Molecular Insight into Acute Limb Ischemia
Abstract
1. Introduction
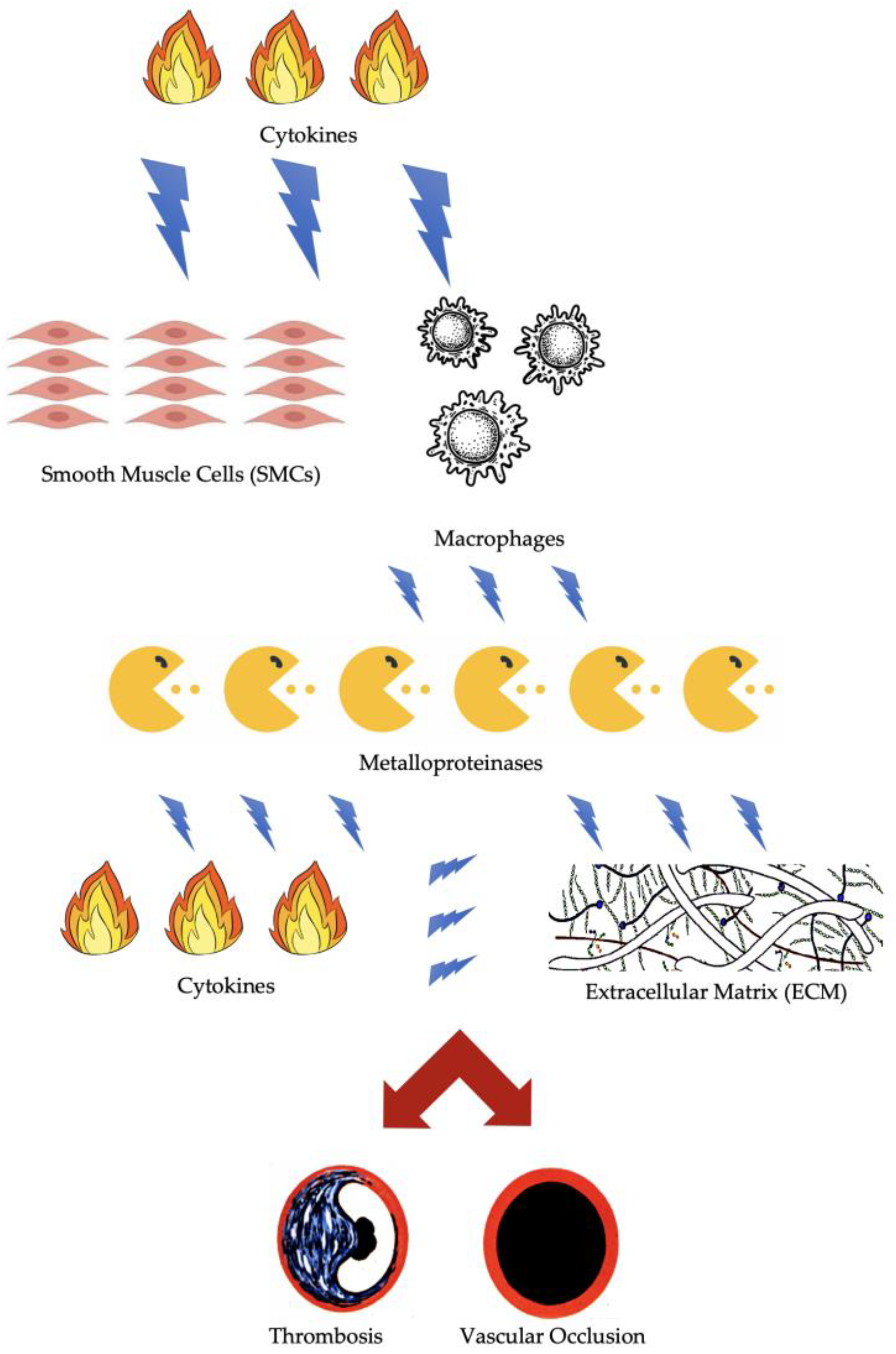
2. Molecular and Cellular Factors of Arterial Thrombosis
3. Molecular and Cellular Factors of Arterial Embolism
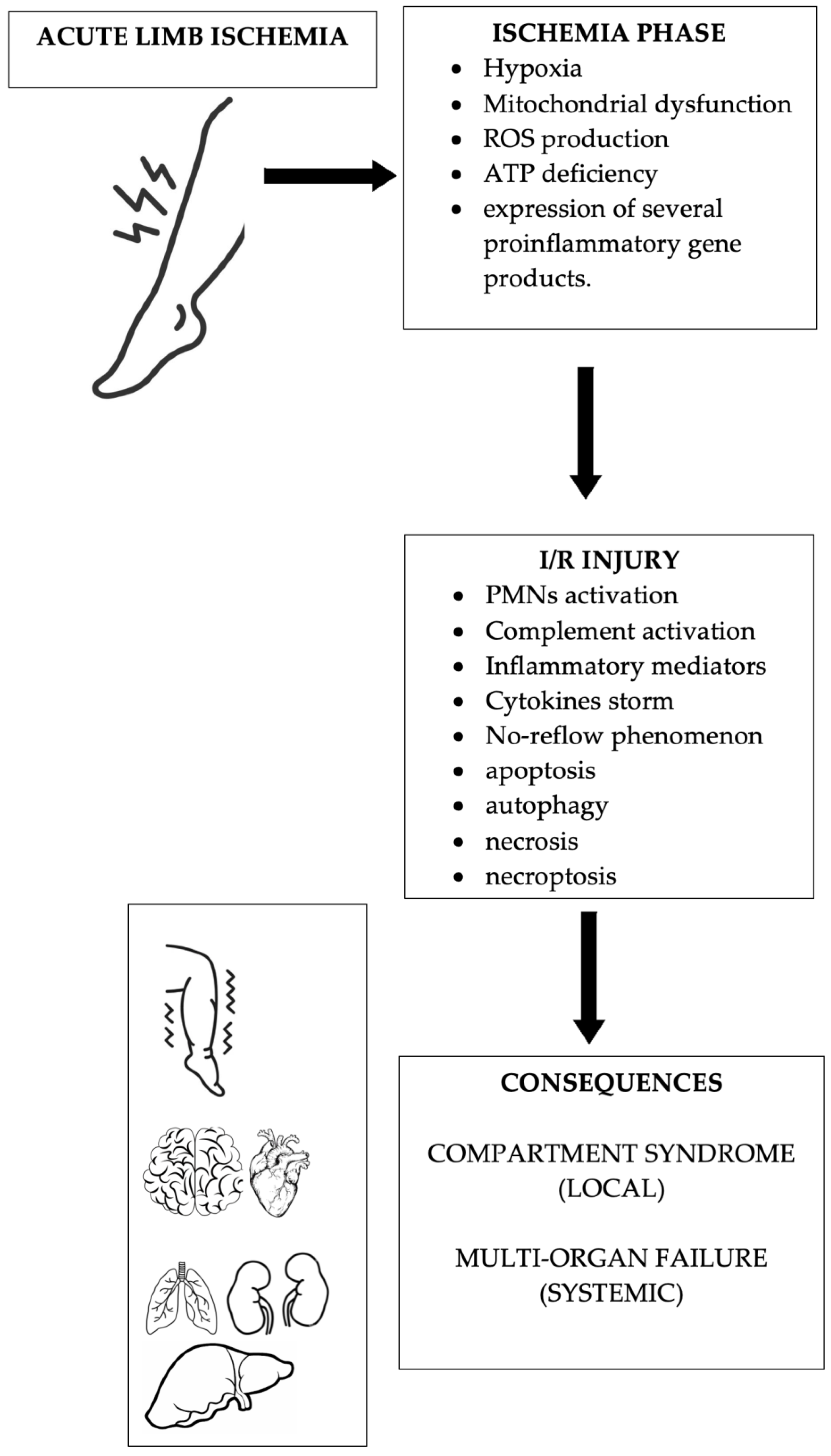
4. Molecular and Cellular Factors of Acute Limb Ischemia
5. Molecular and Cellular Factors of Ischemia/Reperfusion Syndrome
6. The Role of Biomarkers in Clinical Practice
7. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Creager, M.A.; Kaufman, J.A.; Conte, M.S. Clinical practice. Acute limb ischemia. N. Engl. J. Med. 2012, 366, 2198–2206. [Google Scholar] [CrossRef]
- Arnold, J.; Koyfman, A.; Long, B. High risk and low prevalence diseases: Acute limb ischemia. Am. J. Emerg. Med. 2023, 74, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Oberhuber, A.; Floros, N.; Busch, A.; Wagenhäuser, M.U.; Schelzig, H.; Duran, M. Acute Limb Ischemia-Much More Than Just a Lack of Oxygen. Int. J. Mol. Sci. 2018, 19, 374. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G. TASC II Working Group Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S), S5–S67. [Google Scholar] [CrossRef] [PubMed]
- Baril, D.T.; Ghosh, K.; Rosen, A.B. Trends in the incidence, treatment, and outcomes of acute lower extremity ischemia in the United States Medicare population. J. Vasc. Surg. 2014, 60, 669–677.e2. [Google Scholar] [CrossRef] [PubMed]
- Apichartpiyakul, P.; Shinlapawittayatorn, K.; Rerkasem, K.; Chattipakorn, S.C.; Chattipakorn, N. Mechanisms and Interventions on Acute Lower Limb Ischemia/Reperfusion Injury: A Review and Insights from Cell to Clinical Investigations. Ann. Vasc. Surg. 2022, 86, 452–481. [Google Scholar] [CrossRef] [PubMed]
- de Franciscis, S.; De Caridi, G.; Massara, M.; Spinelli, F.; Gallelli, L.; Buffone, G.; Caliò, F.G.; Butrico, L.; Grande, R.; Serra, R. Biomarkers in post-reperfusion syndrome after acute lower limb ischaemia. Int. Wound J. 2016, 13, 854–859. [Google Scholar] [CrossRef]
- Lorentzen, L.G.; Yeung, K.; Eldrup, N.; Eiberg, J.P.; Sillesen, H.H.; Davies, M.J. Proteomic analysis of the extracellular matrix of human atherosclerotic plaques shows marked changes between plaque types. Matrix Biol. Plus 2024, 21, 100141. [Google Scholar] [CrossRef] [PubMed]
- Koga, J.; Aikawa, M. Crosstalk between macrophages and smooth muscle cells in atherosclerotic vascular diseases. Vasc. Pharmacol. 2012, 57, 24–28. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Moreno, P.R.; Purushothaman, M.; Purushothaman, K.R. Plaque neovascularization: Defense mechanisms, betrayal, or a war in progress. Ann. N. Y. Acad. Sci. 2012, 1254, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.P.; Farb, A.; Malcom, G.T.; Liang, Y.; Smialek, J.E.; Virmani, R. Plaque rupture and sudden death related to exertion in men with coronary artery disease. JAMA 1999, 281, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Tedgui, A.; Mallat, Z. Apoptosis as a determinant of atherothrombosis. Thromb. Haemost. 2001, 86, 420–426. [Google Scholar] [PubMed]
- Lyaker, M.R.; Tulman, D.B.; Dimitrova, G.T.; Pin, R.H.; Papadimos, T.J. Arterial embolism. Int. J. Crit. Illn. Inj. Sci. 2013, 3, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Alkarithi, G.; Duval, C.; Shi, Y.; Macrae, F.L.; Ariëns, R.A.S. Thrombus Structural Composition in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2370–2383. [Google Scholar] [CrossRef] [PubMed]
- Lejay, A.; Meyer, A.; Schlagowski, A.I.; Charles, A.L.; Singh, F.; Bouitbir, J.; Pottecher, J.; Chakfé, N.; Zoll, J.; Geny, B. Mitochondria: Mitochondrial participation in ischemia-reperfusion injury in skeletal muscle. Int. J. Biochem. Cell Biol. 2014, 50, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, T.F.; Liauw, S.; Romaschin, A.D.; Walker, P.M. The effect of ischemia/reperfusion on adenine nucleotide metabolism and xanthine oxidase production in skeletal muscle. J. Vasc. Surg. 1990, 12, 8–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Wilson, H.M.; Welikson, R.E.; Luo, J.; Kean, T.J.; Cao, B.; Dennis, J.E.; Allen, M.D. Can Cytoprotective Cobalt Protoporphyrin Protect Skeletal Muscle and Muscle-derived Stem Cells From Ischemic Injury? Clin. Orthop. Relat. Res. 2015, 473, 2908–2919. [Google Scholar] [CrossRef][Green Version]
- Chambers, D.E.; Parks, D.A.; Patterson, G.; Roy, R.; McCord, J.M.; Yoshida, S.; Parmley, L.F.; Downey, J.M. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J. Mol. Cell. Cardiol. 1985, 17, 145–152. [Google Scholar] [CrossRef]
- Gao, X.; Bi, Y.; Chi, K.; Liu, Y.; Yuan, T.; Li, X.; Bi, W. Glycine-nitronyl nitroxide conjugate protects human umbilical vein endothelial cells against hypoxia/reoxygenation injury via multiple mechanisms and ameliorates hind limb ischemia/reperfusion injury in rats. Biochem. Biophys. Res. Commun. 2017, 488, 239–246. [Google Scholar] [CrossRef]
- McCully, J.D.; Wakiyama, H.; Hsieh, Y.J.; Jones, M.; Levitsky, S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am. J. Physiol.-Heart Circ. Physiol. 2004, 286, H1923–H1935. [Google Scholar] [CrossRef]
- Serra, R.; Ciranni, S.; Molinari, V.; Mastroroberto, P.; de Franciscis, S. Fatal early peripheral post-reperfusion syndrome and the role of cutaneous signs. Int. Wound J. 2016, 13, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Nanobashvili, J.; Neumayer, C.; Fuegl, A.; Blumer, R.; Prager, M.; Sporn, E.; Polterauer, P.; Malinski, T.; Huk, I. Development of ‘no-reflow’ phenomenon in ischemia/reperfusion injury: Failure of active vasomotility and not simply passive vasoconstriction. Eur. Surg. Res. 2003, 35, 417–424. [Google Scholar] [CrossRef]
- Chappell, D.; Heindl, B.; Jacob, M.; Annecke, T.; Chen, C.; Rehm, M.; Conzen, P.; Becker, B.F. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology 2011, 115, 483–491. [Google Scholar] [CrossRef]
- Abela, C.B.; Homer-Vanniasinkham, S. Clinical implications of ischaemia-reperfusion injury. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2003, 9, 229–240. [Google Scholar] [CrossRef]
- Szijártó, A.; Turóczi, Z.; Szabó, J.; Kaliszky, P.; Gyurkovics, E.; Arányi, P.; Regáli, L.; Harsányi, L.; Lotz, G. Rapidly progressing fatal reperfusion syndrome caused by acute critical ischemia of the lower limb. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2013, 22, 493–500. [Google Scholar] [CrossRef]
- Cruz, C.P.; Eidt, J.F.; Capps, C.; Kirtley, L.; Moursi, M.M. Major lower extremity amputations at a Veterans Affairs hospital. Am. J. Surg. 2003, 186, 449–454. [Google Scholar] [CrossRef]
- Klausner, J.M.; Paterson, I.S.; Kobzik, L.; Valeri, C.R.; Shepro, D.; Hechtman, H.B. Oxygen free radicals mediate ischemia-induced lung injury. Surgery 1989, 105 Pt 1, 192–199. [Google Scholar] [PubMed]
- Allen, D.M.; Chen, L.E.; Seaber, A.V.; Urbaniak, J.R. Pathophysiology and related studies of the no reflow phenomenon in skeletal muscle. Clin. Orthop. Relat. Res. 1995, 314, 122–133. [Google Scholar] [CrossRef]
- Gute, D.C.; Ishida, T.; Yarimizu, K.; Korthuis, R.J. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Mol. Cell. Biochem. 1998, 179, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [PubMed]
- Broughton, B.R.; Reutens, D.C.; Sobey, C.G. Apoptotic mechanisms after cerebral ischemia. Stroke 2009, 40, e331–e339. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Whelan, R.S.; Kaplinskiy, V.; Kitsis, R.N. Cell death in the pathogenesis of heart disease: Mechanisms and significance. Annu. Rev. Physiol. 2010, 72, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.A.; Mentzer, R.M. Autophagy during cardiac stress: Joys and frustrations of autophagy. Annu. Rev. Physiol. 2010, 72, 45–59. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Kim, Y.S.; Liu, Z.G. TNFalpha and reactive oxygen species in necrotic cell death. Cell Res. 2008, 18, 343–349. [Google Scholar] [CrossRef]
- Watson, J.D.; Gifford, S.M.; Clouse, W.D. Biochemical markers of acute limb ischemia, rhabdomyolysis, and impact on limb salvage. Semin. Vasc. Surg. 2014, 27, 176–181. [Google Scholar] [CrossRef]
- Ielapi, N.; Andreucci, M.; Licastro, N.; Faga, T.; Grande, R.; Buffone, G.; Mellace, S.; Sapienza, P.; Serra, R. Precision Medicine and Precision Nursing: The Era of Biomarkers and Precision Health. Int. J. Gen. Med. 2020, 13, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Ielapi, N.; Licastro, N.; Provenzano, M.; Andreucci, M.; Bracale, U.M.; Jiritano, F.; de Franciscis, S.; Mastroroberto, P.; Serraino, G.F. Neutrophil-to-lymphocyte Ratio and Platelet-to-lymphocyte Ratio as Biomarkers for Cardiovascular Surgery Procedures: A Literature Review. Rev. Recent Clin. Trials 2021, 16, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, H.; Agha, R.; Wong, J.; Tang, T.Y.; Wilson, Y.G.; Walsh, S.R. Neutrophil-lymphocyte ratio predicts medium-term survival following elective major vascular surgery: A cross-sectional study. Vasc. Endovasc. Surg. 2011, 45, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Puckridge, P.; Ullah, S.; Delaney, C.; Spark, J.I. Neutrophil-lymphocyte ratio as a prognostic marker of outcome in infrapopliteal percutaneous interventions for critical limb ischemia. J. Vasc. Surg. 2014, 60, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Taurino, M.; Aloisi, F.; Del Porto, F.; Nespola, M.; Dezi, T.; Pranteda, C.; Rizzo, L.; Sirignano, P. Neutrophil-to-Lymphocyte Ratio Could Predict Outcome in Patients Presenting with Acute Limb Ischemia. J. Clin. Med. 2021, 10, 4343. [Google Scholar] [CrossRef] [PubMed]
- Busceti, M.T.; Grande, R.; Amato, B.; Gasbarro, V.; Buffone, G.; Amato, M.; Gallelli, L.; Serra, R.; de Franciscis, S. Pulmonary embolism, metalloproteinsases and neutrophil gelatinase associated lipocalin. Acta Phlebol. 2013, 14, 115–121. [Google Scholar]
- Costa, D.; Andreucci, M.; Ielapi, N.; Serraino, G.F.; Mastroroberto, P.; Bracale, U.M.; Serra, R. Vascular Biology of Arterial Aneurysms. Ann. Vasc. Surg. 2023, 94, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Scalise, E.; Ielapi, N.; Bracale, U.M.; Andreucci, M.; Serra, R. Metalloproteinases as Biomarkers and Sociomarkers in Human Health and Disease. Biomolecules 2024, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Gremmels, H.; Teraa, M.; de Jager, S.C.A.; Pasterkamp, G.; de Borst, G.J.; Verhaar, M.C. A Pro-Inflammatory Biomarker-Profile Predicts Amputation-Free Survival in Patients with Severe Limb Ischemia. Sci. Rep. 2019, 9, 10740. [Google Scholar] [CrossRef]
- Hasan, S.A.; Haque, A.; Nazir, F. Acute Limb Ischemia: A Rare Complication of COVID-19. Cureus 2020, 12, e11488. [Google Scholar] [CrossRef]
- Grosse, G.M.; Werlein, C.; Blume, N.; Abu-Fares, O.; Götz, F.; Gabriel, M.M.; Ernst, J.; Leotescu, A.; Worthmann, H.; Kühnel, M.P.; et al. Circulating Cytokines and Growth Factors in Acute Cerebral Large Vessel Occlusion-Association with Success of Endovascular Treatment. Thromb. Haemost. 2022, 122, 623–632. [Google Scholar]
- Saenz-Pipaon, G.; Martinez-Aguilar, E.; Orbe, J.; González Miqueo, A.; Fernandez-Alonso, L.; Paramo, J.A.; Roncal, C. The Role of Circulating Biomarkers in Peripheral Arterial Disease. Int. J. Mol. Sci. 2021, 22, 3601. [Google Scholar] [CrossRef] [PubMed]
- Sapalidis, K.; Papavramidis, T.S.; Gialamas, E.; Deligiannidis, N.; Tzioufa, V.; Papavramidis, S. The role of allopurinol’s timing in the ischemia reperfusion injury of small intestine. J. Emergencies Trauma Shock. 2013, 6, 203–208. [Google Scholar]
- Halestrap, A.P. A pore way to die: The role of mitochondria in reperfusion injury and cardioprotection. Biochem. Soc. Trans. 2010, 38, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Coppen, S.R.; Varela-Carver, A.; Yamahara, K.; Sarathchandra, P.; Smolenski, R.T.; Yacoub, M.H.; Suzuki, K. A novel strategy for myocardial protection by combined antibody therapy inhibiting both P-selectin and intercellular adhesion molecule-1 via retrograde intracoronary route. Circulation 2006, 114 (Suppl. 1), I251–I256. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Nojima, T.; Fujisaki, N.; Tsukahara, K.; Yamamoto, H.; Yamada, T.; Aokage, T.; Yumoto, T.; Osako, T.; Nakao, A. Therapeutic strategies for ischemia reperfusion injury in emergency medicine. Acute Med. Surg. 2020, 7, e501. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Kawamura, T.; Toyoda, Y.; Nakao, A. Recent advances in hydrogen research as a therapeutic medical gas. Free. Radic. Res. 2010, 44, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Sugimoto, R.; Billiar, T.R.; McCurry, K.R. Therapeutic antioxidant medical gas. J. Clin. Biochem. Nutr. 2009, 44, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Husted, T.L.; Lentsch, A.B. The role of cytokines in pharmacological modulation of hepatic ischemia/reperfusion injury. Curr. Pharm. Des. 2006, 12, 2867–2873. [Google Scholar] [CrossRef]
- Sukhera, J. Narrative Reviews: Flexible, Rigorous, and Practical. J. Grad. Med. Educ. 2022, 14, 414–417. [Google Scholar] [CrossRef]
| Author | Article Title | Topics |
|---|---|---|
| Simon, F., et al. [3] | Acute Limb Ischemia-Much More Than Just a Lack of Oxygen. | ALI: mechanisms of acute tissue damage combined with end-organ loss of function. |
| Apichartpiyakul, P., et al. [6] | Mechanisms and Interventions on Acute Lower Limb Ischemia/Reperfusion Injury: A Review and Insights from Cell to Clinical Investigations. | Cellular mechanisms of ALI and I/R injury. |
| de Franciscis, S., et al. [7] | Biomarkers in post-reperfusion syndrome after acute lower limb ischaemia. | Biomarkers in I/R injury. |
| Lorentzen, L. G., et al. [8] | Proteomic analysis of the extracellular matrix of human atherosclerotic plaques shows marked changes between plaque types. | Plaque Destabilization: plaque compositions and plaques types in atherosclerosis. |
| Koga, J., et al. [9] | Crosstalk between macrophages and smooth muscle cells in atherosclerotic vascular diseases. | Plaque Destabilization: role of macrophages and smooth muscle cells (SMCs) in atherosclerotic lesions and plaque destabilization. |
| Virmani, R., et al. [10] | Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. | Plaque Destabilization: morphological insight of atherosclerotic plaques. |
| Moreno, P. R., et al. [11] | Plaque neovascularization: defense mechanisms, betrayal, or a war in progress. | Plaque Destabilization: role of plaque neovascularization. |
| Burke, A. P., et al. [12] | Plaque rupture and sudden death related to exertion in men with coronary artery disease. | Plaque Destabilization: mechanims of plaque rupture. |
| Tedgui, A., et al. [13] | Apoptosis as a determinant of atherothrombosis. | Apoptosis: role in atherothrombosis. |
| Lyaker, et al. [14] | Arterial embolism. | Arterial embolism: pathophysiology. |
| Alkarithi, G., et al. [15] | Thrombus Structural Composition in Cardiovascular Disease. | Arterial embolism: relationship with thrombus structure. |
| Lejay, A., et al. [16] | Mitochondria: mitochondrial participation in ischemia-reperfusion injury in skeletal muscle. | Cellular factors and components related to ischemia: the role of mitochondria. |
| Lindsay, T. F., et al. [17] | The effect of ischemia/reperfusion on adenine nucleotide metabolism and xanthine oxidase production in skeletal muscle. | Cellular factors and components related to ischemia: the role of cellular metabolism. |
| Youle, R. J., et al. [18] | Mitochondrial fission, fusion, and stress. | Cellular factors and components related to ischemia: the role of mitochondria. |
| Wilson, H. M., et al. [19] | Can Cytoprotective Cobalt Protoporphyrin Protect Skeletal Muscle and Muscle-derived Stem Cells From Ischemic Injury? | Cellular factors and components related to ischemia: the role of muscle tissues. |
| Chambers, D. E., et al. [20] | Xanthine oxidase as a source of free radical damage in myocardial ischemia. | Cellular factors and components related to ischemia: mechanisms of damage. |
| Gao, X., et al. [21] | Glycine-nitronyl nitroxide conjugate protects human umbilical vein endothelial cells against hypoxia/reoxygenation injury via multiple mechanisms and ameliorates hind limb ischemia/reperfusion injury in rats. | Cellular factors and components related to ischemia: mechanisms of damage. |
| McCully JD, et al. [22] | Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. | Apoptosis: role in I/R injury. |
| Serra, R., et al. [23] | Fatal early peripheral post-reperfusion syndrome and the role of cutaneous signs. | Cutaneous signs in post-reperfusion syndrome. |
| Nanobashvili, J., et al. [24] | Development of ‘no-reflow’ phenomenon in ischemia/reperfusion injury: failure of active vasomotility and not simply passive vasoconstriction. | I/R injury: the role of No-reflow’ phenomenon. |
| Chappell, D., et al. [25] | Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. | I/R injury: the role of endothelial glycocalyx. |
| Abela, C. B., et al. [26] | Clinical implications of ischaemia-reperfusion injury. | I/R injury: pathophysiology. |
| Szijártó, A., et al. [27] | Rapidly progressing fatal reperfusion syndrome caused by acute critical ischemia of the lower limb. | I/R injury: and severity of symptoms. |
| Cruz, C. P., et al. [28] | Major lower extremity amputations at a Veterans Affairs hospital. | I/R injury and severity of symptoms. |
| Klausner, J. M., et al. [29] | Oxygen free radicals mediate ischemia-induced lung injury. | I/R injury and severity of symptoms. |
| Allen, D. M., et al. [30] | Pathophysiology and related studies of the no reflow phenomenon in skeletal muscle. | I/R injury: the role of No-reflow’ phenomenon. |
| Gute, D. C., et al. [31] | Inflammatory responses to ischemia and reperfusion in skeletal muscle. | I/R injury: the role of inflammation. |
| Kalogeris, T., et al. [32] | Cell biology of ischemia/reperfusion injury. | I/R injury: cellular mechanisms. |
| Broughton, B. R., et al. [33] | Apoptotic mechanisms after cerebral ischemia. | Apoptosis: cellular mechanisms. |
| Kroemer, G., et al. [34] | Mitochondrial membrane permeabilization in cell death. | Cellular factors and components related to ischemia: the role of mitochondria. |
| Whelan, R. S., et al. [35] | Cell death in the pathogenesis of heart disease: mechanisms and significance. | Apoptosis: cellular mechanisms. |
| Gottlieb, R. A., et al. [36] | Autophagy during cardiac stress: joys and frustrations of autophagy. | Autophagy and I/R injury. |
| He, C., et al. [37] | Regulation mechanisms and signaling pathways of autophagy. | Autophagy and I/R injury. |
| Levine, B., et al. [38] | Autophagy in the pathogenesis of disease. | Autophagy and I/R injury. |
| Morgan, M. J., et al. [39] | TNFalpha and reactive oxygen species in necrotic cell death. | Autophagy and cytokines |
| Watson, J. D., et al. [40] | Biochemical markers of acute limb ischemia, rhabdomyolysis, and impact on limb salvage. | Biomarkers in I/R injury. |
| Ielapi, N., et al. [41] | Precision Medicine and Precision Nursing: The Era of Biomarkers and Precision Health. | Biomarkers and Precision Medicine. |
| Serra, R., et al. [42] | Neutrophil-to-lymphocyte Ratio and Platelet-to-lymphocyte Ratio as Biomarkers for Cardiovascular Surgery Procedures: A Literature Review. | Biomarkers: role of inflammation biomarkers. |
| Bhutta, H., et al. [43] | Neutrophil-lymphocyte ratio predicts medium-term survival following elective major vascular surgery: a cross-sectional study. | Biomarkers: role of inflammation biomarkers. |
| Chan, C., et al. [44] | Neutrophil-lymphocyte ratio as a prognostic marker of outcome in infrapopliteal percutaneous interventions for critical limb ischemia. | Biomarkers: role of inflammation biomarkers. |
| Taurino, M., et al. [45] | Neutrophil-to-Lymphocyte Ratio Could Predict Outcome in Patients Presenting with Acute Limb Ischemia. | Biomarkers: role of inflammation biomarkers. |
| Busceti, M.T., et al. [46] | Pulmonary embolism, metalloproteinsases and neutrophil gelatinase associated lipocalin. | Biomarkers: role of inflammation biomarkers. |
| Costa, D., et al. [47] | Vascular Biology of Arterial Aneurysms. | Biomarkers: role of inflammation biomarkers. |
| Costa, D., et al. [48] | Metalloproteinases as Biomarkers and Sociomarkers in Human Health and Disease. | Biomarkers: role of inflammation biomarkers. |
| Gremmels, H., et al. [49] | A Pro-Inflammatory Biomarker-Profile Predicts Amputation-Free Survival in Patients with Severe Limb Ischemia. | Biomarkers: role of inflammation biomarkers. |
| Hasan, S. A., et al. [50] | Acute Limb Ischemia: A Rare Complication of COVID-19. | Biomarkers: role of inflammation biomarkers. |
| Grosse, G. M., at al. [51] | Circulating Cytokines and Growth Factors in Acute Cerebral Large Vessel Occlusion-Association with Success of Endovascular Treatment. | Biomarkers: role of inflammation biomarkers. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, D.; Ielapi, N.; Perri, P.; Minici, R.; Faga, T.; Michael, A.; Bracale, U.M.; Andreucci, M.; Serra, R. Molecular Insight into Acute Limb Ischemia. Biomolecules 2024, 14, 838. https://doi.org/10.3390/biom14070838
Costa D, Ielapi N, Perri P, Minici R, Faga T, Michael A, Bracale UM, Andreucci M, Serra R. Molecular Insight into Acute Limb Ischemia. Biomolecules. 2024; 14(7):838. https://doi.org/10.3390/biom14070838
Chicago/Turabian StyleCosta, Davide, Nicola Ielapi, Paolo Perri, Roberto Minici, Teresa Faga, Ashour Michael, Umberto Marcello Bracale, Michele Andreucci, and Raffaele Serra. 2024. "Molecular Insight into Acute Limb Ischemia" Biomolecules 14, no. 7: 838. https://doi.org/10.3390/biom14070838
APA StyleCosta, D., Ielapi, N., Perri, P., Minici, R., Faga, T., Michael, A., Bracale, U. M., Andreucci, M., & Serra, R. (2024). Molecular Insight into Acute Limb Ischemia. Biomolecules, 14(7), 838. https://doi.org/10.3390/biom14070838









