małodobra-Mazur, M.; Dobosz, T. PPARs in Clinical Experimental Medicine after 35 Years of Worldwide Scientific Investigations and Medical Experiments. Biomolecules 2024, 14, 786. https://doi.org/10.3390/biom14070786
Skoczyńska A, Ołdakowska M, Dobosz A, Adamiec R, Gritskevich S, Jonkisz A, Lebioda A, Adamiec-Mroczek J, Małodobra-Mazur M, Dobosz T. PPARs in Clinical Experimental Medicine after 35 Years of Worldwide Scientific Investigations and Medical Experiments. Biomolecules. 2024; 14(7):786. https://doi.org/10.3390/biom14070786
Chicago/Turabian StyleSkoczyńska, Anna, Monika Ołdakowska, Agnieszka Dobosz, Rajmund Adamiec, Sofya Gritskevich, Anna Jonkisz, Arleta Lebioda, Joanna Adamiec-Mroczek, Małgorzata Małodobra-Mazur, and Tadeusz Dobosz. 2024. "PPARs in Clinical Experimental Medicine after 35 Years of Worldwide Scientific Investigations and Medical Experiments" Biomolecules 14, no. 7: 786. https://doi.org/10.3390/biom14070786
APA StyleSkoczyńska, A., Ołdakowska, M., Dobosz, A., Adamiec, R., Gritskevich, S., Jonkisz, A., Lebioda, A., Adamiec-Mroczek, J., Małodobra-Mazur, M., & Dobosz, T. (2024). PPARs in Clinical Experimental Medicine after 35 Years of Worldwide Scientific Investigations and Medical Experiments. Biomolecules, 14(7), 786. https://doi.org/10.3390/biom14070786








