Investigating the Antifibrotic Effects of β-Citronellol on a TGF-β1-Stimulated LX-2 Hepatic Stellate Cell Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Crude Extract and Active Compound
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. RNA Extraction and Gene Expression Analysis
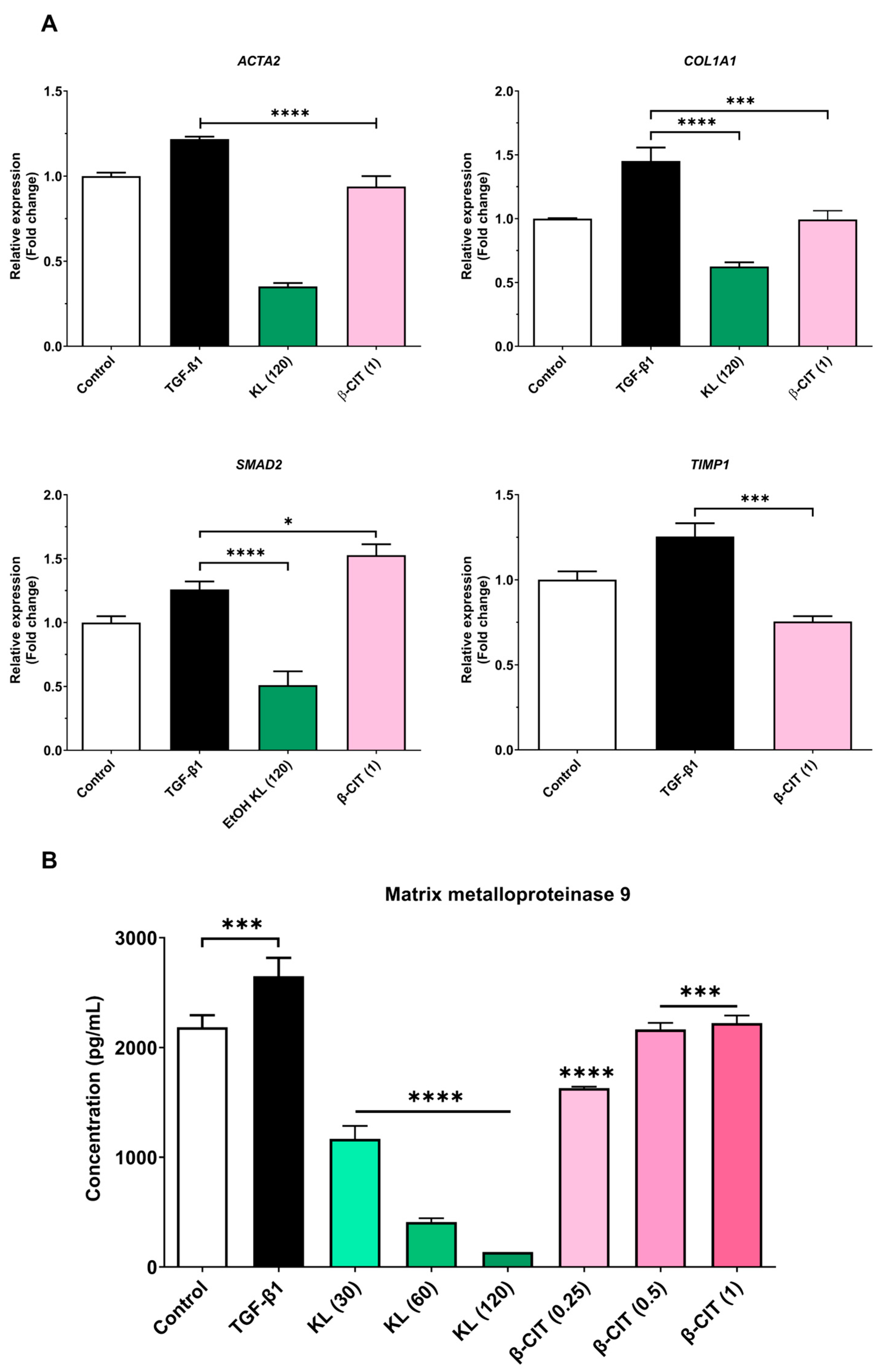
| Gene | Description | Forward Primer (3′ → 5′) | Reverse Primer (3′ → 5′) | Ref |
|---|---|---|---|---|
| ACTA2 | actin alpha 2, smooth muscle | CATCCTCATCCTCCCTTGAG | ATGAAGGATGGCTGGAACAG | [25] |
| COL1A1 | Collagen type I alpha 1 chain | CCGGCTCCTGCTCCTCTTAGCG | CGTTCTGTACGCAGGTGATTGGTGG | |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | CAAGATGTATAAAGGGTTCCAAGC | TCCATCCTGCAGTTTTCCAG | |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | ATGACATCAAGAAGGTGGTG | CATACCAGGAAATGAGCTTG | |
| SMAD2 | SMAD family member 2 | TGCTCTGAAATTTGGGGACTGA | GACGACCATCAAGAGACCTGG | [26] |
2.5. Measurement of the MMP-9 Production
2.6. LC-MS/MS Analysis
2.6.1. Preparation of Protein Sample
2.6.2. Proteomic Data Analysis
2.7. Molecular Docking Analysis
2.8. Statistical Analysis
3. Results
3.1. Cytotoxicity of Crude Extract and Active Compound on the LX-2 Cell Line
3.2. The Inhibition of Activated HSCs’ Genes’ Expression and MMP-9 Production
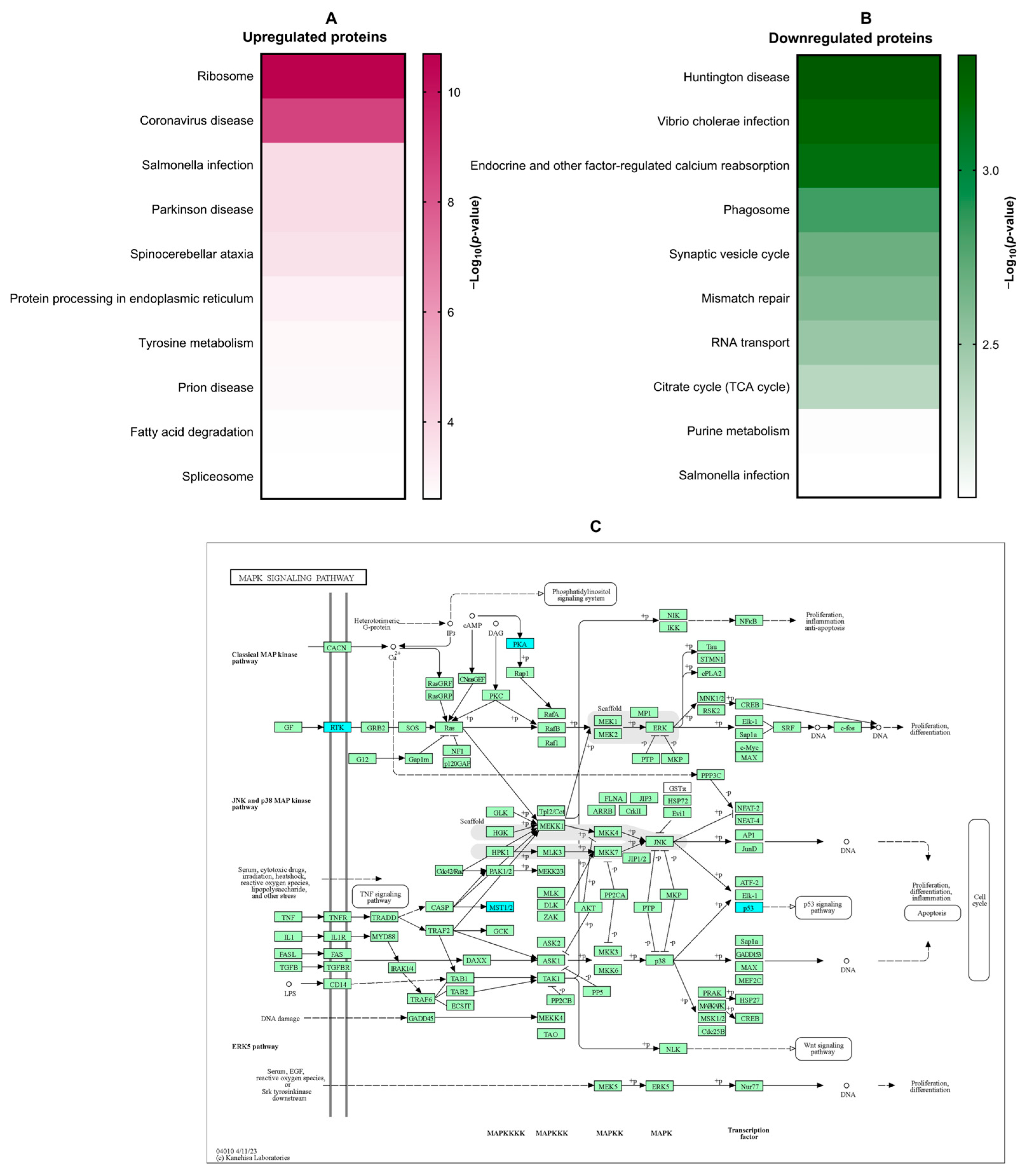
3.3. Proteomic Analysis of the Effect of β-Citronellol on LX-2 Cell
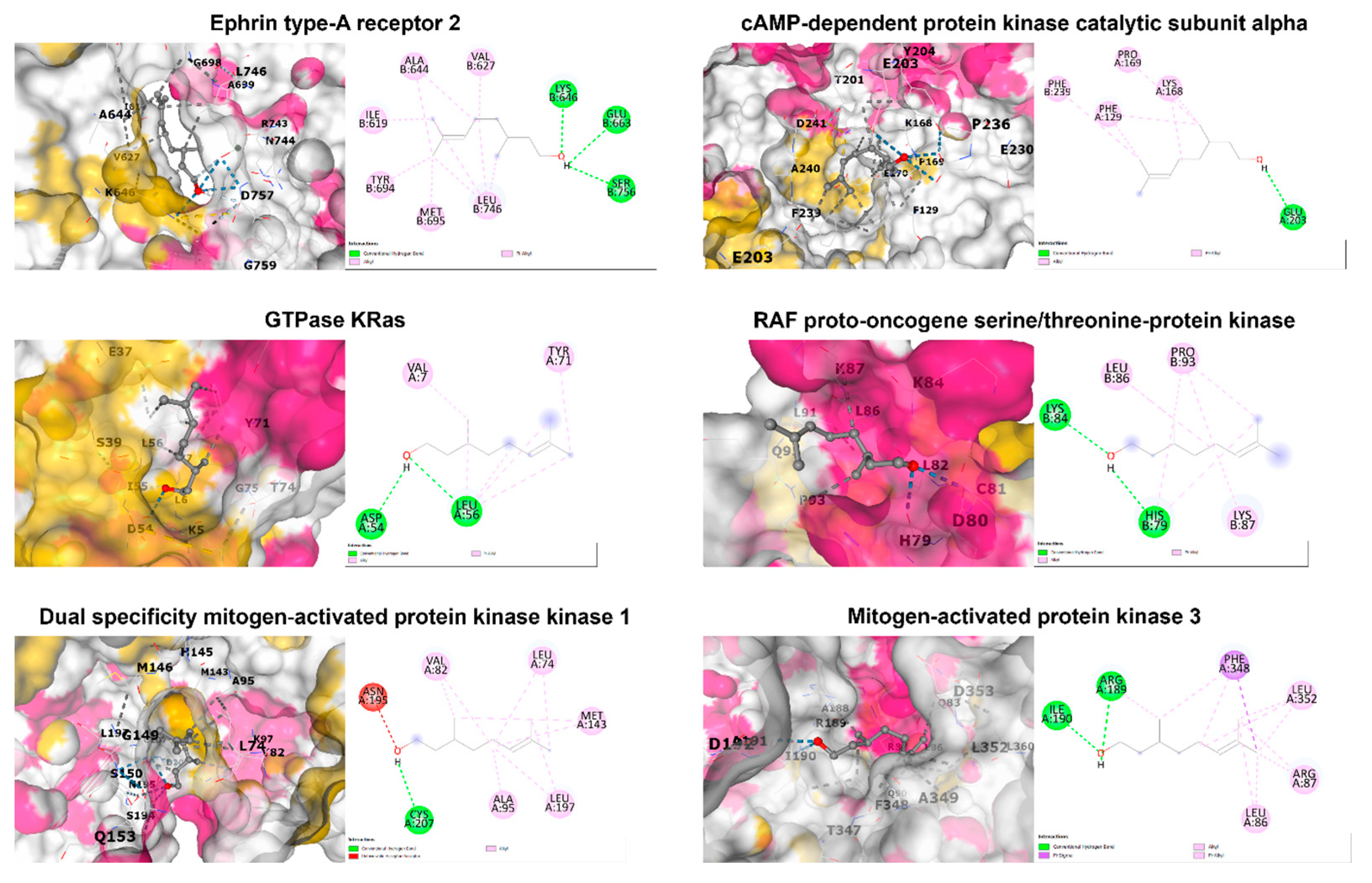
3.4. In Silico Molecular Docking Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Luangmonkong, T.; Parichatikanond, W.; Olinga, P. Targeting collagen homeostasis for the treatment of liver fibrosis: Opportunities and challenges. Biochem. Pharmacol. 2023, 215, 115740. [Google Scholar] [CrossRef]
- Acharya, P.; Chouhan, K.; Weiskirchen, S.; Weiskirchen, R. Cellular Mechanisms of Liver Fibrosis. Front. Pharmacol. 2021, 12, 671640. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Meng, X.M.; Huang, C.; Xu, F.; Li, J. Smad2 protects against TGF-β1/Smad3-mediated collagen synthesis in human hepatic stellate cells during hepatic fibrosis. Mol. Cell. Biochem. 2015, 400, 17–28. [Google Scholar] [CrossRef]
- Rockey, D.C.; Du, Q.; Weymouth, N.D.; Shi, Z. Smooth Muscle α-Actin Deficiency Leads to Decreased Liver Fibrosis via Impaired Cytoskeletal Signaling in Hepatic Stellate Cells. Am. J. Pathol. 2019, 189, 2209–2220. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, B.; Chen, L.; Chen, G.; Zhu, D.; Chen, J.; Duan, L.; Duan, Y. rSjP40 Inhibited the Activity of Collagen Type I Promoter via Ets-1 in HSCs. Front. Cell Dev. Biol. 2021, 9, 765616. [Google Scholar] [CrossRef]
- Wang, K.; Lin, B.; Brems, J.J.; Gamelli, R.L. Hepatic apoptosis can modulate liver fibrosis through TIMP1 pathway. Apoptosis 2013, 18, 566–577. [Google Scholar] [CrossRef]
- Affo, S.; Yu, L.-X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu. Rev. Pathol. 2017, 12, 153–186. [Google Scholar] [CrossRef]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Liu, S.; Yang, M. Treatment of liver fibrosis: Past, current, and future. World J. Hepatol. 2023, 15, 755–774. [Google Scholar] [CrossRef] [PubMed]
- Agouillal, F.; Taher, Z.; Moghrani, H.; Nasrallah, N.; El Enshasy, H. A Review of Genetic Taxonomy, Biomolecules Chemistry and Bioactivities of Citrus hystrix DC. Biosci. Biotechnol. Res. Asia 2017, 14, 285–305. [Google Scholar] [CrossRef]
- Kidarn, S.; Saenjum, C.; Hongwiset, D.; Phrutivorapongkul, A. Furanocoumarins from Kaffir lime and their inhibitory effects on inflammatory mediator production. Cogent Chem. 2018, 4, 1529259. [Google Scholar] [CrossRef]
- Buakaew, W.; Pankla Sranujit, R.; Noysang, C.; Thongsri, Y.; Potup, P.; Nuengchamnong, N.; Suphrom, N.; Usuwanthim, K. Phytochemical Constituents of Citrus hystrix DC. Leaves Attenuate Inflammation via NF-κB Signaling and NLRP3 Inflammasome Activity in Macrophages. Biomolecules 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Ratanachamnong, P.; Chunchaowarit, Y.; Namchaiw, P.; Niwaspragrit, C.; Rattanacheeworn, P.; Jaisin, Y. HPLC analysis and in vitro antioxidant mediated through cell migration effect of C. hystrix water extract on human keratinocytes and fibroblasts. Heliyon 2023, 9, 13068. [Google Scholar] [CrossRef]
- Abirami, A.; Nagarani, G.; Siddhuraju, P. In vitro antioxidant, anti-diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C. maxima fruits. Food Sci. Hum. Wellness 2014, 3, 16–25. [Google Scholar] [CrossRef]
- Srifuengfung, S.; Bunyapraphatsara, N.; Satitpatipan, V.; Tribuddharat, C.; Junyaprasert, V.B.; Tungrugsasut, W.; Srisukh, V. Antibacterial oral sprays from kaffir lime (Citrus hystrix DC.) fruit peel oil and leaf oil and their activities against respiratory tract pathogens. J. Tradit. Complement. Med. 2020, 10, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Sreepian, P.M.; Rattanasinganchan, P.; Sreepian, A. Antibacterial efficacy of Citrus hystrix (makrut lime) essential oil against clinical multidrug-resistant methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates. Saudi Pharm. J. 2023, 31, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Tunjung, W.A.S.; Cinatl, J.; Michaelis, M.; Smales, C.M. Anti-Cancer Effect of Kaffir Lime (Citrus hystrix DC) Leaf Extract in Cervical Cancer and Neuroblastoma Cell Lines. Procedia Chem. 2015, 14, 465–468. [Google Scholar] [CrossRef]
- Ho, Y.; Suphrom, N.; Daowtak, K.; Potup, P.; Thongsri, Y.; Usuwanthim, K. Anticancer Effect of Citrus hystrix DC. Leaf Extract and Its Bioactive Constituents Citronellol and, Citronellal on the Triple Negative Breast Cancer MDA-MB-231 Cell Line. Pharmaceuticals 2020, 13, 476. [Google Scholar] [CrossRef]
- Abolmaesoomi, M.; Mat Junit, S.; Mohd Ali, J.; Chik, Z.B.; Abdul Aziz, A. Effects of polyphenolic-rich extracts from Citrus hystrix on proliferation and oxidative stress in breast and colorectal cancer. Turk. J. Biochem. 2023, 48, 110–118. [Google Scholar] [CrossRef]
- Abirami, A.; Nagarani, G.; Siddhuraju, P. Hepatoprotective effect of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rats. Food Sci. Hum. Wellness 2015, 4, 35–41. [Google Scholar] [CrossRef]
- Buakaew, W.; Pankla Sranujit, R.; Noysang, C.; Krobthong, S.; Yingchutrakul, Y.; Thongsri, Y.; Potup, P.; Daowtak, K.; Usuwanthim, K. Proteomic Analysis Reveals Proteins Involved in the Mode of Action of β-Citronellol Identified From Citrus hystrix DC. Leaf Against Candida albicans. Front. Microbiol. 2022, 13, 894637. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2-ΔΔCT method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma Biomath. 2013, 3, 71–85. [Google Scholar] [PubMed]
- Robert, S.; Gicquel, T.; Bodin, A.; Lagente, V.; Boichot, E. Characterization of the MMP/TIMP Imbalance and Collagen Production Induced by IL-1β or TNF-α Release from Human Hepatic Stellate Cells. PLoS ONE 2016, 11, e153118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, W.; Yang, L.; Chen, L.; Stimpson, S.A.; Diehl, A.M. PPARγ agonists prevent TGFβ1/Smad3-signaling in human hepatic stellate cells. Biochem. Biophys. Res. Commun. 2006, 350, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Krobthong, S.; Yingchutrakul, Y.; Samutrtai, P.; Hitakarun, A.; Siripattanapipong, S.; Leelayoova, S.; Mungthin, M.; Choowongkomon, K. Utilizing Quantitative Proteomics to Identify Species-Specific Protein Therapeutic Targets for the Treatment of Leishmaniasis. ACS Omega 2022, 7, 12580–12588. [Google Scholar] [CrossRef]
- Griss, J.; Viteri, G.; Sidiropoulos, K.; Nguyen, V.; Fabregat, A.; Hermjakob, H. ReactomeGSA—Efficient Multi-Omics Comparative Pathway Analysis. Mol. Cell. Proteom. 2020, 19, 2115–2125. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.-X.; Cao, Y. CB-Dock2: Improved protein–ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis—Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Y.; Sun, B. The Molecular Mechanisms of Liver Fibrosis and Its Potential Therapy in Application. Int. J. Mol. Sci. 2022, 23, 12572. [Google Scholar] [CrossRef] [PubMed]
- Westenberger, G.; Sellers, J.; Fernando, S.; Junkins, S.; Han, S.M.; Min, K.; Lawan, A. Function of Mitogen-Activated Protein Kinases in Hepatic Inflammation. J. Cell. Signal. 2021, 2, 172–180. [Google Scholar]
- Darling, T.K.; Lamb, T.J. Emerging Roles for Eph Receptors and Ephrin Ligands in Immunity. Front. Immunol. 2019, 10, 1473. [Google Scholar] [CrossRef]
- Mekala, S.; Dugam, P.; Das, A. Ephrin–Eph receptor tyrosine kinases for potential therapeutics against hepatic pathologies. J. Cell Commun. Signal. 2023, 17, 549–561. [Google Scholar] [CrossRef]
- Creeden, J.F.; Kipp, Z.A.; Xu, M.; Flight, R.M.; Moseley, H.N.B.; Martinez, G.J.; Lee, W.H.; Alganem, K.; Imami, A.S.; McMullen, M.R.; et al. Hepatic kinome atlas: An in-depth identification of kinase pathways in liver fibrosis of humans and rodents. Hepatology 2022, 76, 1376–1388. [Google Scholar] [CrossRef]

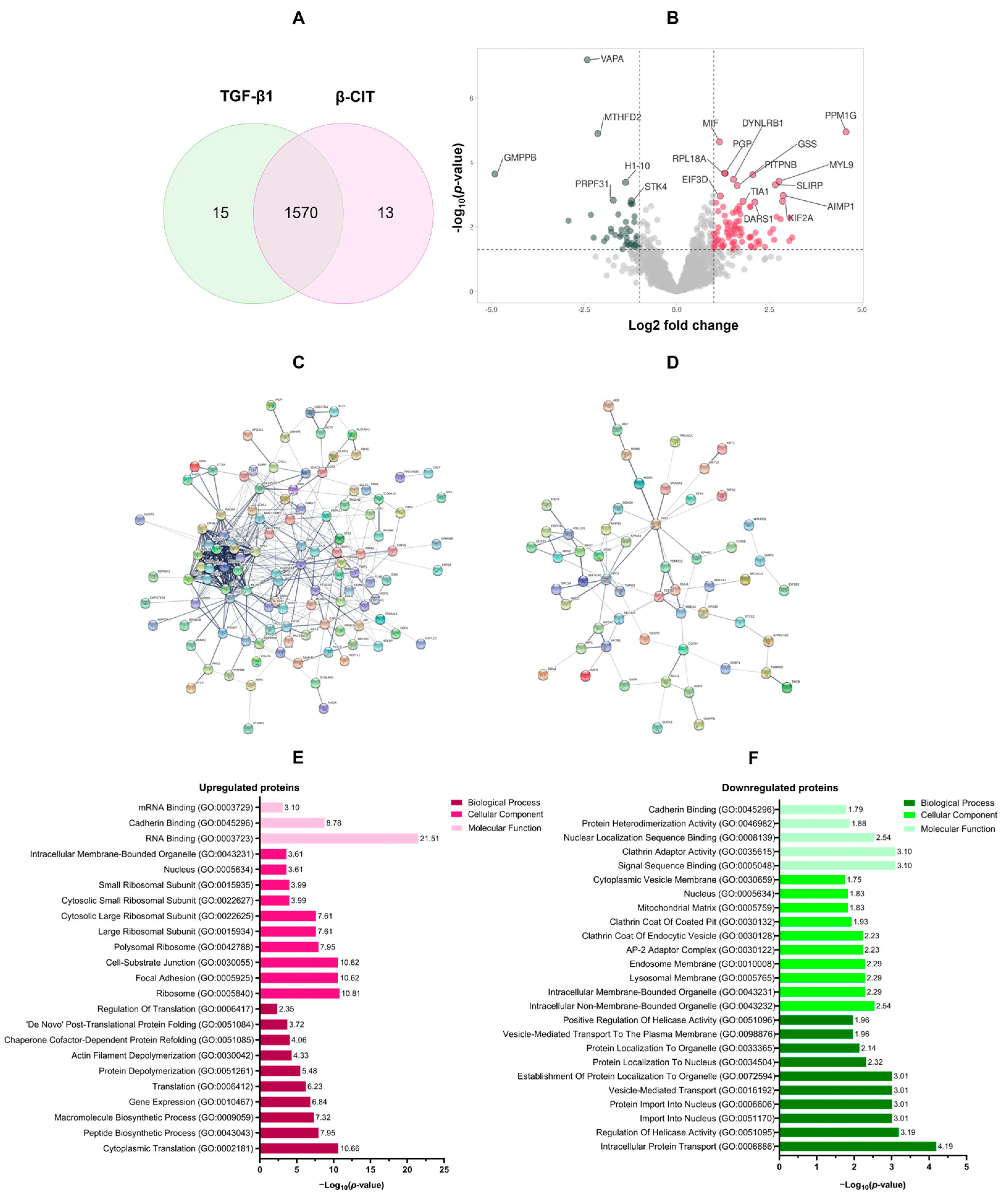
| Uniprot ID | Gene ID | Description | Log2 Fold Change | −Log10 p-Value |
|---|---|---|---|---|
| Top 10 upregulated proteins | ||||
| O15355 | PPM1G | Protein phosphatase, Mg2+/Mn2+ dependent 1G | 4.57 | 4.95 |
| Q9Y4W6 | AFG3L2 | AFG3-like protein 2 | 3.11 | 1.68 |
| P04066 | FUCA1 | Tissue alpha-L-fucosidase | 3.05 | 2.29 |
| Q16537 | PPP2R5E | Serine/threonine-protein phosphatase 2A 56 kDa regulatory subunit epsilon isoform | 3.04 | 1.57 |
| Q12904 | AIMP1 | Aminoacyl tRNA synthase complex-interacting multifunctional protein 1 | 2.87 | 2.98 |
| O00139 | KIF2A | Kinesin-like protein KIF2A | 2.85 | 2.80 |
| Q16186 | ADRM1 | Proteasomal ubiquitin receptor ADRM1 | 2.80 | 2.24 |
| P24844 | MYL9 | Myosin regulatory light polypeptide 9 | 2.76 | 3.42 |
| Q0VDF9 | HSPA14 | Heat shock 70 kDa protein 14 | 2.69 | 2.32 |
| Q9GZT3 | SLIRP | SRA stem-loop-interacting RNA-binding protein, mitochondrial | 2.66 | 3.31 |
| Top 10 downregulated proteins | ||||
| Q9Y5P6 | GMPPB | Mannose-1-phosphate guanyltransferase beta | −4.91 | 3.65 |
| O75431 | MTX2 | Metaxin-2 | −2.92 | 2.20 |
| Q9P0L0 | VAPA | Vesicle-associated membrane protein-associated protein A | −2.42 | 7.19 |
| P04264 | KRT1 | Keratin, type II cytoskeletal 1 | −2.32 | 2.38 |
| O76021 | RSL1D1 | Ribosomal L1 domain-containing protein 1 | −2.24 | 1.67 |
| P13995 | MTHFD2 | Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial | −2.14 | 4.90 |
| P51398 | DAP3 | 28S ribosomal protein S29, mitochondrial | −1.96 | 1.56 |
| Q9P2R3 | ANKFY1 | Rabankyrin-5 | −1.89 | 1.66 |
| O00291 | HIP1 | Huntingtin-interacting protein 1 | −1.78 | 1.95 |
| PDB ID | Protein Name | Binding Score (Kcal/mol) | Interacting Amino Acid |
|---|---|---|---|
| 1MQB | Ephrin type-A receptor 2 | −5.0 | Ile619, Val627, Ala644, Lys646, Glu663, Tyr694, Met695, Leu746, Ser756 |
| 7Y1G | cAMP-dependent protein kinase catalytic subunit alpha | −5.4 | Phe129, Lys168, Pro169, Glu203, Phe239 |
| 8FMI | GTPase KRas | −4.8 | Val7, Asp54, Leu56, Tyr71 |
| 1C1Y | RAF proto-oncogene serine/threonine-protein kinase | −4.2 | His79, Lys84, Leu86, Lys87, Pro93 |
| 1S9J | Dual specificity mitogen-activated protein kinase kinase 1 | −5.3 | Leu74, Val82, Ala95, Met143, Asn195, Leu197, Cys207 |
| 2ZOQ | Mitogen-activated protein kinase 3 | −5.7 | Leu86, Arg87, Arg189, Ile190, Phe348, Leu352 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buakaew, W.; Krobthong, S.; Yingchutrakul, Y.; Potup, P.; Thongsri, Y.; Daowtak, K.; Ferrante, A.; Usuwanthim, K. Investigating the Antifibrotic Effects of β-Citronellol on a TGF-β1-Stimulated LX-2 Hepatic Stellate Cell Model. Biomolecules 2024, 14, 800. https://doi.org/10.3390/biom14070800
Buakaew W, Krobthong S, Yingchutrakul Y, Potup P, Thongsri Y, Daowtak K, Ferrante A, Usuwanthim K. Investigating the Antifibrotic Effects of β-Citronellol on a TGF-β1-Stimulated LX-2 Hepatic Stellate Cell Model. Biomolecules. 2024; 14(7):800. https://doi.org/10.3390/biom14070800
Chicago/Turabian StyleBuakaew, Watunyoo, Sucheewin Krobthong, Yodying Yingchutrakul, Pachuen Potup, Yordhathai Thongsri, Krai Daowtak, Antonio Ferrante, and Kanchana Usuwanthim. 2024. "Investigating the Antifibrotic Effects of β-Citronellol on a TGF-β1-Stimulated LX-2 Hepatic Stellate Cell Model" Biomolecules 14, no. 7: 800. https://doi.org/10.3390/biom14070800
APA StyleBuakaew, W., Krobthong, S., Yingchutrakul, Y., Potup, P., Thongsri, Y., Daowtak, K., Ferrante, A., & Usuwanthim, K. (2024). Investigating the Antifibrotic Effects of β-Citronellol on a TGF-β1-Stimulated LX-2 Hepatic Stellate Cell Model. Biomolecules, 14(7), 800. https://doi.org/10.3390/biom14070800








