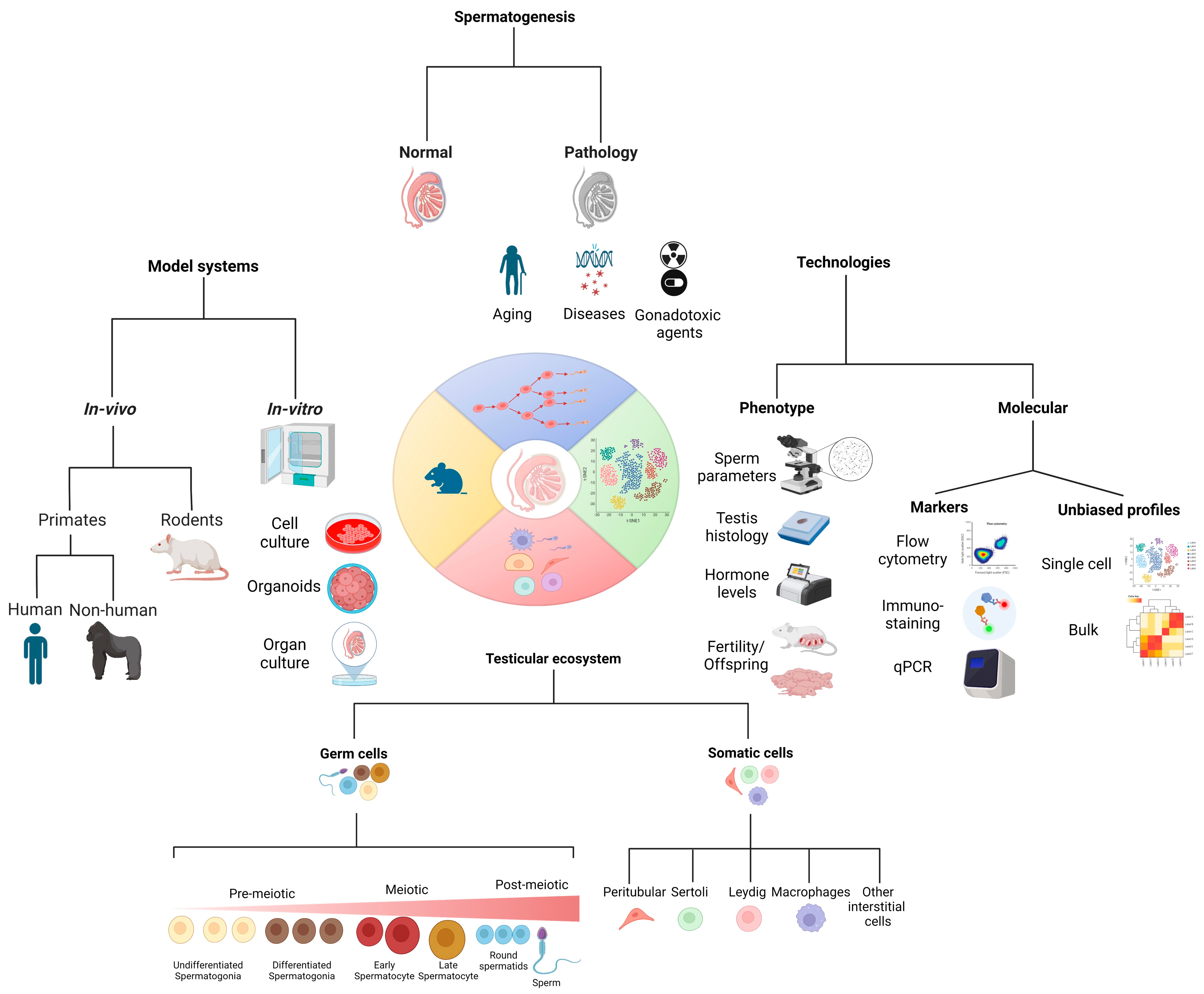
Elucidating the Transcriptional States of Spermatogenesis—Joint Analysis of Germline and Supporting Cell, Mice and Human, Normal and Perturbed, Bulk and Single-Cell RNA-Seq
Abstract
:1. Introduction
1.1. Spermatogenesis
1.2. Gene Expression Profiles in Spermatogenic Cells by scRNA-Seq
2. Normal Physiology
2.1. Stages of Maturation
2.2. Testicular Cells
3. Pathology
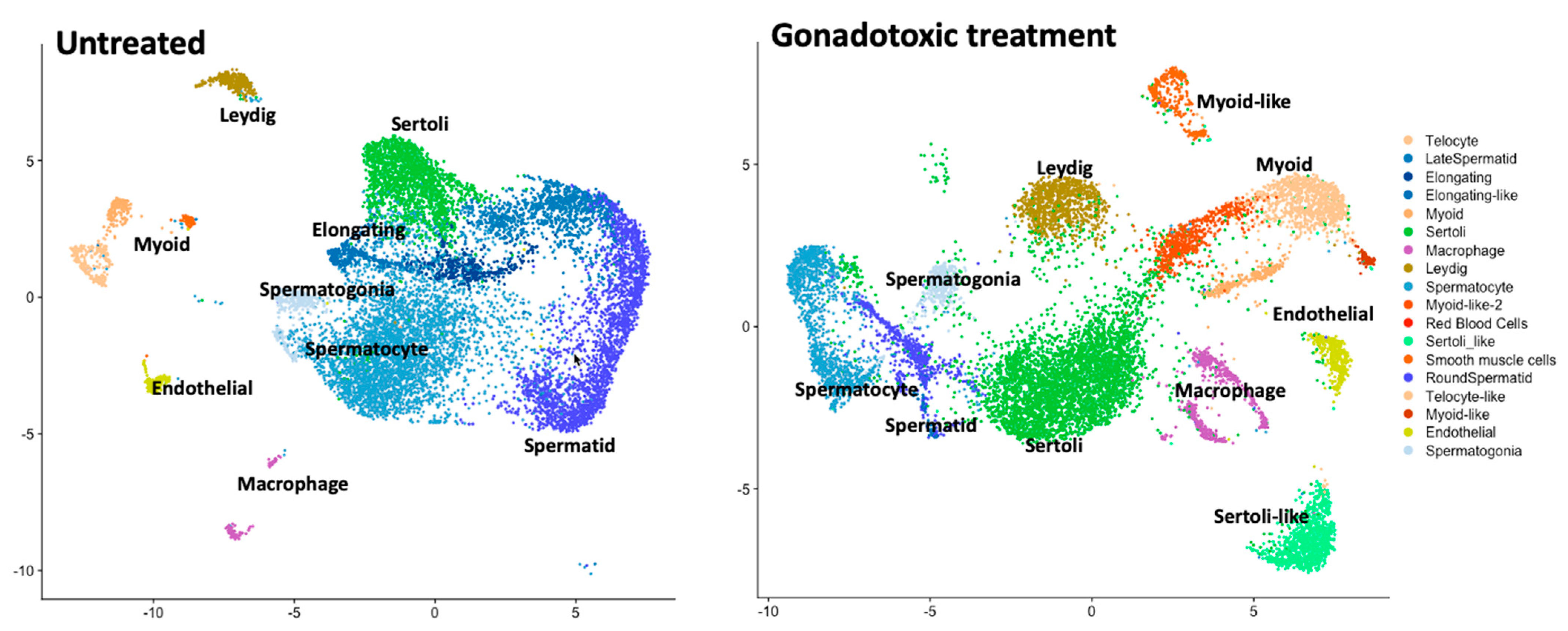
3.1. Gonadotoxic Treatments (Chemo, Radiation, and Others)
3.1.1. Effect on Adults
3.1.2. Effect on Prepubertal Age
4. Model Systems to Study Spermatogenesis (Lessons Insights Gleaned from Model Systems)
4.1. Primates (Non-Human)
4.2. Rodents
4.3. In Vitro and Ex Vivo
5. Clinical Importance (Motivation to Improve Our Understanding)
6. Technologies to Measure Phenotype and Molecular Underpinning to Study Spermatogenesis
6.1. Measured Phenotypes—Fertility, Sperm Parameters, Sperm Maturation, etc.
Selected Molecular Markers
6.2. Unbiased Profiles with Genomic Techniques-Bulk and Single-Cell RNA-Seq
6.3. Building the Atlas of the Testicular Cellular Composition in Human and Murine Models
6.3.1. Spermatogonial Stem Cells (SSCs)
6.3.2. Sertoli Cells
6.3.3. Peritubular Myoid Cells
6.3.4. Leydig Cells
7. Limitations of the Mouse Model for Studying Human Spermatogenesis
8. Discussion, Conclusions, and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huleihel, M.; Abuelhija, M.; Lunenfeld, E. In Vitro Culture of Testicular Germ Cells: Regulatory Factors and Limitations. Growth Factors 2007, 25, 236–252. [Google Scholar] [CrossRef] [PubMed]
- de Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13 (Suppl. S1), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T. Spermatogonial Transplantation: The Principle and Possible Applications. J. Mol. Med. 2001, 79, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Auharek, S.A.; de França, L.R. Postnatal Testis Development, Sertoli Cell Proliferation and Number of Different Spermatogonial Types in C57BL/6J Mice Made Transiently Hypo- and Hyperthyroidic during the Neonatal Period. J. Anat. 2010, 216, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H. Concise Review: Spermatogenesis in an Artificial Three-Dimensional System. Stem Cells 2012, 30, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Fayomi, A.P.; Orwig, K.E. Spermatogonial Stem Cells and Spermatogenesis in Mice, Monkeys and Men. Stem Cell Res. 2018, 29, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, A.; Bacher, M.; Wennemuth, G.; Eickhoff, R.; Hedger, M. Macrophage Migration Inhibitory Factor (MIF) as a Paracrine Mediator in the Interaction of Testicular Somatic Cells. Andrologia 2000, 32, 46–48. [Google Scholar] [PubMed]
- França, L.R.; Hess, R.A.; Dufour, J.M.; Hofmann, M.C.; Griswold, M.D. The Sertoli Cell: One Hundred Fifty Years of Beauty and Plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, P.J.; Hu, L.; Baker, P.J. Effect of Germ Cell Depletion on Levels of Specific MRNA Transcripts in Mouse Sertoli Cells and Leydig Cells. Reproduction 2008, 135, 839–850. [Google Scholar] [CrossRef]
- Smith, L.B.; O’Shaughnessy, P.J.; Rebourcet, D. Cell-Specific Ablation in the Testis: What Have We Learned? Andrology 2015, 3, 1035–1049. [Google Scholar] [CrossRef]
- Garcia, T.X.; Hofmann, M.C. Regulation of Germ Line Stem Cell Homeostasis. Anim. Reprod. 2015, 12, 35–45. [Google Scholar] [PubMed]
- Green, C.D.; Ma, Q.; Manske, G.L.; Shami, A.N.; Zheng, X.; Marini, S.; Moritz, L.; Sultan, C.; Gurczynski, S.J.; Moore, B.B.; et al. A Comprehensive Roadmap of Murine Spermatogenesis Defined by Single-Cell RNA-Seq. Dev. Cell 2018, 46, 651–667.e10. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, X.; Chang, G.; Chen, Y.; An, G.; Yan, L.; Gao, S.; Xu, Y.; Cui, Y.; Dong, J.; et al. Single-Cell RNA Sequencing Analysis Reveals Sequential Cell Fate Transition during Human Spermatogenesis. Cell Stem Cell 2018, 23, 599–614.e4. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Diaz, V.D.; Hermann, B.P. What Has Single-Cell RNA-Seq Taught Us about Mammalian Spermatogenesis? Biol. Reprod. 2019, 101, 617–634. [Google Scholar] [CrossRef] [PubMed]
- Salehi, N.; Karimi-Jafari, M.H.; Totonchi, M.; Amiri-Yekta, A. Integration and Gene Co-Expression Network Analysis of ScRNA-Seq Transcriptomes Reveal Heterogeneity and Key Functional Genes in Human Spermatogenesis. Sci. Rep. 2021, 11, 19089. [Google Scholar] [CrossRef] [PubMed]
- Shami, A.N.; Zheng, X.; Munyoki, S.K.; Ma, Q.; Manske, G.L.; Green, C.D.; Sukhwani, M.; Orwig, K.E.; Li, J.Z.; Hammoud, S.S. Single-Cell RNA Sequencing of Human, Macaque, and Mouse Testes Uncovers Conserved and Divergent Features of Mammalian Spermatogenesis. Dev. Cell 2020, 54, 529–547.e12. [Google Scholar] [CrossRef] [PubMed]
- Di Persio, S.; Tekath, T.; Siebert-Kuss, L.M.; Cremers, J.-F.; Wistuba, J.; Li, X.; Meyer Zu Hörste, G.; Drexler, H.C.A.; Wyrwoll, M.J.; Tüttelmann, F.; et al. Single-Cell RNA-Seq Unravels Alterations of the Human Spermatogonial Stem Cell Compartment in Patients with Impaired Spermatogenesis. Cell Rep. Med. 2021, 2, 100395. [Google Scholar] [CrossRef]
- Wu, X.; Lu, M.; Yun, D.; Gao, S.; Chen, S.; Hu, L.; Wu, Y.; Wang, X.; Duan, E.; Cheng, C.Y.; et al. Single-Cell ATAC-Seq Reveals Cell Type-Specific Transcriptional Regulation and Unique Chromatin Accessibility in Human Spermatogenesis. Hum. Mol. Genet. 2022, 31, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, S.; Meistrich, M.L. The Radiation-Induced Block in Spermatogonial Differentiation Is Due to Damage to the Somatic Environment, Not the Germ Cells. J. Cell. Physiol. 2007, 211, 149–158. [Google Scholar] [CrossRef]
- Stukenborg, J.-B.; Wistuba, J.; Luetjens, C.M.; Elhija, M.A.; Huleihel, M.; Lunenfeld, E.; Gromoll, J.; Nieschlag, E.; Schlatt, S. Coculture of Spermatogonia with Somatic Cells in a Novel Three-Dimensional Soft-Agar-Culture-System. J. Androl. 2008, 29, 312–329. [Google Scholar] [CrossRef]
- Huleihel, M.; Abofoul-Azab, M.; Abarbanel, Y.; Einav, I.; Levitas, E.; Lunenfeld, E. Production of Macrophage Inhibitory Factor (MIF) by Primary Sertoli Cells; Its Possible Involvement in Migration of Spermatogonial Cells. J. Cell. Physiol. 2017, 232, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Male Infertility; Evidences, Risk Factors, Causes, Diagnosis and Management in Human. Ann. Clin. Lab. Res. 2017, 5, 188. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S. Hormones in Male Reproduction and Fertility. Asian Pac. J. Reprod. 2019, 8, 187–188. [Google Scholar] [CrossRef]
- Hussein Ali, N. Celiac Disease Occurrence with Autoimmune Infertility in Infertile Men. Fam. Med. Med. Sci. Res. 2016, 5, 1000209. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Staraj, S. Testicular Size: The Effects of Aging, Malnutrition, and Illness. J. Androl. 1985, 6, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Dakouane Giudicelli, M.; Serazin, V.; Le Sciellour, C.R.; Albert, M.; Selva, J.; Giudicelli, Y. Increased Achondroplasia Mutation Frequency with Advanced Age and Evidence for G1138A Mosaicism in Human Testis Biopsies. Fertil. Steril. 2008, 89, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Goemaere, S.; El-Garem, Y.; Van Pottelbergh, I.; Comhaire, F.H.; Kaufman, J.M. Testicular Volume in Relation to Hormonal Indices of Gonadal Function in Community-Dwelling Elderly Men. J. Clin. Endocrinol. Metab. 2003, 88, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Morpurgo, P.S.; Corsi, A.; Vaghi, I.; Fanelli, M.; Cremonesi, G.; Vaninetti, S.; Beck-Peccoz, P.; Spada, A. Activin A Serum Levels and Aging of the Pituitary-Gonadal Axis: A Cross-Sectional Study in Middle-Aged and Elderly Healthy Subjects. Exp. Gerontol. 2001, 36, 1403–1412. [Google Scholar] [CrossRef]
- Wu, F.C.W.; Tajar, A.; Beynon, J.M.; Pye, S.R.; Silman, A.J.; Finn, J.D.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Forti, G.; et al. Identification of Late-Onset Hypogonadism in Middle-Aged and Elderly Men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar] [CrossRef]
- Zitzmann, M. Testosterone Deficiency, Insulin Resistance and the Metabolic Syndrome. Nat. Rev. Endocrinol. 2009, 5, 673–681. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone: A Metabolic Hormone in Health and Disease. J. Endocrinol. 2013, 217, R25–R45. [Google Scholar] [CrossRef]
- Katz-Jaffe, M.G.; Parks, J.; McCallie, B.; Schoolcraft, W.B. Aging Sperm Negatively Impacts in vivo and in vitro Reproduction: A Longitudinal Murine Study. Fertil. Steril. 2013, 100, 262–268.e2. [Google Scholar] [CrossRef]
- Jenkins, T.G.; Aston, K.I.; Cairns, B.R.; Carrell, D.T. Paternal Aging and Associated Intraindividual Alterations of Global Sperm 5-Methylcytosine and 5-Hydroxymethylcytosine Levels. Fertil. Steril. 2013, 100, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Paduch, D.A.; Fine, R.G.; Bolyakov, A.; Kiper, J. New Concepts in Klinefelter Syndrome. Curr. Opin. Urol. 2008, 18, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ruan, Y.C.; Xu, W.M.; Chen, J.; Chan, H.C. Regulation of Male Fertility by CFTR and Implications in Male Infertility. Hum. Reprod. Update 2012, 18, 703–713. [Google Scholar] [CrossRef]
- Ayensu-Coker, L.; Bishop, C.; Rohozinski, J. The Genetics of Male Infertility; Ayensu-Coker, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Ravel, C.; Chantot-Bastaraud, S.; El Houate, B.; Berthaut, I.; Verstraete, L.; De Larouziere, V.; Lourenço, D.; Dumaine, A.; Antoine, J.M.; Mandelbaum, J.; et al. Mutations in the Protamine 1 Gene Associated with Male Infertility. Mol. Hum. Reprod. 2007, 13, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Arafat, M.; Kleiman, S.E.; AbuMadighem, A.; Zeadna, A.; Levitas, E.; Vardi, I.H.; Barda, S.; Lehavi, O.; Hauser, R.; Lunenfeld, E.; et al. Pathogenic Variations in Germ Cell Nuclear Acidic Peptidase (GCNA) Are Associated with Human Male Infertility. Eur. J. Hum. Genet. 2021, 29, 1781–1788. [Google Scholar] [CrossRef]
- Wormser, O.; Levy, Y.; Bakhrat, A.; Bonaccorsi, S.; Graziadio, L.; Gatti, M.; AbuMadighem, A.; McKenney, R.J.; Okada, K.; El Riati, S.; et al. Absence of SCAPER Causes Male Infertility in Humans and Drosophila by Modulating Microtubule Dynamics during Meiosis. J. Med. Genet. 2021, 58, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.A. Mumps Orchitis and Testicular Atrophy; a Factor in Male Sterility. Ann. Intern. Med. 1950, 32, 1075–1086. [Google Scholar] [CrossRef]
- Mesbah, N.; Salem, H.K. Genital Tract Infection as a Cause of Male Infertility. In Genital Infections and Infertility; InTech: Houston, TX, USA, 2016. [Google Scholar]
- Ochsendorf, F.R. Sexually Transmitted Infections: Impact on Male Fertility. Andrologia 2008, 40, 72–75. [Google Scholar] [CrossRef]
- Bretveld, R.; Brouwers, M.; Ebisch, I.; Roeleveld, N. Influence of Pesticides on Male Fertility. Scand. J. Work. Environ. Health 2007, 33, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Roeleveld, N.; Bretveld, R. The Impact of Pesticides on Male Fertility. Curr. Opin. Obstet. Gynecol. 2008, 20, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Wettemann, R.P.; Boehmer, B.H. Influence of Heat Stress on Male Fertility; K-State Research and Extension: Stillwater, OK, USA, 2014. [Google Scholar]
- Kesari, K.K.; Agarwal, A.; Henkel, R. Radiations and Male Fertility. Reprod. Biol. Endocrinol. 2018, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, I.; Sainova, I.; Yosifcheva, K. Toxic Effects of Heavy Metals (Lead and Cadmium) on Sperm Quality and Male Fertility. Acta Morphol. Et Anthropol. 2020, 27, 63–75. [Google Scholar]
- Meistrich, M.L.; Wilson, G.; Mathur, K.; Fuller, L.M.; Rodriguez, M.A.; McLaughlin, P.; Romaguera, J.E.; Cabanillas, F.F.; Ha, C.S.; Lipshultz, L.I.; et al. Rapid Recovery of Spermatogenesis after Mitoxantrone, Vincristine, Vinblastine, and Prednisone Chemotherapy for Hodgkin’s Disease. J. Clin. Oncol. 1997, 15, 3488–3495. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, M.F.; Meistrich, M.L.; Haq, M.M.; Gordon, L.A.; Wyrobek, A.J. Temporary Effects of AMSA (4’-(9-Acridinylamino) Methanesulfon-m-Anisidide) Chemotherapy on Spermatogenesis. Cancer 1982, 49, 2459–2462. [Google Scholar] [CrossRef] [PubMed]
- Meistrich, M.L. Effects of Chemotherapy and Radiotherapy on Spermatogenesis in Humans. Fertil. Steril. 2013, 100, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.L.; Constine, L.S. Late Effects of Treatment for Hodgkin Lymphoma. J. Natl. Compr. Cancer Netw. 2006, 4, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Longhi, A.; Macchiagodena, M.; Vitali, G.; Bacci, G. Fertility in Male Patients Treated with Neoadjuvant Chemotherapy for Osteosarcoma. J. Pediatr. Hematol. Oncol. 2003, 25, 292–296. [Google Scholar] [CrossRef]
- Anserini, P.; Chiodi, S.; Spinelli, S.; Costa, M.; Conte, N.; Copello, F.; Bacigalupo, A. Semen Analysis Following Allogeneic Bone Marrow Transplantation. Additional Data for Evidence-Based Counselling. Bone Marrow Transplant. 2002, 30, 447–451. [Google Scholar] [CrossRef]
- Fung, C.; Dinh, P.; Ardeshir-Rouhani-Fard, S.; Schaffer, K.; Fossa, S.D.; Travis, L.B. Toxicities Associated with Cisplatin-Based Chemotherapy and Radiotherapy in Long-Term Testicular Cancer Survivors. Adv. Urol. 2018, 2018, 8671832. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.D.; Fairley, K.F.; Barrie, J.U. Return of Spermatogenesis after Stopping Cyclophosphamide Therapy. Lancet 1975, 2, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Kawashima, T.; Stovall, M.; Leisenring, W.; Sklar, C.A.; Mertens, A.C.; Donaldson, S.S.; Byrne, J.; Robison, L.L. Fertility of Male Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2010, 28, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, O.; Eberhard, J.; Jepson, K.; Spano, M.; Cwikiel, M.; Cavallin-Ståhl, E.; Giwercman, A. Sperm DNA Integrity in Testicular Cancer Patients. Hum. Reprod. 2006, 21, 3199–3205. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.J.; Shalet, S.M. Spermatogenesis after Cancer Treatment: Damage and Recovery. J. Natl. Cancer Inst. Monogr. 2005, 2005, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.M. Reproductive Outcomes for Survivors of Childhood Cancer. Obstet. Gynecol. 2010, 116, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Müller, J. Impact of Cancer Therapy on the Reproductive Axis. Horm. Res. 2003, 59 (Suppl. S1), 12–20. [Google Scholar] [CrossRef]
- Thomson, A.B.; Critchley, H.O.D.; Kelnar, C.J.H.; Wallace, W.H.B. Late Reproductive Sequelae Following Treatment of Childhood Cancer and Options for Fertility Preservation. Best. Pract. Res. Clin. Endocrinol. Metab. 2002, 16, 311–334. [Google Scholar] [CrossRef]
- Afify, Z.; Shaw, P.J.; Clavano-Harding, A.; Cowell, C.T. Growth and Endocrine Function in Children with Acute Myeloid Leukaemia after Bone Marrow Transplantation Using Busulfan/Cyclophosphamide. Bone Marrow Transplant. 2000, 25, 1087–1092. [Google Scholar] [CrossRef]
- Brennan, B.M.D.; Shalet, S.M. Endocrine Late Effects after Bone Marrow Transplant. Br. J. Haematol. 2002, 118, 58–66. [Google Scholar] [CrossRef]
- Ishiguro, H.; Yasuda, Y.; Tomita, Y.; Shinagawa, T.; Shimizu, T.; Morimoto, T.; Hattori, K.; Matsumoto, M.; Inoue, H.; Yabe, H.; et al. Gonadal Shielding to Irradiation Is Effective in Protecting Testicular Growth and Function in Long-Term Survivors of Bone Marrow Transplantation during Childhood or Adolescence. Bone Marrow Transplant. 2007, 39, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Meistrich, M.L.; Byrne, J. Genetic Disease in Offspring of Long-Term Survivors of Childhood and Adolescent Cancer Treated with Potentially Mutagenic Therapies. Am. J. Hum. Genet. 2002, 70, 1069–1071. [Google Scholar] [CrossRef]
- Witt, K.L.; Bishop, J.B. Mutagenicity of Anticancer Drugs in Mammalian Germ Cells. Mutat. Res. 1996, 355, 209–234. [Google Scholar] [CrossRef]
- Kaplanis, J.; Ide, B.; Sanghvi, R.; Neville, M.; Danecek, P.; Coorens, T.; Prigmore, E.; Short, P.; Gallone, G.; McRae, J.; et al. Genetic and Chemotherapeutic Influences on Germline Hypermutation. Nature 2022, 605, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Maher, G.J.; Bernkopf, M.; Koelling, N.; Wilkie, A.O.M.; Meistrich, M.L.; Goriely, A. The Impact of Chemo- and Radiotherapy Treatments on Selfish de Novo FGFR2 Mutations in Sperm of Cancer Survivors. Hum. Reprod. 2019, 34, 1404–1415. [Google Scholar] [CrossRef]
- Sasani, T.A.; Pedersen, B.S.; Gao, Z.; Baird, L.; Przeworski, M.; Jorde, L.B.; Quinlan, A.R. Large, Three-Generation Human Families Reveal Post-Zygotic Mosaicism and Variability in Germline Mutation Accumulation. Elife 2019, 8, e46922. [Google Scholar] [CrossRef]
- Lindsay, S.J.; Rahbari, R.; Kaplanis, J.; Keane, T.; Hurles, M.E. Similarities and Differences in Patterns of Germline Mutation between Mice and Humans. Nat. Commun. 2019, 10, 4053. [Google Scholar] [CrossRef] [PubMed]
- Harris, K. Evidence for Recent, Population-Specific Evolution of the Human Mutation Rate. Proc. Natl. Acad. Sci. USA 2015, 112, 3439–3444. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Huang, J.; Zhang, X.; Yuan, Y.; Chen, J.-Q.; Hurst, L.D.; Tian, D. Parent-Progeny Sequencing Indicates Higher Mutation Rates in Heterozygotes. Nature 2015, 523, 463–467. [Google Scholar] [CrossRef]
- Amos, W. Flanking Heterozygosity Influences the Relative Probability of Different Base Substitutions in Humans. R. Soc. Open Sci. 2019, 6, 191018. [Google Scholar] [CrossRef]
- Kilpivaara, O.; Aaltonen, L.A. Diagnostic Cancer Genome Sequencing and the Contribution of Germline Variants. Science 2013, 339, 1559–1562. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, A.; Higuchi, M.; Minakuchi, Y.; Ohno, M.; Toyoda, A.; Fujiyama, A.; Miura, I.; Wakana, S.; Nishino, J.; Yagi, T. Germline Mutation Rates and the Long-Term Phenotypic Effects of Mutation Accumulation in Wild-Type Laboratory Mice and Mutator Mice. Genome Res. 2015, 25, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M.; Meeks, H.D.; Sasani, T.A.; Smith, K.R.; Kerber, R.A.; O’Brien, E.; Baird, L.; Dixon, M.M.; Peiffer, A.P.; Leppert, M.F.; et al. Germline Mutation Rates in Young Adults Predict Longevity and Reproductive Lifespan. Sci. Rep. 2020, 10, 10001. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K.M.; Lyon, M.F. Induction of Congenital Malformations in the Offspring of Male Mice Treated with X-rays at Pre-Meiotic and Post-Meiotic Stages. Mutat. Res. 1984, 125, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Generoso, W.M.; Cain, K.T.; Cacheiro, N.L.; Cornett, C.V.; Gossle, D.G. Response of Mouse Spermatogonial Stem Cells to X-ray Induction of Heritable Reciprocal Translocations. Mutat. Res. 1984, 126, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Adewoye, A.B.; Lindsay, S.J.; Dubrova, Y.E.; Hurles, M.E. The Genome-Wide Effects of Ionizing Radiation on Mutation Induction in the Mammalian Germline. Nat. Commun. 2015, 6, 6684. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-W.; Liu, J.; Juay, L.; Czene, K.; Miao, H.; Salim, A.; Verkooijen, H.M.; Hartman, M. Birth Rates among Male Cancer Survivors and Mortality Rates among Their Offspring: A Population-Based Study from Sweden. BMC Cancer 2016, 16, 196. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.; Rasmussen, S.A.; Steinhorn, S.C.; Connelly, R.R.; Myers, M.H.; Lynch, C.F.; Flannery, J.; Austin, D.F.; Holmes, F.F.; Holmes, G.E.; et al. Genetic Disease in Offspring of Long-Term Survivors of Childhood and Adolescent Cancer. Am. J. Hum. Genet. 1998, 62, 45–52. [Google Scholar] [CrossRef]
- Winther, J.F.; Boice, J.D.; Svendsen, A.L.; Frederiksen, K.; Stovall, M.; Olsen, J.H. Spontaneous Abortion in a Danish Population-Based Cohort of Childhood Cancer Survivors. J. Clin. Oncol. 2008, 26, 4340–4346. [Google Scholar] [CrossRef]
- Winther, J.F.; Boice, J.D.; Mulvihill, J.J.; Stovall, M.; Frederiksen, K.; Tawn, E.J.; Olsen, J.H. Chromosomal Abnormalities among Offspring of Childhood-Cancer Survivors in Denmark: A Population-Based Study. Am. J. Hum. Genet. 2004, 74, 1282–1285. [Google Scholar] [CrossRef]
- Boice, J.D.; Tawn, E.J.; Winther, J.F.; Donaldson, S.S.; Green, D.M.; Mertens, A.C.; Mulvihill, J.J.; Olsen, J.H.; Robison, L.L.; Stovall, M. Genetic Effects of Radiotherapy for Childhood Cancer. Health Phys. 2003, 85, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Kenney, L.B.; Nicholson, H.S.; Brasseux, C.; Mills, J.L.; Robison, L.L.; Zeltzer, L.K.; Meadows, A.T.; Reaman, G.H.; Byrne, J. Birth Defects in Offspring of Adult Survivors of Childhood Acute Lymphoblastic Leukemia. A Childrens Cancer Group/National Institutes of Health Report. Cancer 1996, 78, 169–176. [Google Scholar] [CrossRef]
- Madanat-Harjuoja, L.-M.S.; Malila, N.; Lähteenmäki, P.; Pukkala, E.; Mulvihill, J.J.; Boice, J.D.; Sankila, R. Risk of Cancer among Children of Cancer Patients—A Nationwide Study in Finland. Int. J. Cancer 2010, 126, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Winther, J.F.; Boice, J.D.; Frederiksen, K.; Bautz, A.; Mulvihill, J.J.; Stovall, M.; Olsen, J.H. Radiotherapy for Childhood Cancer and Risk for Congenital Malformations in Offspring: A Population-Based Cohort Study. Clin. Genet. 2009, 75, 50–56. [Google Scholar] [CrossRef] [PubMed]
- van Gerwen, M.; Maggen, C.; Cardonick, E.; Verwaaijen, E.J.; van den Heuvel-Eibrink, M.; Shmakov, R.G.; Boere, I.; Gziri, M.M.; Ottevanger, P.B.; Lok, C.A.R.; et al. Association of Chemotherapy Timing in Pregnancy With Congenital Malformation. JAMA Netw. Open 2021, 4, e2113180. [Google Scholar] [CrossRef] [PubMed]
- Stensheim, H.; Klungsøyr, K.; Skjaerven, R.; Grotmol, T.; Fosså, S.D. Birth Outcomes among Offspring of Adult Cancer Survivors: A Population-Based Study. Int. J. Cancer 2013, 133, 2696–2705. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, G.V.; Bielski, C.M.; Samocha, K.; Fromer, M.; Seepo, S.; Gentry, C.; Neale, B.; Garraway, L.A.; Sweeney, C.J.; Taplin, M.-E.; et al. Genetic Effect of Chemotherapy Exposure in Children of Testicular Cancer Survivors. Clin. Cancer Res. 2016, 22, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Makino, S.; Kimura, H.; Ota, T.; Furuhashi, T.; Nagamura, Y. Sperm Motion Analysis in Rats Treated with Adriamycin and Its Applicability to Male Reproductive Toxicity Studies. J. Toxicol. Sci. 2001, 26, 51–59. [Google Scholar] [CrossRef]
- Vaisheva, F.; Delbes, G.; Hales, B.F.; Robaire, B. Effects of the Chemotherapeutic Agents for Non-Hodgkin Lymphoma, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP), on the Male Rat Reproductive System and Progeny Outcome. J. Androl. 2007, 28, 578–587. [Google Scholar] [CrossRef]
- Delbès, G.; Vaisheva, F.; Luu, T.; Marcon, L.; Hales, B.F.; Robaire, B. Reversibility of the Effects of the Chemotherapeutic Regimen for Non-Hodgkin Lymphoma, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone, on the Male Rat Reproductive System and Progeny Outcome. Reprod. Toxicol. 2010, 29, 332–338. [Google Scholar] [CrossRef]
- Marcon, L.; Hales, B.F.; Robaire, B. Reversibility of the Effects of Subchronic Exposure to the Cancer Chemotherapeutics Bleomycin, Etoposide, and Cisplatin on Spermatogenesis, Fertility, and Progeny Outcome in the Male Rat. J. Androl. 2008, 29, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Harrouk, W.; Khatabaksh, S.; Robaire, B.; Hales, B.F. Paternal Exposure to Cyclophosphamide Dysregulates the Gene Activation Program in Rat Preimplantation Embryos. Mol. Reprod. Dev. 2000, 57, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Auroux, M.R.; Dulioust, E.J.; Nawar, N.N.; Yacoub, S.G.; Mayaux, M.J.; Schwartz, D.; David, G. Antimitotic Drugs in the Male Rat Behavioral Abnormalities in the Second Generation. J. Androl. 1988, 9, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Seethalakshmi, L.; Flores, C.; Kinkead, T.; Carboni, A.A.; Malhotra, R.K.; Menon, M. Effects of Subchronic Treatment with Cis-Platinum on Testicular Function, Fertility, Pregnancy Outcome, and Progeny. J. Androl. 1992, 13, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rappolee, D.A.; Ruden, D.M. Epigenetic Reprogramming in Mice and Humans: From Fertilization to Primordial Germ Cell Development. Cells 2023, 12, 1874. [Google Scholar] [CrossRef] [PubMed]
- Shnorhavorian, M.; Schwartz, S.M.; Stansfeld, B.; Sadler-Riggleman, I.; Beck, D.; Skinner, M.K. Differential DNA Methylation Regions in Adult Human Sperm Following Adolescent Chemotherapy: Potential for Epigenetic Inheritance. PLoS ONE 2017, 12, e0170085. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Manikkam, M.; Haque, M.M.; Zhang, B.; Savenkova, M.I. Epigenetic Transgenerational Inheritance of Somatic Transcriptomes and Epigenetic Control Regions. Genome Biol. 2012, 13, R91. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Larsen, G.; Manikkam, M.; Guerrero-Bosagna, C.; Savenkova, M.I.; Skinner, M.K. Environmentally Induced Epigenetic Transgenerational Inheritance of Ovarian Disease. PLoS ONE 2012, 7, e36129. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Bosagna, C.; Savenkova, M.; Haque, M.M.; Nilsson, E.; Skinner, M.K. Environmentally Induced Epigenetic Transgenerational Inheritance of Altered Sertoli Cell Transcriptome and Epigenome: Molecular Etiology of Male Infertility. PLoS ONE 2013, 8, e59922. [Google Scholar] [CrossRef]
- Hermann, B.P.; Sukhwani, M.; Winkler, F.; Pascarella, J.N.; Peters, K.A.; Sheng, Y.; Valli, H.; Rodriguez, M.; Ezzelarab, M.; Dargo, G.; et al. Spermatogonial Stem Cell Transplantation into Rhesus Testes Regenerates Spermatogenesis Producing Functional Sperm. Cell Stem Cell 2012, 11, 715–726. [Google Scholar] [CrossRef]
- Arslan, M.; Weinbauer, G.F.; Schlatt, S.; Shahab, M.; Nieschlag, E. FSH and Testosterone, Alone or in Combination, Initiate Testicular Growth and Increase the Number of Spermatogonia and Sertoli Cells in a Juvenile Non-Human Primate (Macaca mulatta). J. Endocrinol. 1993, 136, 235–243. [Google Scholar] [CrossRef]
- Marshall, G.R.; Wickings, E.J.; Nieschlag, E. Testosterone Can Initiate Spermatogenesis in an Immature Nonhuman Primate, Macaca Fascicularis. Endocrinology 1984, 114, 2228–2233. [Google Scholar] [CrossRef]
- Stanton, P.G.; Sluka, P.; Foo, C.F.H.; Stephens, A.N.; Smith, A.I.; McLachlan, R.I.; O’Donnell, L. Proteomic Changes in Rat Spermatogenesis in Response to in Vivo Androgen Manipulation; Impact on Meiotic Cells. PLoS ONE 2012, 7, e41718. [Google Scholar] [CrossRef] [PubMed]
- Troiano, L.; Fustini, M.F.; Lovato, E.; Frasoldati, A.; Malorni, W.; Capri, M.; Grassilli, E.; Marrama, P.; Franceschi, C. Apoptosis and Spermatogenesis: Evidence from an In Vivo Model of Testosterone Withdrawal in the Adult Rat. Biochem. Biophys. Res. Commun. 1994, 202, 1315–1321. [Google Scholar] [CrossRef]
- Stukenborg, J.-B.; Schlatt, S.; Simoni, M.; Yeung, C.-H.; Elhija, M.A.; Luetjens, C.M.; Huleihel, M.; Wistuba, J. New Horizons for In Vitro Spermatogenesis? An Update on Novel Three-Dimensional Culture Systems as Tools for Meiotic and Post-Meiotic Differentiation of Testicular Germ Cells. Mol. Hum. Reprod. 2009, 15, 521–529. [Google Scholar] [CrossRef]
- Huleihel, M.; Lunenfeld, E. Approaches and Technologies in Male Fertility Preservation. Int. J. Mol. Sci. 2020, 21, 5471. [Google Scholar] [CrossRef] [PubMed]
- Gassei, K.; Orwig, K.E. Experimental Methods to Preserve Male Fertility and Treat Male Factor Infertility. Fertil. Steril. 2016, 105, 256–266. [Google Scholar] [CrossRef]
- Pelzman, D.L.; Orwig, K.E.; Hwang, K. Progress in Translational Reproductive Science: Testicular Tissue Transplantation and In Vitro Spermatogenesis. Fertil. Steril. 2020, 113, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yuan, Q.; Niu, M.; Wang, H.; Wen, L.; Yao, C.; Hou, J.; Chen, Z.; Fu, H.; Zhou, F.; et al. Efficient Generation of Functional Haploid Spermatids from Human Germline Stem Cells by Three-Dimensional-Induced System. Cell Death Differ. 2018, 25, 749–766. [Google Scholar] [CrossRef]
- Feng, L.-X.; Chen, Y.; Dettin, L.; Pera, R.A.R.; Herr, J.C.; Goldberg, E.; Dym, M. Generation and In Vitro Differentiation of a Spermatogonial Cell Line. Science 2002, 297, 392–395. [Google Scholar] [CrossRef]
- Abu Elhija, M.; Lunenfeld, E.; Schlatt, S.; Huleihel, M. Differentiation of Murine Male Germ Cells to Spermatozoa in a Soft Agar Culture System. Asian J. Androl. 2012, 14, 285–293. [Google Scholar] [CrossRef] [PubMed]
- AbuMadighem, A.; Solomon, R.; Stepanovsky, A.; Kapelushnik, J.; Shi, Q.; Meese, E.; Lunenfeld, E.; Huleihel, M. Development of Spermatogenesis In Vitro in Three-Dimensional Culture from Spermatogonial Cells of Busulfan-Treated Immature Mice. Int. J. Mol. Sci. 2018, 19, 3804. [Google Scholar] [CrossRef] [PubMed]
- Abofoul-Azab, M.; Lunenfeld, E.; Levitas, E.; Zeadna, A.; Younis, J.S.; Bar-Ami, S.; Huleihel, M. Identification of Premeiotic, Meiotic, and Postmeiotic Cells in Testicular Biopsies Without Sperm from Sertoli Cell-Only Syndrome Patients. Int. J. Mol. Sci. 2019, 20, 470. [Google Scholar] [CrossRef] [PubMed]
- AbuMadighem, A.; Shuchat, S.; Lunenfeld, E.; Yossifon, G.; Huleihel, M. Testis on a Chip-a Microfluidic Three-Dimensional Culture System for the Development of Spermatogenesisin-Vitro. Biofabrication 2022, 14, 035004. [Google Scholar] [CrossRef] [PubMed]
- Ishikura, Y.; Ohta, H.; Sato, T.; Murase, Y.; Yabuta, Y.; Kojima, Y.; Yamashiro, C.; Nakamura, T.; Yamamoto, T.; Ogawa, T.; et al. In Vitro Reconstitution of the Whole Male Germ-Cell Development from Mouse Pluripotent Stem Cells. Cell Stem Cell 2021, 28, 2167–2179.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, M.; Yuan, Y.; Wang, X.; Fu, R.; Wan, H.; Xie, M.; Liu, M.; Guo, X.; Zheng, Y.; et al. Complete Meiosis from Embryonic Stem Cell-Derived Germ Cells In Vitro. Cell Stem Cell 2016, 18, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Abofoul-Azab, M.; AbuMadighem, A.; Lunenfeld, E.; Kapelushnik, J.; Shi, Q.; Pinkas, H.; Huleihel, M. Development of Postmeiotic Cells In Vitro from Spermatogonial Cells of Prepubertal Cancer Patients. Stem Cells Dev. 2018, 27, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Lai, X.; Eliveld, J.; Chuva de Sousa Lopes, S.M.; van Pelt, A.M.M.; Hamer, G. In Vitro Meiosis of Male Germline Stem Cells. Stem Cell Rep. 2020, 15, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Nolte, J.; Michelmann, H.W.; Wolf, M.; Wulf, G.; Nayernia, K.; Meinhardt, A.; Zechner, U.; Engel, W. PSCDGs of Mouse Multipotent Adult Germline Stem Cells Can Enter and Progress through Meiosis to Form Haploid Male Germ Cells In Vitro. Differentiation 2010, 80, 184–194. [Google Scholar] [CrossRef]
- Solomon, R.; AbuMadighem, A.; Kapelushnik, J.; Amano, B.-C.; Lunenfeld, E.; Huleihel, M. Involvement of Cytokines and Hormones in the Development of Spermatogenesis In Vitro from Spermatogonial Cells of Cyclophosphamide-Treated Immature Mice. Int. J. Mol. Sci. 2021, 22, 1672. [Google Scholar] [CrossRef] [PubMed]
- Stukenborg, J.B.; Colón, E.; Söder, O. Ontogenesis of Testis Development and Function in Humans. Sex. Dev. 2010, 4, 199–212. [Google Scholar] [CrossRef]
- Handel, M.A.; Eppig, J.J.; Schimenti, J.C. Applying “Gold Standards” to In-Vitro-Derived Germ Cells. Cell 2014, 157, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Pendergraft, S.S.; Sadri-Ardekani, H.; Atala, A.; Bishop, C.E. Three-Dimensional Testicular Organoid: A Novel Tool for the Study of Human Spermatogenesis and Gonadotoxicity in Vitro. Biol. Reprod. 2017, 96, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Baert, Y.; Rombaut, C.; Goossens, E. Scaffold-Based and Scaffold-Free Testicular Organoids from Primary Human Testicular Cells. Methods Mol. Biol. 2019, 1576, 283–290. [Google Scholar] [CrossRef]
- Baert, Y.; De Kock, J.; Alves-Lopes, J.P.; Söder, O.; Stukenborg, J.-B.; Goossens, E. Primary Human Testicular Cells Self-Organize into Organoids with Testicular Properties. Stem Cell Rep. 2017, 8, 30–38. [Google Scholar] [CrossRef]
- Oliver, E.; Alves-Lopes, J.P.; Harteveld, F.; Mitchell, R.T.; Åkesson, E.; Söder, O.; Stukenborg, J.-B. Self-Organising Human Gonads Generated by a Matrigel-Based Gradient System. BMC Biol. 2021, 19, 212. [Google Scholar] [CrossRef] [PubMed]
- Alves-Lopes, J.P.; Söder, O.; Stukenborg, J.-B. Testicular Organoid Generation by a Novel In Vitro Three-Layer Gradient System. Biomaterials 2017, 130, 76–89. [Google Scholar] [CrossRef]
- Alves-Lopes, J.P.; Söder, O.; Stukenborg, J.-B. Use of a Three-Layer Gradient System of Cells for Rat Testicular Organoid Generation. Nat. Protoc. 2018, 13, 248–259. [Google Scholar] [CrossRef]
- Sakib, S.; Uchida, A.; Valenzuela-Leon, P.; Yu, Y.; Valli-Pulaski, H.; Orwig, K.; Ungrin, M.; Dobrinski, I. Formation of Organotypic Testicular Organoids in Microwell Culture. Biol. Reprod. 2019, 100, 1648–1660. [Google Scholar] [CrossRef]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A Unique View on Male Infertility around the Globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Aston, K.I.; Conrad, D.F. A Review of Genome-Wide Approaches to Study the Genetic Basis for Spermatogenic Defects. Methods Mol. Biol. 2013, 927, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Sohni, A.; Tan, K.; Song, H.-W.; Burow, D.; de Rooij, D.G.; Laurent, L.; Hsieh, T.-C.; Rabah, R.; Hammoud, S.S.; Vicini, E.; et al. The Neonatal and Adult Human Testis Defined at the Single-Cell Level. Cell Rep. 2019, 26, 1501–1517.e4. [Google Scholar] [CrossRef] [PubMed]
- Lukassen, S.; Bosch, E.; Ekici, A.B.; Winterpacht, A. Single-Cell RNA Sequencing of Adult Mouse Testes. Sci. Data 2018, 5, 180192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ren, X.; Zhang, T.; Zhou, X.; Chen, X.; Lu, H.; Zhou, X.; Zhang, X.; Wang, S.; Qin, C. Comprehensive Analysis of the Association Between Human Non-Obstructive Azoospermia and Plasticisers via Single-Cell and Traditional RNA Sequencing Methods. Expo. Health 2022, 14, 829–842. [Google Scholar] [CrossRef]
- Warren, A.; Chen, Y.; Jones, A.; Shibue, T.; Hahn, W.C.; Boehm, J.S.; Vazquez, F.; Tsherniak, A.; McFarland, J.M. Global Computational Alignment of Tumor and Cell Line Transcriptional Profiles. Nat. Commun. 2021, 12, 22. [Google Scholar] [CrossRef]
- Farah, O.I.; Cuiling, L.; Jiaojiao, W.; Huiping, Z. Use of Fluorescent Dyes for Readily Recognizing Sperm Damage. J. Reprod. Infertil. 2013, 14, 120–125. [Google Scholar] [PubMed]
- Buageaw, A.; Sukhwani, M.; Ben-Yehudah, A.; Ehmcke, J.; Rawe, V.Y.; Pholpramool, C.; Orwig, K.E.; Schlatt, S. GDNF Family Receptor Alpha1 Phenotype of Spermatogonial Stem Cells in Immature Mouse Testes. Biol. Reprod. 2005, 73, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Kang, H.-G.; Kim, B.-J.; Jung, S.-E.; Karmakar, P.C.; Kim, S.-M.; Hwang, S.; Ryu, B.-Y. Enrichment and In Vitro Culture of Spermatogonial Stem Cells from Pre-Pubertal Monkey Testes. Tissue Eng. Regen. Med. 2017, 14, 557–566. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Toyokuni, S.; Shinohara, T. CD9 Is a Surface Marker on Mouse and Rat Male Germline Stem Cells. Biol. Reprod. 2004, 70, 70–75. [Google Scholar] [CrossRef]
- Evenson, D.; Jost, L. Sperm Chromatin Structure Assay Is Useful for Fertility Assessment. Methods Cell Sci. 2000, 22, 169–189. [Google Scholar] [CrossRef]
- Domínguez-Fandos, D.; Camejo, M.I.; Ballescà, J.L.; Oliva, R. Human Sperm DNA Fragmentation: Correlation of TUNEL Results as Assessed by Flow Cytometry and Optical Microscopy. Cytom. A 2007, 71, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Oatley, M.J.; Racicot, K.E.; Oatley, J.M. Sertoli Cells Dictate Spermatogonial Stem Cell Niches in the Mouse Testis. Biol. Reprod. 2011, 84, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Dorval-Coiffec, I.; Delcros, J.-G.; Hakovirta, H.; Toppari, J.; Jégou, B.; Piquet-Pellorce, C. Identification of the Leukemia Inhibitory Factor Cell Targets within the Rat Testis. Biol. Reprod. 2005, 72, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Hermann, B.P.; Cheng, K.; Singh, A.; Roa-De La Cruz, L.; Mutoji, K.N.; Chen, I.-C.; Gildersleeve, H.; Lehle, J.D.; Mayo, M.; Westernströer, B.; et al. The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids. Cell Rep. 2018, 25, 1650–1667.e8. [Google Scholar] [CrossRef] [PubMed]
- Sisakhtnezhad, S.; Heshmati, P. Comparative Analysis of Single-Cell RNA Sequencing Data from Mouse Spermatogonial and Mesenchymal Stem Cells to Identify Differentially Expressed Genes and Transcriptional Regulators of Germline Cells. J. Cell. Physiol. 2018, 233, 5231–5242. [Google Scholar] [CrossRef] [PubMed]
- Di Persio, S.; Neuhaus, N. Human Spermatogonial Stem Cells and Their Niche in Male (in)Fertility: Novel Concepts from Single-Cell RNA-Sequencing. Hum. Reprod. 2023, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Luo, L.; Alamdar, A.; Zhang, J.; Liu, L.; Tian, M.; Eqani, S.A.M.A.S.; Shen, H. Integrated Proteomics and Metabolomics Analysis of Rat Testis: Mechanism of Arsenic-Induced Male Reproductive Toxicity. Sci. Rep. 2016, 6, 32518. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Ye, Y.; Yang, F.; Wang, F.; Shen, Y.; Chang, D.; You, Y. Integrative Proteomics and Metabolomics Approach to Identify the Key Roles of Icariin-Mediated Protective Effects against Cyclophosphamide-Induced Spermatogenesis Dysfunction in Mice. Front. Pharmacol. 2022, 13, 1040544. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Carvalho, B.; Carrageta, D.F.; Crisóstomo, L.; Carvalho, R.A.; Alves, M.G.; Oliveira, P.F. Molecular Mechanisms Regulating Spermatogenesis in Vertebrates: Environmental, Metabolic, and Epigenetic Factor Effects. Anim. Reprod. Sci. 2022, 246, 106896. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, Z.; Cui, H.; Wang, J.; Wang, Y.; Tang, Y.; Yang, W.; Zou, P.; Ling, X.; Han, F.; et al. Analysis by Transcriptomics and Metabolomics for the Proliferation Inhibition and Dysfunction through Redox Imbalance-Mediated DNA Damage Response and Ferroptosis in Male Reproduction of Mice and TM4 Sertoli Cells Exposed to PM2.5. Ecotoxicol. Environ. Saf. 2022, 238, 113569. [Google Scholar] [CrossRef]
- Rabbani, M.; Zheng, X.; Manske, G.L.; Vargo, A.; Shami, A.N.; Li, J.Z.; Hammoud, S.S. Decoding the Spermatogenesis Program: New Insights from Transcriptomic Analyses. Annu. Rev. Genet. 2022, 56, 339–368. [Google Scholar] [CrossRef]
- Aramaki, S.; Hayashi, K.; Kurimoto, K.; Ohta, H.; Yabuta, Y.; Iwanari, H.; Mochizuki, Y.; Hamakubo, T.; Kato, Y.; Shirahige, K.; et al. A Mesodermal Factor, T, Specifies Mouse Germ Cell Fate by Directly Activating Germline Determinants. Dev. Cell 2013, 27, 516–529. [Google Scholar] [CrossRef]
- Ball, R.L.; Fujiwara, Y.; Sun, F.; Hu, J.; Hibbs, M.A.; Handel, M.A.; Carter, G.W. Regulatory Complexity Revealed by Integrated Cytological and RNA-Seq Analyses of Meiotic Substages in Mouse Spermatocytes. BMC Genom. 2016, 17, 628. [Google Scholar] [CrossRef]
- Gill, M.E.; Hu, Y.-C.; Lin, Y.; Page, D.C. Licensing of Gametogenesis, Dependent on RNA Binding Protein DAZL, as a Gateway to Sexual Differentiation of Fetal Germ Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 7443–7448. [Google Scholar] [CrossRef] [PubMed]
- Gkountela, S.; Zhang, K.X.; Shafiq, T.A.; Liao, W.-W.; Hargan-Calvopiña, J.; Chen, P.-Y.; Clark, A.T. DNA Demethylation Dynamics in the Human Prenatal Germline. Cell 2015, 161, 1425–1436. [Google Scholar] [CrossRef]
- Grivna, S.T.; Pyhtila, B.; Lin, H. MIWI Associates with Translational Machinery and PIWI-Interacting RNAs (PiRNAs) in Regulating Spermatogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 13415–13420. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, S.S.; Low, D.H.P.; Yi, C.; Carrell, D.T.; Guccione, E.; Cairns, B.R. Chromatin and Transcription Transitions of Mammalian Adult Germline Stem Cells and Spermatogenesis. Cell Stem Cell 2014, 15, 239–253. [Google Scholar] [CrossRef]
- Sanz, E.; Evanoff, R.; Quintana, A.; Evans, E.; Miller, J.A.; Ko, C.; Amieux, P.S.; Griswold, M.D.; McKnight, G.S. RiboTag Analysis of Actively Translated MRNAs in Sertoli and Leydig Cells In Vivo. PLoS ONE 2013, 8, e66179. [Google Scholar] [CrossRef]
- Soumillon, M.; Necsulea, A.; Weier, M.; Brawand, D.; Zhang, X.; Gu, H.; Barthès, P.; Kokkinaki, M.; Nef, S.; Gnirke, A.; et al. Cellular Source and Mechanisms of High Transcriptome Complexity in the Mammalian Testis. Cell Rep. 2013, 3, 2179–2190. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ahn, H.W.; Chu, T.; Bowden, W.; Gassei, K.; Orwig, K.; Rajkovic, A. SOHLH1 and SOHLH2 Coordinate Spermatogonial Differentiation. Dev. Biol. 2012, 361, 301–312. [Google Scholar] [CrossRef]
- Hammoud, S.S.; Low, D.H.P.; Yi, C.; Lee, C.L.; Oatley, J.M.; Payne, C.J.; Carrell, D.T.; Guccione, E.; Cairns, B.R. Transcription and Imprinting Dynamics in Developing Postnatal Male Germline Stem Cells. Genes. Dev. 2015, 29, 2312–2324. [Google Scholar] [CrossRef] [PubMed]
- Irie, N.; Weinberger, L.; Tang, W.W.C.; Kobayashi, T.; Viukov, S.; Manor, Y.S.; Dietmann, S.; Hanna, J.H.; Surani, M.A. SOX17 Is a Critical Specifier of Human Primordial Germ Cell Fate. Cell 2015, 160, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Kitadate, Y.; Jörg, D.J.; Tokue, M.; Maruyama, A.; Ichikawa, R.; Tsuchiya, S.; Segi-Nishida, E.; Nakagawa, T.; Uchida, A.; Kimura-Yoshida, C.; et al. Competition for Mitogens Regulates Spermatogenic Stem Cell Homeostasis in an Open Niche. Cell Stem Cell 2019, 24, 79–92.e6. [Google Scholar] [CrossRef]
- Oatley, J.M.; Avarbock, M.R.; Telaranta, A.I.; Fearon, D.T.; Brinster, R.L. Identifying Genes Important for Spermatogonial Stem Cell Self-Renewal and Survival. Proc. Natl. Acad. Sci. USA 2006, 103, 9524–9529. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Hogarth, C.; Mitchell, D.; Griswold, M. Riding the Spermatogenic Wave: Profiling Gene Expression within Neonatal Germ and Sertoli Cells during a Synchronized Initial Wave of Spermatogenesis in Mice. Biol. Reprod. 2014, 90, 108. [Google Scholar] [CrossRef]
- Ge, R.-S.; Dong, Q.; Sottas, C.M.; Chen, H.; Zirkin, B.R.; Hardy, M.P. Gene Expression in Rat Leydig Cells during Development from the Progenitor to Adult Stage: A Cluster Analysis. Biol. Reprod. 2005, 72, 1405–1415. [Google Scholar] [CrossRef]
- Jauregui, E.J.; Mitchell, D.; Garza, S.M.; Topping, T.; Hogarth, C.A.; Griswold, M.D. Leydig Cell Genes Change Their Expression and Association with Polysomes in a Stage-Specific Manner in the Adult Mouse Testis. Biol. Reprod. 2018, 98, 722–738. [Google Scholar] [CrossRef]
- Johnston, D.S.; Wright, W.W.; Dicandeloro, P.; Wilson, E.; Kopf, G.S.; Jelinsky, S.A. Stage-Specific Gene Expression Is a Fundamental Characteristic of Rat Spermatogenic Cells and Sertoli Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 8315–8320. [Google Scholar] [CrossRef]
- Kliesch, S.; Penttilä, T.L.; Gromoll, J.; Saunders, P.T.; Nieschlag, E.; Parvinen, M. FSH Receptor MRNA Is Expressed Stage-Dependently during Rat Spermatogenesis. Mol. Cell. Endocrinol. 1992, 84, R45–R49. [Google Scholar] [CrossRef]
- McClelland, K.S.; Bell, K.; Larney, C.; Harley, V.R.; Sinclair, A.H.; Oshlack, A.; Koopman, P.; Bowles, J. Purification and Transcriptomic Analysis of Mouse Fetal Leydig Cells Reveals Candidate Genes for Specification of Gonadal Steroidogenic Cells. Biol. Reprod. 2015, 92, 145. [Google Scholar] [CrossRef]
- Parvinen, M.; Pelto-Huikko, M.; Söder, O.; Schultz, R.; Kaipia, A.; Mali, P.; Toppari, J.; Hakovirta, H.; Lönnerberg, P.; Ritzén, E.M. Expression of Beta-Nerve Growth Factor and Its Receptor in Rat Seminiferous Epithelium: Specific Function at the Onset of Meiosis. J. Cell Biol. 1992, 117, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Söderström, K.O.; Parvinen, M. RNA Synthesis in Different Stages of Rat Seminiferous Epithelial Cycle. Mol. Cell. Endocrinol. 1976, 5, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Grow, E.J.; Mlcochova, H.; Maher, G.J.; Lindskog, C.; Nie, X.; Guo, Y.; Takei, Y.; Yun, J.; Cai, L.; et al. The Adult Human Testis Transcriptional Cell Atlas. Cell Res. 2018, 28, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Sisakhtnezhad, S. In Silico Analysis of Single-Cell RNA Sequencing Data from 3 and 7 Days Old Mouse Spermatogonial Stem Cells to Identify Their Differentially Expressed Genes and Transcriptional Regulators. J. Cell. Biochem. 2018, 119, 7556–7569. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, Y.; Gao, Y.; Lin, Z.; Yang, S.; Wang, T.; Wang, Q.; Xie, N.; Hua, R.; Liu, M.; et al. Single-Cell RNA-Seq Uncovers Dynamic Processes and Critical Regulators in Mouse Spermatogenesis. Cell Res. 2018, 28, 879–896. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Song, H.-W.; Wilkinson, M.F. Single-Cell RNAseq Analysis of Testicular Germ and Somatic Cell Development during the Perinatal Period. Development 2020, 147, dev183251. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Wistuba, J.; Pock, T.; Schlatt, S.; Neuhaus, N. Spermatogonial Stem Cells: Updates from Specification to Clinical Relevance. Hum. Reprod. Update 2019, 25, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Valli, H.; Phillips, B.T.; Shetty, G.; Byrne, J.A.; Clark, A.T.; Meistrich, M.L.; Orwig, K.E. Germline Stem Cells: Toward the Regeneration of Spermatogenesis. Fertil. Steril. 2014, 101, 3–13. [Google Scholar] [CrossRef]
- Clermont, Y. The Cycle of the Seminiferous Epithelium in Man. Am. J. Anat. 1963, 112, 35–51. [Google Scholar] [CrossRef]
- Caldeira-Brant, A.L.; Martinelli, L.M.; Marques, M.M.; Reis, A.B.; Martello, R.; Almeida, F.R.C.L.; Chiarini-Garcia, H. A Subpopulation of Human Adark Spermatogonia Behaves as the Reserve Stem Cell. Reproduction 2020, 159, 437–451. [Google Scholar] [CrossRef]
- Jan, S.Z.; Vormer, T.L.; Jongejan, A.; Röling, M.D.; Silber, S.J.; de Rooij, D.G.; Hamer, G.; Repping, S.; van Pelt, A.M.M. Unraveling Transcriptome Dynamics in Human Spermatogenesis. Development 2017, 144, 3659–3673. [Google Scholar] [CrossRef]
- Tan, K.; Song, H.-W.; Thompson, M.; Munyoki, S.; Sukhwani, M.; Hsieh, T.-C.; Orwig, K.E.; Wilkinson, M.F. Transcriptome Profiling Reveals Signaling Conditions Dictating Human Spermatogonia Fate in Vitro. Proc. Natl. Acad. Sci. USA 2020, 117, 17832–17841. [Google Scholar] [CrossRef]
- Guo, J.; Sosa, E.; Chitiashvili, T.; Nie, X.; Rojas, E.J.; Oliver, E.; DonorConnect; Plath, K.; Hotaling, J.M.; Stukenborg, J.-B.; et al. Single-Cell Analysis of the Developing Human Testis Reveals Somatic Niche Cell Specification and Fetal Germline Stem Cell Establishment. Cell Stem Cell 2021, 28, 764–778.e4. [Google Scholar] [CrossRef]
- Brawley, C.; Matunis, E. Regeneration of Male Germline Stem Cells by Spermatogonial Dedifferentiation In Vivo. Science 2004, 304, 1331–1334. [Google Scholar] [CrossRef]
- Sheng, X.R.; Brawley, C.M.; Matunis, E.L. Dedifferentiating Spermatogonia Outcompete Somatic Stem Cells for Niche Occupancy in the Drosophila Testis. Cell Stem Cell 2009, 5, 191–203. [Google Scholar] [CrossRef]
- Hara, K.; Nakagawa, T.; Enomoto, H.; Suzuki, M.; Yamamoto, M.; Simons, B.D.; Yoshida, S. Mouse Spermatogenic Stem Cells Continually Interconvert between Equipotent Singly Isolated and Syncytial States. Cell Stem Cell 2014, 14, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Sharma, M.; Nabeshima, Y.; Braun, R.E.; Yoshida, S. Functional Hierarchy and Reversibility within the Murine Spermatogenic Stem Cell Compartment. Science 2010, 328, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Nie, X.; Giebler, M.; Mlcochova, H.; Wang, Y.; Grow, E.J.; DonorConnect; Kim, R.; Tharmalingam, M.; Matilionyte, G.; et al. The Dynamic Transcriptional Cell Atlas of Testis Development during Human Puberty. Cell Stem Cell 2020, 26, 262–276.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yao, C.; Xing, X.; Jing, T.; Li, P.; Zhu, Z.; Yang, C.; Zhai, J.; Tian, R.; Chen, H.; et al. Single-Cell Analysis of Developing and Azoospermia Human Testicles Reveals Central Role of Sertoli Cells. Nat. Commun. 2020, 11, 5683. [Google Scholar] [CrossRef]
- Alfano, M.; Tascini, A.S.; Pederzoli, F.; Locatelli, I.; Nebuloni, M.; Giannese, F.; Garcia-Manteiga, J.M.; Tonon, G.; Amodio, G.; Gregori, S.; et al. Aging, Inflammation and DNA Damage in the Somatic Testicular Niche with Idiopathic Germ Cell Aplasia. Nat. Commun. 2021, 12, 5205. [Google Scholar] [CrossRef]
- Mayerhofer, A. Human Testicular Peritubular Cells: More than Meets the Eye. Reproduction 2013, 145, R107–R116. [Google Scholar] [CrossRef] [PubMed]
- Mahyari, E.; Guo, J.; Lima, A.C.; Lewinsohn, D.P.; Stendahl, A.M.; Vigh-Conrad, K.A.; Nie, X.; Nagirnaja, L.; Rockweiler, N.B.; Carrell, D.T.; et al. Comparative Single-Cell Analysis of Biopsies Clarifies Pathogenic Mechanisms in Klinefelter Syndrome. Am. J. Hum. Genet. 2021, 108, 1924–1945. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AbuMadighem, A.; Cohen, O.; Huleihel, M. Elucidating the Transcriptional States of Spermatogenesis—Joint Analysis of Germline and Supporting Cell, Mice and Human, Normal and Perturbed, Bulk and Single-Cell RNA-Seq. Biomolecules 2024, 14, 840. https://doi.org/10.3390/biom14070840
AbuMadighem A, Cohen O, Huleihel M. Elucidating the Transcriptional States of Spermatogenesis—Joint Analysis of Germline and Supporting Cell, Mice and Human, Normal and Perturbed, Bulk and Single-Cell RNA-Seq. Biomolecules. 2024; 14(7):840. https://doi.org/10.3390/biom14070840
Chicago/Turabian StyleAbuMadighem, Ali, Ofir Cohen, and Mahmoud Huleihel. 2024. "Elucidating the Transcriptional States of Spermatogenesis—Joint Analysis of Germline and Supporting Cell, Mice and Human, Normal and Perturbed, Bulk and Single-Cell RNA-Seq" Biomolecules 14, no. 7: 840. https://doi.org/10.3390/biom14070840





