Ion Signaling in Cell Motility and Development in Dictyostelium discoideum
Abstract
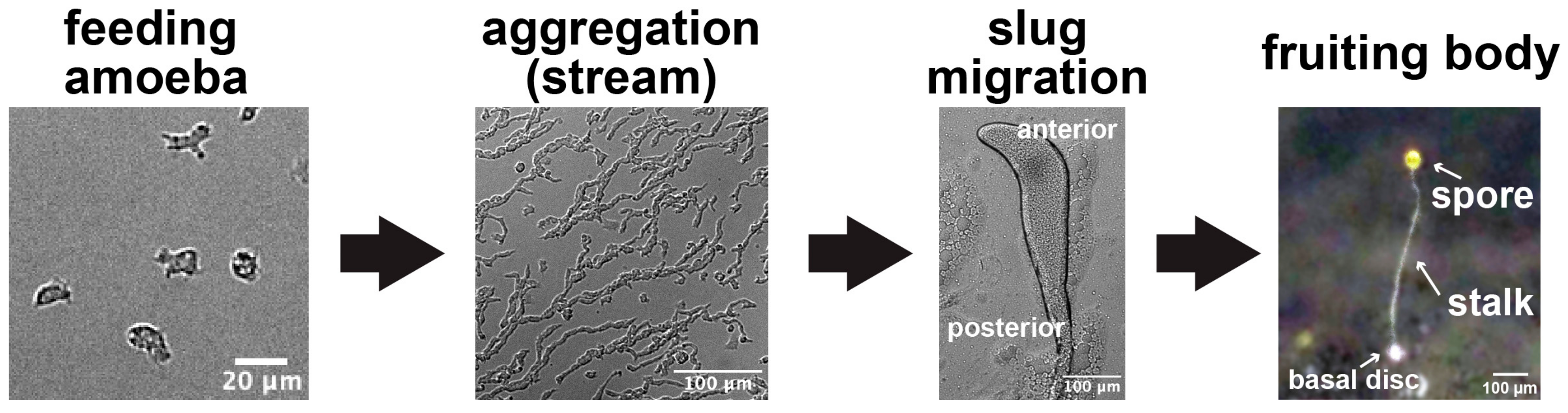
1. Introduction
2. Ion Signals in Dictyostelium
2.1. Calcium Signals
2.1.1. Ca2+ in Chemotaxis and Cell Motility
2.1.2. Ca2+ Signaling during Development
2.1.3. Ca2+ Signaling in Mechanosensation
2.1.4. Determining Ca2+ Signals
2.2. pH Signals
2.2.1. pH in Cell Motility
2.2.2. pH Signaling during Development
2.2.3. Measurement Techniques for Intracellular pH
2.3. K+, Na+, and Fe2+ Signals and Membrane Potential
3. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Basson, M.A. Signaling in cell differentiation and morphogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008151. [Google Scholar] [CrossRef] [PubMed]
- Armingol, E.; Officer, A.; Harismendy, O.; Lewis, N.E. Deciphering cell-cell interactions and communication from gene expression. Nat. Rev. Genet. 2021, 22, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009, 10, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Culhane, K.J.; Liu, Y.; Cai, Y.; Yan, E.C. Transmembrane signal transduction by peptide hormones via family B G protein-coupled receptors. Front. Pharmacol. 2015, 6, 264. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C.; Bootman, M.D.; Scott, J.D. Second Messengers. Cold Spring Harb. Perspect. Biol. 2016, 8, a005926. [Google Scholar] [CrossRef] [PubMed]
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, M.; Fee, D.; Barkhaus, P.E. Generation and propagation of the action potential. Handb. Clin. Neurol. 2019, 160, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.H. Generation of resting membrane potential. Adv. Physiol. Educ. 2004, 28, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Calcium signal transduction and cellular control mechanisms. Biochim. Biophys. Acta 2004, 1742, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M. Calcium at fertilization and in early development. Physiol. Rev. 2006, 86, 25–88. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E.; Runnels, L.W.; Strubing, C. The TRP ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Deitmer, J.W.; Rose, C.R. Ion changes and signalling in perisynaptic glia. Brain Res. Rev. 2010, 63, 113–129. [Google Scholar] [CrossRef]
- Biquet-Bisquert, A.; Labesse, G.; Pedaci, F.; Nord, A.L. The dynamic ion motive force powering the bacterial flagellar motor. Front. Microbiol. 2021, 12, 659464. [Google Scholar] [CrossRef] [PubMed]
- Galera-Laporta, L.; Comerci, C.J.; Garcia-Ojalvo, J.; Suel, G.M. IonoBiology: The functional dynamics of the intracellular metallome, with lessons from bacteria. Cell Syst. 2021, 12, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, E.J.; Rodan, A.R. Intracellular ion control of WNK signaling. Annu. Rev. Physiol. 2023, 85, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.V.; Minamino, T. Measurements of the ion channel activity of the transmembrane stator complex in the bacterial flagellar motor. Methods Mol. Biol. 2023, 2646, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Weijer, C.J. Dictyostelium morphogenesis. Curr. Opin. Genet. Dev. 2004, 14, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Loomis, W.F. Genetic control of morphogenesis in Dictyostelium. Dev. Biol. 2015, 402, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Kin, K.; Schaap, P. Evolution of multicellular complexity in the Dictyostelid social amoebas. Genes 2021, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Hashimura, H.; Morimoto, Y.V.; Yasui, M.; Ueda, M. Collective cell migration of Dictyostelium without cAMP oscillations at multicellular stages. Commun. Biol. 2019, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, A.R.; Parent, C.A. The signal to move: D. discoideum go orienteering. Science 2003, 300, 1525–1527. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, Y.; Ueda, M. Different heterotrimeric G protein dynamics for wide-range chemotaxis in eukaryotic cells. Front. Cell Dev. Biol. 2021, 9, 724797. [Google Scholar] [CrossRef] [PubMed]
- Hashimura, H.; Morimoto, Y.V.; Hirayama, Y.; Ueda, M. Calcium responses to external mechanical stimuli in the multicellular stage of Dictyostelium discoideum. Sci. Rep. 2022, 12, 12428. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.R.; Kay, R.R. The role of DIF-1 signaling in Dictyostelium development. Mol. Cell 2000, 6, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Schaap, P. The prokaryote messenger c-di-GMP triggers stalk cell differentiation in Dictyostelium. Nature 2012, 488, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Ide, H.; Hayashida, Y.; Morimoto, Y.V. Visualization of c-di-GMP in multicellular Dictyostelium stages. Front. Cell Dev. Biol. 2023, 11, 1237778. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Fey, P.; Jimenez-Morales, D.; Dodson, R.J.; Chisholm, R.L. dictyBase 2015: Expanding data and annotations in a new software environment. Genesis 2015, 53, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Kanemaru, K.; Iino, M. Genetically encoded fluorescent indicators for organellar calcium imaging. Biophys. J. 2016, 111, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Europe-Finner, G.N.; Newell, P.C. Calcium transport in the cellular slime mould Dictyostelium discoideum. FEBS Lett. 1985, 186, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Nebl, T.; Fisher, P.R. Intracellular Ca2+ signals in Dictyostelium chemotaxis are mediated exclusively by Ca2+ influx. J. Cell Sci. 1997, 110 Pt 22, 2845–2853. [Google Scholar] [CrossRef] [PubMed]
- Schaloske, R.; Malchow, D. Mechanism of cAMP-induced Ca2+ influx in Dictyostelium: Role of phospholipase A2. Biochem. J. 1997, 327 Pt 1, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Nebl, T.; Kotsifas, M.; Schaap, P.; Fisher, P.R. Multiple signalling pathways connect chemoattractant receptors and calcium channels in Dictyostelium. J. Muscle Res. Cell Motil. 2002, 23, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Schlatterer, C.; Happle, K.; Lusche, D.F.; Sonnemann, J. Cytosolic [Ca2+] transients in dictyostelium discoideum depend on the filling state of internal stores and on an active sarco/endoplasmic reticulum calcium ATPase (SERCA) Ca2+ pump. J. Biol. Chem. 2004, 279, 18407–18414. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.S.; Wang, Y.; Dmitriev, P.; Gross, J.; Galione, A.; Pears, C. A two-pore channel protein required for regulating mTORC1 activity on starvation. BMC Biol. 2020, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Traynor, D.; Piel, M.; Kabla, A.J.; Kay, R.R. Pressure sensing through Piezo channels controls whether cells migrate with blebs or pseudopods. Proc. Natl. Acad. Sci. USA 2020, 117, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Wick, U.; Malchow, D.; Gerisch, G. Cyclic-AMP stimulated calcium influx into aggregating cells of Dictyostelium discoideum. Cell Biol. Int. Rep. 1978, 2, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Maeda, Y.; Iijima, T. Transient increase of the intracellular Ca2+ concentration during chemotactic signal transduction in Dictyostelium discoideum cells. Differentiation 1988, 39, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Yumura, S.; Furuya, K.; Takeuchi, I. Intracellular free calcium responses during chemotaxis of Dictyostelium cells. J. Cell Sci. 1996, 109 Pt 11, 2673–2678. [Google Scholar] [CrossRef] [PubMed]
- Gregor, T.; Fujimoto, K.; Masaki, N.; Sawai, S. The onset of collective behavior in social amoebae. Science 2010, 328, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, K.; Yamada, Y.; Matsuda, T.; Kobayashi, K.; Hashimoto, M.; Matsu-ura, T.; Miyawaki, A.; Michikawa, T.; Mikoshiba, K.; Nagai, T. Spontaneous network activity visualized by ultrasensitive Ca2+ indicators, yellow Cameleon-Nano. Nat. Methods 2010, 7, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.L.; Devreotes, P.N. The surface cyclic AMP receptors, cAR1, cAR2, and cAR3, promote Ca2+ influx in Dictyostelium discoideum by a G alpha 2-independent mechanism. Mol. Biol. Cell 1993, 4, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.L.; Wu, L.; Caterina, M.J.; Devreotes, P.N. Seven helix cAMP receptors stimulate Ca2+ entry in the absence of functional G proteins in Dictyostelium. J. Biol. Chem. 1995, 270, 5926–5931. [Google Scholar] [CrossRef] [PubMed]
- Traynor, D.; Kay, R.R. A polycystin-type transient receptor potential (Trp) channel that is activated by ATP. Biol. Open 2017, 6, 200–209. [Google Scholar] [CrossRef]
- Anjard, C.; Loomis, W.F. GABA induces terminal differentiation of Dictyostelium through a GABAB receptor. Development 2006, 133, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Taylor, G.W.; Yang, J.C.; Neuhaus, D.; Stetsenko, D.; Kato, A.; Kay, R.R. Identification of new differentiation inducing factors from Dictyostelium discoideum. Biochim. Biophys. Acta 2006, 1760, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, M.J.; Traynor, D.; Fisher, P.R.; Ennion, S.J. Purinergic-mediated Ca2+ influx in Dictyostelium discoideum. Cell Calcium 2008, 44, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, M.J.; Durai, L.; Ennion, S.J. Functional characterization of intracellular Dictyostelium discoideum P2X receptors. J. Biol. Chem. 2009, 284, 35227–35239. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, V.; Fountain, S.J. Intracellular P2X receptors as novel calcium release channels and modulators of osmoregulation in Dictyostelium: A comparison of two common laboratory strains. Channels 2013, 7, 43–46. [Google Scholar] [CrossRef]
- Parkinson, K.; Baines, A.E.; Keller, T.; Gruenheit, N.; Bragg, L.; North, R.A.; Thompson, C.R. Calcium-dependent regulation of Rab activation and vesicle fusion by an intracellular P2X ion channel. Nat. Cell Biol. 2014, 16, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Traynor, D.; Milne, J.L.; Insall, R.H.; Kay, R.R. Ca2+ signalling is not required for chemotaxis in Dictyostelium. EMBO J. 2000, 19, 4846–4854. [Google Scholar] [CrossRef] [PubMed]
- Malchow, D.; Schaloske, R.; Schlatterer, C. An increase in cytosolic Ca2+ delays cAMP oscillations in Dictyostelium cells. Biochem. J. 1996, 319 Pt 1, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Malchow, D.; Lusche, D.F.; Schlatterer, C. A link of Ca2+ to cAMP oscillations in Dictyostelium: The calmodulin antagonist W-7 potentiates cAMP relay and transiently inhibits the acidic Ca2+-store. BMC Dev. Biol. 2004, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Schaloske, R.H.; Lusche, D.F.; Bezares-Roder, K.; Happle, K.; Malchow, D.; Schlatterer, C. Ca2+ regulation in the absence of the iplA gene product in Dictyostelium discoideum. BMC Cell Biol. 2005, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Malchow, D.; Lusche, D.F.; De Lozanne, A.; Schlatterer, C. A fast Ca2+-induced Ca2+-release mechanism in Dictyostelium discoideum. Cell Calcium 2008, 43, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Lusche, D.F.; Wessels, D.; Scherer, A.; Daniels, K.; Kuhl, S.; Soll, D.R. The IplA Ca2+ channel of Dictyostelium discoideum is necessary for chemotaxis mediated through Ca2+, but not through cAMP, and has a fundamental role in natural aggregation. J. Cell Sci. 2012, 125, 1770–1783. [Google Scholar] [CrossRef] [PubMed]
- Maruta, H.; Baltes, W.; Dieter, P.; Marme, D.; Gerisch, G. Myosin heavy chain kinase inactivated by Ca2+/calmodulin from aggregating cells of Dictyostelium discoideum. EMBO J. 1983, 2, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Small, N.V.; Europe-Firmer, G.N.; Newell, P.C. Calcium induces cyclic GMP formation in Dictyostelium. FEBS Lett. 1986, 203, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Menz, S.; Bumann, J.; Jaworski, E.; Malchow, D. Mutant analysis suggests that cyclic GMP mediates the cyclic AMP-induced Ca2+ uptake in Dictyostelium. J. Cell Sci. 1991, 99 Pt 1, 187–191. [Google Scholar] [CrossRef]
- Flaadt, H.; Jaworski, E.; Schlatterer, C.; Malchow, D. Cyclic AMP- and Ins(1,4,5)P3-induced Ca2+ fluxes in permeabilised cells of Dictyostelium discoideum: cGMP regulates Ca2+ entry across the plasma membrane. J. Cell Sci. 1993, 105, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Kuwayama, H.; van Haastert, P.J. cGMP potentiates receptor-stimulated Ca2+ influx in Dictyostelium discoideum. Biochim. Biophys. Acta 1998, 1402, 102–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lusche, D.F.; Kaneko, H.; Malchow, D. cGMP-phosphodiesterase antagonists inhibit Ca2+-influx in Dictyostelium discoideum and bovine cyclic-nucleotide-gated-channel. Eur. J. Pharmacol. 2005, 513, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Lusche, D.F.; Malchow, D. Developmental control of cAMP-induced Ca2+-influx by cGMP: Influx is delayed and reduced in a cGMP-phosphodiesterase D deficient mutant of Dictyostelium discoideum. Cell Calcium 2005, 37, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Sonnemann, J.; Knoll, G.; Schlatterer, C. cAMP-induced changes in the cytosolic free Ca2+ concentration in Dictyostelium discoideum are light sensitive. Cell Calcium 1997, 22, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Schaloske, R.; Schlatterer, C.; Malchow, D. A Xestospongin C-sensitive Ca2+ store is required for cAMP-induced Ca2+ influx and cAMP oscillations in Dictyostelium. J. Biol. Chem. 2000, 275, 8404–8408. [Google Scholar] [CrossRef] [PubMed]
- Wilczynska, Z.; Happle, K.; Muller-Taubenberger, A.; Schlatterer, C.; Malchow, D.; Fisher, P.R. Release of Ca2+ from the endoplasmic reticulum contributes to Ca2+ signaling in Dictyostelium discoideum. Eukaryot. Cell 2005, 4, 1513–1525. [Google Scholar] [CrossRef]
- Bohme, R.; Bumann, J.; Aeckerle, S.; Malchow, D. A high-affinity plasma membrane Ca2+-ATPase in Dictyostelium discoideum: Its relation to cAMP-induced Ca2+ fluxes. Biochim. Biophys. Acta 1987, 904, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Malchow, D.; Lusche, D.F.; Schlatterer, C.; De Lozanne, A.; Muller-Taubenberger, A. The contractile vacuole in Ca2+-regulation in Dictyostelium: Its essential function for cAMP-induced Ca2+-influx. BMC Dev. Biol. 2006, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Moniakis, J.; Coukell, M.B.; Janiec, A. Involvement of the Ca2+-ATPase PAT1 and the contractile vacuole in calcium regulation in Dictyostelium discoideum. J. Cell Sci. 1999, 112 Pt 3, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Shanley, L.J.; Walczysko, P.; Bain, M.; MacEwan, D.J.; Zhao, M. Influx of extracellular Ca2+ is necessary for electrotaxis in Dictyostelium. J. Cell Sci. 2006, 119, 4741–4748. [Google Scholar] [CrossRef] [PubMed]
- Lusche, D.F.; Bezares-Roder, K.; Happle, K.; Schlatterer, C. cAMP controls cytosolic Ca2+ levels in Dictyostelium discoideum. BMC Cell Biol. 2005, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Lusche, D.F.; Wessels, D.; Soll, D.R. The effects of extracellular calcium on motility, pseudopod and uropod formation, chemotaxis, and the cortical localization of myosin II in Dictyostelium discoideum. Cell Motil. Cytoskelet. 2009, 66, 567–587. [Google Scholar] [CrossRef] [PubMed]
- Wessels, D.; Lusche, D.F.; Steimle, P.A.; Scherer, A.; Kuhl, S.; Wood, K.; Hanson, B.; Egelhoff, T.T.; Soll, D.R. Myosin heavy chain kinases play essential roles in Ca2+, but not cAMP, chemotaxis and the natural aggregation of Dictyostelium discoideum. J. Cell Sci. 2012, 125, 4934–4944. [Google Scholar] [CrossRef] [PubMed]
- Saito, M. Effect of extracellular Ca2+ on the morphogenesis of Dictyostelium discoideum. Exp. Cell Res. 1979, 123, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Malchow, D.; Bohme, R.; Gras, U. On the role of calcium in chemotaxis and oscillations of dictyostelium cells. Biophys. Struct. Mech. 1982, 9, 131–136. [Google Scholar] [CrossRef]
- Kubohara, Y.; Okamoto, K. Cytoplasmic Ca2+ and H+ concentrations determine cell fate in Dictyostelium discoideum. FASEB J. 1994, 8, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Newell, P.C.; Malchow, D.; Gross, J.D. The role of calcium in aggregation and development of Dictyostelium. Experientia 1995, 51, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.; Manogaran, P.S.; Kennady, P.K.; Pande, G.; Nanjundiah, V. A Ca2+-dependent early functional heterogeneity in amoebae of Dictyostelium discoideum, revealed by flow cytometry. Exp. Cell Res. 1996, 227, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Malchow, D.; Mutzel, R.; Schlatterer, C. On the role of calcium during chemotactic signalling and differentiation of the cellular slime mould Dictyostelium discoideum. Int. J. Dev. Biol. 1996, 40, 135–139. [Google Scholar] [PubMed]
- Cubitt, A.B.; Reddy, I.; Lee, S.; McNally, J.G.; Firtel, R.A. Coexpression of a constitutively active plasma membrane calcium pump with GFP identifies roles for intracellular calcium in controlling cell sorting during morphogenesis in Dictyostelium. Dev. Biol. 1998, 196, 77–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Azhar, M.; Kennady, P.K.; Pande, G.; Espiritu, M.; Holloman, W.; Brazill, D.; Gomer, R.H.; Nanjundiah, V. Cell cycle phase, cellular Ca2+ and development in Dictyostelium discoideum. Int. J. Dev. Biol. 2001, 45, 405–414. [Google Scholar]
- Coukell, B.; Li, Y.; Moniakis, J.; Cameron, A. The Ca2+/calcineurin-regulated cup gene family in Dictyostelium discoideum and its possible involvement in development. Eukaryot. Cell 2004, 3, 61–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Milne, J.L.; Coukell, M.B. A Ca2+ transport system associated with the plasma membrane of Dictyostelium discoideum is activated by different chemoattractant receptors. J. Cell Biol. 1991, 112, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Saran, S.; Nakao, H.; Tasaka, M.; Iida, H.; Tsuji, F.I.; Nanjundiah, V.; Takeuchi, I. Intracellular free calcium level and its response to cAMP stimulation in developing Dictyostelium cells transformed with jellyfish apoaequorin cDNA. FEBS Lett. 1994, 337, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Schlatterer, C.; Walther, P.; Muller, M.; Mendgen, K.; Zierold, K.; Knoll, G. Calcium stores in differentiated Dictyostelium discoideum: Prespore cells sequester calcium more efficiently than prestalk cells. Cell Calcium 2001, 29, 171–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dohrmann, U.; Fisher, P.R.; Bruderlein, M.; Williams, K.L. Transitions in Dictyostelium discoideum behaviour: Influence of calcium and fluoride on slug phototaxis and thermotaxis. J. Cell Sci. 1984, 65, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.; Kennady, P.K.; Pande, G.; Nanjundiah, V. Stimulation by DIF causes an increase of intracellular Ca2+ in Dictyostelium discoideum. Exp. Cell Res. 1997, 230, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Schaap, P.; Nebl, T.; Fisher, P.R. A slow sustained increase in cytosolic Ca2+ levels mediates stalk gene induction by differentiation inducing factor in Dictyostelium. EMBO J. 1996, 15, 5177–5183. [Google Scholar] [CrossRef] [PubMed]
- Kubohara, Y.; Arai, A.; Gokan, N.; Hosaka, K. Pharmacological evidence that stalk cell differentiation involves increases in the intracellular Ca2+ and H+ concentrations in Dictyostelium discoideum. Dev. Growth Differ. 2007, 49, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Poloz, Y.; O’Day, D.H. Ca2+ signaling regulates ecmB expression, cell differentiation and slug regeneration in Dictyostelium. Differentiation 2012, 84, 163–175. [Google Scholar] [CrossRef]
- Horn, F.; Gross, J. A role for calcineurin in Dictyostelium discoideum development. Differentiation 1996, 60, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Nishio, K.; Tomisako, M.; Kuwayama, H.; Tanaka, Y.; Suetake, I.; Tajima, S.; Ogihara, S.; Coukell, B.; Maeda, M. Identification and characterization of novel calcium-binding proteins of Dictyostelium and their spatial expression patterns during development. Dev. Growth Differ. 2003, 45, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Lydan, M.A.; Cotter, D.A. The role of Ca2+ during spore germination in Dictyostelium: Autoactivation is mediated by the mobilization of Ca2+ while amoebal emergence requires entry of external Ca2+. J. Cell Sci. 1995, 108 Pt 5, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Sameshima, M.; Kishi, Y.; Osumi, M.; Minamikawa-Tachino, R.; Mahadeo, D.; Cotter, D.A. The formation of actin rods composed of actin tubules in Dictyostelium discoideum spores. J. Struct. Biol. 2001, 136, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Doring, V.; Veretout, F.; Albrecht, R.; Muhlbauer, B.; Schlatterer, C.; Schleicher, M.; Noegel, A.A. The in vivo role of annexin VII (synexin): Characterization of an annexin VII-deficient Dictyostelium mutant indicates an involvement in Ca2+-regulated processes. J. Cell Sci. 1995, 108 Pt 5, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- Okafuji, T.; Abe, F.; Maeda, Y. Antisense-mediated regulation of Annexin VII gene expression during the transition from growth to differentiation in Dictyostelium discoideum. Gene 1997, 189, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Sesaki, H.; Siu, C.H. Novel redistribution of the Ca2+-dependent cell adhesion molecule DdCAD-1 during development of Dictyostelium discoideum. Dev. Biol. 1996, 177, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Brar, S.K.; Desbarats, L.; Siu, C.H. Synthesis of the Ca2+-dependent cell adhesion molecule DdCAD-1 is regulated by multiple factors during Dictyostelium development. Differentiation 1997, 61, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Sriskanthadevan, S.; Brar, S.K.; Manoharan, K.; Siu, C.H. Ca2+ -calmodulin interacts with DdCAD-1 and promotes DdCAD-1 transport by contractile vacuoles in Dictyostelium cells. FEBS J. 2013, 280, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Kuebler, W.M. Mechanotransduction by TRP channels: General concepts and specific role in the vasculature. Cell Biochem. Biophys. 2010, 56, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Volkers, L.; Mechioukhi, Y.; Coste, B. Piezo channels: From structure to function. Pflug. Arch. 2015, 467, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Bruni, G.N.; Weekley, R.A.; Dodd, B.J.T.; Kralj, J.M. Voltage-gated calcium flux mediates Escherichia coli mechanosensation. Proc. Natl. Acad. Sci. USA 2017, 114, 9445–9450. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Fisher, P.R.; Wilczynska, Z. Contribution of endoplasmic reticulum to Ca2+ signals in Dictyostelium depends on extracellular Ca2+. FEMS Microbiol. Lett. 2006, 257, 268–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lima, W.C.; Vinet, A.; Pieters, J.; Cosson, P. Role of PKD2 in rheotaxis in Dictyostelium. PLoS ONE 2014, 9, e88682. [Google Scholar] [CrossRef]
- Lombardi, M.L.; Knecht, D.A.; Lee, J. Mechano-chemical signaling maintains the rapid movement of Dictyostelium cells. Exp. Cell Res. 2008, 314, 1850–1859. [Google Scholar] [CrossRef] [PubMed]
- Allan, C.Y.; Fisher, P.R. In vivo measurements of cytosolic calcium in Dictyostelium discoideum. Methods Mol. Biol. 2009, 571, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Schlatterer, C.; Knoll, G.; Malchow, D. Intracellular calcium during chemotaxis of Dictyostelium discoideum: A new fura-2 derivative avoids sequestration of the indicator and allows long-term calcium measurements. Eur. J. Cell Biol. 1992, 58, 172–181. [Google Scholar] [PubMed]
- Cubitt, A.B.; Firtel, R.A.; Fischer, G.; Jaffe, L.F.; Miller, A.L. Patterns of free calcium in multicellular stages of Dictyostelium expressing jellyfish apoaequorin. Development 1995, 121, 2291–2301. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.V.; Kami-Ike, N.; Miyata, T.; Kawamoto, A.; Kato, T.; Namba, K.; Minamino, T. High-resolution pH imaging of living bacterial cells to detect local pH differences. mBio 2016, 7, e01911-16. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.J.; Durston, A.J.; Moolenaar, W.H. Cytoplasmic pH and the regulation of the Dictyostelium cell cycle. Cell 1985, 43, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.B.; Klein, G.; Satre, M. 23Na NMR study of intracellular sodium ions in Dictyostelium discoideum amoeba. Arch. Biochem. Biophys. 1987, 254, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, R.; Wampler, J.E.; Fechheimer, M. Measurement of the cytoplasmic pH of Dictyostelium discoideum using a low light level microspectrofluorometer. J. Cell Biol. 1988, 107, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Van Duijn, B.; Inouye, K. Regulation of movement speed by intracellular pH during Dictyostelium discoideum chemotaxis. Proc. Natl. Acad. Sci. USA 1991, 88, 4951–4955. [Google Scholar] [CrossRef]
- Liu, T.; Mirschberger, C.; Chooback, L.; Arana, Q.; Dal Sacco, Z.; MacWilliams, H.; Clarke, M. Altered expression of the 100 kDa subunit of the Dictyostelium vacuolar proton pump impairs enzyme assembly, endocytic function and cytosolic pH regulation. J. Cell Sci. 2002, 115, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.D.; Peacey, M.J.; von Strandmann, R.P. Plasma membrane proton pump inhibition and stalk cell differentiation in Dictyostelium discoideum. Differentiation 1988, 38, 91–98. [Google Scholar] [CrossRef]
- van Duijn, B.; Vogelzang, S.A. The membrane potential of the cellular slime mold Dictyostelium discoideum is mainly generated by an electrogenic proton pump. Biochim. Biophys. Acta 1989, 983, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Padh, H.; Lavasa, M.; Steck, T.L. Characterization of a vacuolar proton ATPase in Dictyostelium discoideum. Biochim. Biophys. Acta 1989, 982, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Heuser, J.; Zhu, Q.; Clarke, M. Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J. Cell Biol. 1993, 121, 1311–1327. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Clarke, M. The vacuolar proton pump of Dictyostelium discoideum: Molecular cloning and analysis of the 100 kDa subunit. J. Cell Sci. 1996, 109 Pt 5, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Rooney, E.K.; Gross, J.D. ATP-driven Ca2+/H+ antiport in acid vesicles from Dictyostelium. Proc. Natl. Acad. Sci. USA 1992, 89, 8025–8029. [Google Scholar] [CrossRef] [PubMed]
- Rooney, E.K.; Gross, J.D.; Satre, M. Characterisation of an intracellular Ca2+ pump in Dictyostelium. Cell Calcium 1994, 16, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, B.T.; Murray, J.; Condeelis, J. pH regulation of the F-actin binding properties of Dictyostelium elongation factor 1 alpha. J. Biol. Chem. 1995, 270, 15222–15230. [Google Scholar] [CrossRef] [PubMed]
- Hanakam, F.; Eckerskorn, C.; Lottspeich, F.; Muller-Taubenberger, A.; Schafer, W.; Gerish, G. The pH-sensitive actin-binding protein hisactophilin of Dictyostelium exists in two isoforms which both are myristoylated and distributed between plasma membrane and cytoplasm. J. Biol. Chem. 1995, 270, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Stoeckelhuber, M.; Noegel, A.A.; Eckerskorn, C.; Kohler, J.; Rieger, D.; Schleicher, M. Structure/function studies on the pH-dependent actin-binding protein hisactophilin in Dictyostelium mutants. J. Cell Sci. 1996, 109 Pt 7, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Patel, H.; Barber, D.L. Expression of actin-interacting protein 1 suppresses impaired chemotaxis of Dictyostelium cells lacking the Na+-H+ exchanger NHE1. Mol. Biol. Cell 2010, 21, 3162–3170. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Barber, D.L. A developmentally regulated Na-H exchanger in Dictyostelium discoideum is necessary for cell polarity during chemotaxis. J. Cell Biol. 2005, 169, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Malchow, D.; Nanjundiah, V.; Wurster, B.; Eckstein, F.; Gerisch, G. Cyclic AMP-induced pH changes in Dictyostelium discoideum and their control by calcium. Biochim. Biophys. Acta 1978, 538, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.J.; Durston, A.J.; Konijn, T.M. Cytoplasmic pH at the onset of development in Dictyostelium. J. Cell Sci. 1987, 87 Pt 3, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Malchow, D.; Nanjundiah, V.; Gerisch, G. PH oscillations in cell suspensions of Dictyostelium discoideum: Their relation to cyclic-amp signals. J. Cell Sci. 1978, 30, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Gottmann, K.; Weijer, C.J. In situ measurements of external pH and optical density oscillations in Dictyostelium discoideum aggregates. J. Cell Biol. 1986, 102, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Coukell, M.B.; Gombos, Z. Antisense RNA inhibition of the putative vacuolar H+-ATPase proteolipid of Dictyostelium reduces intracellular Ca2+ transport and cell viability. J. Cell Sci. 1996, 109 Pt 2, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, G.A., Jr.; Frazier, W.A.; Schlesinger, P.H. Transient increase in intracellular pH during Dictyostelium differentiation. J. Cell Biol. 1984, 99, 1883–1887. [Google Scholar] [CrossRef] [PubMed]
- Inouye, K. Measurements of intracellular pH and its relevance to cell differentiation in Dictyostelium discoideum. J. Cell Sci. 1985, 76, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.J. Changes in cytoplasmic pH are involved in the cell type regulation of Dictyostelium. Cell Differ. 1988, 23, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Van Lookeren Campagne, M.M.; Aerts, R.J.; Spek, W.; Firtel, R.A.; Schaap, P. Cyclic-AMP-induced elevation of intracellular pH precedes, but does not mediate, the induction of prespore differentiation in Dictyostelium discoideum. Development 1989, 105, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Gruenheit, N.; Parkinson, K.; Brimson, C.A.; Kuwana, S.; Johnson, E.J.; Nagayama, K.; Llewellyn, J.; Salvidge, W.M.; Stewart, B.; Keller, T.; et al. Cell cycle heterogeneity can generate robust cell type proportioning. Dev. Cell 2018, 47, 494–508.e494. [Google Scholar] [CrossRef]
- Town, C.D.; Dominov, J.A.; Karpinski, B.A.; Jentoft, J.E. Relationships between extracellular pH, intracellular pH, and gene expression in Dictyostelium discoideum. Dev. Biol. 1987, 122, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Chhabra, P.; Mascarenhas, P.; Nanjundiah, V. A cell type-specific effect of calcium on pattern formation and differentiation in dictyostelium discoideum. Int. J. Dev. Biol. 2000, 44, 491–498. [Google Scholar] [PubMed]
- Gross, J.D.; Bradbury, J.; Kay, R.R.; Peacey, M.J. Intracellular pH and the control of cell differentiation in Dictyostelium discoideum. Nature 1983, 303, 244–245. [Google Scholar] [CrossRef]
- Inouye, K. Differences in cytoplasmic pH and the sensitivity to acid load between prespore cells and prestalk cells of Dictyostelium. J. Cell Sci. 1988, 91, 109–115. [Google Scholar] [CrossRef]
- Inouye, K. Induction by acid load of the maturation of prestalk cells in Dictyostelium discoideum. Development 1988, 104, 669–681. [Google Scholar] [CrossRef]
- Davies, L.; Satre, M.; Martin, J.B.; Gross, J.D. The target of ammonia action in dictyostelium. Cell 1993, 75, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Neave, N.; Sobolewski, A.; Weeks, G. The effect of ammonia on stalk cell formation in submerged monolayers of Dictyostelium discoideum. Cell Differ. 1983, 13, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Dominov, J.A.; Town, C.D. Regulation of stalk and spore antigen expression in monolayer cultures of Dictyostelium discoideum by pH. J. Embryol. Exp. Morphol. 1986, 96, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Sawai, S.; Hirano, T.; Maeda, Y.; Sawada, Y. Rapid patterning and zonal differentiation in a two-dimensional Dictyostelium cell mass: The role of pH and ammonia. J. Exp. Biol. 2002, 205, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Takeuchi, I. Vital staining of autophagic vacuoles in differentiating cells of Dictyostelium discoideum. Differentiation 1983, 24, 83–87. [Google Scholar] [CrossRef]
- Kay, R.R.; Gadian, D.G.; Williams, S.R. Intracellular pH in Dictyostelium: A 31P nuclear magnetic resonance study of its regulation and possible role in controlling cell differentiation. J. Cell Sci. 1986, 83, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.T.; Hay, A.; John, D.G.; Suthers, H.B. pH affects fruiting and slug orientation in Dictyostelium discoideum. J. Embryol. Exp. Morphol. 1985, 87, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.D. Acidic Ca2+ stores, excitability, and cell patterning in Dictyostelium discoideum. Eukaryot. Cell 2009, 8, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Jentoft, J.E.; Town, C.D. Intracellular pH in Dictyostelium discoideum: A 31P nuclear magnetic resonance study. J. Cell Biol. 1985, 101, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Satre, M.; Martin, J.B. 31P-nuclear magnetic resonance analysis of the intracellular pH in the slime mold Dictyostelium discoideum. Biochem. Biophys. Res. Commun. 1985, 132, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Satre, M.; Klein, G.; Martin, J.B. Intracellular pH control in Dictyostelium discoideum: A 31P-NMR analysis. Biochimie 1986, 68, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Fechheimer, M.; Denny, C.; Murphy, R.F.; Taylor, D.L. Measurement of cytoplasmic pH in Dictyostelium discoideum by using a new method for introducing macromolecules into living cells. Eur. J. Cell Biol. 1986, 40, 242–247. [Google Scholar] [PubMed]
- Furukawa, R.; Wampler, J.E.; Fechheimer, M. Cytoplasmic pH of Dictyostelium discoideum amebae during early development: Identification of two cell subpopulations before the aggregation stage. J. Cell Biol. 1990, 110, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Cardone, R.A.; Casavola, V.; Reshkin, S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 2005, 5, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.H.; von Lersner, A.; Guerrero, J.; Krystofiak, E.S.; Inman, D.; Pelletier, R.; Zijlstra, A.; Ponik, S.M.; Weaver, A.M. A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat. Commun. 2020, 11, 2092. [Google Scholar] [CrossRef] [PubMed]
- Lusche, D.F.; Wessels, D.; Ryerson, D.E.; Soll, D.R. Nhe1 is essential for potassium but not calcium facilitation of cell motility and the monovalent cation requirement for chemotactic orientation in Dictyostelium discoideum. Eukaryot. Cell 2011, 10, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Aeckerle, S.; Malchow, D. Calcium regulates cAMP-induced potassium ion efflux in Dictyostelium discoideum. Biochim. Biophys. Acta 1989, 1012, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Aeckerle, S.; Wurster, B.; Malchow, D. Oscillations and cyclic AMP-induced changes of the K+ concentration in Dictyostelium discoideum. EMBO J. 1985, 4, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Van Duijn, B.; Van der Molen, L.G.; Ypey, D.L. Effects of potassium channel blockers on differentiation of Dictyostelium discoideum. Pflugers Arch. 1989, 414 (Suppl. S1), S148–S149. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.K.; Pardee, J.D. Assembly mechanism of Dictyostelium myosin II: Regulation by K+, Mg2+, and actin filaments. Biochemistry 1996, 35, 15504–15514. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Ide, T.; Inouye, K.; Mizuno, K.; Taguchi, T.; Kasai, M. A voltage- and K+-dependent K+ channel from a membrane fraction enriched in contractile vacuole of Dictyostelium discoideum. Biochim. Biophys. Acta 1997, 1325, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Peracino, B.; Buracco, S.; Bozzaro, S. The Nramp (Slc11) proteins regulate development, resistance to pathogenic bacteria and iron homeostasis in Dictyostelium discoideum. J. Cell Sci. 2013, 126, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Van Duijn, B.; Wang, M. Chemoattractant-induced membrane hyperpolarization in Dictyostelium discoideum. A possible role for cyclic GMP. FEBS Lett. 1990, 275, 201–204. [Google Scholar] [CrossRef]
- Gao, R.C.; Zhang, X.D.; Sun, Y.H.; Kamimura, Y.; Mogilner, A.; Devreotes, P.N.; Zhao, M. Different roles of membrane potentials in electrotaxis and chemotaxis of Dictyostelium cells. Eukaryot. Cell 2011, 10, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Gu, Y.; Jiang, W.; Li, Y.; Ayre, W.N.; Liu, Z.; Yin, T.; Janetopoulos, C.; Iijima, M.; Devreotes, P.; et al. Electric signals counterbalanced posterior vs anterior PTEN signaling in directed migration of Dictyostelium. Cell Biosci. 2021, 11, 111. [Google Scholar] [CrossRef]
- Banerjee, T.; Biswas, D.; Pal, D.S.; Miao, Y.; Iglesias, P.A.; Devreotes, P.N. Spatiotemporal dynamics of membrane surface charge regulates cell polarity and migration. Nat. Cell Biol. 2022, 24, 1499–1515. [Google Scholar] [CrossRef]
- Li, S.A.; Meng, X.Y.; Zhang, Y.J.; Chen, C.L.; Jiao, Y.X.; Zhu, Y.Q.; Liu, P.P.; Sun, W. Progress in pH-Sensitive sensors: Essential tools for organelle pH detection, spotlighting mitochondrion and diverse applications. Front. Pharmacol. 2023, 14, 1339518. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.S.; Itoh, G.; Talukder, M.S.U.; Fujimoto, K.; Morimoto, Y.V.; Tanaka, M.; Ueda, M.; Yumura, S. A study of wound repair in Dictyostelium cells by using novel laserporation. Sci. Rep. 2018, 8, 7969. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimoto, Y.V. Ion Signaling in Cell Motility and Development in Dictyostelium discoideum. Biomolecules 2024, 14, 830. https://doi.org/10.3390/biom14070830
Morimoto YV. Ion Signaling in Cell Motility and Development in Dictyostelium discoideum. Biomolecules. 2024; 14(7):830. https://doi.org/10.3390/biom14070830
Chicago/Turabian StyleMorimoto, Yusuke V. 2024. "Ion Signaling in Cell Motility and Development in Dictyostelium discoideum" Biomolecules 14, no. 7: 830. https://doi.org/10.3390/biom14070830
APA StyleMorimoto, Y. V. (2024). Ion Signaling in Cell Motility and Development in Dictyostelium discoideum. Biomolecules, 14(7), 830. https://doi.org/10.3390/biom14070830





