Haplotypes of ATP-Binding Cassette CaABCC6 in Chickpea from Kazakhstan Are Associated with Salinity Tolerance and Leaf Necrosis via Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Salinity Stress Indicators: Leaf Necrosis and Chlorophyll Content
2.3. Na+ and K+ Accumulation and Ratio Na+/K+ in Leaves
2.4. Oxidative Stress Indicator: MDA Content
2.5. Content of Glutathione (GSH) in Reduced Form and Glutathione Disulfide (GSSG) in Oxidized Form and Their Ratio (GSH/GSSG) in Leaves
2.6. 6K DArT Assay
2.7. Marker–Trait Association (MTA)
2.8. DNA Extraction, Sanger Sequencing, and SNP Identification
2.9. RNA Extraction and RT-qPCR Analysis of Gene Expression in Parental and F6 Breeding Lines
2.10. Seed Protein Extraction, Purification, and Mass Spectrometry for Measurement of Enzymes from the Glutathione Pathway
2.11. Statistical Analysis
3. Results
3.1. Salinity Stress: Leaf Necrosis, Chlorophyll Degradation, and Na+ Accumulation
3.2. Oxidative Stress Indicators: Malondialdehyde (MDA) and Ratio between Reduced Glutathione (GSH) and Oxidized Glutathione (GSSG) in Leaves
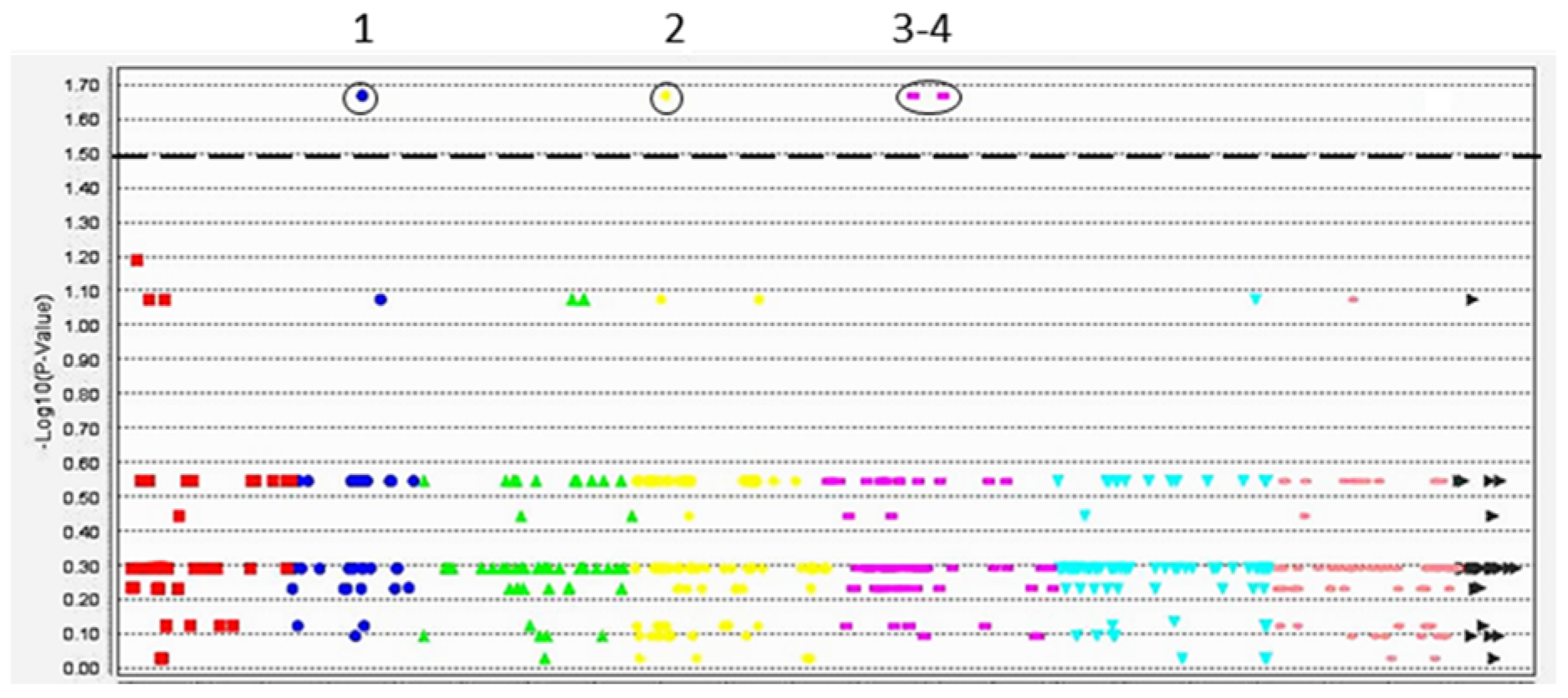
3.3. Marker–Trait Association Analysis for Salinity and Oxidative Stresses Using 6K DArT Assay
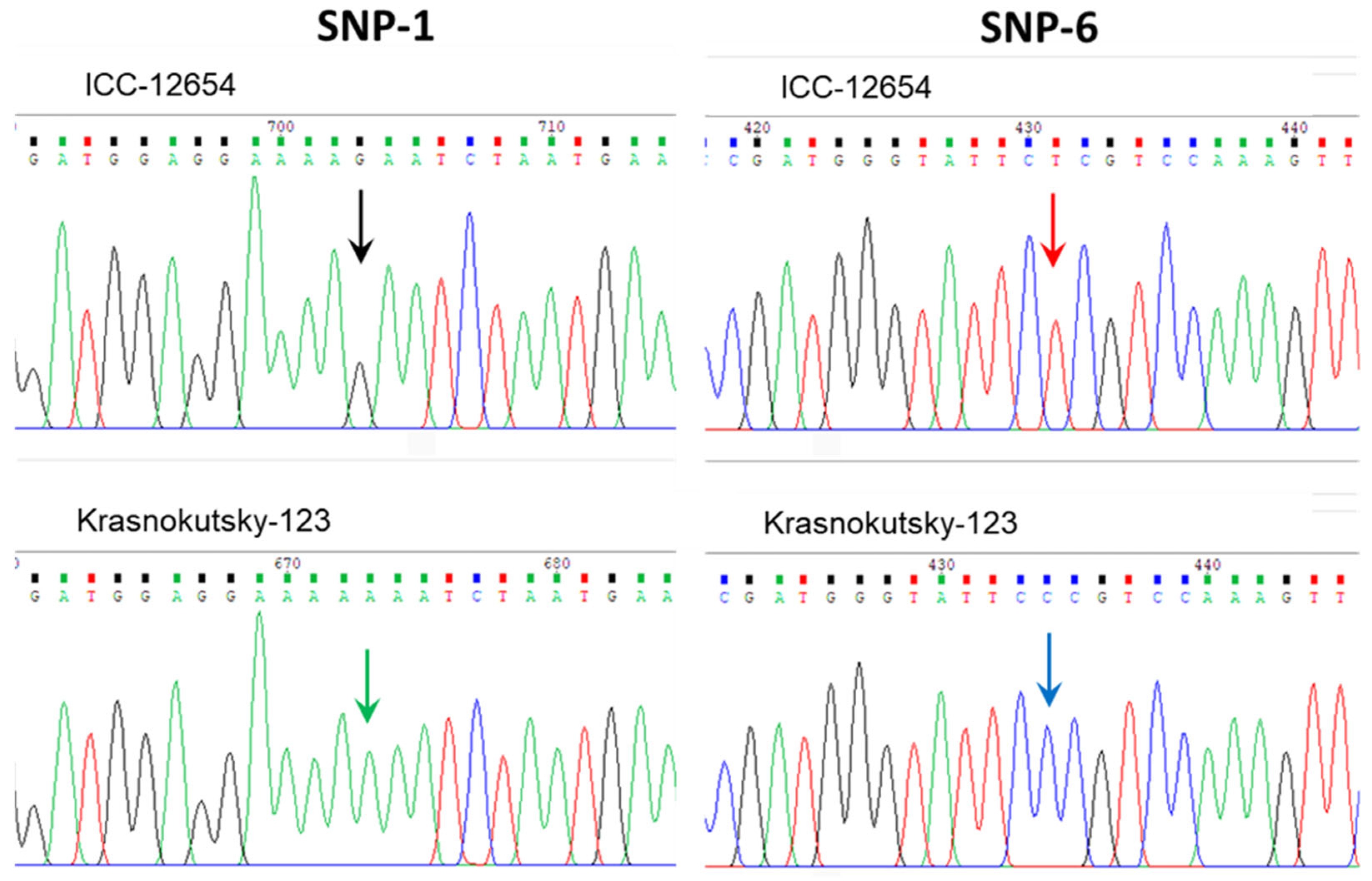
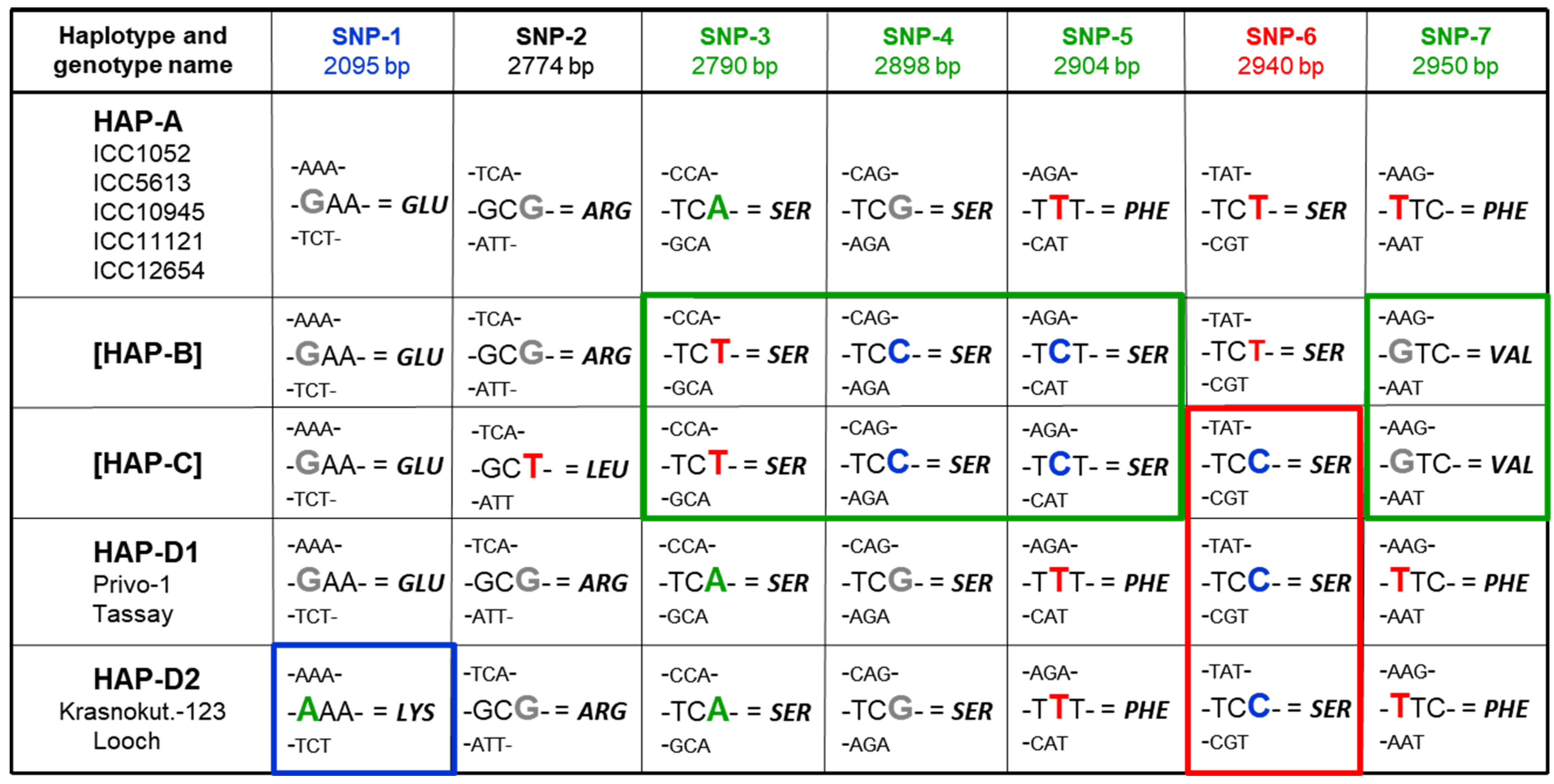
3.4. SNP and Novel Haplotype Identification in the CaABCC6 Gene in Three Groups of Chickpea Genotypes Using Sanger Sequencing
3.5. CaABCC6 Gene Expression in Parents and F6 Breeding Lines
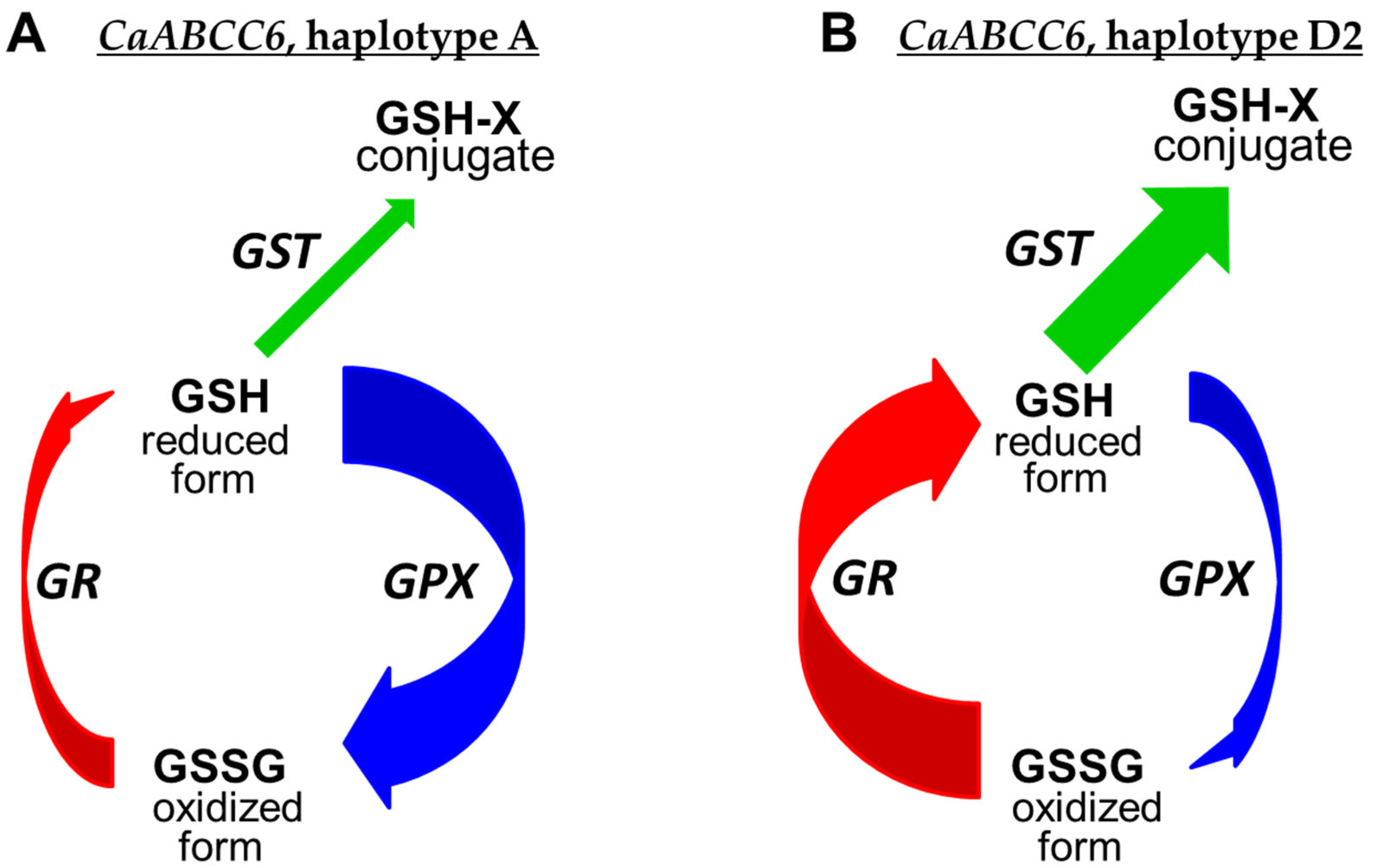
3.6. Mass Spectrometry Measuring of Glutathione Peroxidase (GPX), Glutathione Reductase (GR), and Glutathione S-Transferase (GST) in Mature Seeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat (accessed on 9 April 2024).
- Hossain, M.Z.; Anawar, H.M.; Chaudhary, D.R. Legume plants in the context of global climate change: Challenges and scopes for environmental sustainability. In Climate Change and Legumes. Stress Mitigation for Sustainability and Food Security; Hossain, M.Z., Anawar, H.M., Chaudhary, D.R., Eds.; CRC Press: Boca Raton, FL, USA, 2023; pp. 1–22. [Google Scholar] [CrossRef]
- Oyebamiji, Y.O.; Adigun, B.A.; Shamsudin, N.A.A.; Ikmal, A.M.; Salisu, M.A.; Malike, F.A.; Lateef, A.A. Recent advancements in mitigating abiotic stresses in crops. Horticulturae 2024, 10, 156. [Google Scholar] [CrossRef]
- Munns, R. Physiological processes limiting plant growth in saline soils: Some dogmas and hypotheses. Plant Cell Environ. 1993, 16, 15–24. [Google Scholar] [CrossRef]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; He, X.; Zoghlami, A.; Abdelly, C.; Brestic, M. Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. In Salinity Responses and Tolerance in Plants. Targeting Sensory, Transport and Signaling Mechanisms; Kumar, V., Wani, S.H., Suprasanna, P., Tran, L.S.P., Eds.; Springer: Cham, Switzerland, 2018; Volume 1, pp. 85–136. [Google Scholar] [CrossRef]
- Khan, H.A.; Siddique, K.H.M.; Munir, R.; Colmer, T.D. Salt sensitivity in chickpea: Growth, photosynthesis, seed yield components and tissue ion regulation in contrasting genotypes. J. Plant Physiol. 2015, 182, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Siddique, K.H.M.; Colmer, T. Salt sensitivity in chickpea is determined by sodium toxicity. Planta 2016, 244, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, L.; Vázquez, J.G.; Hernández, L.; Pérez-Bonachea, L.; Campbell, R.; Martínez, J.; Hajari, E.; Zayas, R.G.D.; Acosta, Y.; Lorenzo, J.C. Soluble phenolics, chlorophylls, and malondialdehyde are the best indicators of salt stress in Eichornia crassipes. Vegetos 2023, 36, 1–7. [Google Scholar] [CrossRef]
- Wulandari, Y.R.E.; Sulistyaningsih, Y.C.; Suprayogi, A.; Rahminiwati, M.; Triadiati, T. Morpho-physiology of mulberry (Morus sp.) plant on salinity stress tolerance. HAYATI J. Biosci. 2023, 30, 682–691. [Google Scholar] [CrossRef]
- Paudel, A.; Sun, Y. Effect of salt stress on the growth, physiology, and mineral nutrients of two Penstemon species. HortScience 2024, 59, 209–219. [Google Scholar] [CrossRef]
- Ledesma, F.; Lopez, C.; Ortiz, D.; Chen, P.; Korth, K.L.; Ishibashi, T.; Zeng, A.; Orazaly, M.; Florez-Palacios, L. A simple greenhouse method for screening salt tolerance in soybean. Crop Sci. 2016, 56, 585–594. [Google Scholar] [CrossRef]
- Franzisky, B.L.; Geilfus, C.M.; Kränzlein, M.; Zhang, X.; Zörb, C. Shoot chloride translocation as a determinant for NaCl tolerance in Vicia faba L. J. Plant Physiol. 2019, 236, 23–33. [Google Scholar] [CrossRef]
- Maliro, M.F.A.; McNeil, D.; Redden, B.; Kollmorgen, J.F.; Pittock, C. Sampling strategies and screening of chickpea (Cicer arietinum L.) germplasm for salt tolerance. Genet. Resour. Crop Evol. 2008, 55, 53–63. [Google Scholar] [CrossRef]
- Atieno, J.; Colmer, T.D.; Taylor, J.; Li, Y.; Quealy, J.; Kotula, L.; Nicol, D.; Nguyen, D.T.; Brien, C.; Langridge, P.; et al. Novel salinity tolerance loci in chickpea identified in glasshouse and field environments. Front. Plant Sci. 2021, 12, 667910. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, C.; Soole, K.L. Reactive oxygen species and alternative oxidase in multiple stress tolerance. In Multiple Abiotic Stress Tolerances in Higher Plants. Addressing the Growing Challenges; Gupta, N.K., Shavrukov, Y., Singhai, R.K., Borisjuk, N., Eds.; CRC Press: Boca Raton, FL, USA, 2023; pp. 109–129. [Google Scholar] [CrossRef]
- Rasool, S.; Ahmad, A.; Siddiqi, T.O.; Ahmad, P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 2013, 35, 1039–1050. [Google Scholar] [CrossRef]
- Kaur, G.; Sanwal, S.K.; Sehrawat, N.; Kumar, A.; Kumar, N.; Mann, A. Getting to the roots of Cicer arietinum L. (chickpea) to study the effect of salinity on morpho-physiological, biochemical and molecular traits. Saudi J. Biol. Sci. 2022, 29, 103464. [Google Scholar] [CrossRef] [PubMed]
- Inzé, D.; van Montagu, M. Oxidative stress in plants. Curr. Opin. Biotechnol. 1995, 6, 153–158. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Ishikawa, T.; Li, Z.S.; Lu, Y.P.; Rea, P.A. The GS-X pump in plant, yeast, and animal cells: Structure, function, and gene expression. Biosci. Rep. 1997, 17, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, A.K.; Thakur, A.; Kaur, J.; Zulkifli, M. Glutathione transporters. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3154–3164. [Google Scholar] [CrossRef]
- Dragičević, V.; Kratovalieva, S.; Dumanović, Z.; Dimov, Z.; Kravić, N. Variations in level of oil, protein, and some antioxidants in chickpea and peanut seeds. Chem. Biol. Technol. Agric. 2015, 2, 2. [Google Scholar] [CrossRef]
- Parveen, K.; Saddique, M.A.B.; Ali, Z.; Rehman, S.U.; Nisa, Z.U.; Khan, Z.; Waqas, M.; Munir, M.Z.; Hussain, N.; Muneer, M.A. Genome-wide analysis of glutathione peroxidase (GPX) gene family in chickpea (Cicer arietinum L.) under salinity stress. Gene 2024, 898, 148088. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, R.; Singh, S.; Jagota, N.; Kaur, G.; Manchanda, G.; Bindra, S.; Sharma, A. Morphological and antioxidant responses of Cicer arietinum L. genotypes exposed to combination stress of anthracene and sodium chloride. Chemosphere 2023, 313, 137419. [Google Scholar] [CrossRef] [PubMed]
- Sree, Y.K.; Lakra, N.; Manorama, K.; Ahlawat, Y.; Zaid, A.; Elansary, H.O.; Sayed, S.R.M.; Rashwan, M.A.; Mahmoud, E.A. Drought-induced morpho-physiological, biochemical, metabolite responses and protein profiling of chickpea (Cicer arietinum L.). Agronomy 2023, 13, 1814. [Google Scholar] [CrossRef]
- Nianiou-Obeidat, I.; Madesis, P.; Kissoudis, C.; Voulgari, G.; Chronopoulou, E.; Tsaftaris, A.; Labrou, N.E. Plant glutathione transferase-mediated stress tolerance: Functions and biotechnological applications. Plant Cell Rep. 2017, 36, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges–A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Ann. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, O.; Cacciuttolo, F.; Cabeza, R.A.; Carrasco, B.; Schwember, A.R. A comprehensive review on chickpea (Cicer arietinum L.) breeding for abiotic stress tolerance and climate change resilience. Int. J. Mol. Sci. 2022, 23, 6794. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Li, J.; Yang, Y.; Jiang, C.; Guo, Y. Designing salt stress-resilient crops: Current progress and future challenges. J. Integr. Plant Biol. 2024, 66, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Srungarapu, R.; Mahendrakar, M.D.; Mohammad, L.A.; Chand, U.; Jagarlamudi, V.R.; Kondamudi, K.P.; Kudapa, H.; Samineni, S. Genome-wide association analysis reveals trait-linked markers for grain nutrient and agronomic traits in diverse set of chickpea germplasm. Cells 2022, 11, 2457. [Google Scholar] [CrossRef]
- Susmitha, P.; Kumar, P.; Yadav, P.; Sahoo, S.; Kaur, G.; Pandey, M.K.; Singh, V.; Tseng, T.M.; Gangurde, S.S. Genome-wide association study as a powerful tool for dissecting competitive traits in legumes. Front. Plant Sci. 2023, 14, 1123631. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Jahufer, M.Z.Z.; Brouwer, J.B.; Pang, E.C.K. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica 2005, 142, 169–196. [Google Scholar] [CrossRef]
- Semagn, K.; Bjornstad, Å.; Xu, Y. The genetic dissection of quantitative traits in crops. Electron. J. Biotechnol. 2010, 13, 16–17. [Google Scholar] [CrossRef][Green Version]
- Kumar, R.; Saini, M.; Taku, M.; Debbarma, P.; Mahto, R.K.; Ramlal, A.; Sharma, D.; Rajendran, A.; Pandey, R.; Gaikwad, K.; et al. Identification of quantitative trait loci (QTLs) and candidate genes for seed shape and 100-seed weight in soybean [Glycine max (L.) Merr.]. Front. Plant Sci. 2023, 13, 1074245. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, P.J.; Kumar, A.; Penmetsa, R.V.; Farmer, A.; Schlueter, J.A.; Chamarthi, S.K.; Whaley, A.M.; Carrasquilla-Garcia, N.; Gaur, P.M.; Upadhyaya, H.D.; et al. Large-scale development of cost-effective SNP marker assays for diversity assessment and genetic mapping in chickpea and comparative mapping in legumes. Plant Biotechnol. J. 2012, 10, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Gould, B.A.; Stinchcombe, J.R. Identifying the genes underlying quantitative traits: A rationale for the QTN programme. AoB Plants 2014, 6, plu004. [Google Scholar] [CrossRef] [PubMed]
- Jha, U.C.; Jha, R.; Bohra, A.; Manjunatha, L.; Saabale, P.R.; Parida, S.K.; Chaturvedi, S.K.; Thakro, V.; Singh, N.P. Association mapping of genomic loci linked with Fusarium wilt resistance (Foc2) in chickpea. Plant Genet. Resour. 2021, 19, 195–202. [Google Scholar] [CrossRef]
- Zia, M.A.B.; Yousaf, M.F.; Asim, A.; Naeem, M. An overview of genome-wide association mapping studies in Poaceae species (model crops: Wheat and rice). Mol. Biol. Rep. 2022, 49, 12077–12090. [Google Scholar] [CrossRef] [PubMed]
- Shavrukov, Y.; Suchecki, R.; Eliby, S.; Abugalieva, A.; Kenebayev, S.; Langridge, P. Application of Next-generation sequencing technology to study genetic diversity and identify unique SNP markers in bread wheat from Kazakhstan. BMC Plant Biol. 2014, 14, 258. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Demirel, U.; Yousaf, M.F.; Caliskan, S.; Caliskan, M.E. Overview on domestication, breeding, genetic gain and improvement of tuber quality traits of potato using fast forwarding technique (GWAS): A review. Plant Breed. 2021, 140, 519–542. [Google Scholar] [CrossRef]
- Varshney, R.K.; Kudapa, H.; Roorkiwal, M.; Thudi, M.; Pandey, M.K.; Saxena, R.K.; Chamarthi, S.K.; Murali, M.S.; Mallikarjuna, N.; Upadhyaya, H.; et al. Advances in genetics and molecular breeding of three legume crops of semi-arid tropics using next-generation sequencing and high-throughput genotyping technologies. J. Biosci. 2012, 37, 811–820. [Google Scholar] [CrossRef]
- Kudapa, H.; Azam, S.; Sharpe, A.G.; Taran, B.; Li, R.; Deonovic, B.; Cameron, C.; Farmer, A.D.; Cannon, S.B.; Varshney, R.K. Comprehensive transcriptome assembly of chickpea (Cicer arietinum L.) using Sanger and Next generation sequencing platforms: Development and applications. PLoS ONE 2014, 9, e86039. [Google Scholar] [CrossRef]
- Mazkirat, S.; Baitarakova, K.; Kudaybergenov, M.; Babissekova, D.; Bastaubayeva, S.; Bulatova, K.; Shavrukov, Y. SSR genotyping and marker–trait association with yield components in a Kazakh germplasm collection of chickpea (Cicer arietinum L.). Biomolecules 2023, 13, 1722. [Google Scholar] [CrossRef] [PubMed]
- Kujur, A.; Bajaj, D.; Upadhyaya, H.D.; Das, S.; Ranjan, R.; Shree, T.; Saxena, M.S.; Badoni, S.; Kumar, V.; Tripathi, S.; et al. A genome-wide SNP scan accelerates trait-regulatory genomic loci identification in chickpea. Sci. Rep. 2015, 5, 11166. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Altaf, M.T.; Nadeem, M.A.; Karaköy, T.; Shah, A.N.; Azeem, H.; Baloch, F.S.; Baran, N.; Hussain, T.; Duangpan, S.; et al. Recent advancement in OMICS approaches to enhance abiotic stress tolerance in legumes. Front. Plant Sci. 2022, 13, 952759. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Lamsaadi, N.; Cabassa, C.; Farissi, M.; Savouré, A. Molecular approaches to improve legume salt stress tolerance. Plant Mol. Biol. Rep. 2024, 42, 1–14. [Google Scholar] [CrossRef]
- Rahman, M.A.; Woo, J.H.; Song, Y.; Lee, S.H.; Hasan, M.M.; Azad, M.A.K.; Lee, K.W. Heat shock proteins and antioxidant genes involved in heat combined with drought stress responses in perennial rye grass. Life 2022, 12, 1426. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, D.; Xue, G.; Zheng, X.; Lu, Y.; Shi, J.; Hao, Z.; Chen, J. Characterization of the Liriodendron chinense pentatricopeptide repeat (PPR) gene family and its role in osmotic stress response. Genes 2023, 14, 1125. [Google Scholar] [CrossRef] [PubMed]
- Kyu, K.L.; Taylor, C.M.; Douglas, C.A.; Malik, A.I.; Colmer, T.D.; Siddique, K.H.M.; Erskine, W. Genetic diversity and candidate genes for transient waterlogging tolerance in mungbean at the germination and seedling stages. Front. Plant Sci. 2024, 15, 1297096. [Google Scholar] [CrossRef] [PubMed]
- Pandurangaiah, S.; Ravishankar, K.V.; Shivashankar, K.S.; Sadashiva, A.T.; Pillakenchappa, K.; Narayanan, S.K. Differential expression of carotenoid biosynthetic pathway genes in two contrasting tomato genotypes for lycopene content. J. Biosci. 2016, 41, 257–264. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, G.; Xia, Y.; Liu, X.; Tang, J.; Lu, Y.; Lan, H.; Zhang, S.; Li, C.; Cao, M. Comparative transcriptome analysis reveals that tricarboxylic acid cycle-related genes are associated with maize CMS-C fertility restoration. BMC Plant Biol. 2018, 18, 190. [Google Scholar] [CrossRef]
- Quintero-Soto, M.F.; Chávez-Ontiveros, J.; Garzón-Tiznado, J.A.; Salazar-Salas, N.Y.; Pineda-Hidalgo, K.V.; Delgado-Vargas, F.; López-Valenzuela, J.A. Characterization of peptides with antioxidant activity and antidiabetic potential obtained from chickpea (Cicer arietinum L.) protein hydrolyzates. J. Food Sci. 2021, 86, 2962–2977. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; De Santis, M.A.; Lanzoni, A.; Pittalà, M.G.G.; Saletti, R.; Flagella, Z.; Cunsolo, V. Mass spectrometry characterization of the SDS-PAGE protein profile of legumins and vicilins from chickpea seed. Foods 2024, 13, 887. [Google Scholar] [CrossRef] [PubMed]
- Theodoulou, F.L. Plant ABC transporters. Biochim. Biophys. Acta–Biomembr. 2000, 1465, 79–103. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, T.; Burla, B.; Lee, Y.; Martinoia, E.; Nagy, R. Functions of ABC transporters in plants. Essays Biochem. 2011, 50, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.T.; Martinoia, E.; Lee, Y.; Hwang, J.U. 2021 update on ATP-binding cassette (ABC) transporters: How they meet the needs of plants. Plant Physiol. 2021, 187, 1876–1892. [Google Scholar] [CrossRef] [PubMed]
- Banasiak, J.; Jasiński, M. ATP-binding cassette transporters in nonmodel plants. New Phytol. 2022, 233, 1597–1612. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Burla, B.; Martinoia, E. The multidrug resistance-associated protein (MRP/ABCC) subfamily of ATP-binding cassette transporters in plants. FEBS Lett. 2006, 580, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Wanke, D.; Kolukisaoglu, H.Ü. An update on the ABCC transporter family in plants: Many genes, many proteins, but how many functions? Plant Biol. 2010, 12, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, S.; Jacquet, H.; Vavasseur, A.; Leonhardt, N.; Forestier, C. AtMRP6/AtABCC6, an ATP-binding cassette transporter gene expressed during early steps of seedling development and up-regulated by cadmium in Arabidopsis thaliana. BMC Plant Biol. 2008, 28, 22. [Google Scholar] [CrossRef]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef]
- Brunetti, P.; Zanella, L.; de Paolis, A.; di Litta, D.; Cecchetti, V.; Falasca, G.; Barbieri, M.; Altamura, M.M.; Costantino, P.; Cardarelli, M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 3815–3829. [Google Scholar] [CrossRef] [PubMed]
- Naaz, S.; Ahmad, N.; Jameel, M.R.; Al-Huqail, A.A.; Khan, F.; Qureshi, M.I. Impact of some toxic metals on important ABC transporters in soybean (Glycine max L.). ACS Omega 2023, 8, 27597–27611. [Google Scholar] [CrossRef] [PubMed]
- Sari-Gorla, M.; Ferrario, S.; Rossini, L.; Frova, C.; Villa, M. Developmental expression of glutathione-S-transferase in maize and its possible connection with herbicide tolerance. Euphytica 1993, 67, 221–230. [Google Scholar] [CrossRef]
- Goldberg-Cavalleri, A.; Onkokesung, N.; Franco-Ortega, S.; Edwards, R. ABC transporters linked to multiple herbicide resistance in blackgrass (Alopecurus myosuroides). Front. Plant Sci. 2023, 14, 1082761. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.V.; Willis, M.; Shaban, L. Transport of acylated anthocyanins by the Arabidopsis ATP-binding cassette transporters AtABCC1, AtABCC2, and AtABCC14. Physiol. Plant. 2022, 174, e13780. [Google Scholar] [CrossRef]
- Qian, T.; Wang, X.; Liu, J.; Shi, M.; Zhao, J.; Sun, P.; Zheng, G.; Fang, C.; Xie, X. ATP-binding cassette protein ABCC8 promotes anthocyanin accumulation in strawberry fruits. Plant Physiol. Biochem. 2023, 203, 108037. [Google Scholar] [CrossRef] [PubMed]
- Frelet-Barrand, A.; Kolukisaoglu, H.Ü.; Plaza, S.; Rüffer, M.; Azevedo, L.; Hörtensteiner, S.; Marinova, K.; Weder, B.; Schulz, B.; Klein, M. Comparative mutant analysis of Arabidopsis ABCC-type ABC transporters: AtMRP2 contributes to detoxification, vacuolar organic anion transport and chlorophyll degradation. Plant Cell Physiol. 2008, 49, 557–569. [Google Scholar] [CrossRef]
- Raichaudhuri, A.; Peng, M.; Naponelli, V.; Chen, S.; Sánchez-Fernández, R.; Gu, H.; Gregory, J.F., III; Hanson, A.D.; Rea, P.A. Plant vacuolar ATP-binding cassette transporters that translocate folates and antifolates in vitro and contribute to antifolate tolerance in vivo. J. Biol. Chem. 2009, 284, 8449–8460. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Geisler, M.; Suh, S.J.; Kolukisaoglu, H.Ü.; Azevedo, L.; Plaza, S.; Curtis, M.D.; Richter, A.; Weder, B.; Schulz, B.; et al. Disruption of AtMRP4, a guard cell plasma membrane ABCC-type ABC transporter, leads to deregulation of stomatal opening and increased drought susceptibility. Plant J. 2004, 39, 219–236. [Google Scholar] [CrossRef]
- Nagy, R.; Grob, H.; Weder, B.; Green, P.; Klein, M.; Frelet, A.; Schjoerring, J.K.; Brearley, C.A.; Martinoia, E. The Arabidopsis ATP-binding cassette protein AtMRP5/AtABCC5 is a high affinity inositol hexakisphosphate transporter involved in guard cell signaling and phytate storage. J. Biol. Chem. 2009, 284, 33614–33622. [Google Scholar] [CrossRef]
- Gaedeke, N.; Klein, M.; Kolukisaoglu, U.; Forestier, C.; Müller, A.; Ansorge, M.; Becker, D.; Mamnun, Y.; Kuchler, K.; Schulz, B.; et al. The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO J. 2001, 20, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Wang, C.J.; Li, R.F.; Yi, Y.J.; Zeng, L.; Yang, H.; Zhang, C.F.; Song, K.Y.; Guo, S.J. Transcriptome-based identification and expression characterization of RgABCC transporters in Rehmannia glutinosa. PLoS ONE 2021, 16, e0253188. [Google Scholar] [CrossRef] [PubMed]
- Basu, U.; Upadhyaya, H.D.; Srivastava, R.; Daware, A.; Malik, N.; Sharma, A.; Bajaj, D.; Narnoliya, L.; Thakro, V.; Kujur, A.; et al. ABC transporter-mediated transport of glutathione conjugates enhances seed yield and quality in chickpea. Plant Physiol. 2019, 180, 253–275. [Google Scholar] [CrossRef] [PubMed]
- VIR. Available online: https://www.vir.nw.ru (accessed on 9 April 2024).
- KazRIAPG. Available online: https://kazniizr.kz/nut (accessed on 9 April 2024).
- Khassanova, G.; Oshergina, I.; Ten, E.; Jatayev, S.; Zhanbyrshina, N.; Gabdola, A.; Gupta, N.K.; Schramm, C.; Pupulin, A.; Philp-Dutton, L.; et al. Zinc finger knuckle genes are associated with tolerance to drought and dehydration in chickpea (Cicer arietinum L.). Front. Plant Sci. 2024, 15, 1354413. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, C.; Khassanova, G.; Miller, T.K.; Booth, N.J.; Kurishbayev, A.; Jatayev, S.; Gupta, N.K.; Langridge, P.; Jenkins, C.L.D.; Soole, K.L.; et al. Salt-induced expression of intracellular vesicle trafficking genes, CaRab-GTP, and their association with Na+ accumulation in leaves of chickpea (Cicer arietinum L.). BMC Plant Biol. 2020, 20, 183. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Tari, I.; Csiszár, J.; Horváth, E.; Poór, P.; Takács, Z.; Szepesi, À. The alleviation of the adverse effects of salt stress in the tomato plant by salicylic acid shows a time and organ-specific antioxidant response. Acta Biol. Cracov. Ser. Bot. 2015, 57, 21–30. [Google Scholar] [CrossRef]
- Weining, S.; Langridge, P. Identification and mapping of polymorphisms in cereals based on the polymerase chain reaction. Theor. Appl. Genet. 1991, 82, 209–216. [Google Scholar] [CrossRef]
- DArT: Diversity Array Technology. Available online: https://www.diversityarrays.com (accessed on 9 April 2024).
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- LIS: Legume Information System. Available online: https://www.legumeinfo.org/tools (accessed on 9 April 2024).
- Khassanova, G.; Kurishbayev, A.; Jatayev, S.; Zhubatkanov, A.; Zhumalin, A.; Turbekova, A.; Amantaev, B.; Lopato, S.; Schramm, C.; Jenkins, C.; et al. Intracellular vesicle trafficking genes, RabC-GTP, are highly expressed under salinity and rapid dehydration but down-regulated by drought in leaves of chickpea (Cicer arietinum L.). Front. Genet. 2019, 10, 40. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Bovill, J.; Afzal, I.; Hayes, J.E.; Roy, S.J.; Tester, M.; Collins, N.C. HVP10 encoding V-PPase is a prime candidate for the barley HvNax3 sodium exclusion gene: Evidence from fine mapping and expression analysis. Planta 2013, 237, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Sahoo, A.; Tyagi, A.K.; Jain, M. Validation of internal control genes for quantitative gene expression studies in chickpea (Cicer arietinum L.). Biochem. Biophys. Res. Commun. 2010, 396, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.Y.; Sosulski, F.W. Dispersibility and isolation of proteins from legume flours. Can. Inst. Food Sci. Technol. J. 1974, 7, 256–259. [Google Scholar] [CrossRef]
- Chang, Y.W.; Alli, I.; Molina, A.; Konishi, Y.; Boye, J.I. Isolation and characterization of chickpea (Cicer arietinum L.) seed protein fractions. Food Bioprocess. Technol. 2012, 5, 618–625. [Google Scholar] [CrossRef]
- Hoepner, C.M.; Stewart, Z.K.; Qiao, R.; Fobert, E.K.; Prentis, P.J.; Colella, A.; Chataway, T.; da Silva, K.B.; Abbott, C.A. Proteotransciptomics of the most popular host sea anemone Entacmaea quadricolor reveals not all toxin genes expressed by tentacles are recruited into its venom arsenal. Toxins 2024, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Harcourt-Smith, E.A.; Krstic, E.T.; Soekov-Pearce, B.J.; Colella, A.D.; Chegeni, N.; Chataway, T.K.; Woods, C.M.; Aliakbari, K.; Carney, A.S. The nasal innate immune proteome after saline irrigation: A pilot study in healthy individuals. Am. J. Rhinol. Allergy 2023, 37, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Biognosys. Available online: https://biognosys.com/software/spectronaut (accessed on 9 April 2024).
- Ashraf, M.; Munns, R. Evolution of approaches to increase the salt tolerance of crops. Crit. Rev. Plant Sci. 2022, 41, 128–160. [Google Scholar] [CrossRef]
- Glombitza, S.; Dubuis, P.H.; Thulke, O.; Welzl, G.; Bovet, L.; Götz, M.; Affenzeller, M.; Geist, B.; Hehn, A.; Asnaghi, C.; et al. Crosstalk and differential response to abiotic and biotic stressors reflected at the transcriptional level of effector genes from secondary metabolism. Plant Mol. Biol. 2004, 54, 817–835. [Google Scholar] [CrossRef][Green Version]
- Hou, R.; Yang, L.; Wuyun, T.; Chen, S.; Zhang, L. Genes related to osmoregulation and antioxidation play important roles in the response of Trollius chinensis seedlings to saline-alkali stress. Front. Plant Sci. 2023, 14, 1080504. [Google Scholar] [CrossRef]
- Qian, G.; Wang, M.; Wang, X.; Liu, K.; Li, Y.; Bu, Y.; Li, L. Integrated transcriptome and metabolome analysis of rice leaves response to high saline-alkali stress. Int. J. Mol. Sci. 2023, 24, 4062. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kumar, A.; Kaur, K.; Kaur, S.; Gupta, A.K.; Singh, I. Abiotic stress tolerance of chickpea genotypes depends upon antioxidative potential and nutritional quality of seeds. Proc. Natl. Acad. Sci. India Sec. B Biol. Sci. 2015, 85, 615–623. [Google Scholar] [CrossRef]
- Jin, J.; Wang, J.; Li, K.; Wang, S.; Qin, J.; Zhang, G.; Na, X.; Wang, X.; Bi, Y. Integrated physiological, transcriptomic, and metabolomic analyses revealed molecular mechanism for salt resistance in soybean roots. Int. J. Mol. Sci. 2021, 22, 12848. [Google Scholar] [CrossRef] [PubMed]
- Bach-Pages, M.; Homma, F.; Kourelis, J.; Kaschani, F.; Mohammed, S.; Kaiser, M.; van der Hoorn, R.A.L.; Castello, A.; Preston, G.M. Discovering the RNA-binding proteome of plant leaves with an improved RNA interactome capture method. Biomolecules 2020, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Taji, T.; Seki, M.; Satou, M.; Sakurai, T.; Kobayashi, M.; Ishiyama, K.; Narusaka, Y.; Narusaka, M.; Zhu, J.K.; Shinozaki, K. Comparative genomics in salt tolerance between Arabidopsis and Arabidopsis-related halophyte salt cress using Arabidopsis microarray. Plant Physiol. 2004, 135, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, T.; Okayasu, T.; Kikuraku, K.; Ogawa, T.; Sawa, Y.; Yamamoto, H.; Ishikawa, T.; Maruta, T. Cooperation of chloroplast ascorbate peroxidases and proton gradient regulation 5 is critical for protecting Arabidopsis plants from photo-oxidative stress. Plant J. 2021, 107, 876–892. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F. Role of vacuolar membrane transport systems in plant salinity tolerance. J. Plant Growth Regul. 2023, 42, 1364–1401. [Google Scholar] [CrossRef]
- Rashmi, D.; Ansari, W.A.; Kadoo, N.Y.; Barvkar, V.T.; Deshmukh, R.; Nadaf, A.B. Role of ions and their transporters in combating salt stress in Pandanus odorifer (Forssk.) Kuntze. Acta Physiol. Plant. 2023, 45, 66. [Google Scholar] [CrossRef]
- Wu, Q.; Bai, X.; Zhao, W.; Xiang, D.; Wan, Y.; Yan, J.; Zou, L.; Zhao, G. De novo assembly and analysis of tartary buckwheat (Fagopyrum tataricum Garetn.) transcriptome discloses key regulators involved in salt-stress response. Genes 2017, 8, 255. [Google Scholar] [CrossRef]
- Tommasini, R.; Vogt, E.; Fromenteau, M.; Hörtensteiner, S.; Matile, P.; Amrhein, N.; Martinoia, E. An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J. 1998, 13, 773–780. [Google Scholar] [CrossRef]
- Zuo, J.; Wu, Z.; Li, Y.; Shen, Z.; Feng, X.; Zhang, M.; Ye, H. Mitochondrial ABC transporter ATM3 is essential for cytosolic iron-sulfur cluster assembly. Plant Physiol. 2017, 173, 2096–2109. [Google Scholar] [CrossRef] [PubMed]
- Behrens, C.E.; Smith, K.E.; Iancu, C.V.; Choe, J.Y.; Dean, J.V. Transport of anthocyanins and other flavonoids by the Arabidopsis ATP-binding cassette transporter AtABCC2. Sci. Rep. 2019, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Jha, U.C.; Jha, R.; Thakro, V.; Nayyar, H.; Paul, P.J.; Tripathi, S.; Kumar, Y.; Mondal, B.; Srivastava, A.; Singh, N.P.; et al. Elucidating genetic diversity and association mapping to identify SSR markers linked to 100 seed weight in chickpea (Cicer arietinum L.). Indian J. Genet. Plant Breed. 2022, 82, 193–199. [Google Scholar] [CrossRef]
- Olomitutu, O.E.; Paliwal, R.; Abe, A.; Oluwole, O.O.; Oyatomi, O.A.; Abberton, M.T. Genome-wide association study revealed SNP alleles associated with seed size traits in African yam bean (Sphenostylis stenocarpa (Hochst ex. A. Rich.) Harms). Genes 2022, 13, 2350. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Sharma, A.; Sood, H.; Chauhan, R.S. ABC transporters mined through comparative transcriptomics associate with organ-specific accumulation of picrosides in a medicinal herb, Picrorhiza kurroa. Protoplasma 2023, 260, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Milla, M.A.R.; Maurer, A.; Huete, A.R.; Gustafson, J.P. Glutathione peroxidase genes in Arabidopsis are ubiquitous and regulated by abiotic stresses through diverse signaling pathways. Plant J. 2003, 36, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Eyidogan, F.; Öz, M.T. Effect of salinity on antioxidant responses of chickpea seedlings. Acta Physiol. Plant. 2007, 29, 485–493. [Google Scholar] [CrossRef]
- Cairns, N.G.; Pasternak, M.; Wachter, A.; Cobbett, C.S.; Meyer, A.J. Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiol. 2006, 141, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Stavridou, E.; Voulgari, G.; Michailidis, M.; Kostas, S.; Chronopoulou, E.G.; Labrou, N.E.; Madesis, P.; Nianiou-Obeidat, I. Overexpression of a biotic stress-inducible Pvgstu gene activates early protective responses in tobacco under combined heat and drought. Int. J. Mol. Sci. 2021, 22, 2352. [Google Scholar] [CrossRef]
- Shao, D.; Li, Y.; Zhu, Q.; Zhang, X.; Liu, F.; Xue, F.; Sun, J. GhGSTF12, a glutathione S-transferase gene, is essential for anthocyanin accumulation in cotton (Gossypium hirsutum L.). Plant Sci. 2021, 30, 110827. [Google Scholar] [CrossRef]
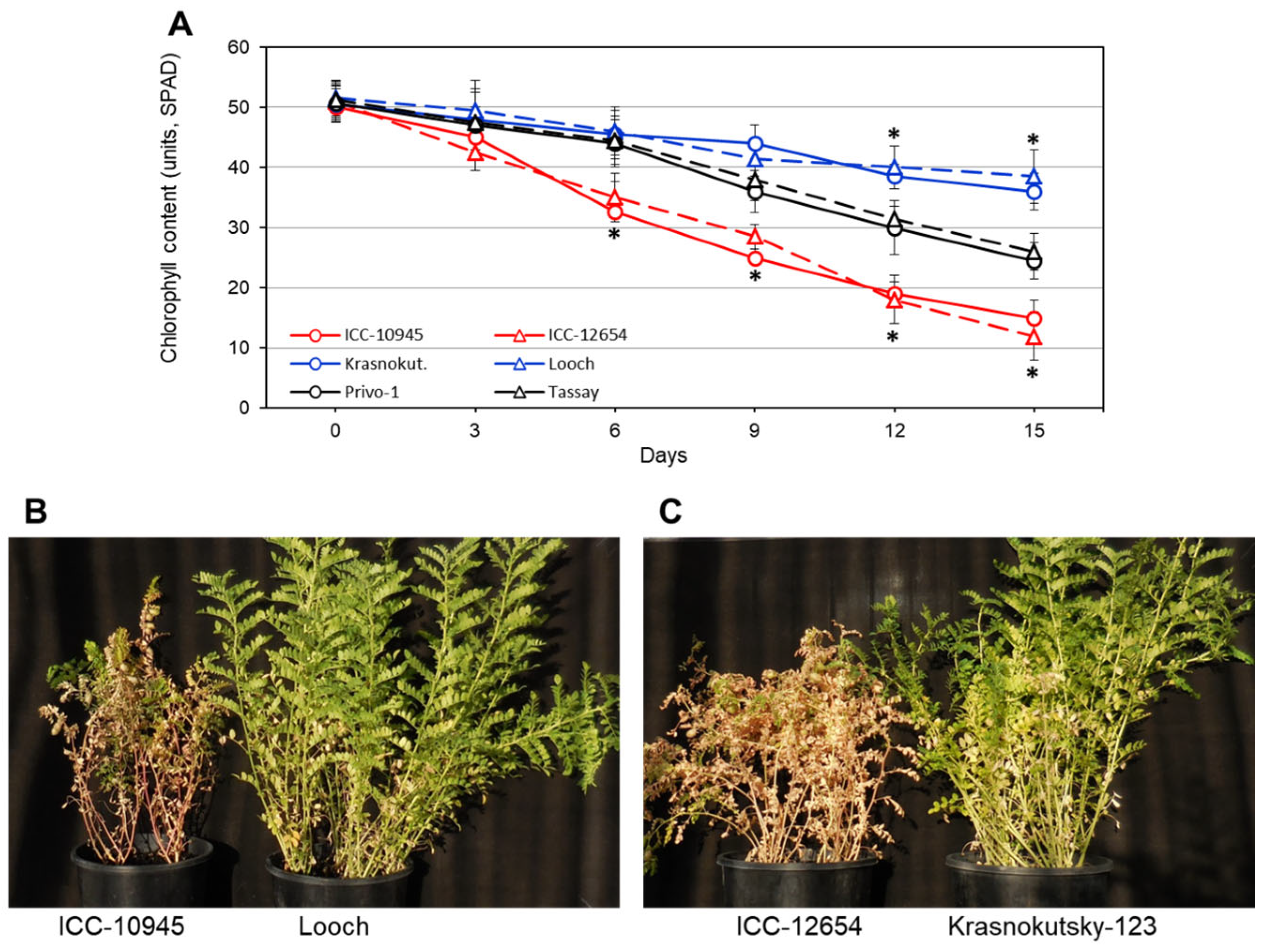
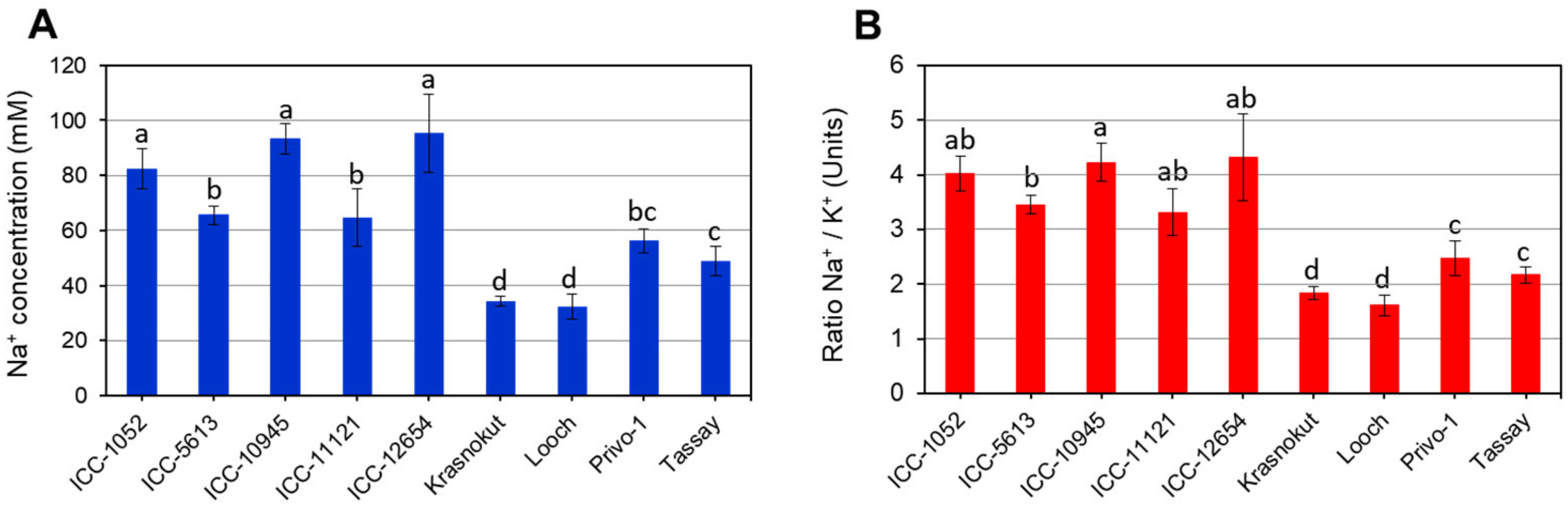

| Accession | Leaf Necrosis, Rate | Chlorophyll Content, Units-SPAD | ||
|---|---|---|---|---|
| Average | SE | Average | SE | |
| ICC-1052 | 7.8 a | 0.3 | 13.5 a | 1.9 |
| ICC-5613 | 7.6 a | 0.4 | 12.8 a | 2.0 |
| ICC-10945 | 8.4 a | 0.5 | 15.2 a | 2.1 |
| ICC-11121 | 7.8 a | 0.3 | 13.6 a | 2.2 |
| ICC-12654 | 8.0 a | 0.4 | 12.3 a | 2.8 |
| Krasnokutsky-123 | 4.6 c | 0.5 | 36.2 c | 2.6 |
| Looch | 5.0 c | 0.4 | 38.5 c | 3.1 |
| Privo-1 | 6.2 b | 0.3 | 24.5 b | 2.2 |
| Tassay | 6.4 b | 0.4 | 26.2 b | 2.3 |
| Accession | MDA, nmol/mL | GSH/GSSG Ratio | ||
|---|---|---|---|---|
| Average | SE | Average | SE | |
| ICC-1052 | 99.2 a | 7.2 | 8.6 a | 0.7 |
| ICC-5613 | 87.9 a | 5.5 | 7.9 a | 1.1 |
| ICC-10945 | 78.3 a | 6.1 | 7.2 a | 1.3 |
| ICC-11121 | 89.2 a | 6.9 | 8.7 a | 0.8 |
| ICC-12654 | 73.6 a | 6.4 | 6.9 a | 1.2 |
| Krasnokutsky-123 | 35.5 c | 2.4 | 16.2 c | 2.1 |
| Looch | 32.3 c | 2.6 | 15.5 c | 1.8 |
| Privo-1 | 56.4 b | 4.2 | 12.1 b | 1.2 |
| Tassay | 54.9 b | 4.1 | 11.4 b | 1.0 |
| Chromosome | DArT Marker and Position on Reference Genome | p-Value for MTA, Four Traits | Closest Candidate Gene and Position on Reference Genome | Annotation of Candidate Gene |
|---|---|---|---|---|
| Ca2 | 10265114 35,466,143–35,466,206 | LN = 0.0076 ** Na+ = 0.025 * MDA = 0.0065 ** GSH = 0.0029 ** | Ca09705 35,445,660–35,452,426 | ATP-binding cassette, ABC transporter. GO:0005524 (ATP binding) |
| Ca4 | 10259848 10,526,726–10,526,760 | LN = 0.0062 ** Na+ = 0.046 * MDA = 0.018 * GSH = 0.0082 ** | Ca04289 10,526,277–10,528,094 | Pentatricopeptide repeat (PPR) superfamily protein. GO:0005515 (protein binding) |
| Ca5 | 23885575 23,699,770–23,699,833 | LN = 0.0065 ** Na+ = 0.028 * MDA = 0.0076 ** GSH = 0.012 * | Ca17680 23,697,998–23,700,690 | Heat shock protein 81.4. GO:0005524 (ATP binding) |
| Ca5 | 10265729 43,890,086–43,890,149 | LN = 0.0025 ** Na+ = 0.042 * MDA = 0.0085 ** GSH = 0.017 * | Ca12664 43,878,837–43,880,175 | Photosystem II 22 kDa protein. IPR022796 (Chlorophyll A-B binding protein) |
| Comparison (Group 1/Group 2) | GPX-3 (Q8L5Q6) | GR-chl/mit (A0A1S3E1Y6) | GST-L3 (A0A1S2XSM5) | |||
|---|---|---|---|---|---|---|
| % Change | Log2 Ratio | % Change | Log2 Ratio | % Change | Log2 Ratio | |
| ICC-1052/Looch | 184.4 ** | 1.5 | −85.2 ** | −2.8 | −42.4 * | −0.8 |
| ICC-5613/Looch | 179.5 ** | 1.5 | −48.2 * | −0.9 | −37.1 * | −0.7 |
| ICC-10945/Looch | 288.2 ** | 2.0 | −64.0 ** | −1.5 | −55.7 * | −1.2 |
| ICC-11121/Looch | 236.9 ** | 1.8 | −91.6 ** | −3.6 | −73.6 ** | −1.9 |
| ICC-12654/Looch | 272.9 ** | 1.9 | −75.7 ** | −2.0 | −38.1 * | −0.7 |
| Privo-1/Looch | 87.8 * | 0.9 | −41.9 * | −0.8 | −50.9 * | −1.0 |
| Tassay/Looch | 108.6 * | 1.1 | −47.4 * | −0.9 | −47.9 * | −1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khassanova, G.; Jatayev, S.; Gabdola, A.; Kuzbakova, M.; Zailasheva, A.; Kylyshbayeva, G.; Schramm, C.; Schleyer, K.; Philp-Dutton, L.; Sweetman, C.; et al. Haplotypes of ATP-Binding Cassette CaABCC6 in Chickpea from Kazakhstan Are Associated with Salinity Tolerance and Leaf Necrosis via Oxidative Stress. Biomolecules 2024, 14, 823. https://doi.org/10.3390/biom14070823
Khassanova G, Jatayev S, Gabdola A, Kuzbakova M, Zailasheva A, Kylyshbayeva G, Schramm C, Schleyer K, Philp-Dutton L, Sweetman C, et al. Haplotypes of ATP-Binding Cassette CaABCC6 in Chickpea from Kazakhstan Are Associated with Salinity Tolerance and Leaf Necrosis via Oxidative Stress. Biomolecules. 2024; 14(7):823. https://doi.org/10.3390/biom14070823
Chicago/Turabian StyleKhassanova, Gulmira, Satyvaldy Jatayev, Ademi Gabdola, Marzhan Kuzbakova, Aray Zailasheva, Gulnar Kylyshbayeva, Carly Schramm, Kathryn Schleyer, Lauren Philp-Dutton, Crystal Sweetman, and et al. 2024. "Haplotypes of ATP-Binding Cassette CaABCC6 in Chickpea from Kazakhstan Are Associated with Salinity Tolerance and Leaf Necrosis via Oxidative Stress" Biomolecules 14, no. 7: 823. https://doi.org/10.3390/biom14070823
APA StyleKhassanova, G., Jatayev, S., Gabdola, A., Kuzbakova, M., Zailasheva, A., Kylyshbayeva, G., Schramm, C., Schleyer, K., Philp-Dutton, L., Sweetman, C., Anderson, P., Jenkins, C. L. D., Soole, K. L., & Shavrukov, Y. (2024). Haplotypes of ATP-Binding Cassette CaABCC6 in Chickpea from Kazakhstan Are Associated with Salinity Tolerance and Leaf Necrosis via Oxidative Stress. Biomolecules, 14(7), 823. https://doi.org/10.3390/biom14070823








