HL156A, an AMP-Activated Protein Kinase Activator, Inhibits Cyst Growth in Autosomal Dominant Polycystic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. MTT Assay in Renal Cyst Cells Treated with HL156A
2.2. Generation of Collecting Duct-Specific Pkd1 KO Mice
2.3. Genotyping
2.4. Administration of HL156A
2.5. Western Blot
2.6. Histology and Immunofluorescence
2.7. Analysis of Blood Urea Nitrogen
2.8. Statistics
3. Results
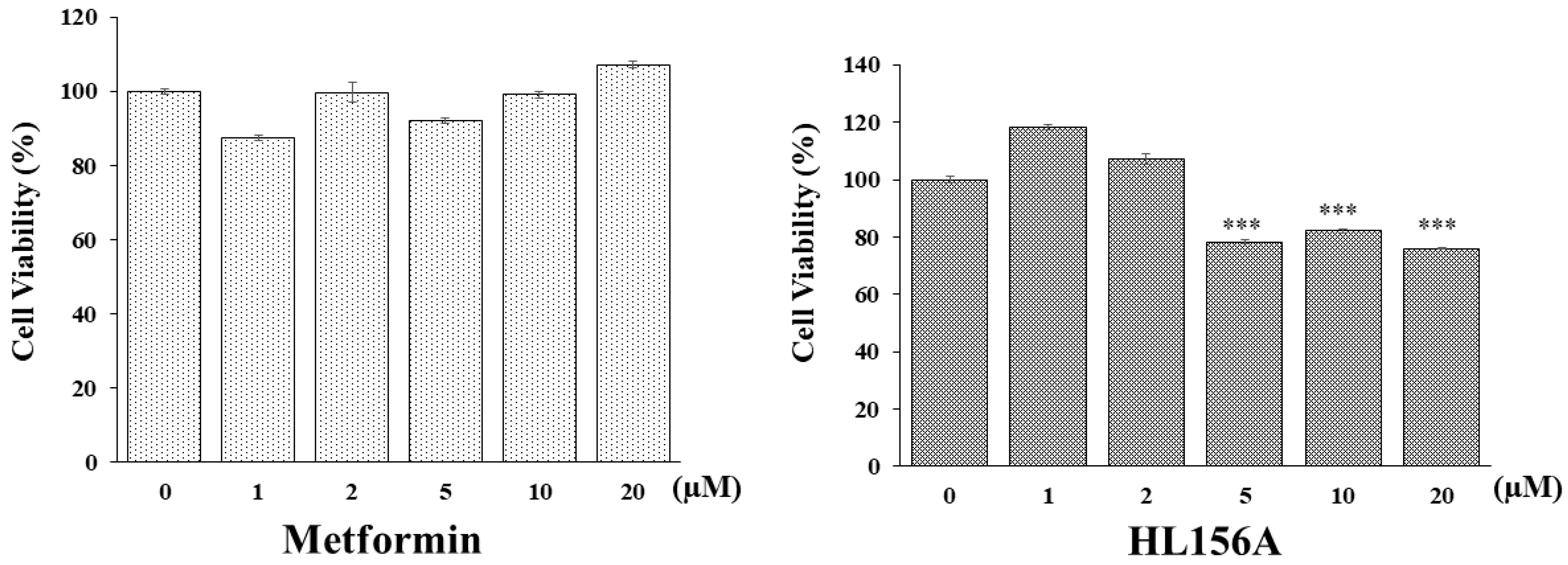
3.1. HL156A Inhibits ADPKD Renal Cyst Cell Proliferation More Effectively Than Metformin
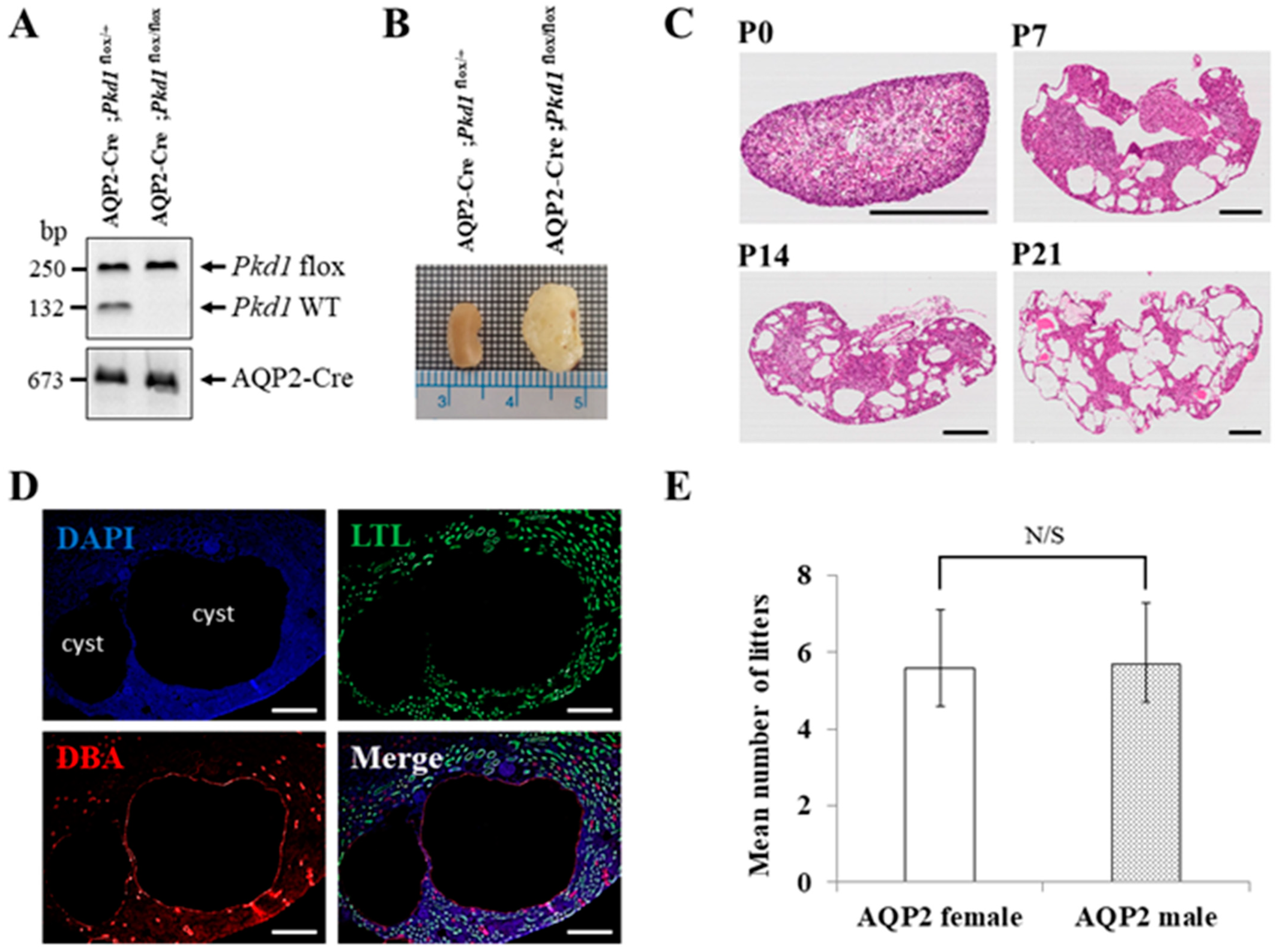
3.2. Characterization of the Collecting Duct-Specific Pkd1 KO Mice
3.3. HL156A Reduces the Kidney Weight-to-Body Weight Ratio in the Collecting Duct-Specific Pkd1 KO Mice
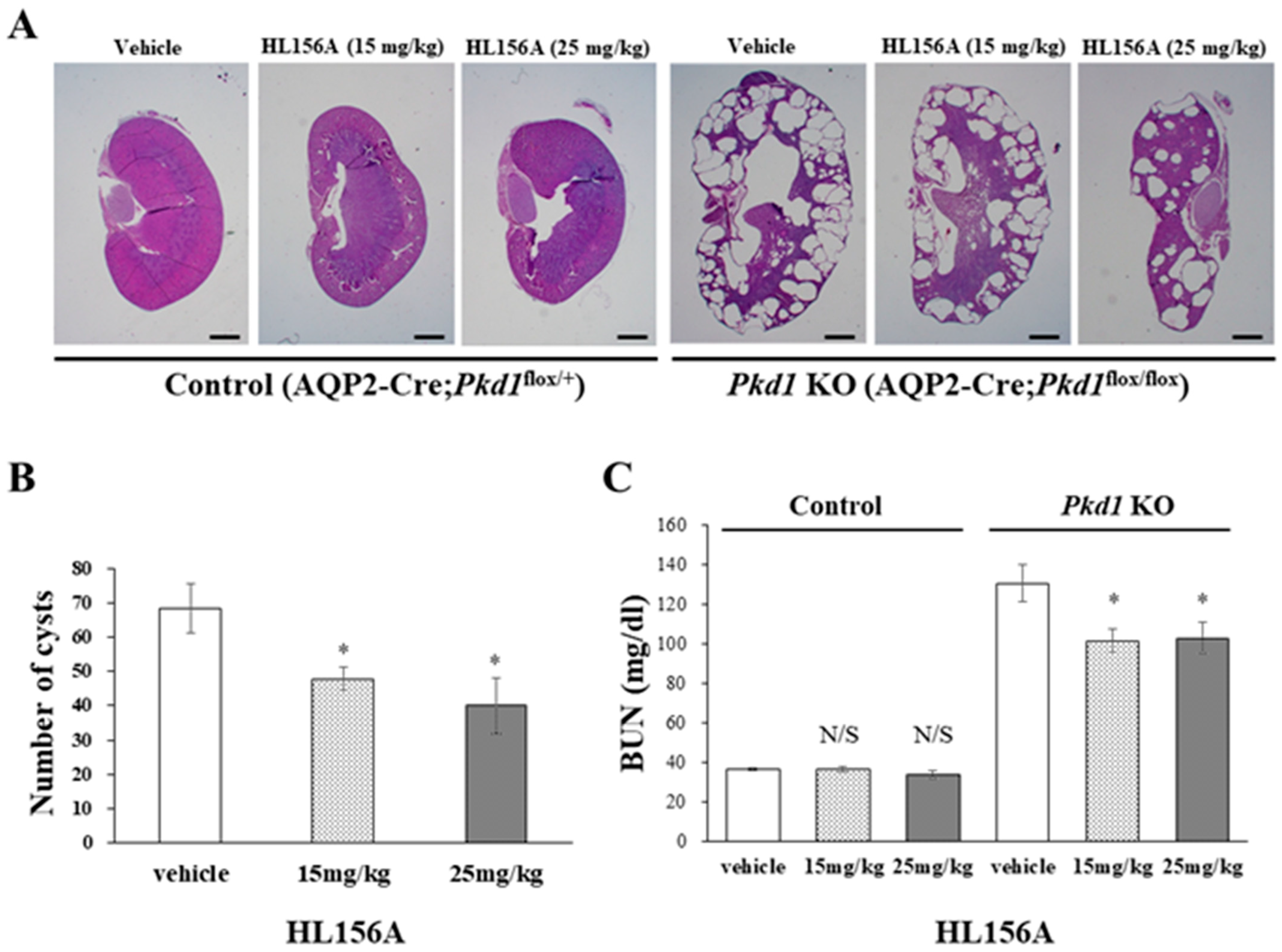
3.4. HL156A Reduces the Number and Size of Cysts and Restores the Kidney Function of the Collecting Duct-Specific Pkd1 KO Mice
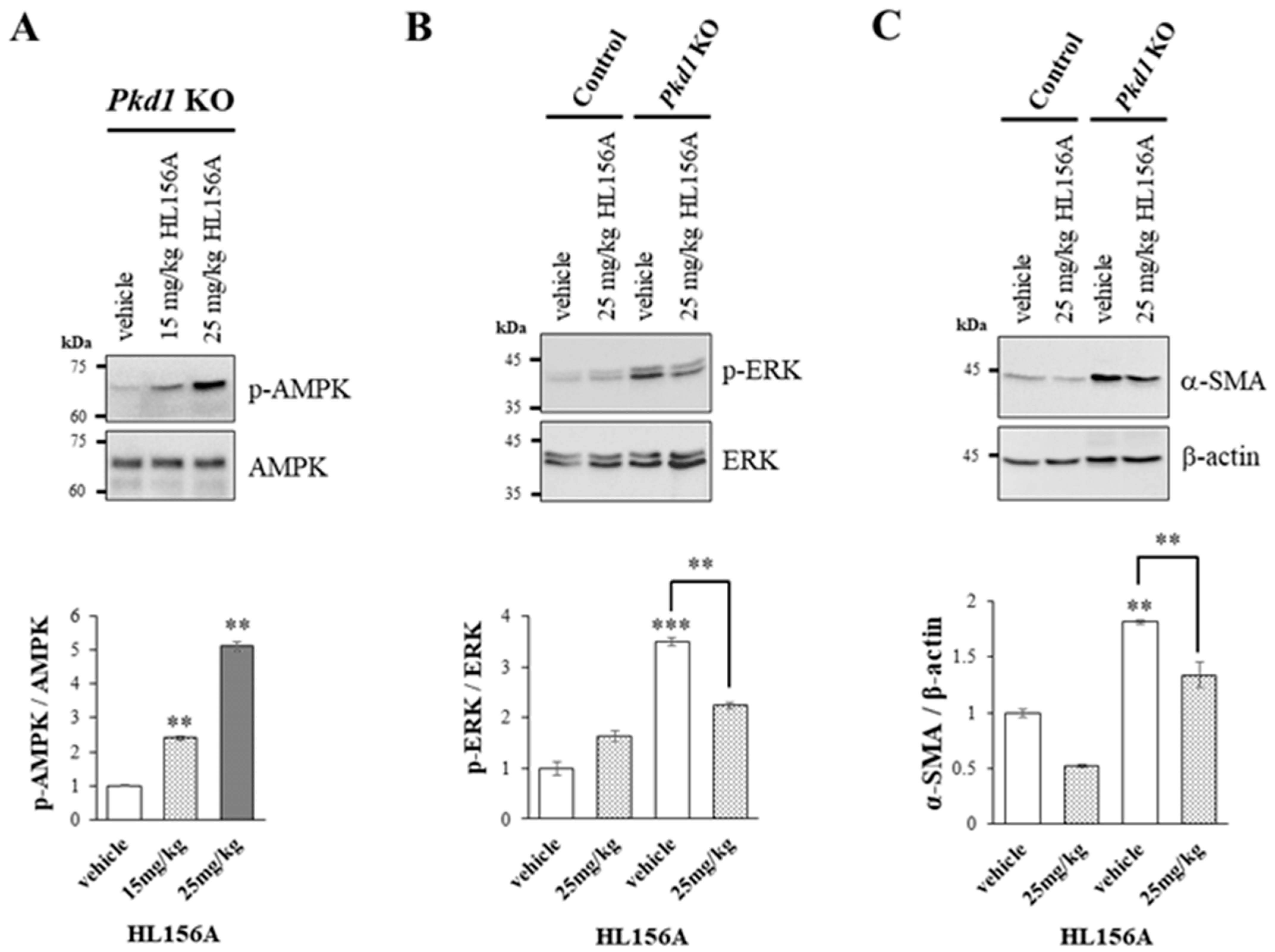
3.5. Increased AMPK Phosphorylation by HL156A Suppresses the Phosphorylation of ERK and α-SMA Expression
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cornec-Le Gall, E.; Alam, A.; Perrone, R.D. Autosomal dominant polycystic kidney disease. Lancet 2019, 393, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Soler, N.M.; Li, H.; Pham, J.; Rivera, D.; Ho, P.-Y.; Mancino, V.; Saitta, B.; Hallows, K.R. Metformin improves relevant disease parameters in an autosomal dominant polycystic kidney disease mouse model. Am. J. Physiol.-Renal 2022, 322, F27–F41. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, P.; Somlo, S. Genetics and pathogenesis of polycystic kidney disease. J. Am. Soc. Nephrol. 2002, 13, 2384–2398. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H.C.; Ryu, H.; Kim, K.; Kim, H.S.; Oh, K.-H.; Yu, S.J.; Chung, J.W.; Cho, J.Y.; Kim, S.H. Clinical correlates of mass effect in autosomal dominant polycystic kidney disease. PLoS ONE 2015, 10, e0144526. [Google Scholar] [CrossRef] [PubMed]
- de Faria, V.P.; Dias, J.P.; Bessa, M.B.; Martingo, M.A.; Lopes, D.; Almeida, C.; Gomes, A.M. Polycystic kidney disease—Beyond PKD1 and PKD2. Nephrol. Dial. Transpl. 2024, 39, gfae069–0225-1148. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.C.; Ryu, H.; Kim, H.; Lee, H.-S.; Heo, J.; Lee, C.; Kim, N.K.; Park, W.-Y.; Hwang, Y.-H. Genetic characteristics of Korean patients with autosomal dominant polycystic kidney disease by targeted exome sequencing. Sci. Rep. 2019, 9, 16952. [Google Scholar] [CrossRef] [PubMed]
- Leonhard, W.N.; Song, X.; Kanhai, A.A.; Iliuta, I.-A.; Bozovic, A.; Steinberg, G.R.; Peters, D.J.; Pei, Y. Salsalate, but not metformin or canagliflozin, slows kidney cyst growth in an adult-onset mouse model of polycystic kidney disease. eBioMedicine 2019, 47, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.C.; Torres, V.E. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J. Clin. Investig. 2014, 124, 2315–2324. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, Y.-H. Clinical trials and a view toward the future of ADPKD. In Cystogenesis; Springer: Singapore, 2016; pp. 105–121. [Google Scholar]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Koch, G.; Ouyang, J.; McQuade, R.D.; Blais, J.D.; Czerwiec, F.S. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2017, 377, 1930–1942. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Bio. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Daugan, M.; Wojcicki, A.D.; d’Hayer, B.; Boudy, V. Metformin: An anti-diabetic drug to fight cancer. Pharmacol. Res. 2016, 113, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.G.; Jeong, Y.S.; Kim, S.A.; Ahn, S.G. New metformin derivative HL 156A prevents oral cancer progression by inhibiting the insulin-like growth factor/AKT/mammalian target of rapamycin pathways. Cancer Sci. 2018, 109, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Takiar, V.; Nishio, S.; Seo-Mayer, P.; King, J.D., Jr.; Li, H.; Zhang, L.; Karihaloo, A.; Hallows, K.R.; Somlo, S.; Caplan, M.J. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 2462–2467. [Google Scholar] [CrossRef] [PubMed]
- Perrone, R.D.; Abebe, K.Z.; Watnick, T.J.; Althouse, A.D.; Hallows, K.R.; Lalama, C.M.; Miskulin, D.C.; Seliger, S.L.; Tao, C.; Harris, P.C. Primary results of the randomized trial of metformin administration in polycystic kidney disease (TAME PKD). Kidney Int. 2021, 100, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.C.; Gansevoort, R.T. TAMEing ADPKD with metformin: Safe and effective? Kidney Int. 2021, 100, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.D.; Kim, H.J.; Tsogbadrakh, B.; Lee, J.; Ryu, H.; Cho, E.J.; Hwang, Y.-H.; Kim, K.; Yang, J.; Ahn, C. HL156A, a novel AMP-activated protein kinase activator, is protective against peritoneal fibrosis in an in vivo and in vitro model of peritoneal fibrosis. Am. J. Physiol.-Renal 2016, 310, F342–F350. [Google Scholar] [CrossRef] [PubMed]
- Tsogbadrakh, B.; Ju, K.D.; Lee, J.; Han, M.; Koh, J.; Yu, Y.; Lee, H.; Yu, K.-S.; Oh, Y.K.; Kim, H.J. HL156A, a novel pharmacological agent with potent adenosine-monophosphate-activated protein kinase (AMPK) activator activity ameliorates renal fibrosis in a rat unilateral ureteral obstruction model. PLoS ONE 2018, 13, e0201692. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.-H.; Koh, I.; Shim, J.-K.; Park, J.; Jeon, J.Y.; Yun, M.; Kim, S.H.; Yook, J.I.; Kim, E.H. Inhibiting stemness and invasive properties of glioblastoma tumorsphere by combined treatment with temozolomide and a newly designed biguanide (HL156A). Oncotarget 2016, 7, 65643–65659. [Google Scholar] [CrossRef]
- Lee, H.S.; Shin, H.-S.; Choi, J.; Bae, S.-J.; Wee, H.-J.; Son, T.; Seo, J.H.; Park, J.-H.; Kim, S.-W.; Kim, K.-W. AMP-activated protein kinase activator, HL156A reduces thioacetamide-induced liver fibrosis in mice and inhibits the activation of cultured hepatic stellate cells and macrophages. Int. J. Oncol. 2016, 49, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Izreig, S.; Gariepy, A.; Kaymak, I.; Bridges, H.R.; Donayo, A.O.; Bridon, G.; DeCamp, L.M.; Kitchen-Goosen, S.M.; Avizonis, D.; Sheldon, R.D. Repression of LKB1 by miR-17~92 sensitizes MYC-dependent lymphoma to biguanide treatment. Cell Rep. Med. 2020, 1. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, B.; Pafundi, D.; Buras, M.; Ashman, J.; Bendok, B.; Laack, N.; Breen, W.; Ruff, M.; Mahajan, A.; Mrugala, M. Impact of 18f-dopa pet on radiation treatment planning from phase ii study of short course hypofractionated proton beam therapy for elderly patients with glioblastoma. Neuro-Oncology 2023, 25, v54. [Google Scholar] [CrossRef]
- Kim, H.; Sung, J.; Kim, H.; Ryu, H.; Park, H.C.; Oh, Y.K.; Lee, H.-S.; Oh, K.-H.; Ahn, C. Expression and secretion of CXCL12 are enhanced in autosomal dominant polycystic kidney disease. BMB Rep. 2019, 52, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.L.; Strait, K.A.; Stricklett, P.K.; Miller, R.L.; Nelson, R.D.; Piontek, K.B.; Germino, G.G.; Kohan, D.E. Inactivation of Pkd1 in principal cells causes a more severe cystic kidney disease than in intercalated cells. Kidney Int. 2009, 75, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Stricklett, P.K.; Hughes, A.K.; Yanagisawa, M.; Kohan, D.E. Collecting duct-specific knockout of the endothelin A receptor alters renal vasopressin responsiveness, but not sodium excretion or blood pressure. Am. J. Physiol.-Renal 2005, 289, F692–F698. [Google Scholar] [CrossRef]
- Raman, A.; Reif, G.A.; Dai, Y.; Khanna, A.; Li, X.; Astleford, L.; Parnell, S.C.; Calvet, J.P.; Wallace, D.P. Integrin-linked kinase signaling promotes cyst growth and fibrosis in polycystic kidney disease. J. Am. Soc. Nephrol. 2017, 28, 2708–2719. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, H.; Jia, C.; Song, Q.; Chen, J.; Zhang, Y.; Lai, P.; Fan, X.; Zhou, X.; Liu, M. Activation of mTORC1 in collecting ducts causes hyperkalemia. J. Am. Soc. Nephrol. 2014, 25, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Rowe, I.; Chiaravalli, M.; Mannella, V.; Ulisse, V.; Quilici, G.; Pema, M.; Song, X.W.; Xu, H.; Mari, S.; Qian, F. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 2013, 19, 488–493. [Google Scholar] [CrossRef]
- Warner, G.; Hein, K.Z.; Nin, V.; Edwards, M.; Chini, C.C.; Hopp, K.; Harris, P.C.; Torres, V.E.; Chini, E.N. Food restriction ameliorates the development of polycystic kidney disease. J. Am. Soc. Nephrol. 2016, 27, 1437–1447. [Google Scholar] [CrossRef]
- Janku, F.; Beom, S.-H.; Moon, Y.W.; Kim, T.W.; Shin, Y.G.; Yim, D.-S.; Kim, G.M.; Kim, H.S.; Kim, S.Y.; Cheong, J.-H. First-in-human study of IM156, a novel potent biguanide oxidative phosphorylation (OXPHOS) inhibitor, in patients with advanced solid tumors. Investig. New Drug 2022, 40, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-H.; Yuan, P.; Perez-Lorenzo, R.; Zhang, Y.; Lee, S.X.; Ou, Y.; Asara, J.M.; Cantley, L.C.; Zheng, B. Phosphorylation of BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation. Mol. Cell 2013, 52, 161–172. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, S.; Kim, H.; Hwang, C.; Kim, J.E.; Kim, J.; Jeon, S.; Song, Y.-j.; Choi, K.-h.; Sim, G.; Cho, M.; et al. HL156A, an AMP-Activated Protein Kinase Activator, Inhibits Cyst Growth in Autosomal Dominant Polycystic Kidney Disease. Biomolecules 2024, 14, 806. https://doi.org/10.3390/biom14070806
Seo S, Kim H, Hwang C, Kim JE, Kim J, Jeon S, Song Y-j, Choi K-h, Sim G, Cho M, et al. HL156A, an AMP-Activated Protein Kinase Activator, Inhibits Cyst Growth in Autosomal Dominant Polycystic Kidney Disease. Biomolecules. 2024; 14(7):806. https://doi.org/10.3390/biom14070806
Chicago/Turabian StyleSeo, Sujung, Hyunho Kim, Chungtaek Hwang, Jin Eop Kim, Jisu Kim, Sohyun Jeon, Young-jin Song, Kwang-ho Choi, Gwangeon Sim, Myunkyu Cho, and et al. 2024. "HL156A, an AMP-Activated Protein Kinase Activator, Inhibits Cyst Growth in Autosomal Dominant Polycystic Kidney Disease" Biomolecules 14, no. 7: 806. https://doi.org/10.3390/biom14070806




