Forces Bless You: Mechanosensitive Piezo Channels in Gastrointestinal Physiology and Pathology
Abstract
1. Introduction
| Items | Piezo1 | Piezo2 | Reference |
|---|---|---|---|
| Gene | Fam38A | Fam38B | [12] |
| Chromosomal localization | human chromosome 16 | human chromosome 18 | NCBI database (https://www.ncbi.nlm.nih.gov/gene/9780 accessed on 17 June 2024. https://www.ncbi.nlm.nih.gov/gene/63895 accessed on 17 June 2024.) |
| Gene region | 16q24.3 | 18p11.22-p11.21 | |
| Amino acid size in human | 2521 amino acids | 2752 amino acids | [16] |
| Amino acid size in mice | 2547 amino acids | 2822 amino acids | [17,18] |
| Tissue distribution | skin, bladder, kidney, lung, endothelial cells, erythrocytes, periodontal ligament cells, etc. | trigeminal sensory neurons, dorsal root ganglion, Merkel cells, somatic neuron cells, etc. | [19] |
| Delection threshold (fJ) | 213.7 ± 16.6 | 86.8 ± 7.1 | [20] |
| Work resolution (fJ) | 1.2 + 0.4 | 1.0 + 0.2 | [20] |
| Transduction speed (ms) | 8.2 ± 2.2 | 1.5 ± 0.5 | [20] |
| Inactivation kinetics (ms) | 16.5 ± 1.4 | 7.3 ± 0.7 | [12] |
| Structure | a homotrimer structure resembling a three—bladed propeller | [18,21] | |
| Function | involving in mechanotransduction in various non-excitable cell types | sensing slight touch and proprioception | [22] |
| Activator | Yoda1, Jedi1/2 | not found yet | [23,24] |
| Inhibitor | Ruthenium red (RR), Gadolinium (Gd3+), Dooku1, and GsMTx4 | RR, Gd3+, and GsMTx4 | [25,26,27,28,29] |
| Hereditary human disorders | dehydrated hereditary stomatocytosis, generalized lymphatic dysplasia, etc. | distal arthrogryposis, Gordon syndrome, Marden-Walker Syndrome, etc. | [30,31,32,33] |
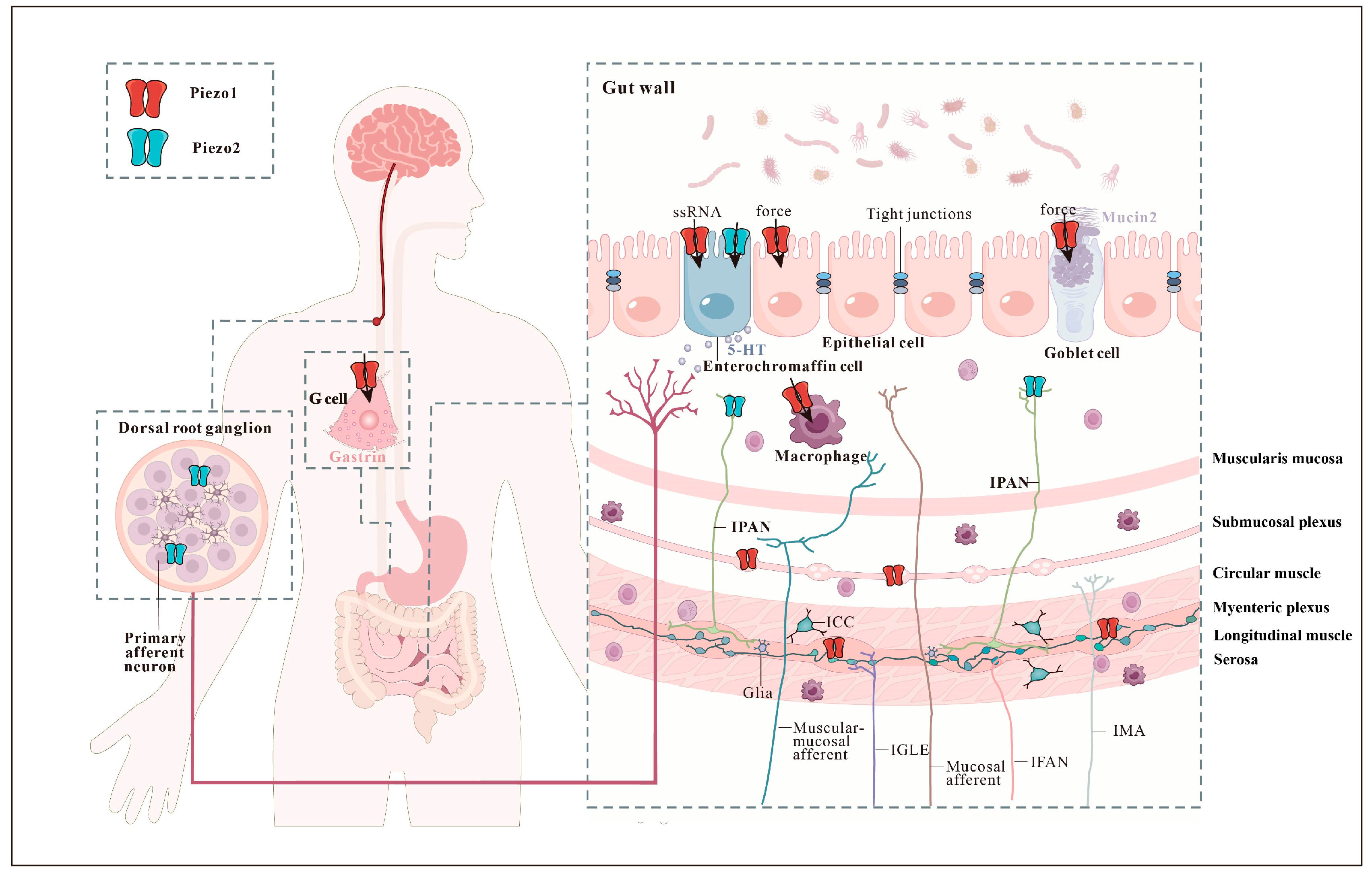
2. Piezo Channels in Mechanosensory Cells That Detect Gastrointestinal Forces
2.1. Epithelial Touch Sensors
2.2. Mechanosensitive Enteric Neurons
2.3. Intrinsic Primary Afferent Neurons
2.4. Extrinsic Sensory Neurons
3. Mechanosensitive Piezo Channels Affect the Gastrointestinal Function
3.1. Intestinal Barrier
3.2. Gastrointestinal Motility
3.3. Intestinal Mechanosensation
4. Mechanosensitive Piezo Channels in the Gastrointestinal Disorders
4.1. Irritable Bowel Syndrome
4.2. Inflammatory Bowel Disease
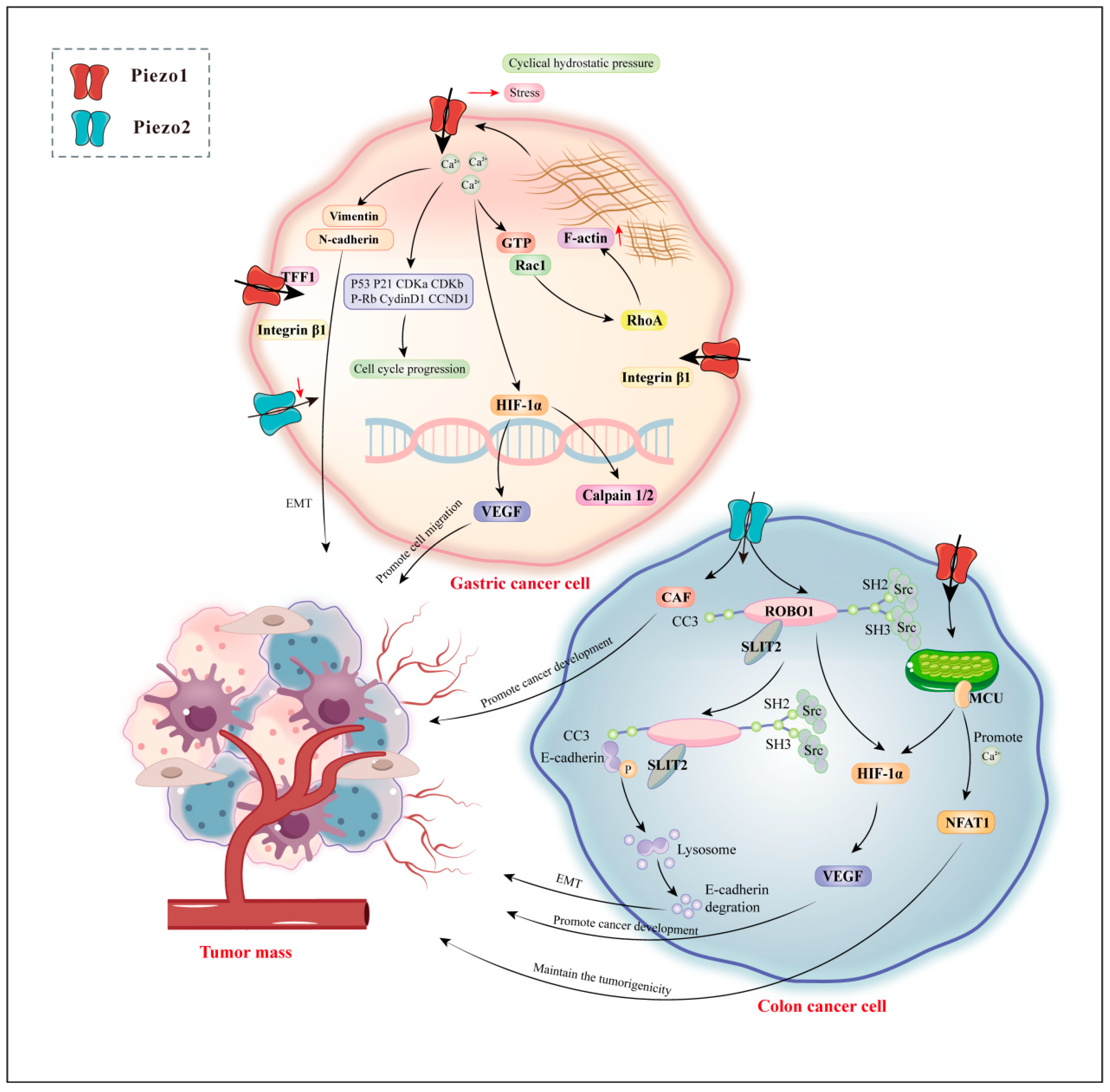
4.3. Gastrointestinal Cancers
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. Faseb. J. 2006, 20, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Perez, A.; Beyder, A. Gut feelings: Mechanosensing in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Alcaino, C.; Farrugia, G.; Beyder, A. Mechanosensitive Piezo Channels in the Gastrointestinal Tract. Curr. Top. Membr. 2017, 79, 219–244. [Google Scholar] [CrossRef] [PubMed]
- Gayer, C.P.; Basson, M.D. The effects of mechanical forces on intestinal physiology and pathology. Cell Signal 2009, 21, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Kola, J.B.; Docsa, T.; Uray, K. Mechanosensing in the Physiology and Pathology of the Gastrointestinal Tract. Int. J. Mol. Sci. 2022, 24, 177. [Google Scholar] [CrossRef] [PubMed]
- Brookes, S.J.; Spencer, N.J.; Costa, M.; Zagorodnyuk, V.P. Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Scholz, N.; Monk, K.R.; Kittel, R.J.; Langenhan, T. Adhesion GPCRs as a Putative Class of Metabotropic Mechanosensors. Handb. Exp. Pharmacol. 2016, 234, 221–247. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Xu, J.; Mathur, J.; Vessières, E.; Hammack, S.; Nonomura, K.; Favre, J.; Grimaud, L.; Petrus, M.; Francisco, A.; Li, J.; et al. GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell 2018, 173, 762–775.e716. [Google Scholar] [CrossRef]
- Chalfie, M. Neurosensory mechanotransduction. Nat. Rev. Mol. Cell Biol. 2009, 10, 44–52. [Google Scholar] [CrossRef]
- Jin, P.; Jan, L.Y.; Jan, Y.N. Mechanosensitive Ion Channels: Structural Features Relevant to Mechanotransduction Mechanisms. Annu. Rev. Neurosci. 2020, 43, 207–229. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Liddle, R.A. Mechanosensing Piezo channels in gastrointestinal disorders. J. Clin. Investig. 2023, 133, e171955. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xie, X.; Xiao, Z.; Qian, W.; Zhang, L.; Hou, X. Piezo1 in Digestive System Function and Dysfunction. Int. J. Mol. Sci. 2023, 24, 12953. [Google Scholar] [CrossRef]
- Yang, H.; Hou, C.; Xiao, W.; Qiu, Y. The role of mechanosensitive ion channels in the gastrointestinal tract. Front. Physiol. 2022, 13, 904203. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lewis, A.H.; Grandl, J. Touch, Tension, and Transduction—The Function and Regulation of Piezo Ion Channels. Trends Biochem. Sci. 2017, 42, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Li, W.; Zhao, Q.; Li, N.; Chen, M.; Zhi, P.; Li, R.; Gao, N.; Xiao, B.; Yang, M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 2015, 527, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, H.; Zhang, M.; Liu, W.; Deng, T.; Zhao, Q.; Li, Y.; Lei, J.; Li, X.; Xiao, B. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature 2019, 573, 225–229. [Google Scholar] [CrossRef]
- Bagriantsev, S.N.; Gracheva, E.O.; Gallagher, P.G. Piezo proteins: Regulators of mechanosensation and other cellular processes. J. Biol. Chem. 2014, 289, 31673–31681. [Google Scholar] [CrossRef]
- Young, M.N.; Sindoni, M.J.; Lewis, A.H.; Zauscher, S.; Grandl, J. The energetics of rapid cellular mechanotransduction. Proc. Natl. Acad. Sci. USA 2023, 120, e2215747120. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, H.; Chi, S.; Wang, Y.; Wang, J.; Geng, J.; Wu, K.; Liu, W.; Zhang, T.; Dong, M.Q.; et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature 2018, 554, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, X.; Jiang, J.; Xiao, B. Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels. Trends Biochem. Sci. 2021, 46, 472–488. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical activation of the mechanotransduction channel Piezo1. eLife 2015, 4, e07369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chi, S.; Guo, H.; Li, G.; Wang, L.; Zhao, Q.; Rao, Y.; Zu, L.; He, W.; Xiao, B. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat. Commun. 2018, 9, 1300. [Google Scholar] [CrossRef]
- Copp, S.W.; Kim, J.S.; Ruiz-Velasco, V.; Kaufman, M.P. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J. Physiol. 2016, 594, 641–655. [Google Scholar] [CrossRef]
- Bae, C.; Sachs, F.; Gottlieb, P.A. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 2011, 50, 6295–6300. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Knutson, K.; Alcaino, C.; Linden, D.R.; Gibbons, S.J.; Kashyap, P.; Grover, M.; Oeckler, R.; Gottlieb, P.A.; Li, H.J.; et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J. Physiol. 2017, 595, 79–91. [Google Scholar] [CrossRef]
- Yarishkin, O.; Phuong, T.T.T.; Baumann, J.M.; De Ieso, M.L.; Vazquez-Chona, F.; Rudzitis, C.N.; Sundberg, C.; Lakk, M.; Stamer, W.D.; Križaj, D. Piezo1 channels mediate trabecular meshwork mechanotransduction and promote aqueous fluid outflow. J. Physiol. 2021, 599, 571–592. [Google Scholar] [CrossRef]
- Evans, E.L.; Cuthbertson, K.; Endesh, N.; Rode, B.; Blythe, N.M.; Hyman, A.J.; Hall, S.J.; Gaunt, H.J.; Ludlow, M.J.; Foster, R.; et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br. J. Pharmacol. 2018, 175, 1744–1759. [Google Scholar] [CrossRef]
- Fotiou, E.; Martin-Almedina, S.; Simpson, M.A.; Lin, S.; Gordon, K.; Brice, G.; Atton, G.; Jeffery, I.; Rees, D.C.; Mignot, C.; et al. Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non-immune hydrops fetalis. Nat. Commun. 2015, 6, 8085. [Google Scholar] [CrossRef]
- McMillin, M.J.; Beck, A.E.; Chong, J.X.; Shively, K.M.; Buckingham, K.J.; Gildersleeve, H.I.; Aracena, M.I.; Aylsworth, A.S.; Bitoun, P.; Carey, J.C.; et al. Mutations in PIEZO2 cause Gordon syndrome, Marden-Walker syndrome, and distal arthrogryposis type 5. Am. J. Hum. Genet. 2014, 94, 734–744. [Google Scholar] [CrossRef]
- Coste, B.; Houge, G.; Murray, M.F.; Stitziel, N.; Bandell, M.; Giovanni, M.A.; Philippakis, A.; Hoischen, A.; Riemer, G.; Steen, U.; et al. Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proc. Natl. Acad. Sci. USA 2013, 110, 4667–4672. [Google Scholar] [CrossRef] [PubMed]
- Albuisson, J.; Murthy, S.E.; Bandell, M.; Coste, B.; Louis-Dit-Picard, H.; Mathur, J.; Fénéant-Thibault, M.; Tertian, G.; de Jaureguiberry, J.P.; Syfuss, P.Y.; et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat. Commun. 2013, 4, 1884. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Breer, H.; Frick, C. Mechanosensitive ion channel Piezo1 is expressed in antral G cells of murine stomach. Cell Tissue Res. 2018, 371, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Sugisawa, E.; Takayama, Y.; Takemura, N.; Kondo, T.; Hatakeyama, S.; Kumagai, Y.; Sunagawa, M.; Tominaga, M.; Maruyama, K. RNA Sensing by Gut Piezo1 Is Essential for Systemic Serotonin Synthesis. Cell 2020, 182, 609–624. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, T.; Xiong, Y.; Liu, C.; Liu, Y.; Hou, X.; Song, J. Mechanical stimulation activates Piezo1 to promote mucin2 expression in goblet cells. J. Gastroenterol. Hepatol. 2021, 36, 3127–3139. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiong, Y.; Liu, Y.; Li, G.; Bai, T.; Zheng, G.; Hou, X.; Song, J. Activation of goblet cell Piezo1 alleviates mucus barrier damage in mice exposed to WAS by inhibiting H3K9me3 modification. Cell Biosci. 2023, 13, 7. [Google Scholar] [CrossRef]
- Song, Y.; Fothergill, L.J.; Lee, K.S.; Liu, B.Y.; Koo, A.; Perelis, M.; Diwakarla, S.; Callaghan, B.; Huang, J.; Wykosky, J.; et al. Stratification of enterochromaffin cells by single-cell expression analysis. bioRxiv 2023, preprint. [Google Scholar] [CrossRef]
- Alcaino, C.; Knutson, K.R.; Treichel, A.J.; Yildiz, G.; Strege, P.R.; Linden, D.R.; Li, J.H.; Leiter, A.B.; Szurszewski, J.H.; Farrugia, G.; et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc. Natl. Acad. Sci. USA 2018, 115, E7632–E7641. [Google Scholar] [CrossRef]
- Dickson, I. Gut mechanosensors: Enterochromaffin cells feel the force via PIEZO2. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 519. [Google Scholar] [CrossRef] [PubMed]
- Beech, D.J.; Lichtenstein, L. RNA and the PIEZO force sensor. Cell Res. 2020, 30, 829–830. [Google Scholar] [CrossRef]
- Matute, J.D.; Duan, J.; Blumberg, R.S. Microbial RNAs Pressure Piezo1 to Respond. Cell 2020, 182, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Mazzuoli-Weber, G.; Kugler, E.M.; Bühler, C.I.; Kreutz, F.; Demir, I.E.; Ceyhan, O.G.; Zeller, F.; Schemann, M. Piezo proteins: Incidence and abundance in the enteric nervous system. Is there a link with mechanosensitivity? Cell Tissue Res. 2019, 375, 605–618. [Google Scholar] [CrossRef]
- Madar, J.; Tiwari, N.; Smith, C.; Sharma, D.; Shen, S.; Elmahdi, A.; Qiao, L.Y. Piezo2 regulates colonic mechanical sensitivity in a sex specific manner in mice. Nat. Commun. 2023, 14, 2158. [Google Scholar] [CrossRef]
- Melo, C.G.S.; Nicolai, E.N.; Alcaino, C.; Cassmann, T.J.; Whiteman, S.T.; Wright, A.M.; Miller, K.E.; Gibbons, S.J.; Beyder, A.; Linden, D.R. Identification of intrinsic primary afferent neurons in mouse jejunum. Neurogastroenterol. Motil. 2020, 32, e13989. [Google Scholar] [CrossRef] [PubMed]
- Morarach, K.; Mikhailova, A.; Knoflach, V.; Memic, F.; Kumar, R.; Li, W.; Ernfors, P.; Marklund, U. Diversification of molecularly defined myenteric neuron classes revealed by single-cell RNA sequencing. Nat. Neurosci. 2021, 24, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Ray, K. A prime role for PIEZO2 in DRG neurons in mechanosensation in the gut. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 693. [Google Scholar] [CrossRef]
- Lou, S.; Duan, B.; Vong, L.; Lowell, B.B.; Ma, Q. Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J. Neurosci. 2013, 33, 870–882. [Google Scholar] [CrossRef]
- Handler, A.; Ginty, D.D. The mechanosensory neurons of touch and their mechanisms of activation. Nat. Rev. Neurosci. 2021, 22, 521–537. [Google Scholar] [CrossRef]
- Woo, S.H.; Lukacs, V.; de Nooij, J.C.; Zaytseva, D.; Criddle, C.R.; Francisco, A.; Jessell, T.M.; Wilkinson, K.A.; Patapoutian, A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 2015, 18, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lönnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggström, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Yang, H.; Li, K.; Lei, X.; Xu, C. The potential role of Piezo2 in the mediation of visceral sensation. Neurosci. Lett. 2016, 630, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; McGinnis, A.; Ji, R.R. Piezo2 mediates visceral mechanosensation: A new therapeutic target for gut pain? Neuron 2023, 111, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Hockley, J.R.F.; Taylor, T.S.; Callejo, G.; Wilbrey, A.L.; Gutteridge, A.; Bach, K.; Winchester, W.J.; Bulmer, D.C.; McMurray, G.; Smith, E.S.J. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 2019, 68, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Servin-Vences, M.R.; Lam, R.M.; Koolen, A.; Wang, Y.; Saade, D.N.; Loud, M.; Kacmaz, H.; Frausto, S.; Zhang, Y.; Beyder, A.; et al. PIEZO2 in somatosensory neurons controls gastrointestinal transit. Cell 2023, 186, 3386–3399. [Google Scholar] [CrossRef]
- Xie, Z.; Feng, J.; Hibberd, T.J.; Chen, B.N.; Zhao, Y.; Zang, K.; Hu, X.; Yang, X.; Chen, L.; Brookes, S.J.; et al. Piezo2 channels expressed by colon-innervating TRPV1-lineage neurons mediate visceral mechanical hypersensitivity. Neuron 2023, 111, 526–538.e524. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Abdelaziz, A.; Rankin, G.; Kushner, S.; Qi, L.; Mazor, O.; Choi, S.; Sharma, N.; Ginty, D.D. DRG afferents that mediate physiologic and pathologic mechanosensation from the distal colon. Cell 2023, 186, 3368–3385.e3318. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, J.; Xu, Y.; Liu, C.; Qian, W.; Bai, T.; Hou, X. Piezo1 regulates intestinal epithelial function by affecting the tight junction protein claudin-1 via the ROCK pathway. Life Sci. 2021, 275, 119254. [Google Scholar] [CrossRef] [PubMed]
- Niu, R. The effect of GZMA-PIEZO1 Regulate Intestinal Epithelial Cell Autophagy on Intestinal Epithelial Barrier Function. Guangzhou Medical University, Guangzhou, China, 2022.
- Papatriantafyllou, M. Musical chairs at the epithelium. Nat. Rev. Mol. Cell Biol. 2012, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Eisenhoffer, G.T.; Loftus, P.D.; Yoshigi, M.; Otsuna, H.; Chien, C.B.; Morcos, P.A.; Rosenblatt, J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 2012, 484, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalez, R.; Zallen, J.A. Feeling the squeeze: Live-cell extrusion limits cell density in epithelia. Cell 2012, 149, 965–967. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Santacroce, G.; Rossi, C.M.; Broglio, G.; Lenti, M.V. Role of mucosal immunity and epithelial-vascular barrier in modulating gut homeostasis. Intern. Emerg. Med. 2023, 18, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef]
- Recktenwald, C.V.; Hansson, G.C. The Reduction-insensitive Bonds of the MUC2 Mucin Are Isopeptide Bonds. J. Biol. Chem. 2016, 291, 13580–13590. [Google Scholar] [CrossRef]
- Arike, L.; Hansson, G.C.; Recktenwald, C.V. Identifying transglutaminase reaction products via mass spectrometry as exemplified by the MUC2 mucin—Pitfalls and traps. Anal. Biochem. 2020, 597, 113668. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, F.; Xiong, Y.; Wu, J.; Li, X.; Li, G.; Bai, T.; Hou, X.; Song, J. Reprogrammed fecal and mucosa-associated intestinal microbiota and weakened mucus layer in intestinal goblet cell- specific Piezo1-deficient mice. Front. Cell Infect. Microbiol. 2022, 12, 1035386. [Google Scholar] [CrossRef] [PubMed]
- Tadala, L.; Langenbach, D.; Dannborg, M.; Cervantes-Rivera, R.; Sharma, A.; Vieth, K.; Rieckmann, L.M.; Wanders, A.; Cisneros, D.A.; Puhar, A. Infection-induced membrane ruffling initiates danger and immune signaling via the mechanosensor PIEZO1. Cell Rep. 2022, 40, 111173. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Hong, Z.; Zhong, M.; Klomp, J.; Bayless, K.J.; Mehta, D.; Karginov, A.V.; Hu, G.; Malik, A.B. Piezo1 mediates angiogenesis through activation of MT1-MMP signaling. Am. J. Physiol. Cell Physiol. 2019, 316, C92–C103. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.E.; Buechler, M.B.; Gressier, E.; Turley, S.J.; Carroll, M.C. Mechanosensing by Peyer’s patch stroma regulates lymphocyte migration and mucosal antibody responses. Nat. Immunol. 2019, 20, 1506–1516. [Google Scholar] [CrossRef]
- Geng, J.; Shi, Y.; Zhang, J.; Yang, B.; Wang, P.; Yuan, W.; Zhao, H.; Li, J.; Qin, F.; Hong, L.; et al. TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection. Nat. Commun. 2021, 12, 3519. [Google Scholar] [CrossRef]
- Leng, S.; Zhang, X.; Wang, S.; Qin, J.; Liu, Q.; Liu, A.; Sheng, Z.; Feng, Q.; Hu, X.; Peng, J. Ion channel Piezo1 activation promotes aerobic glycolysis in macrophages. Front. Immunol. 2022, 13, 976482. [Google Scholar] [CrossRef]
- Atcha, H.; Jairaman, A.; Holt, J.R.; Meli, V.S.; Nagalla, R.R.; Veerasubramanian, P.K.; Brumm, K.T.; Lim, H.E.; Othy, S.; Cahalan, M.D.; et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 2021, 12, 3256. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Treichel, A.J.; Finholm, I.; Knutson, K.R.; Alcaino, C.; Whiteman, S.T.; Brown, M.R.; Matveyenko, A.; Wegner, A.; Kacmaz, H.; Mercado-Perez, A.; et al. Specialized Mechanosensory Epithelial Cells in Mouse Gut Intrinsic Tactile Sensitivity. Gastroenterology 2022, 162, 535–547.e513. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.; Knight, Z.A. Interoception: Spinal sensory neurons that innervate the intestines. Curr. Biol. 2023, 33, R945–R947. [Google Scholar] [CrossRef]
- Greenwood-van Meerveld, B. Importance of 5-hydroxytryptamine receptors on intestinal afferents in the regulation of visceral sensitivity. Neurogastroenterol. Motil. 2007, 19 (Suppl. 2), 13–18. [Google Scholar] [CrossRef]
- Beumer, J.; Clevers, H. How the Gut Feels, Smells, and Talks. Cell 2017, 170, 10–11. [Google Scholar] [CrossRef][Green Version]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198.e116. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.W.; Chang, L. Functional Bowel Disorders. Gastroenterology 2018, 155, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Black, C.J.; Ford, A.C. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef]
- Vergnolle, N. Abdominal pain in irritable bowel syndrome. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 350. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Li, Y.; Xia, J.; Jiang, Y.; Zhang, L.; Wang, H.; Qian, W.; Song, J.; Hou, X. Piezo2: A Candidate Biomarker for Visceral Hypersensitivity in Irritable Bowel Syndrome? J. Neurogastroenterol. Motil. 2017, 23, 453–463. [Google Scholar] [CrossRef]
- Zhai, L.; Huang, C.; Ning, Z.; Zhang, Y.; Zhuang, M.; Yang, W.; Wang, X.; Wang, J.; Zhang, L.; Xiao, H.; et al. Ruminococcus gnavus plays a pathogenic role in diarrhea-predominant irritable bowel syndrome by increasing serotonin biosynthesis. Cell Host Microbe 2023, 31, 33–44.e35. [Google Scholar] [CrossRef]
- Thijssen, A.Y.; Mujagic, Z.; Jonkers, D.M.; Ludidi, S.; Keszthelyi, D.; Hesselink, M.A.; Clemens, C.H.; Conchillo, J.M.; Kruimel, J.W.; Masclee, A.A. Alterations in serotonin metabolism in the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 43, 272–282. [Google Scholar] [CrossRef]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Bayrer, J.R.; Castro, J.; Venkataraman, A.; Touhara, K.K.; Rossen, N.D.; Morrie, R.D.; Maddern, J.; Hendry, A.; Braverman, K.N.; Garcia-Caraballo, S.; et al. Gut enterochromaffin cells drive visceral pain and anxiety. Nature 2023, 616, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut—Functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Reed, D. Enterochromaffin Cells: Small in Number but Big in Impact. Gastroenterology 2023, 165, 1090–1091. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Guo, T. Visceral pain from colon and rectum: The mechanotransduction and biomechanics. J. Neural Transm. 2020, 127, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, L.; Wang, Y.H.; Song, Y.F.; Zhao, Z.H.; Zhao, T.T.; Lin, Z.Y.; Gu, D.M.; Liu, Y.Q.; Peng, Y.J.; et al. Electroacupuncture Attenuates Post-Inflammatory IBS-Associated Visceral and Somatic Hypersensitivity and Correlates with the Regulatory Mechanism of Epac1-Piezo2 Axis. Front. Endocrinol. 2022, 13, 918652. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohns. Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, D.; Yang, X.; Ma, F.; Han, W.; Hu, J.; Mei, Q. The Mechanosensitive Ion Channel PIEZO1 in Intestinal Epithelial Cells Mediates Inflammation through the NOD-Like Receptor 3 Pathway in Crohn’s Disease. Inflamm. Bowel Dis. 2023, 29, 103–115. [Google Scholar] [CrossRef]
- Choi, S.H.; Aguilar, A.; Myers, J.; Kim, B.-G.; Eid, S.; Tomchuck, S.; Kingsley, D.; Huang, A. Mechanosensory channel Piezo1 is essential in pathogenic T cell-mediated intestinal inflammation. J. Immunol. 2022, 208, 113–116. [Google Scholar] [CrossRef]
- De Felice, D.; Alaimo, A. Mechanosensitive Piezo Channels in Cancer: Focus on altered Calcium Signaling in Cancer Cells and in Tumor Progression. Cancers 2020, 12, 1780. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, V.; Patel, N.; Grover, D.; Ali, F.S.; Thosani, N. Interventional gastroenterology in oncology. CA Cancer J. Clin. 2023, 73, 286–319. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Liang, T.; Da, M.X. Expression and Clinical Significance of PIEZO2 in Gastric Cancer. Comb. Chem. High. Throughput Screen 2023, 26, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, Y.; Huang, T.; Wu, F.; Liu, L.; Kwan, J.S.H.; Cheng, A.S.L.; Yu, J.; To, K.F.; Kang, W. PIEZO1 functions as a potential oncogene by promoting cell proliferation and migration in gastric carcinogenesis. Mol. Carcinog. 2018, 57, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, G.; Miao, Y.; Qiu, F.; Bai, L.; Gao, Z.; Huang, Y.; Dong, L.; Niu, X.; Wang, X.; et al. Piezo type mechanosensitive ion channel component 1 facilitates gastric cancer omentum metastasis. J. Cell Mol. Med. 2021, 25, 2238–2253. [Google Scholar] [CrossRef]
- Yang, X.N.; Lu, Y.P.; Liu, J.J.; Huang, J.K.; Liu, Y.P.; Xiao, C.X.; Jazag, A.; Ren, J.L.; Guleng, B. Piezo1 is as a novel trefoil factor family 1 binding protein that promotes gastric cancer cell mobility in vitro. Dig. Dis. Sci. 2014, 59, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995-2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef]
- Sun, Y.; Li, M.; Liu, G.; Zhang, X.; Zhi, L.; Zhao, J.; Wang, G. The function of Piezo1 in colon cancer metastasis and its potential regulatory mechanism. J. Cancer Res. Clin. Oncol. 2020, 146, 1139–1152. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Xu, A.; Yan, H.; Xu, D.; Zhang, J.; Fang, X. PIEZO2 promotes cell proliferation and metastasis in colon carcinoma through the SLIT2/ROBO1/VEGFC pathway. Adv. Clin. Exp. Med. 2023, 32, 763–776. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.V.; Vanner, R.; Dirks, P.; Eaves, C.J. Cancer stem cells: An evolving concept. Nat. Rev. Cancer 2012, 12, 133–143. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.L.; Francescangeli, F.; Zeuner, A.; Baiocchi, M. Colorectal Cancer Stem Cells: An Overview of Evolving Methods and Concepts. Cancers 2021, 13, 5910. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, D.; Li, H.; Lei, X.; Liao, W.; Liu, X.Y. Identification of Piezo1 as a potential target for therapy of colon cancer stem-like cells. Discov. Oncol. 2023, 14, 95. [Google Scholar] [CrossRef]
- Guo, J.; Gu, D.; Zhao, T.; Zhao, Z.; Xiong, Y.; Sun, M.; Xin, C.; Zhang, Y.; Pei, L.; Sun, J. Trends in Piezo Channel Research Over the Past Decade: A Bibliometric Analysis. Front. Pharmacol. 2021, 12, 668714. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.; Herget, R.; Lapatsina, L.; Ngo, H.D.; Lewin, G.R. Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat. Commun. 2014, 5, 3520. [Google Scholar] [CrossRef]
- Shin, K.C.; Park, H.J.; Kim, J.G.; Lee, I.H.; Cho, H.; Park, C.; Sung, T.S.; Koh, S.D.; Park, S.W.; Bae, Y.M. The Piezo2 ion channel is mechanically activated by low-threshold positive pressure. Sci. Rep. 2019, 9, 6446. [Google Scholar] [CrossRef]
- Szczot, M.; Nickolls, A.R.; Lam, R.M.; Chesler, A.T. The Form and Function of PIEZO2. Annu. Rev. Biochem. 2021, 90, 507–534. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Li, L.; Chen, F.; Fu, M.; Cheng, C.; Wang, M.; Hu, J.; Pei, L.; Sun, J. Forces Bless You: Mechanosensitive Piezo Channels in Gastrointestinal Physiology and Pathology. Biomolecules 2024, 14, 804. https://doi.org/10.3390/biom14070804
Guo J, Li L, Chen F, Fu M, Cheng C, Wang M, Hu J, Pei L, Sun J. Forces Bless You: Mechanosensitive Piezo Channels in Gastrointestinal Physiology and Pathology. Biomolecules. 2024; 14(7):804. https://doi.org/10.3390/biom14070804
Chicago/Turabian StyleGuo, Jing, Li Li, Feiyi Chen, Minhan Fu, Cheng Cheng, Meizi Wang, Jun Hu, Lixia Pei, and Jianhua Sun. 2024. "Forces Bless You: Mechanosensitive Piezo Channels in Gastrointestinal Physiology and Pathology" Biomolecules 14, no. 7: 804. https://doi.org/10.3390/biom14070804
APA StyleGuo, J., Li, L., Chen, F., Fu, M., Cheng, C., Wang, M., Hu, J., Pei, L., & Sun, J. (2024). Forces Bless You: Mechanosensitive Piezo Channels in Gastrointestinal Physiology and Pathology. Biomolecules, 14(7), 804. https://doi.org/10.3390/biom14070804






