Abstract
Background: Autosomal dominant polycystic kidney disease (ADPKD) is the most prevalent genetic kidney disorder. While metformin has demonstrated the ability to inhibit cyst growth in animal models of ADPKD via activation of adenosine monophosphate-activated protein kinase (AMPK), its effectiveness in humans is limited due to its low potency. This study explored the impact of HL156A, a new and more potent AMPK activator, in a mouse model of ADPKD. Methods: To investigate whether HL156A inhibits the proliferation of renal cyst cells in ADPKD in vitro, exogenous human telomerase reverse transcriptase (hTERT)-immortalized renal cyst cells from ADPKD patients were treated with HL156A, and an MTT (dimethylthiazol-diphenyltetrazolium bromide) assay was performed. To assess the cyst-inhibitory effect of HL156A in vivo, we generated Pkd1 conditional knockout (KO) mice with aquaporin 2 (AQP2)-Cre, which selectively expresses Cre recombinase in the collecting duct. The effectiveness of HL156A in inhibiting cyst growth and improving renal function was confirmed by measuring the number of cysts and blood urea nitrogen (BUN) levels in the collecting duct-specific Pkd1 KO mice. Results: When cyst cells were treated with up to 20 µM of metformin or HL156A, HL156A reduced cell viability by 25% starting at a concentration of 5 µM, whereas metformin showed no effect. When AQP2-Cre male mice were crossed with Pkd1flox/flox female mice, and when AQP2-Cre female mice were crossed with Pkd1flox/flox male mice, the number of litters produced by both groups was comparable. In collecting duct-specific Pkd1 KO mice, HL156A was found to inhibit cyst growth, reducing both the number and size of cysts. Furthermore, it was confirmed that kidney function improved as HL156A treatment led to a reduction in elevated BUN levels. Lastly, it was observed that the increase in AMPK phosphorylation induced by HL156A decreased ERK phosphorylation and α-SMA expression. Conclusion: HL156A has potential as a drug that can restore kidney function in ADPKD patients by inhibiting cyst growth.
1. Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most prevalent genetic kidney disease, affecting from 1 in 500 to 1 in 1000 individuals. It is a severe condition, with 50% of patients over the age of 60 requiring renal replacement therapy [1,2]. In ADPKD, numerous fluid-filled cysts form in the kidneys, compressing the renal parenchyma and leading to progressive renal failure [3]. In addition, ADPKD is a systemic disease that can cause a variety of organ-related issues, including cardiovascular disease and cysts in the liver and pancreas [3,4]. The disease is caused by mutations in the PKD1 and PKD2 genes, which encode polycystin-1 (PC1) and 2 (PC2) proteins; besides PKD1 and PKD2, other genes are associated with PKD [5]. Mutations in PKD1 and PKD2 account for 85% and 15% of ADPKD patients, respectively [6,7]. The phenotype associated with PKD1 mutations is known to be more severe than that associated with PKD2 mutations [6].
The study of ADPKD has advanced significantly since the discovery of the PKD1 and PKD2 genes. Several signaling pathways related to cyst growth have been identified, leading to the development of drugs that target these pathways. There is considerable potential for therapeutic development and clinical translation by focusing on these key signaling pathways [8]. Increased cAMP signaling has been identified as a key mechanism for cystic growth and fluid secretion. This understanding has led to the development of treatments based on vasopressin V2 receptor (V2R) antagonism. Tolvaptan, the primary therapeutic drug in this category, works by reducing cAMP levels, which slows cyst growth and delays the loss of renal function [9]. However, tolvaptan is an expensive medication and is associated with several side effects, including polyuria, fatigue, and reversible transaminase elevations. Consequently, its use is generally restricted to high-risk ADPKD patients [10,11].
Adenosine monophosphate-activated protein kinase (AMPK) is a crucial cellular sensor that regulates a variety of cellular processes, including cellular energy and metabolic homeostasis. AMPK is a heterotrimeric enzyme complex consisting of α, β, and δ subunits, and it is activated through the phosphorylation of Thr172 on the α subunit. The activation of AMPK plays a role in cytokine production, the regulation of inflammation, cell proliferation, and cell death [12].
Metformin, an antidiabetic drug, indirectly activates AMPK by inhibiting mitochondrial function and reducing cellular adenylate charge through its interaction with the AMPK gamma isoform. It also inhibits tumor growth by inhibiting mTOR activity and has demonstrated anti-fibrotic effects in animal models of liver fibrosis [13,14]. Studies on the effects of metformin in ADPKD have shown a reduction in cystic disease in a tamoxifen-inducible PKD1del conditional KO model administered high-dose metformin (300 mg/kg) [15]. In clinical trials, metformin slowed the rate of glomerular filtration rate (GFR) decline in adults with ADPKD, although the results were not significant [16,17]. However, despite its numerous beneficial properties, metformin’s hydrophilic nature limits its cellular penetration, posing challenges for its use as an anti-cancer drug [13].
HL156A is a novel derivative of metformin designed with increased hydrophobicity to address the limitations of metformin, enabling it to induce AMPK phosphorylation more effectively than either metformin or AICAR (5-aminoimidazole-r-carboxamide ribonucleotide) [18,19,20]. HL156A has been reported to have protective effects against peritoneal, liver, and renal fibrosis [18,19,21]. Additionally, HL156A has demonstrated potential effects against MYC-dependent lymphoma [22] and glioblastoma [23]. Given its superior AMPK activation compared to metformin and its lower side effects despite its higher potency, HL156A is a promising candidate drug for treating human ADPKD. The aim of this study was to evaluate the efficacy of HL156A, a potent AMPK activator, in a mouse model of ADPKD.
2. Materials and Methods
2.1. MTT Assay in Renal Cyst Cells Treated with HL156A
To confirm the viability of human telomerase reverse transcriptase (hTERT)-immortalized renal cyst cells [24] by treatment with HL156A, an MTT (dimethylthiazol-diphenyltetrazolium bromide) assay was performed (Sigma, St. Louis, MO, USA). Cells were seeded into a 96-well plate in 100 µL of growth media at 0.5 × 105 cells/well for 24 h. Cells were then incubated with a range of HL156A concentrations—from 0 µM to 20 µM—for 24 h. Then, after removing the medium from each well, 100 µL of MTT solution (mg/mL in serum-free media) was added to each well and incubated for 2 h at 37 °C. After removing the MTT solution, 100 µL of DMSO (dimethyl sulfoxide) was added to each well to dissolve the formazan crystal and measure the absorbance at 540 nm by using a GloMax® Discover multimode plate reader (Promega, Madison, WI, USA).
2.2. Generation of Collecting Duct-Specific Pkd1 KO Mice
Pkd1flox/flox mice (B6.129S4-Pkd1tm2Ggg/J) and AQP2-Cre mice (B6.Cg-Tg(Aqp2-cre)1Dek/J) were purchased from Jackson Laboratories. The collecting duct-specific Pkd1 KO mice were generated in a manner similar to that previously described [25,26]. Floxed Pkd1 mice have loxP sites on exons 1 and 4 of the Pkd1 gene, which allow for the selective deletion of essential coding regions when exposed to Cre recombinase. AQP2-Cre mice were used to create a Pkd1 KO model specific for the collecting duct. First, AQP2-Cre mice were mated with floxed Pkd1 mice, and offspring that were positive for AQP2-Cre and heterozygous for Pkd1 (Pkd1flox/+) were selected. These mice were mated with homozygous floxed Pkd1 (Pkd1flox/flox) to obtain an ADPKD model in Pkd1 KO mice [25]. This animal study was conducted according to the guidelines of the Institutional Animal Care and Use Committees (IACUC) of Hallym University (HallymR12021-32) after receiving approval.
2.3. Genotyping
Genomic DNA was extracted from mouse tails using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). The samples were then stored at −80 °C until further analysis. Polymerase chain reaction was performed on the tail genomic DNA using the following primers: AQP2-Cre forward: 5′-CTC TGC AGG AAC TGG TGC TGG-3′; AQP2-Cre reverse: 5′-GCG AAC ATC TTC AGG TTC TGC GG-3′; Pkd1 forward: 5′-CCT GCC TTG CTC TAC TTT CC-3′; Pkd1 reverse: 5′-AGG GCT TTT CTT GCT GGT CT-3′ [27,28].
2.4. Administration of HL156A
The mice were divided into a control group (AQP2-Cre;Pkd1flox/+), which did not develop cysts in the kidneys, and a Pkd1 KO group (AQP2-Cre;Pkd1flox/flox), which developed cysts in the kidneys. When mice were injected with a dose of 30 mg/kg of HL156A starting from P2, they did not survive, so the experiment was conducted with a dose of 25 mg/kg or less. HL156A (15 mg/kg or 25 mg/kg) was administered orally to mice every other day from P2 to P28. Distilled water was used as the vehicle.
2.5. Western Blot
The kidney tissues of mice were homogenized using a lysis buffer that included complete protease inhibitor (Roche, Basel, Switzerland) and phosphatase inhibitors (Sigma). The homogenates were then centrifuged at 12,000× g for 20 min at 4 °C, and the supernatants were collected for further use. Protein concentrations in the lysates were determined using the Bradford method. The protein samples were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred onto polyvinylidene difluoride (PVDF) membranes. These membranes were incubated with primary antibodies, including anti-AMPK, anti-phospho-AMPK, anti-smooth muscle actin (SMA), anti-extracellular signal-regulated kinase (ERK), and anti-phospho-ERK (Cell Signaling, Danvers, MA, USA). Horseradish peroxidase (HRP)-conjugated secondary antibodies (ACE Biolabs, Jacksonville, FL, USA) were then applied, and the proteins were visualized using chemiluminescence (Amersham Biosciences, Amersham, UK).
2.6. Histology and Immunofluorescence
The mice were sacrificed at P28. The kidney tissues were fixed in 4% paraformaldehyde, and any remaining paraformaldehyde in the tissue was removed through a series of hydration and dehydration steps. The kidney tissue block was then created by embedding it in paraffin. Sections of the kidney tissue, 5 µm thick, were cut from the paraffin-embedded blocks and mounted on coated slides. Subsequently, hematoxylin and eosin (H&E, Baton Rouge, LA, USA) staining was performed to assess the number and size of cysts, following the manufacturer’s instructions.
To confirm whether cysts were generated only in the collecting duct of the Pkd1 KO mice, fixed kidney tissue slides were permeabilized with 0.1% Triton X-100 in PBS for 10 min, followed by blocking with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 30 min. Thereafter, the kidney tissue slides were incubated with primary antibodies in a blocking buffer (5% BSA/0.05% Triton X-100 in PBS) overnight. We used FITC-conjugated anti-lotus tetragonolobus lectin (LTL, Vector Laboratories, Newark, CA, USA) as a proximal tubule marker and rhodamine-conjugated anti-dolichos biflorus agglutinin (DBA, Vector Laboratories) as a collecting duct marker.
2.7. Analysis of Blood Urea Nitrogen
For the analysis of blood urea nitrogen (BUN), blood samples were collected from the intraorbital vein using a microcapillary tube at the time of sacrifice. These samples were centrifuged at 1500× g for 15 min within 1 h of collection to separate the plasma. The plasma samples were then stored at −80 °C until further analysis. BUN levels were determined using a BUN Colorimetric Detection Kit (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. The absorbance of each sample was measured at 450 nm using a GloMax® Discover multimode plate reader (Promega).
2.8. Statistics
All statistical analyses were conducted using IBM SPSS Statistics ver.25 (IBM Corp., Armonk, NY, USA). One-way analysis, the Mann–Whitney test, and the Jonckheere–Terpstra test were used to compare values as appropriate.
3. Results
3.1. HL156A Inhibits ADPKD Renal Cyst Cell Proliferation More Effectively Than Metformin
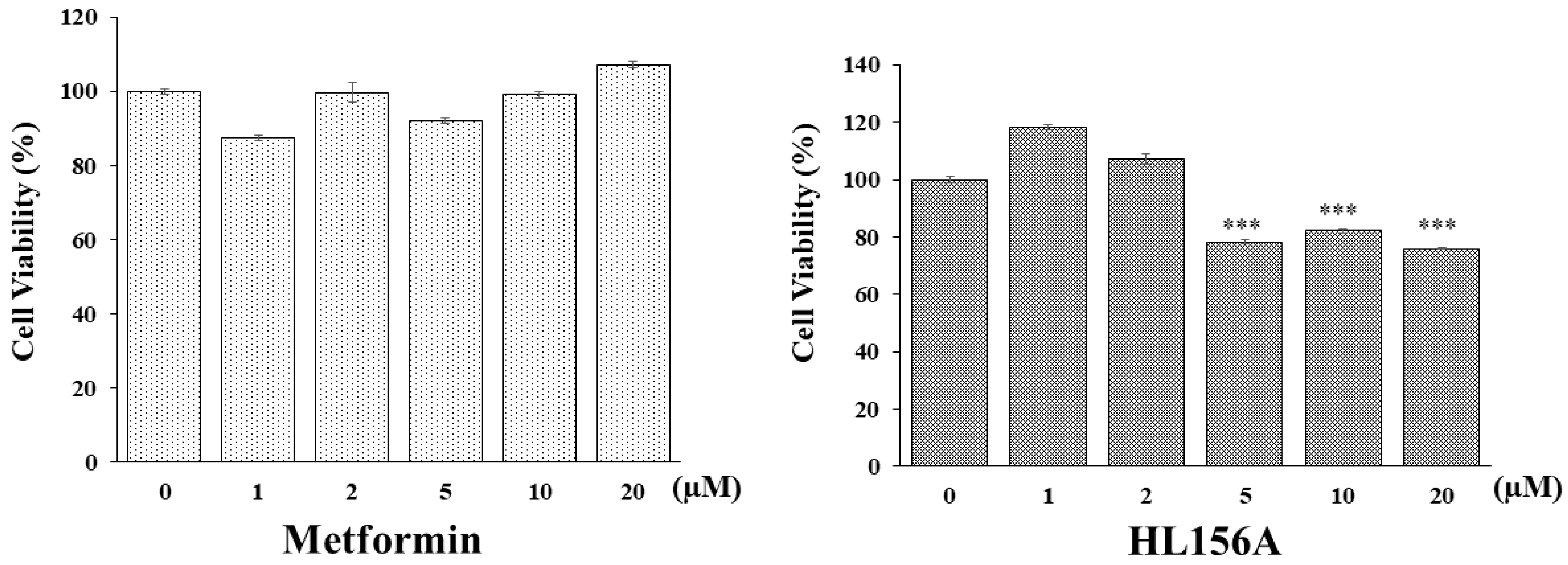
An MTT assay was conducted to determine if HL156A, a novel AMPK activator, can inhibit the proliferation of renal cyst cells effectively in smaller quantities compared to metformin, which is typically used in larger amounts for ADPKD. hTERT-immortalized renal cyst cells were exposed to varying concentrations of metformin or HL156A (Figure 1). While metformin did not decrease cell viability, HL156A reduced it by approximately 25% at a concentration of 5 µM. Thus, HL156A is capable of inhibiting cyst cell proliferation at significantly lower concentrations than metformin.
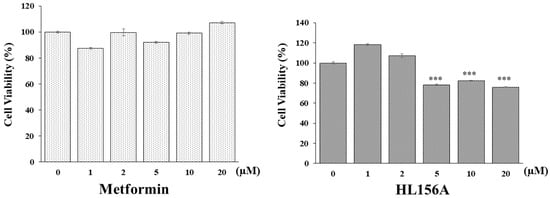
Figure 1.
MTT assay for cell viability. Comparison of effects of metformin and HL156A on inhibition of cyst cell proliferation using exogenous human telomerase reverse transcriptase (hTERT)-immortalized renal cyst cells. HL156A inhibited cell proliferation by approximately 25% starting from 5 µM. *** p < 0.001.
3.2. Characterization of the Collecting Duct-Specific Pkd1 KO Mice
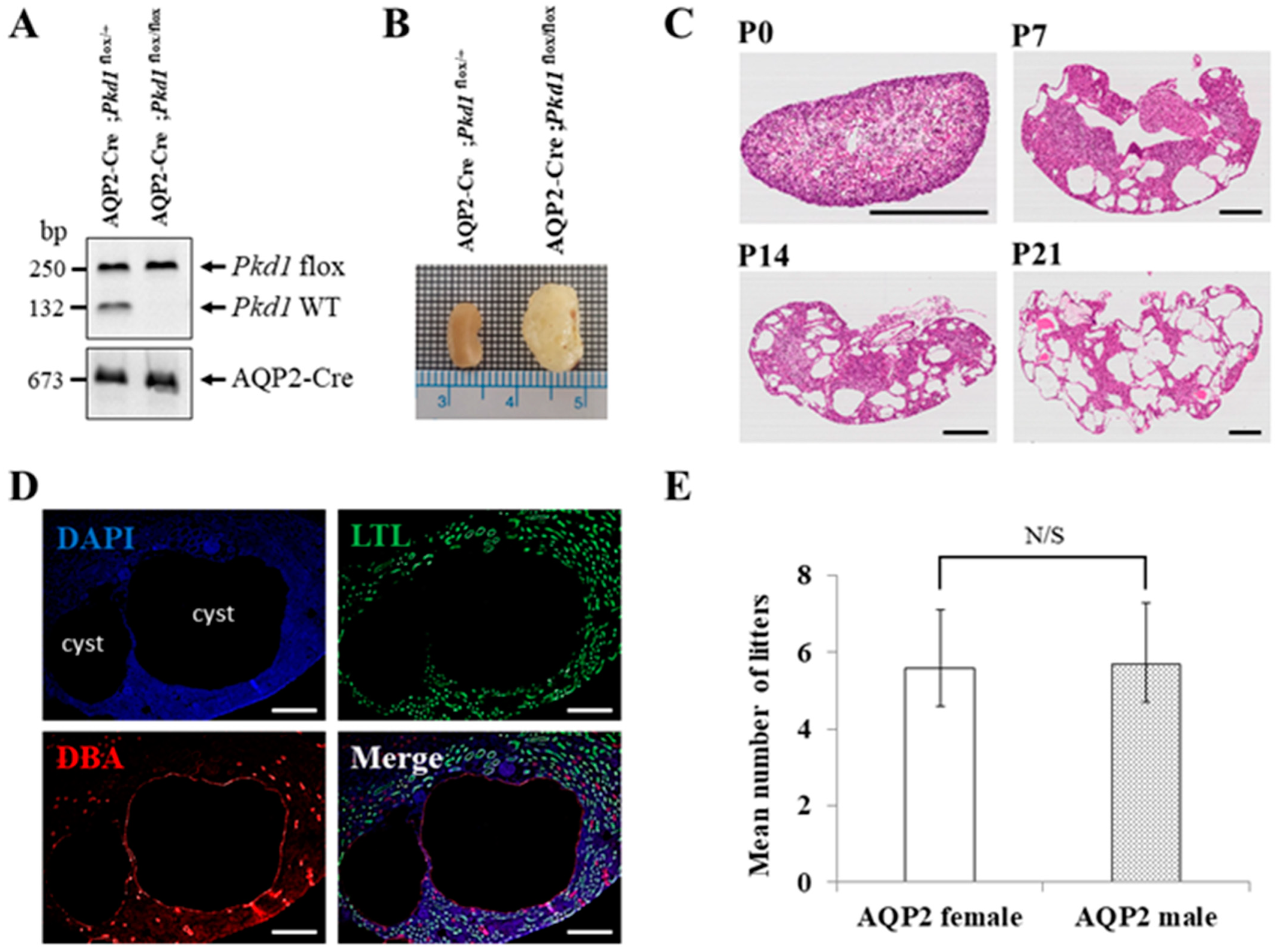
Since mice with homozygous mutations of Pkd1 die in utero, we generated an ADPKD mouse model in which Pkd1 is conditionally knocked out only in the collecting duct by conjugating Cre recombinase to the promoter of the AQP2 gene, which is expressed only in the collecting duct of the kidney [25]. We generated the collecting duct-specific Pkd1 KO mice by crossing two types of B6 mice (AQP2-Cre and Pkd1flox/flox). Crossbreeding AQP2-Cre;Pkd1flox/+ mice with Pkd1flox/flox mice has a 25% chance of producing an ADPKD mouse model (AQP2-Cre;Pkd1flox/flox) (Figure 2A). The collecting duct-specific Pkd1 KO mice (AQP2-Cre;Pkd1flox/flox) developed an overt ADPKD phenotype with kidney enlargement due to postnatal cysts (Figure 2B).
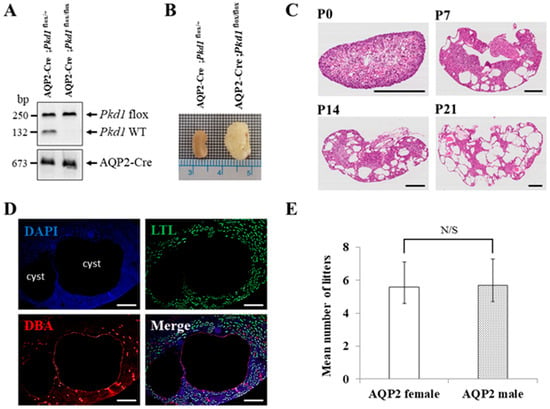
Figure 2.
The characteristics of the collecting duct-specific Pkd1 knockout (KO) mice. (A) Agarose gel analysis of PCR products amplified from genomic DNA of AQP2-Cre;Pkd1flox/+ and AQP2-Cre;Pkd1flox/flox mice. Floxed Pkd1: 250 bp, Pkd1 wild type (WT): 132 bp, and AQP2-Cre: 673 bp. (B) Gross morphology of the kidney. Scale bar, 1 mm. (C) Hematoxylin- and eosin-stained kidney tissues of AQP2-Cre;Pkd1flox/flox mice for each period. (D) The origin of cysts. Immunofluorescence of kidney tissues of AQP2-Cre;Pkd1flox/flox at P14 with LTL, a proximal tubule marker, and DBA, a collecting duct marker. Scale bar, 100 μm. (E) The fertility of female AQP2-Cre mice vs. male AQP2-Cre mice with Pkd1flox/flox mice. N/S: not significant.
H&E staining was performed to evaluate cyst formation in each period. The staining revealed an increase in both the number and size of cysts in the kidney over time (Figure 2C). Immunofluorescence staining of kidney tissues confirmed that cyst formation was exclusively in the collecting duct (Figure 2D). To assess the fertility of AQP2-Cre mice by sex, 20 pairs of mice were used in the following configurations: AQP2-Cre;Pkd1flox/+ female and Pkd1flox/flox male or AQP2-Cre;Pkd1flox/+ male and Pkd1flox/flox female. When a Pkd1 female and an AQP2-Cre male or a Pkd1 male and an AQP2-Cre female were crossed, there was no difference in the number of litters between the two groups (Figure 2E).
3.3. HL156A Reduces the Kidney Weight-to-Body Weight Ratio in the Collecting Duct-Specific Pkd1 KO Mice
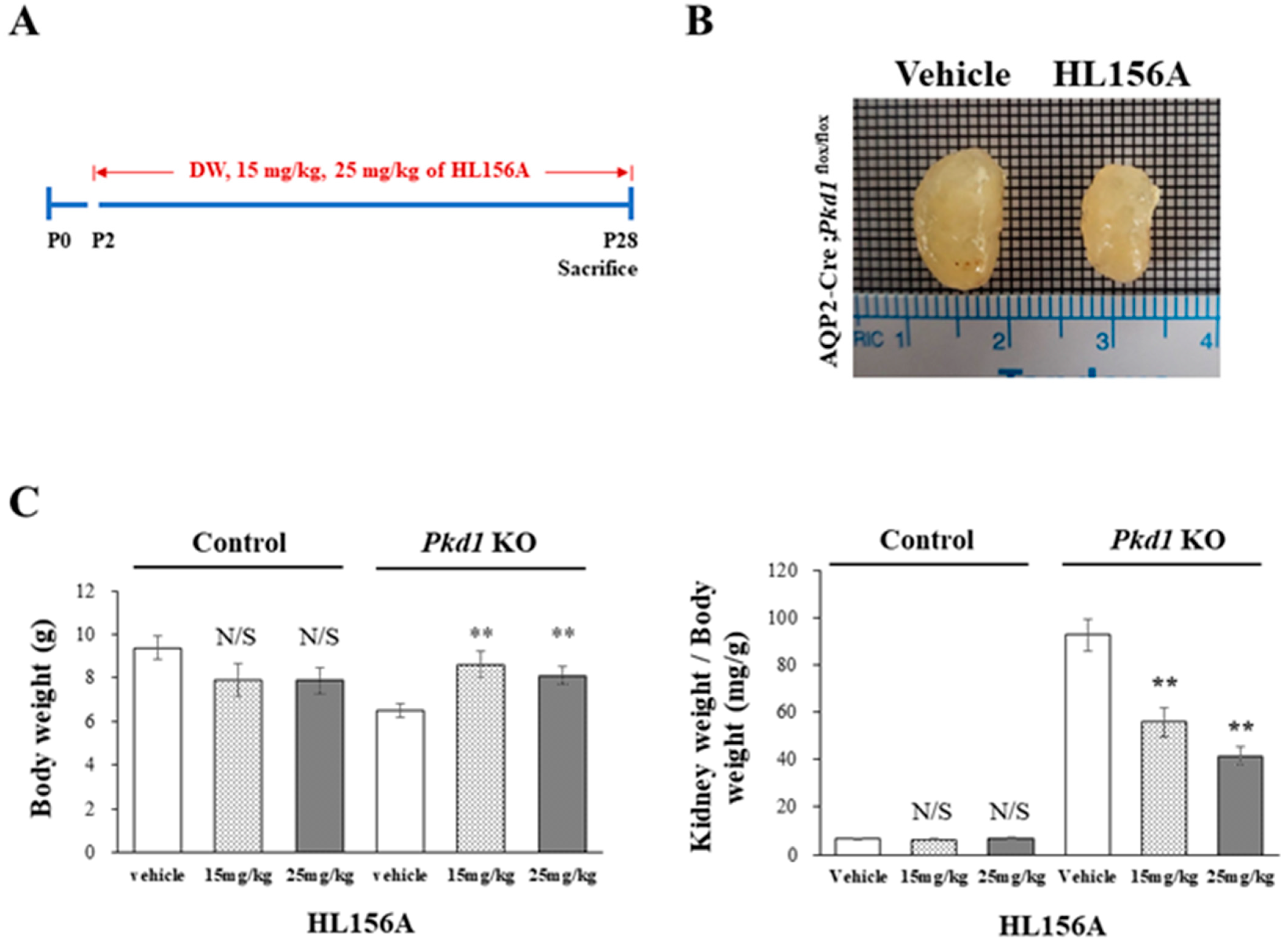
HL156A was administered orally on P2, before cyst formation, and then, the collecting duct-specific Pkd1 KO mice were sacrificed on P28 to measure BUN levels (Figure 3A). In the collecting duct-specific Pkd1 KO mice (AQP2-Cre;Pkd1flox/flox) that were administered 25 mg/kg HL156A from P2 to P28, the overall kidney size decreased (Figure 3B). Body weight (BW) and the ratio of kidney weight to body weight (KW/BW) are shown in Figure 3C. When normalized to body weight, there was no significant difference in the kidney weight in the control group (AQP2-Cre;Pkd1flox/+) according to the administration of HL156A. However, in the Pkd1 KO group (AQP2-Cre;Pkd1flox/flox), the kidney weight decreased depending on the amount of HL156A. Therefore, HL156A reduced the kidney mass of the collecting duct-specific Pkd1 KO mice.
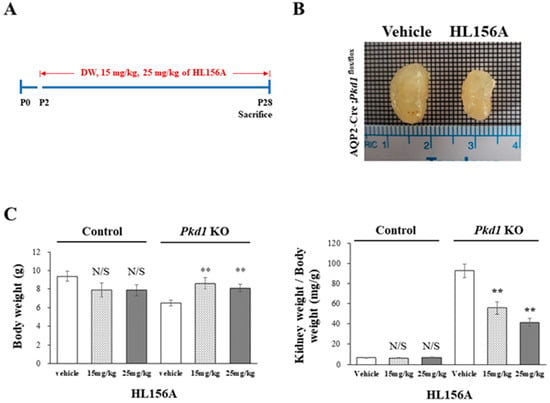
Figure 3.
HL156A reduces the kidney mass of the collecting duct-specific Pkd1 knockout (KO) mice. (A) A diagram of the HL156A schedule for the control (AQP2-Cre;Pkd1flox/+) and the Pkd1 KO (AQP2-Cre;Pkd1flox/flox) mice. P: postnatal day. (B) Gross kidney morphology of P28 AQP2-Cre;Pkd1flox/flox mice administered distilled water (DW) as the vehicle or 25 mg/kg of HL156A. (C) Body weight (BW) and the ratio of kidney weight to body weight (KW/BW) of the control (AQP2-Cre;Pkd1flox/+) and Pkd1 KO (AQP2-Cre;Pkd1flox/flox) mice administered DW, 15 mg/kg of HL156A, or 25 mg/kg of HL156A. N/S: not significant. ** p < 0.01.
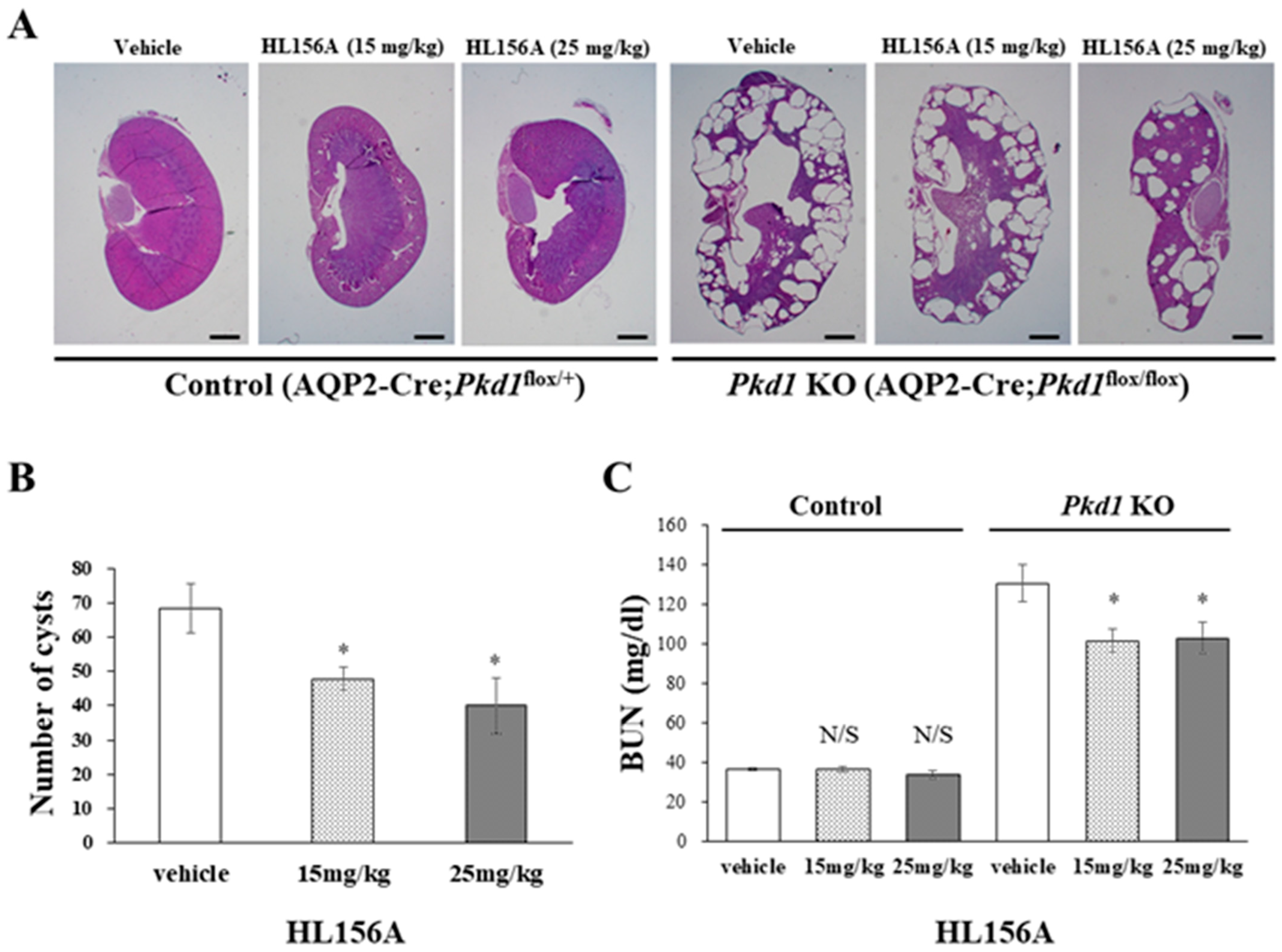
3.4. HL156A Reduces the Number and Size of Cysts and Restores the Kidney Function of the Collecting Duct-Specific Pkd1 KO Mice
In the Pkd1 KO group, the kidney weight of mice treated with HL156A decreased compared to the vehicle group. The number and size of cysts in the collecting duct-specific Pkd1 KO mice significantly decreased after treatment with HL156A (Figure 4A,B). To confirm the effect of HL156A on improving renal function in the collecting duct-specific Pkd1 KO mice, we analyzed BUN as a renal function-related marker. In the control group, there was no significant difference in BUN levels according to the administration of HL156A. In contrast, the Pkd1 KO mice that received distilled water as the vehicle showed higher BUN levels than the control group. When HL156A was administered to the Pkd1 KO group, the BUN levels decreased compared to the group that received the vehicle (Figure 4C). These results indicate that the kidney function of the collecting duct-specific Pkd1 KO mice was restored.
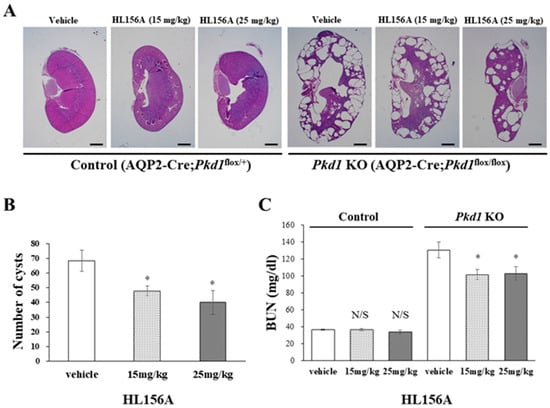
Figure 4.
HL156A reduces the number and size of cysts in the collecting duct-specific Pkd1 knockout (KO) mice. (A) Hematoxylin- and eosin-stained kidney sections from control (AQP2-Cre;Pkd1flox/+) and Pkd1 KO (AQP2-Cre;Pkd1flox/flox) mice administered distilled water (DW) as the vehicle or HL156A. Scale bar, 1 mm. (B) The number of cysts from Pkd1 KO (AQP2-Cre;Pkd1flox/flox) mice administered HL156A (15 mg/kg or 25 mg/kg). (C) Blood urea nitrogen (BUN) levels were measured in control (AQP2-Cre;Pkd1flox/+) and Pkd1 KO (AQP2-Cre;Pkd1flox/flox) mice administered DW as the vehicle or HL156A. N/S: not significant. * p < 0.05.
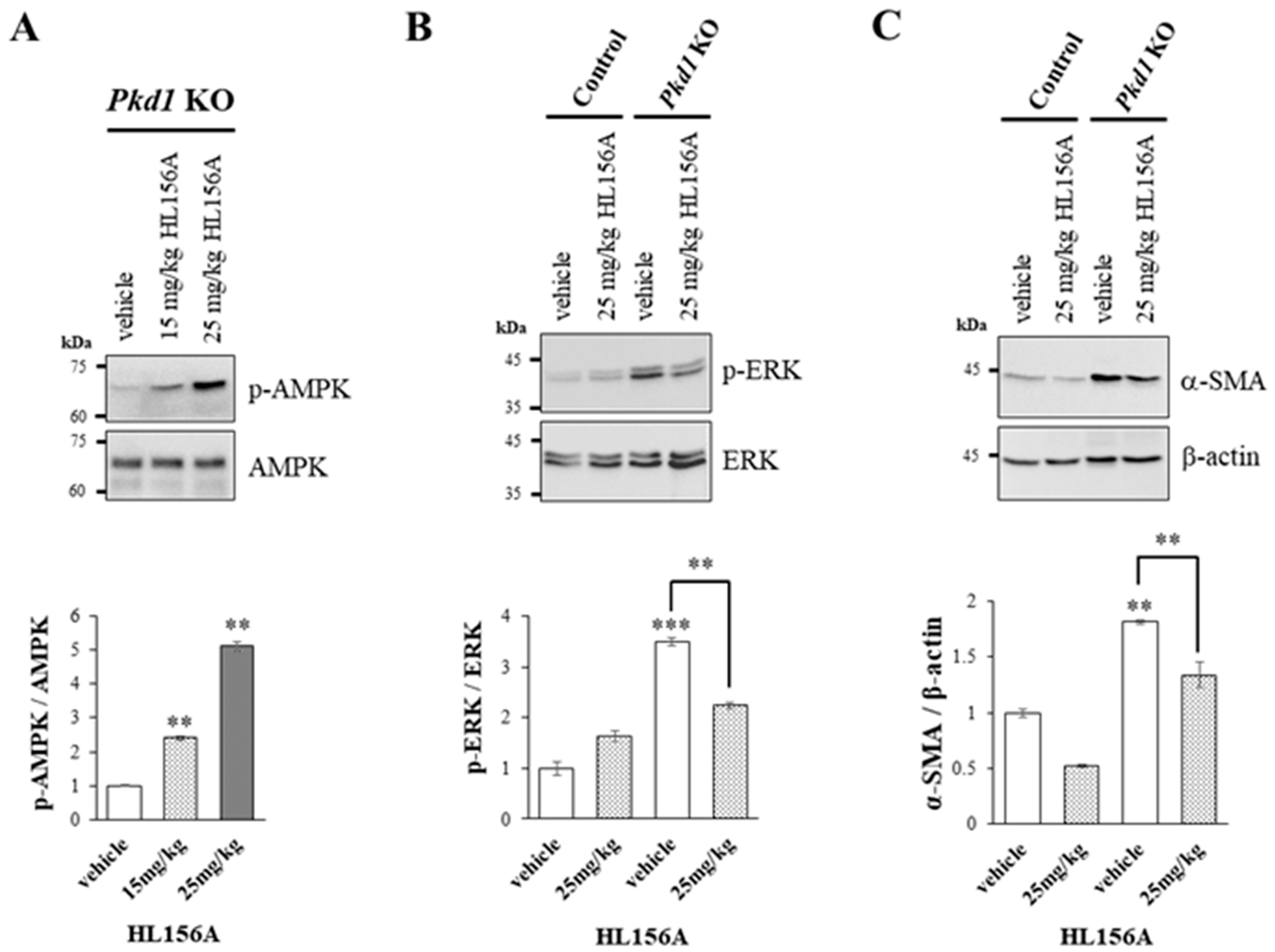
3.5. Increased AMPK Phosphorylation by HL156A Suppresses the Phosphorylation of ERK and α-SMA Expression
To explore the signaling mechanisms involved in reducing the number and size of cysts and restoring kidney function, we investigated the role of ERK, which is associated with cyst proliferation, and α-SMA, which is linked to fibrosis. This was conducted through Western blot analysis using kidney lysates from collecting duct-specific Pkd1 KO mice.
As the concentration of HL156A, a novel AMPK activator, increased, there was a corresponding increase in AMPK phosphorylation in the kidneys of collecting duct-specific Pkd1 KO mice (Figure 5A). This finding suggests that oral administration of HL156A effectively induces AMPK phosphorylation in the kidneys of these mice.
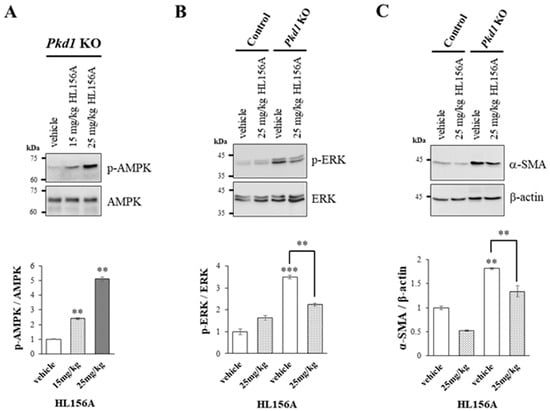
Figure 5.
Increased phosphorylation of AMPK by HL156A reduces the phosphorylation of ERK and the expression of SMA. (A) Immunoblot of the phosphorylation of AMPK by HL156A in Pkd1 knockout (KO) (AQP2-Cre;Pkd1flox/flox) mice. (B) Immunoblot of phospho-ERK and ERK from control (AQP2-Cre;Pkd1flox/+) and Pkd1 KO (AQP2-Cre;Pkd1flox/flox) mice administered with distilled water (DW) as the vehicle or 25 mg/kg of HL156A. (C) Immunoblot of α-SMA from control (AQP2-Cre;Pkd1flox/+) and Pkd1 KO (AQP2-Cre;Pkd1flox/flox) mice administered distilled water (DW) as the vehicle or 25 mg/kg of HL156A. β-actin was used as the loading control. The intensity of protein bands was quantified using image J. ** p < 0.01; *** p < 0.001.
We found that phosphorylation of ERK increased in the kidney of the collecting duct-specific Pkd1 KO mice compared to control mice, but decreased after treatment with HL156A (Figure 5B). This result suggests that the reduction in the number and size of cysts after HL156A treatment may be related to the ERK signaling pathway. Additionally, the expression of α-SMA, which is related to fibrosis, increased in the collecting duct-specific Pkd1 KO mice compared to control mice and then decreased after HL156A treatment (Figure 5C). Therefore, it can be inferred that HL156A suppressed fibrosis and restored kidney function in the collecting duct-specific Pkd1 KO mice.
4. Discussion and Conclusions
In this study, we developed collecting duct-specific Pkd1 knockout (KO) mice, which developed cysts exclusively in the collecting duct. We demonstrated that HL156A, a novel AMPK activator, not only inhibited cyst growth but also improved kidney function in these mice. In the collecting duct-specific Pkd1 KO mice, treatment with HL156A led to a reduction in kidney mass, indicative of decreased cyst number and size, and restored kidney function, as evidenced by reduced BUN levels. Additionally, treatment with HL156A resulted in decreased phosphorylation of ERK, which is related to growth, and reduced expression of α-SMA, which is associated with fibrosis.
The role of AMPK in ADPKD has been extensively studied. In an ADPKD mouse model treated with 2-deoxyglucose, there was a notable reduction in kidney weight, volume, cystic index, and proliferation rate compared to untreated mice. This effect is attributed to the restoration of the ERK pathway by activated AMPK, which simultaneously inhibits the liver kinase B1 (LKB1)-AMP-AMPK axis and activates the mTOR complex 1 (mTORC1) [29]. Metformin significantly inhibited cystic growth in both in vitro and ex vivo models of renal cystogenesis and significantly reduced the cystic index in two mouse models of ADPKD [15]. In addition, studies have been conducted on salsalate [7], an ADaM site ligand, and caloric restriction [30]. However, no drugs are currently in clinical use, and the application of metformin in humans is challenging due to its minimal effect.
HL156A, which is a novel derivative of metformin, is a more powerful AMPK activator that complements the shortcomings of metformin [20]. A phase 1 study of HL156A in solid tumors has been completed and HL156A, also named IM156, demonstrated a good safety profile and tolerability at the recommended phase 2 clinical dose [31]. HL156A was shown to induce AMPK activation more strongly than metformin or AICAR [18,19]. HL156A reduced tubulointerstitial fibrosis in a unilateral ureteral obstruction model and inhibited the p-Smad3 downstream signaling cascade after TGF-β1 stimulation in vitro [19]. HL156A has the potential to be a candidate drug for ADPKD in humans due to its superior AMPK activation and fewer side effects compared to metformin.
In this study, we confirmed the effectiveness of HL156A in both in vitro and in vivo experiments for ADPKD. In vitro testing with hTERT-immortalized renal cyst cells demonstrated that HL156A more effectively inhibited cell viability than metformin. In vivo testing using collecting duct-specific Pkd1 KO mice showed that HL156A reduced kidney mass by decreasing the number and size of renal cysts, thereby restoring kidney function. Additionally, an analysis of kidney protein expression levels related to cyst proliferation and fibrosis revealed that HL156A treatment resulted in decreased phosphorylation of ERK and reduced expression of α-SMA.
The ERK signaling pathway is known to be associated with cyst growth in ADPKD. It can be inferred that the significant increase in ERK phosphorylation observed in Pkd1 KO mice, compared to control mice, contributes to cyst development in these mice, potentially related to the increase in kidney mass. It is reported that activated AMPK phosphorylates BRAF on serine 729 (S729). This phosphorylation disrupts their interaction with the scaffold protein Kinase Suppressor of RAS 1 (KSR1) and promotes the association of BRAF with 14-3-3 proteins, thereby attenuating the mitogen-activated protein kinase (MEK)/ERK signaling pathway [32]. Therefore, it is reasonable to conclude that activated AMPK by HL156A, an AMPK activator, phosphorylates BRAF and blunts the BRAF/MEK/ERK signaling pathway, and that the reduction in ERK phosphorylation by HL156A inhibits cyst growth and decreases kidney mass in Pkd1 KO mice.
Renal fibrosis is known to impair kidney function, and our findings indicate that BUN levels in Pkd1 KO mice were significantly higher than in the control group, suggesting diminished kidney function in these mice. Additionally, the increased expression of α-SMA, a marker associated with fibrosis, in Pkd1 KO mice implies a link between fibrosis and elevated BUN levels. The observed reduction in α-SMA expression following treatment with HL156A suggests a decrease in fibrosis, potentially explaining the lowered BUN levels and indicating a restoration of kidney function in Pkd1 KO mice. Therefore, we propose that HL156A may be a promising new drug alternative to metformin, which has not proven effective in clinical trials for ADPKD.
Author Contributions
Conceptualization, H.K. (Hyunho Kim), S.S. and H.K. (Hyunsuk Kim); methodology, S.S. and H.K. (Hyunsuk Kim); formal analysis, S.S. and H.K. (Hyunho Kim); investigation, J.-T.H., J.E.K., J.K., S.J., Y.-j.S., K.-h.C., G.S., M.C. and J.-w.Y.; resources, H.K. (Hyunho Kim) and H.K. (Hyunsuk Kim); writing—original draft, H.K. (Hyunho Kim), S.S. and H.K. (Hyunsuk Kim); writing—review and editing, H.K. (Hyunho Kim), S.S. and H.K. (Hyunsuk Kim). All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2021R1I1A3057140 and RS-2023-00243582) and the Young Investigator Research Grant from the Korean Nephrology Research Foundation (2019).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committees (IACUC) of Hallym University (HallymR12021-32; 4 June 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included within the article. Raw data requests and further enquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cornec-Le Gall, E.; Alam, A.; Perrone, R.D. Autosomal dominant polycystic kidney disease. Lancet 2019, 393, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Soler, N.M.; Li, H.; Pham, J.; Rivera, D.; Ho, P.-Y.; Mancino, V.; Saitta, B.; Hallows, K.R. Metformin improves relevant disease parameters in an autosomal dominant polycystic kidney disease mouse model. Am. J. Physiol.-Renal 2022, 322, F27–F41. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, P.; Somlo, S. Genetics and pathogenesis of polycystic kidney disease. J. Am. Soc. Nephrol. 2002, 13, 2384–2398. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H.C.; Ryu, H.; Kim, K.; Kim, H.S.; Oh, K.-H.; Yu, S.J.; Chung, J.W.; Cho, J.Y.; Kim, S.H. Clinical correlates of mass effect in autosomal dominant polycystic kidney disease. PLoS ONE 2015, 10, e0144526. [Google Scholar] [CrossRef] [PubMed]
- de Faria, V.P.; Dias, J.P.; Bessa, M.B.; Martingo, M.A.; Lopes, D.; Almeida, C.; Gomes, A.M. Polycystic kidney disease—Beyond PKD1 and PKD2. Nephrol. Dial. Transpl. 2024, 39, gfae069–0225-1148. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.C.; Ryu, H.; Kim, H.; Lee, H.-S.; Heo, J.; Lee, C.; Kim, N.K.; Park, W.-Y.; Hwang, Y.-H. Genetic characteristics of Korean patients with autosomal dominant polycystic kidney disease by targeted exome sequencing. Sci. Rep. 2019, 9, 16952. [Google Scholar] [CrossRef] [PubMed]
- Leonhard, W.N.; Song, X.; Kanhai, A.A.; Iliuta, I.-A.; Bozovic, A.; Steinberg, G.R.; Peters, D.J.; Pei, Y. Salsalate, but not metformin or canagliflozin, slows kidney cyst growth in an adult-onset mouse model of polycystic kidney disease. eBioMedicine 2019, 47, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.C.; Torres, V.E. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J. Clin. Investig. 2014, 124, 2315–2324. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, Y.-H. Clinical trials and a view toward the future of ADPKD. In Cystogenesis; Springer: Singapore, 2016; pp. 105–121. [Google Scholar]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Koch, G.; Ouyang, J.; McQuade, R.D.; Blais, J.D.; Czerwiec, F.S. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2017, 377, 1930–1942. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Bio. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Daugan, M.; Wojcicki, A.D.; d’Hayer, B.; Boudy, V. Metformin: An anti-diabetic drug to fight cancer. Pharmacol. Res. 2016, 113, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.G.; Jeong, Y.S.; Kim, S.A.; Ahn, S.G. New metformin derivative HL 156A prevents oral cancer progression by inhibiting the insulin-like growth factor/AKT/mammalian target of rapamycin pathways. Cancer Sci. 2018, 109, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Takiar, V.; Nishio, S.; Seo-Mayer, P.; King, J.D., Jr.; Li, H.; Zhang, L.; Karihaloo, A.; Hallows, K.R.; Somlo, S.; Caplan, M.J. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 2462–2467. [Google Scholar] [CrossRef] [PubMed]
- Perrone, R.D.; Abebe, K.Z.; Watnick, T.J.; Althouse, A.D.; Hallows, K.R.; Lalama, C.M.; Miskulin, D.C.; Seliger, S.L.; Tao, C.; Harris, P.C. Primary results of the randomized trial of metformin administration in polycystic kidney disease (TAME PKD). Kidney Int. 2021, 100, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.C.; Gansevoort, R.T. TAMEing ADPKD with metformin: Safe and effective? Kidney Int. 2021, 100, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.D.; Kim, H.J.; Tsogbadrakh, B.; Lee, J.; Ryu, H.; Cho, E.J.; Hwang, Y.-H.; Kim, K.; Yang, J.; Ahn, C. HL156A, a novel AMP-activated protein kinase activator, is protective against peritoneal fibrosis in an in vivo and in vitro model of peritoneal fibrosis. Am. J. Physiol.-Renal 2016, 310, F342–F350. [Google Scholar] [CrossRef] [PubMed]
- Tsogbadrakh, B.; Ju, K.D.; Lee, J.; Han, M.; Koh, J.; Yu, Y.; Lee, H.; Yu, K.-S.; Oh, Y.K.; Kim, H.J. HL156A, a novel pharmacological agent with potent adenosine-monophosphate-activated protein kinase (AMPK) activator activity ameliorates renal fibrosis in a rat unilateral ureteral obstruction model. PLoS ONE 2018, 13, e0201692. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.-H.; Koh, I.; Shim, J.-K.; Park, J.; Jeon, J.Y.; Yun, M.; Kim, S.H.; Yook, J.I.; Kim, E.H. Inhibiting stemness and invasive properties of glioblastoma tumorsphere by combined treatment with temozolomide and a newly designed biguanide (HL156A). Oncotarget 2016, 7, 65643–65659. [Google Scholar] [CrossRef]
- Lee, H.S.; Shin, H.-S.; Choi, J.; Bae, S.-J.; Wee, H.-J.; Son, T.; Seo, J.H.; Park, J.-H.; Kim, S.-W.; Kim, K.-W. AMP-activated protein kinase activator, HL156A reduces thioacetamide-induced liver fibrosis in mice and inhibits the activation of cultured hepatic stellate cells and macrophages. Int. J. Oncol. 2016, 49, 1407–1414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Izreig, S.; Gariepy, A.; Kaymak, I.; Bridges, H.R.; Donayo, A.O.; Bridon, G.; DeCamp, L.M.; Kitchen-Goosen, S.M.; Avizonis, D.; Sheldon, R.D. Repression of LKB1 by miR-17~92 sensitizes MYC-dependent lymphoma to biguanide treatment. Cell Rep. Med. 2020, 1. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, B.; Pafundi, D.; Buras, M.; Ashman, J.; Bendok, B.; Laack, N.; Breen, W.; Ruff, M.; Mahajan, A.; Mrugala, M. Impact of 18f-dopa pet on radiation treatment planning from phase ii study of short course hypofractionated proton beam therapy for elderly patients with glioblastoma. Neuro-Oncology 2023, 25, v54. [Google Scholar] [CrossRef]
- Kim, H.; Sung, J.; Kim, H.; Ryu, H.; Park, H.C.; Oh, Y.K.; Lee, H.-S.; Oh, K.-H.; Ahn, C. Expression and secretion of CXCL12 are enhanced in autosomal dominant polycystic kidney disease. BMB Rep. 2019, 52, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.L.; Strait, K.A.; Stricklett, P.K.; Miller, R.L.; Nelson, R.D.; Piontek, K.B.; Germino, G.G.; Kohan, D.E. Inactivation of Pkd1 in principal cells causes a more severe cystic kidney disease than in intercalated cells. Kidney Int. 2009, 75, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Stricklett, P.K.; Hughes, A.K.; Yanagisawa, M.; Kohan, D.E. Collecting duct-specific knockout of the endothelin A receptor alters renal vasopressin responsiveness, but not sodium excretion or blood pressure. Am. J. Physiol.-Renal 2005, 289, F692–F698. [Google Scholar] [CrossRef]
- Raman, A.; Reif, G.A.; Dai, Y.; Khanna, A.; Li, X.; Astleford, L.; Parnell, S.C.; Calvet, J.P.; Wallace, D.P. Integrin-linked kinase signaling promotes cyst growth and fibrosis in polycystic kidney disease. J. Am. Soc. Nephrol. 2017, 28, 2708–2719. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, H.; Jia, C.; Song, Q.; Chen, J.; Zhang, Y.; Lai, P.; Fan, X.; Zhou, X.; Liu, M. Activation of mTORC1 in collecting ducts causes hyperkalemia. J. Am. Soc. Nephrol. 2014, 25, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Rowe, I.; Chiaravalli, M.; Mannella, V.; Ulisse, V.; Quilici, G.; Pema, M.; Song, X.W.; Xu, H.; Mari, S.; Qian, F. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 2013, 19, 488–493. [Google Scholar] [CrossRef]
- Warner, G.; Hein, K.Z.; Nin, V.; Edwards, M.; Chini, C.C.; Hopp, K.; Harris, P.C.; Torres, V.E.; Chini, E.N. Food restriction ameliorates the development of polycystic kidney disease. J. Am. Soc. Nephrol. 2016, 27, 1437–1447. [Google Scholar] [CrossRef]
- Janku, F.; Beom, S.-H.; Moon, Y.W.; Kim, T.W.; Shin, Y.G.; Yim, D.-S.; Kim, G.M.; Kim, H.S.; Kim, S.Y.; Cheong, J.-H. First-in-human study of IM156, a novel potent biguanide oxidative phosphorylation (OXPHOS) inhibitor, in patients with advanced solid tumors. Investig. New Drug 2022, 40, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-H.; Yuan, P.; Perez-Lorenzo, R.; Zhang, Y.; Lee, S.X.; Ou, Y.; Asara, J.M.; Cantley, L.C.; Zheng, B. Phosphorylation of BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation. Mol. Cell 2013, 52, 161–172. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).