Does the XPA–FEN1 Interaction Concern to Nucleotide Excision Repair or Beyond?
Abstract
:1. Introduction
2. Materials and Methods
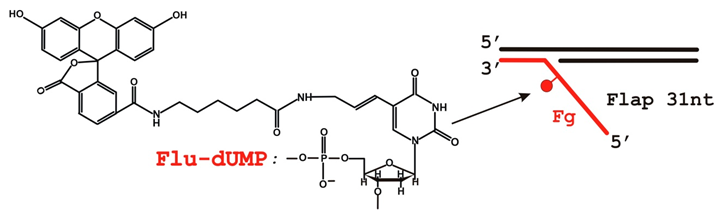
2.1. Reagents and Oligonucleotides
2.2. Protein Purification
2.3. Fluorescent Labeling of XPA
2.4. Preparation of 5′-32P-Labeled Oligonucleotides
2.5. Preparation of DNA Structures
2.6. Endonuclease Assay
2.7. Electrophoretic Mobility Shift Assay (EMSA)
2.8. Far-Western Blotting Assay (Dot Blot Protein–Protein Binding Assay)
2.9. Statistical Analysis
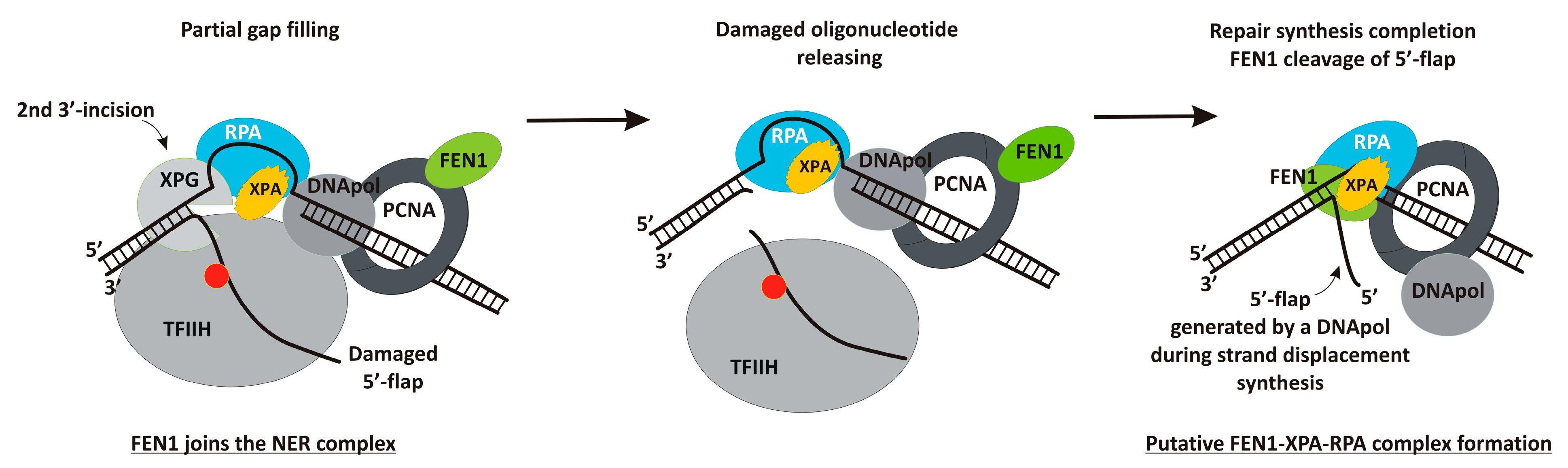
3. Results
3.1. FEN1 Is Able to Cleave Flap Substrates Containing a Bulky Lesion in Combination with a Gap of Different Sizes at Nearly Physiological Salt Conditions
3.2. FEN1 Interacts with XPA in DNA-Independent Manner
3.3. RPA–XPA–FEN1 Complex Formation
3.4. XPA Moderately Inhibits FEN1 Catalytic Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staresincic, L.; Fagbemi, A.F.; Enzlin, J.H.; Gourdin, A.M.; Wijgers, N.; Dunand-Sauthier, I.; Giglia-Mari, G.; Clarkson, S.G.; Vermeulen, W.; Schärer, O.D. Coordination of dual incision and repair synthesis in human nucleotide excision repair. EMBO J. 2009, 28, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Schärer, O.D. Nucleotide excision repair in eukaryotes. Cold Spring Harb. Perspect. Biol. 2013, 5, a012609. [Google Scholar] [CrossRef] [PubMed]
- Fagbemi, A.F.; Orelli, B.; Schärer, O.D. Regulation of endonuclease activity in human nucleotide excision repair. DNA Repair 2011, 10, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Ogi, T.; Limsirichaikul, S.; Overmeer, R.M.; Volker, M.; Takenaka, K.; Cloney, R.; Nakazawa, Y.; Niimi, A.; Miki, Y.; Jaspers, N.G.; et al. Three DNA polymerases, recruited by different mechanisms, carry out NER repair synthesis in human cells. Mol. Cell 2010, 37, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, S.; Kitano, K.; Yamaguchi, H.; Hamada, K.; Okada, K.; Fukuda, K.; Uchida, M.; Ohtsuka, E.; Morioka, H.; Hakoshima, T. Structural basis for recruitment of human flap endonuclease 1 to PCNA. EMBO J. 2005, 24, 683–693. [Google Scholar] [CrossRef]
- Querol-Audí, J.; Yan, C.; Xu, X.; Tsutakawa, S.E.; Tsai, M.S.; Tainer, J.A.; Cooper, P.K.; Nogales, E.; Ivanov, I. Repair complexes of FEN1 endonuclease, DNA, and Rad9-Hus1-Rad1 are distinguished from their PCNA counterparts by functionally important stability. Proc. Natl. Acad. Sci. USA 2012, 109, 8528–8533. [Google Scholar] [CrossRef] [PubMed]
- Lancey, C.; Tehseen, M.; Raducanu, V.S.; Rashid, F.; Merino, N.; Ragan, T.J.; Savva, C.G.; Zaher, M.S.; Shirbini, A.; Blanco, F.J.; et al. Structure of the processive human Pol δ holoenzyme. Nat. Commun. 2020, 11, 1109. [Google Scholar] [CrossRef] [PubMed]
- Blair, K.; Tehseen, M.; Raducanu, V.S.; Shahid, T.; Lancey, C.; Rashid, F.; Crehuet, R.; Hamdan, S.M.; De Biasio, A. Mechanism of human Lig1 regulation by PCNA in Okazaki fragment sealing. Nat. Commun. 2022, 13, 7833, Erratum in Nat. Commun. 2023, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Tsutakawa, S.E.; Tainer, J.A. Double strand binding-single strand incision mechanism for human flap endonuclease: Implications for the superfamily. Mech. Ageing Dev. 2012, 133, 195–202. [Google Scholar] [CrossRef]
- Balakrishnan, L.; Bambara, R.A. Flap endonuclease 1. Annu. Rev. Biochem. 2013, 82, 119–138. [Google Scholar] [CrossRef]
- Fortini, P.; Dogliotti, E. Base damage and singlestrand break repair: Mechanisms and functional significance of short- and long-patch repair subpathways. DNA Repair 2007, 6, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Krokan, H.E.; Bjørås, M. Base excision repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012583. [Google Scholar] [CrossRef] [PubMed]
- Laverde, E.E.; Lai, Y.; Leng, F.; Balakrishnan, L.; Freudenreich, C.H.; Liu, Y. R-loops promote trinucleotide repeat deletion through DNA base excision repair enzymatic activities. J. Biol. Chem. 2020, 295, 13902–13913. [Google Scholar] [CrossRef] [PubMed]
- Laverde, E.E.; Polyzos, A.A.; Tsegay, P.P.; Shaver, M.; Hutcheson, J.D.; Balakrishnan, L.; McMurray, C.T.; Liu, Y. Flap Endonuclease 1 Endonucleolytically Processes RNA to Resolve R-Loops through DNA Base Excision Repair. Genes 2022, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Muse, T.; Aguilera, A. R Loops: From Physiological to Pathological Roles. Cell 2019, 179, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Cristini, A.; Ricci, G.; Britton, S.; Salimbeni, S.; Huang, S.N.; Marinello, J.; Calsou, P.; Pommier, Y.; Favre, G.; Capranico, G.; et al. Dual Processing of R-Loops and Topoisomerase I Induces Transcription-Dependent DNA Double-Strand Breaks. Cell Rep. 2019, 28, 3167–3181.e6. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, N.; Sivley, R.M.; Perry, K.E.; Capra, J.A.; Chazin, W.J. XPA: A key scaffold for human nucleotide excision repair. DNA Repair 2016, 44, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Borszéková Pulzová, L.; Ward, T.A.; Chovanec, M. XPA: DNA Repair Protein of Significant Clinical Importance. Int. J. Mol. Sci. 2020, 21, 2182. [Google Scholar] [CrossRef] [PubMed]
- Krasikova, Y.S.; Lavrik, O.I.; Rechkunova, N.I. The XPA Protein-Life under Precise Control. Cells 2022, 11, 3723. [Google Scholar] [CrossRef] [PubMed]
- Krasikova, Y.S.; Rechkunova, N.I.; Maltseva, E.A.; Lavrik, O.I. RPA and XPA interaction with DNA structures mimicking intermediates of the late stages in nucleotide excision repair. PLoS ONE 2018, 13, e0190782. [Google Scholar] [CrossRef]
- Kim, H.S.; Brill, S.J. Rfc4 interacts with Rpa1 and is required for both DNA replication and DNA damage checkpoints in Saccharomyces cerevisiae. Mol. Cell Biol. 2001, 21, 3725–3737. [Google Scholar] [CrossRef]
- Hayner, J.N.; Douma, L.G.; Bloom, L.B. The interplay of primer-template DNA phosphorylation status and single-stranded DNA binding proteins in directing clamp loaders to the appropriate polarity of DNA. Nucleic Acids Res. 2014, 42, 10655–10667. [Google Scholar] [CrossRef] [PubMed]
- Gilljam, K.M.; Feyzi, E.; Aas, P.A.; Sousa, M.M.; Muller, R.; Vagbo, C.B.; Catterall, T.C.; Liabakk, N.B.; Slupphaug, G.; Drablos, F.; et al. Identification of a novel, widespread, and functionally important PCNA-binding motif. J. Cell Biol. 2009, 186, 645–654. [Google Scholar] [CrossRef]
- Gilljam, K.M.; Müller, R.; Liabakk, N.B.; Otterlei, M. Nucleotide excision repair is associated with the replisome and its efficiency depends on a direct interaction between XPA and PCNA. PLoS ONE 2012, 7, e49199. [Google Scholar] [CrossRef]
- Hara, K.; Uchida, M.; Tagata, R.; Yokoyama, H.; Ishikawa, Y.; Hishiki, A.; Hashimoto, H. Structure of proliferating cell nuclear antigen (PCNA) bound to an APIM peptide reveals the universality of PCNA interaction. Acta Cryst. F Struct. Biol. Commun. 2018, 74, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Topolska-Woś, A.M.; Sugitani, N.; Cordoba, J.J.; Le Meur, K.V.; Le Meur, R.A.; Kim, H.S.; Yeo, J.E.; Rosenberg, D.; Hammel, M.; Schärer, O.D.; et al. A key interaction with RPA orients XPA in NER complexes. Nucleic Acids Res. 2020, 48, 2173–2188. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.S.; D′Souza, A.; Gallagher, K.; Jeong, E.; Topolska-Wós, A.; Ogorodnik Le Meur, K.; Tsai, C.L.; Tsai, M.S.; Kee, M.; et al. Two interaction surfaces between XPA and RPA organize the preincision complex in nucleotide excision repair. Proc. Natl. Acad. Sci. USA 2022, 119, e2207408119. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Henricksen, L.A.; Wold, M.S.; Ingles, C.J. RPA involvement in the damage-recognition and incision steps of nucleotide excision repair. Nature 1995, 374, 566–569. [Google Scholar] [CrossRef]
- Li, L.; Elledge, S.J.; Peterson, C.A.; Bales, E.S.; Legerski, R.J. Specific association between the human DNA repair proteins XPA and ERCC1. Proc. Natl. Acad. Sci. USA 1994, 91, 5012–5016. [Google Scholar] [CrossRef]
- Croteau, D.L.; Peng, Y.; Van Houten, B. DNA repair gets physical: Mapping an XPA-binding site on ERCC1. DNA Repair 2008, 7, 819–826. [Google Scholar] [CrossRef]
- de Laat, W.L.; Appeldoorn, E.; Sugasawa, K.; Weterings, E.; Jaspers, N.G.; Hoeijmakers, J.H. DNA-binding polarity of human replication protein A positions nucleases in nucleotide excision repair. Genes. Dev. 1998, 12, 2598–2609. [Google Scholar] [CrossRef] [PubMed]
- Dunand-Sauthier, I.; Hohl, M.; Thorel, F.; Jaquier-Gubler, P.; Clarkson, S.G.; Scharer, O.D. The spacer region of XPG mediates recruitment to nucleotide excision repair complexes and determines substrate specificity. J. Biol. Chem. 2005, 280, 7030–7037. [Google Scholar] [CrossRef] [PubMed]
- Tsodikov, O.V.; Ivanov, D.; Orelli, B.; Staresincic, L.; Shoshani, I.; Oberman, R.; Schärer, O.D.; Wagner, G.; Ellenberger, T. Structural basis for the recruitment of ERCC1-XPF to nucleotide excision repair complexes by XPA. EMBO J. 2007, 26, 4768–4776. [Google Scholar] [CrossRef] [PubMed]
- Krasikova, Y.S.; Rechkunova, N.I.; Maltseva, E.A.; Petruseva, I.O.; Lavrik, O.I. Localization of xeroderma pigmentosum group A protein and replication protein A on damaged DNA in nucleotide excision repair. Nucleic Acids Res. 2010, 38, 8083–8094. [Google Scholar] [CrossRef]
- Fadda, E. Conformational determinants for the recruitment of ERCC1 by XPA in the nucleotide excision repair (NER) Pathway: Structure and dynamics of the XPA binding motif. Biophys. J. 2013, 104, 2503–2511. [Google Scholar] [CrossRef]
- Mocquet, V.; Lainé, J.P.; Riedl, T.; Yajin, Z.; Lee, M.Y.; Egly, J.M. Sequential recruitment of the repair factors during NER: The role of XPG in initiating the resynthesis step. EMBO J. 2008, 27, 155–167. [Google Scholar] [CrossRef]
- Nazarkina, J.K.; Petrousseva, I.O.; Safronov, I.V.; Lavrik, O.I.; Khodyreva, S.N. Interaction of flap endonuclease-1 and replication protein A with photoreactive intermediates of DNA repair. Biochemistry 2003, 68, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Krasikova, Y.S.; Rechkunova, N.I.; Maltseva, E.A.; Petruseva, I.O.; Silnikov, V.N.; Zatsepin, T.S.; Oretskaya, T.S.; Schärer, O.D.; Lavrik, O.I. Interaction of nucleotide excision repair factors XPC-HR23B, XPA, and RPA with damaged DNA. Biochemistry 2008, 73, 886–896. [Google Scholar] [CrossRef]
- Maltseva, E.A.; Krasikova, Y.S.; Naegeli, H.; Lavrik, O.I.; Rechkunova, N.I. Effect of point substitutions within the minimal DNA-binding domain of xeroderma pigmentosum group A protein on interaction with DNA intermediates of nucleotide excision repair. Biochemistry 2014, 79, 545–554. [Google Scholar] [CrossRef]
- Henricksen, L.A.; Umbricht, C.B.; Wold, M.S. Recombinant replication protein A: Expression, complex formation, and functional characterization. J. Biol. Chem. 1994, 269, 11121–11132, Erratum in J. Biol. Chem. 1994, 269, 16519. [Google Scholar] [CrossRef] [PubMed]
- Moor, N.A.; Vasil′eva, I.A.; Anarbaev, R.O.; Antson, A.A.; Lavrik, O.I. Quantitative characterization of protein-protein complexes involved in base excision DNA repair. Nucleic Acids Res. 2015, 43, 6009–6022. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Tsutakawa, S.E.; Classen, S.; Chapados, B.R.; Arvai, A.S.; Finger, L.D.; Guenther, G.; Tomlinson, C.G.; Thompson, P.; Sarker, A.H.; Shen, B.; et al. Human flap endonuclease structures, DNA double-base flipping, and a unified understanding of the FEN1 superfamily. Cell 2011, 145, 198–211. [Google Scholar] [CrossRef]
- Udensi, U.K.; Tchounwou, P.B. Potassium Homeostasis, Oxidative Stress, and Human Disease. Int. J. Clin. Exp. Physiol. 2017, 4, 111–122. [Google Scholar] [CrossRef]
- Zacchia, M.; Abategiovanni, M.L.; Stratigis, S.; Capasso, G. Potassium: From Physiology to Clinical Implications. Kidney Dis. 2016, 2, 72–79. [Google Scholar] [CrossRef]
- Song, B.; Hamdan, S.M.; Hingorani, M.M. Positioning the 5′-flap junction in the active site controls the rate of flap endonuclease-1-catalyzed DNA cleavage. J. Biol. Chem. 2018, 293, 4792–4804. [Google Scholar] [CrossRef] [PubMed]
- Bornarth, C.J.; Ranalli, T.A.; Henricksen, L.A.; Wahl, A.F.; Bambara, R.A. Effect of flap modifications on human FEN1 cleavage. Biochemistry 1999, 38, 13347–13354. [Google Scholar] [CrossRef]
- Gloor, J.W.; Balakrishnan, L.; Bambara, R.A. Flap endonuclease 1 mechanism analysis indicates flap base binding prior to threading. J. Biol. Chem. 2010, 285, 34922–34931. [Google Scholar] [CrossRef]
- Kaiser, M.; Lyamicheva, N.; Ma, W.; Miller, C.; Neri, B.; Fors, L.; Lyamichev, V. A comparison of eubacterial and archaeal structure-specific 50 -exonucleases. J. Biol. Chem. 1999, 274, 21387–21394. [Google Scholar] [CrossRef]
- Lyamichev, V.; Brow, M.A.; Varvel, V.E.; Dahlberg, J.E. Comparison of the 5′ nuclease activities of taq DNA polymerase and its isolated nuclease domain. Proc. Natl. Acad. Sci. USA 1999, 96, 6143–6148. [Google Scholar] [CrossRef]
- Qiu, J.; Bimston, D.N.; Partikian, A.; Shen, B. Arginine residues 47 and 70 of human flap endonuclease-1 are involved in DNA substrate interactions and cleavage site determination. J. Biol. Chem. 2002, 277, 24659–24666. [Google Scholar] [CrossRef]
- Harrington, J.J.; Lieber, M.R. The characterization of a mammalian DNA structure-specific endonuclease. EMBO J. 1994, 13, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Tsutakawa, S.E.; Thompson, M.J.; Arvai, A.S.; Neil, A.J.; Shaw, S.J.; Algasaier, S.I.; Kim, J.C.; Finger, L.D.; Jardine, E.; Gotham, V.J.B.; et al. Phosphate steering by Flap Endonuclease 1 promotes 5′-flap specificity and incision to prevent genome instability. Nat. Commun. 2017, 8, 15855, Erratum in Nat. Commun. 2017, 8, 16145. [Google Scholar] [CrossRef]
- Bagur, R.; Hajnóczky, G. Intracellular Ca2+ Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Krasikova, Y.; Rechkunova, N.; Lavrik, O. Nucleotide Excision Repair: From Molecular Defects to Neurological Abnormalities. Int. J. Mol. Sci. 2021, 22, 6220. [Google Scholar] [CrossRef] [PubMed]
- Reardon, J.T.; Sancar, A. Recognition and repair of the cyclobutane thymine dimer, a major cause of skin cancers, by the human excision nuclease. Genes. Dev. 2003, 17, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Kesseler, K.J.; Kaufmann, W.K.; Reardon, J.T.; Elston, T.C.; Sancar, A. A mathematical model for human nucleotide excision repair: Damage recognition by random order assembly and kinetic proofreading. J. Theor. Biol. 2007, 249, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Choi, B.S. The protein shuffle. Sequential interactions among components of the human nucleotide excision repair pathway. FEBS J. 2006, 273, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Jia, J.; Finger, L.D.; Guo, Z.; Zer, C.; Shen, B. Functional regulation of FEN1 nuclease and its link to cancer. Nucleic Acids Res. 2011, 39, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Grasby, J.A.; Finger, L.D.; Tsutakawa, S.E.; Atack, J.M.; Tainer, J.A. Unpairing and gating: Sequence-independent substrate recognition by FEN superfamily nucleases. Trends Biochem. Sci. 2012, 37, 74–84. [Google Scholar] [CrossRef]
- Spiro, C.; Pelletier, R.; Rolfsmeier, M.L.; Dixon, M.J.; Lahue, R.S.; Gupta, G.; Park, M.S.; Chen, X.; Mariappan, S.V.; McMurray, C.T. Inhibition of FEN-1 processing by DNA secondary structure at trinucleotide repeats. Mol. Cell 1999, 4, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhou, M.; Chai, Q.; Parrish, J.; Xue, D.; Patrick, S.M.; Turchi, J.J.; Yannone, S.M.; Chen, D.; Shen, B. Novel function of the flap endonuclease 1 complex in processing stalled DNA replication forks. EMBO Rep. 2005, 6, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Freudenreich, C.H. Haploinsufficiency of yeast FEN1 causes instability of expanded CAG/CTG tracts in a length-dependent manner. Gene 2007, 393, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Beaver, J.M.; Lai, Y.; Rolle, S.J.; Liu, Y. Proliferating cell nuclear antigen prevents trinucleotide repeat expansions by promoting repeat deletion and hairpin removal. DNA Repair 2016, 48, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Sampathi, S.; Bhusari, A.; Shen, B.; Chai, W. Human flap endonuclease I is in complex with telomerase and is required for telomerase-mediated telomere maintenance. J. Biol. Chem. 2009, 284, 3682–3690. [Google Scholar] [CrossRef]
- Saharia, A.; Teasley, D.C.; Duxin, J.P.; Dao, B.; Chiappinelli, K.B.; Stewart, S.A. FEN1 ensures telomere stability by facilitating replication fork re-initiation. J. Biol. Chem. 2010, 285, 27057–27066. [Google Scholar] [CrossRef] [PubMed]
- Vallur, A.C.; Maizels, N. Complementary roles for exonuclease 1 and flap endonuclease 1 in maintenance of triplet repeats. J. Biol. Chem. 2010, 285, 28514–28519. [Google Scholar] [CrossRef] [PubMed]
- Teasley, D.C.; Parajuli, S.; Nguyen, M.; Moore, H.R.; Alspach, E.; Lock, Y.J.; Honaker, Y.; Saharia, A.; Piwnica-Worms, H.; Stewart, S.A. Flap Endonuclease 1 Limits Telomere Fragility on the Leading Strand. J. Biol. Chem. 2015, 290, 15133–15145. [Google Scholar] [CrossRef] [PubMed]
- Kokic, G.; Chernev, A.; Tegunov, D.; Dienemann, C.; Urlaub, H.; Cramer, P. Structural basis of TFIIH activation for nucleotide excision repair. Nat. Commun. 2019, 10, 2885. [Google Scholar] [CrossRef]
- Lian, F.M.; Yang, X.; Jiang, Y.L.; Yang, F.; Li, C.; Yang, W.; Qian, C. New structural insights into the recognition of undamaged splayed-arm DNA with a single pair of non-complementary nucleotides by human nucleotide excision repair protein XPA. Int. J. Biol. Macromol. 2020, 148, 466–474. [Google Scholar] [CrossRef]
- Matsuda, T.; Saijo, M.; Kuraoka, I.; Kobayashi, T.; Nakatsu, Y.; Nagai, A.; Enjoji, T.; Masutani, C.; Sugasawa, K.; Hanaoka, F.; et al. DNA repair protein XPA binds replication protein A (RPA). J. Biol. Chem. 1995, 270, 4152–4157. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Mahrenholz, A.; Lee, S.H. RPA stabilizes the XPA-damaged DNA complex through protein-protein interaction. Biochemistry 2000, 39, 6433–6439. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.G.; Liu, Y.; Mao, L.Y.; Zhang, J.T.; Zou, Y. Dimerization of human XPA and formation of XPA(2)-RPA protein complex. Biochemistry 2002, 41, 13012–13020. [Google Scholar] [CrossRef] [PubMed]
- Spegg, V.; Panagopoulos, A.; Stout, M.; Krishnan, A.; Reginato, G.; Imhof, R.; Roschitzki, B.; Cejka, P.; Altmeyer, M. Phase separation properties of RPA combine high-affinity ssDNA binding with dynamic condensate functions at telomeres. Nat. Struct. Mol. Biol. 2023, 30, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Hanawalt, P.C.; Spivak, G. Transcription-coupled DNA repair: Two decades of progress and surprises. Nat. Rev. Mol. Cell Biol. 2008, 9, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Son, K.; Schärer, O.D. Repair, Removal, and Shutdown: It All Hinges on RNA Polymerase II Ubiquitylation. Cell 2020, 180, 1039–1041. [Google Scholar] [CrossRef]
- Belotserkovskii, B.P.; Tornaletti, S.; D’Souza, A.D.; Hanawalt, P.C. R-loop generation during transcription: Formation, processing and cellular outcomes. DNA Repair 2018, 71, 69–81. [Google Scholar] [CrossRef]
- Sollier, J.; Stork, C.T.; García-Rubio, M.L.; Paulsen, R.D.; Aguilera, A.; Cimprich, K.A. Transcription-coupled nucleotide excision repair factors promote R-loop-induced genome instability. Mol. Cell 2014, 56, 777–785. [Google Scholar] [CrossRef]
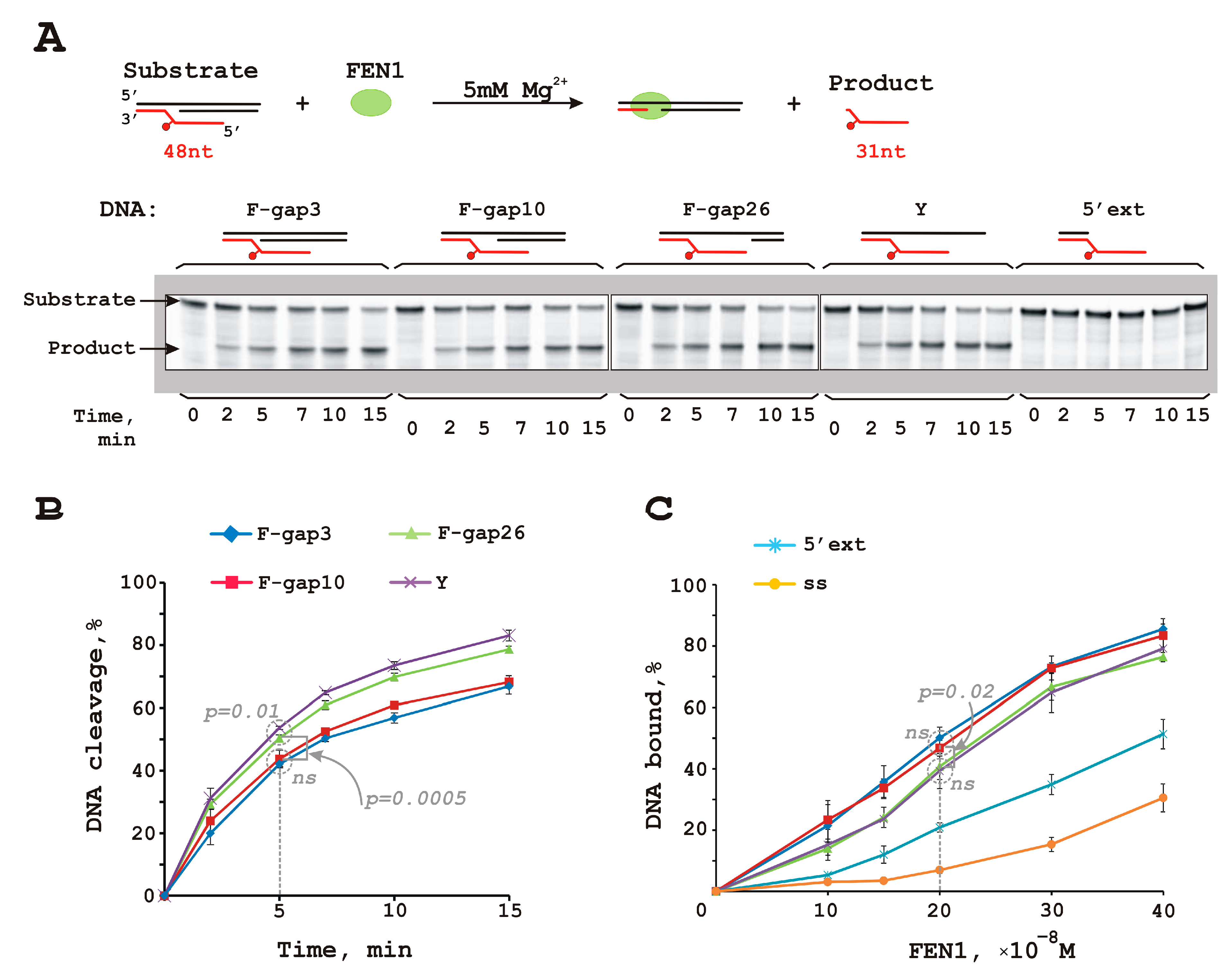
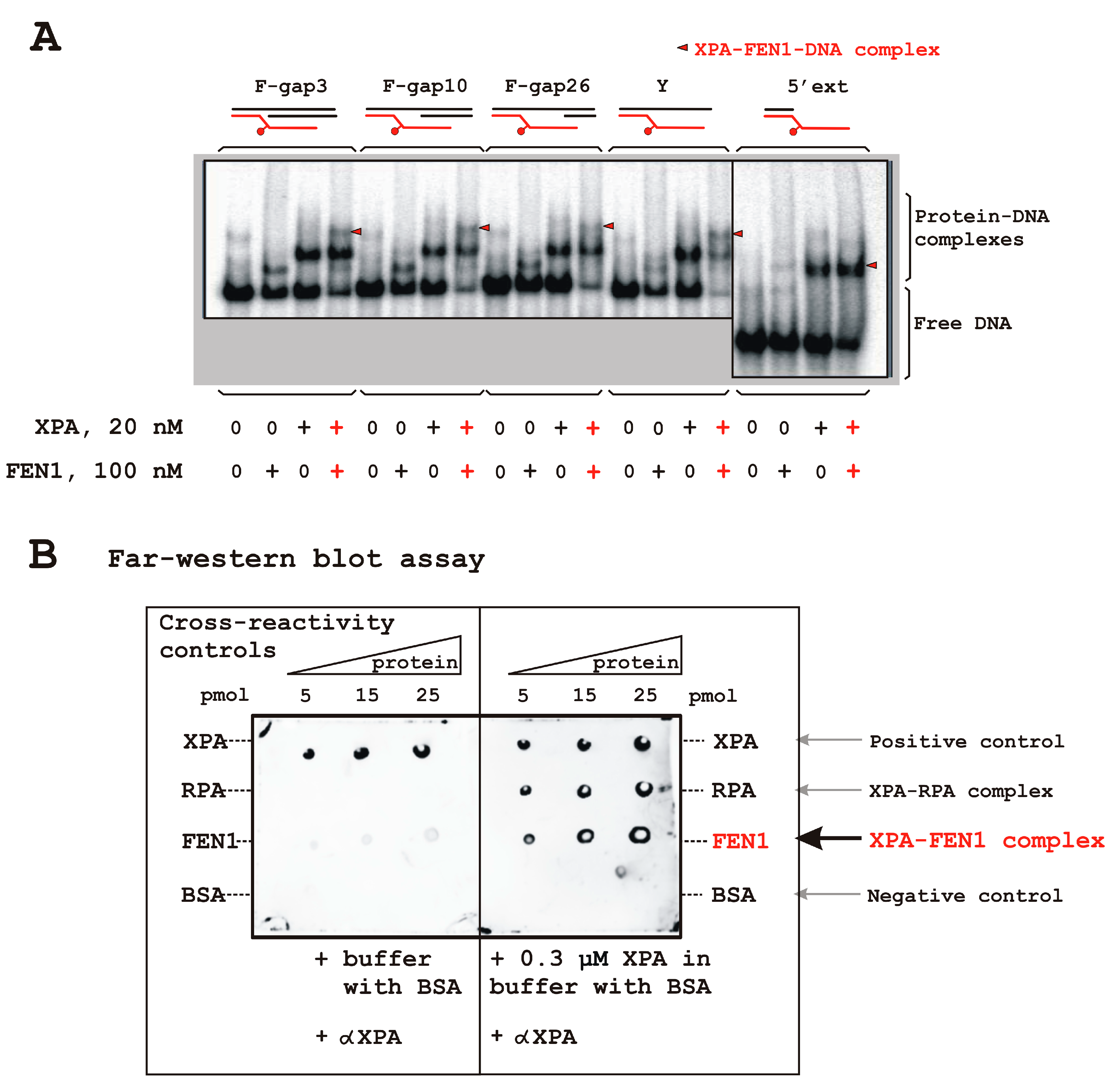
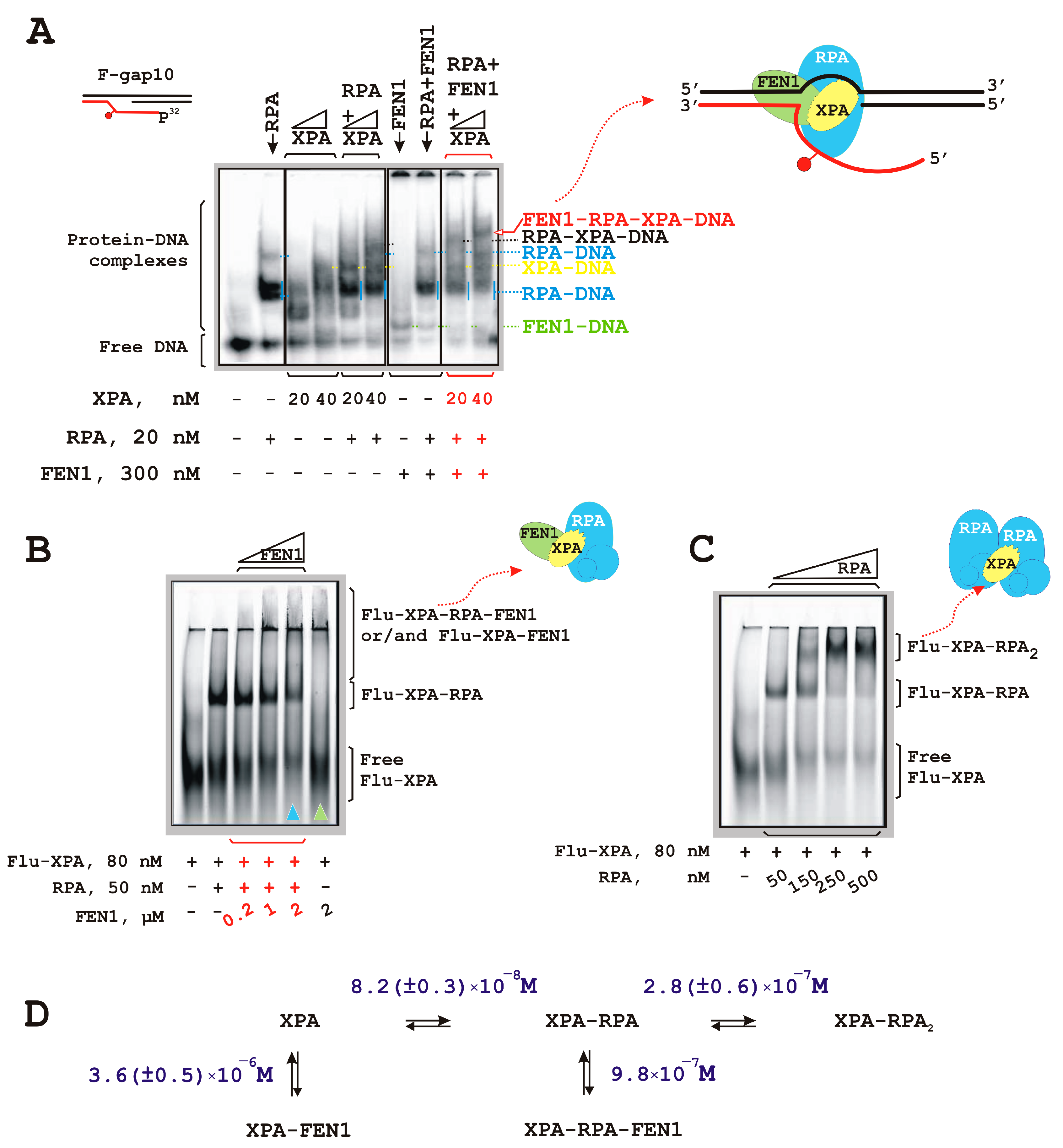
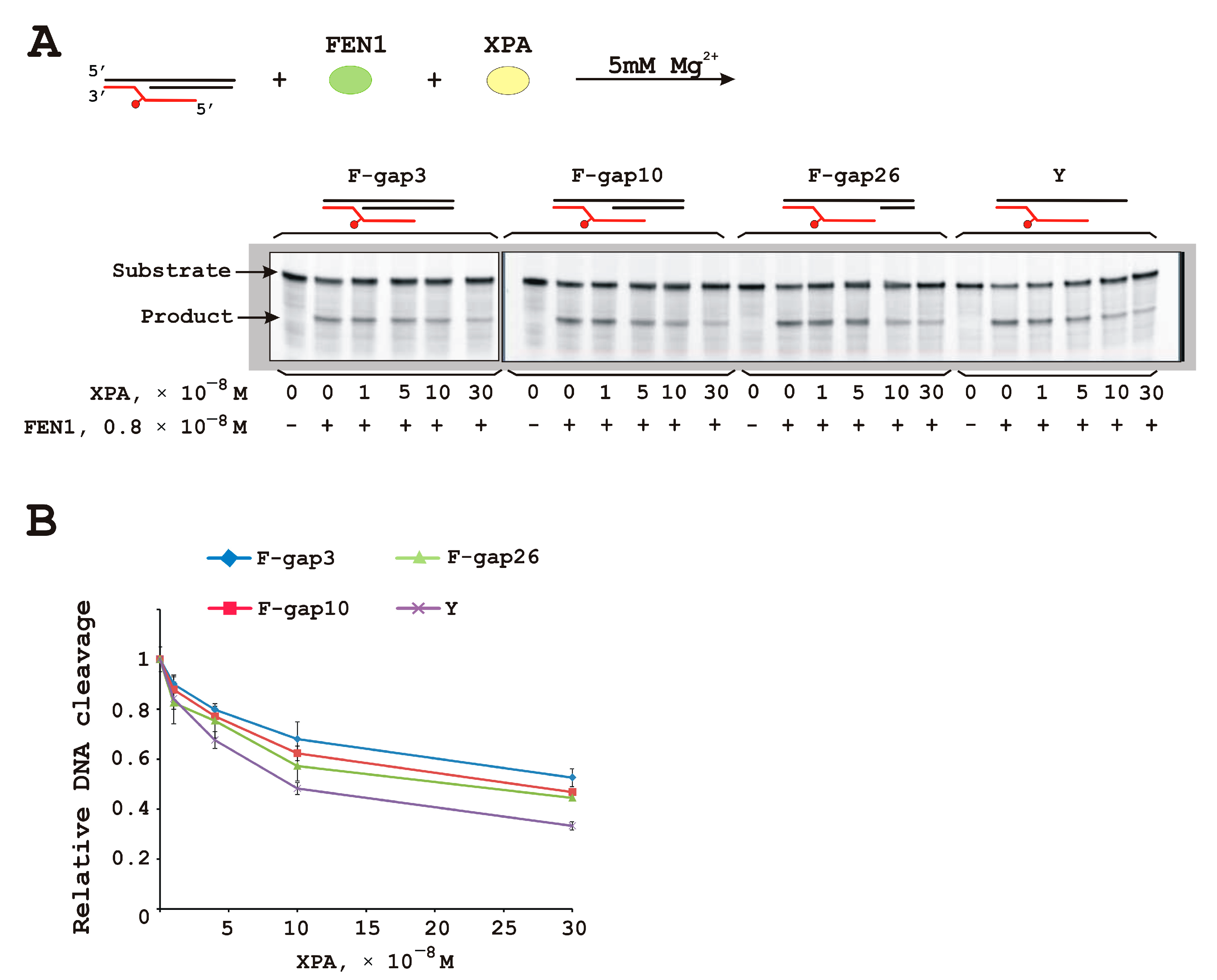
| DNA Designation | Oligo Composition | Schematic View | Gap Size |
|---|---|---|---|
| F-gap3 | 60 Fg 40 | 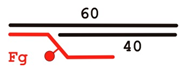 | 3 |
| F-gap10 | 60 Fg 33 | 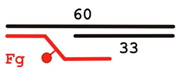 | 10 |
| F-gap26 | 60 Fg 17 | 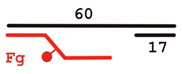 | 26 |
| Y | 60 Fg | 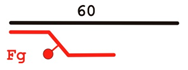 | − |
| 5’ext | 17up Fg |  | − |
 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasikova, Y.S.; Maltseva, E.A.; Khodyreva, S.N.; Evdokimov, A.N.; Rechkunova, N.I.; Lavrik, O.I. Does the XPA–FEN1 Interaction Concern to Nucleotide Excision Repair or Beyond? Biomolecules 2024, 14, 814. https://doi.org/10.3390/biom14070814
Krasikova YS, Maltseva EA, Khodyreva SN, Evdokimov AN, Rechkunova NI, Lavrik OI. Does the XPA–FEN1 Interaction Concern to Nucleotide Excision Repair or Beyond? Biomolecules. 2024; 14(7):814. https://doi.org/10.3390/biom14070814
Chicago/Turabian StyleKrasikova, Yuliya S., Ekaterina A. Maltseva, Svetlana N. Khodyreva, Alexey N. Evdokimov, Nadejda I. Rechkunova, and Olga I. Lavrik. 2024. "Does the XPA–FEN1 Interaction Concern to Nucleotide Excision Repair or Beyond?" Biomolecules 14, no. 7: 814. https://doi.org/10.3390/biom14070814







