Blood-Derived Extracellular Vesicles as a Promising Liquid Biopsy Diagnostic Tool for Early Cancer Detection
Abstract
:1. Introduction
2. Discovery History of EVs
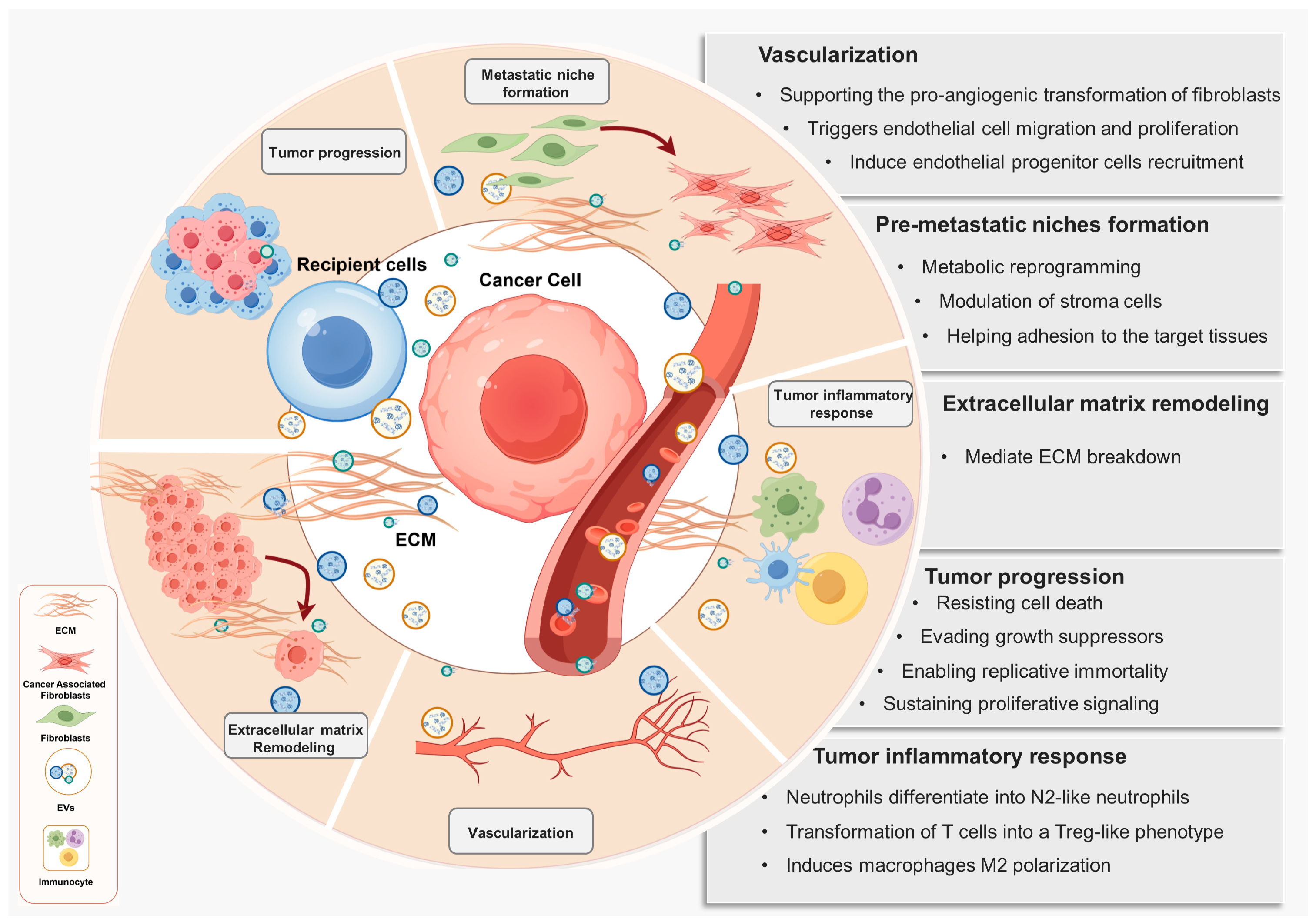
3. Heterogeneity and Features of Tumor-Derived Extracellular Vesicles
3.1. Classification of EVs
3.2. Biogenesis of EVs
3.3. Features of Tumor-Derived EVs
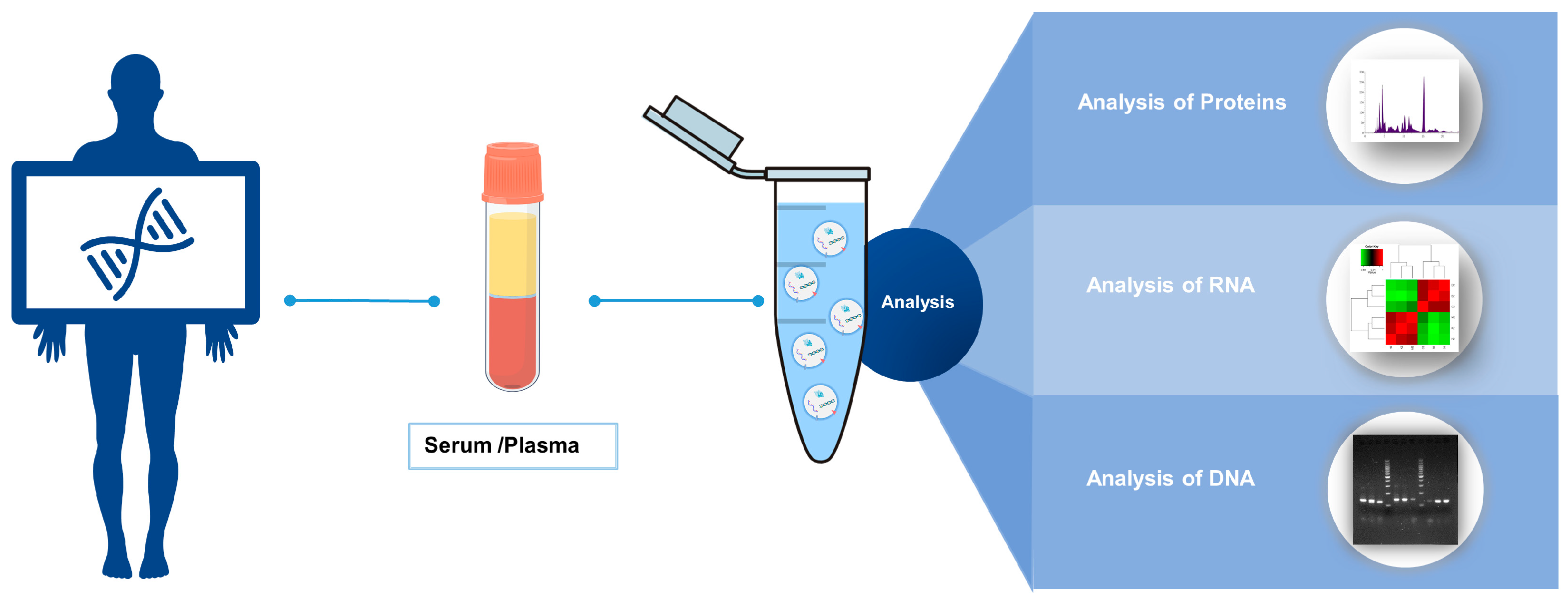
4. Advantages of Blood-Derived EVs as a Biomarker Source in Cancer Diagnosis
5. Progress in Research on Blood-Derived EV Biomarkers for Early Cancer Diagnosis
5.1. The Candidate Proteins in Blood-Derived EVs
5.2. The Candidate Nucleic Acids in Blood-Derived EVs
5.3. Extraction and Quality Control Methods of EVs
6. Limitations and Challenges
7. Discussion
8. Permission to Reuse and Copyright
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Andermann, A.; Blancquaert, I.; Beauchamp, S.; Déry, V. Revisiting Wilson and Jungner in the genomic age: A review of screening criteria over the past 40 years. Bull. World Health Organ. 2008, 86, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Dans, L.F.; Silvestre, M.A.; Dans, A.L. Trade-off between benefit and harm is crucial in health screening recommendations. Part I: General principles. J. Clin. Epidemiol. 2011, 64, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Teng, C.; Wan, W.; Wu, F.; Wu, C.; Ji, W.; Shan, Y. A panel of four plasma amino acids is a promising biomarker for newly diagnosed bladder cancer. Clin. Nutr. 2024, 43, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Bocchetti, M.; Luce, A.; Iannarone, C.; Pasquale, L.S.; Falco, M.; Tammaro, C.; Abate, M.; Ferraro, M.G.; Addeo, R.; Ricciardiello, F.; et al. Exosomes multiplex profiling, a promising strategy for early diagnosis of laryngeal cancer. J. Transl. Med. 2024, 22, 582. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Small extracellular vesicles are rejuvenating factors in young blood. Nat. Aging 2024, 4, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, Y.; Lu, X.; Liu, Y.; Liu, X.; Sun, Q.; Wang, L.; Huang, W.; Liu, A.; Liu, L.; et al. Endothelial cell-derived extracellular vesicles modulate the therapeutic efficacy of mesenchymal stem cells through IDH2/TET pathway in ARDS. Cell Commun. Signal. 2024, 22, 293. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Boyiadzis, M.; Whiteside, T.L. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia 2017, 31, 1259–1268. [Google Scholar] [CrossRef]
- Poupardin, R.; Wolf, M.; Strunk, D. Adherence to minimal experimental requirements for defining extracellular vesicles and their functions. Adv. Drug Deliv. Rev. 2021, 176, 113872. [Google Scholar] [CrossRef]
- Guo, W.; Huai, Q.; Liu, T.; Zhang, G.; Liang, N.; Ma, Q.; Liu, X.; Tan, F.; Xue, Q.; Gao, S.; et al. Plasma extracellular vesicle long RNA profiling identifies a diagnostic signature for stage I lung adenocarcinoma. Transl. Lung Cancer Res. 2022, 11, 572–587. [Google Scholar] [CrossRef]
- Guo, X.; Peng, Y.; Song, Q.; Wei, J.; Wang, X.; Ru, Y.; Xu, S.; Cheng, X.; Li, X.; Wu, D.; et al. A Liquid Biopsy Signature for the Early Detection of Gastric Cancer in Patients. Gastroenterology 2023, 165, 402–413.e13. [Google Scholar] [CrossRef]
- Ruivo, C.F.; Bastos, N.; Adem, B.; Batista, I.; Duraes, C.; Melo, C.A.; Castaldo, S.A.; Campos-Laborie, F.; Moutinho-Ribeiro, P.; Morão, B.; et al. Extracellular Vesicles from Pancreatic Cancer Stem Cells Lead an Intratumor Communication Network (EVNet) to fuel tumour progression. Gut 2022, 71, 2043–2068. [Google Scholar] [CrossRef]
- Caivano, A.; Laurenzana, I.; De Luca, L.; La Rocca, F.; Simeon, V.; Trino, S.; D’Auria, F.; Traficante, A.; Maietti, M.; Izzo, T.; et al. High serum levels of extracellular vesicles expressing malignancy-related markers are released in patients with various types of hematological neoplastic disorders. Tumour Biol. 2015, 36, 9739–9752. [Google Scholar] [CrossRef]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Crawford, N. The presence of contractile proteins in platelet microparticles isolated from human and animal platelet-free plasma. Br. J. Haematol. 1971, 21, 53–69. [Google Scholar] [CrossRef]
- Sun, C.N. Lattice structures and osmiophilic bodies in the developing respiratory tissue of rats. J. Ultrastruct. Res. 1966, 15, 380–388. [Google Scholar] [CrossRef]
- Anderson, H.C. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J. Cell Biol. 1969, 41, 59–72. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Mathew, A.; Mason, A.B.; Teng, K. Exosome formation during maturation of mammalian and avian reticulocytes: Evidence that exosome release is a major route for externalization of obsolete membrane proteins. J. Cell. Physiol. 1991, 147, 27–36. [Google Scholar] [CrossRef]
- Couzin, J. Cell biology: The ins and outs of exosomes. Science 2005, 308, 1862–1863. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Kim, Y.B.; Lee, G.B.; Moon, M.H. Size Separation of Exosomes and Microvesicles Using Flow Field-Flow Fractionation/Multiangle Light Scattering and Lipidomic Comparison. Anal. Chem. 2022, 94, 8958–8965. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Tosar, J.P.; Cayota, A.; Witwer, K. Exomeres and Supermeres: Monolithic or diverse? J. Extracell. Biol. 2022, 1, e45. [Google Scholar] [CrossRef]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020, 182, 1044–1061.e18. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Graves-Deal, R.; Trinh, V.Q.; Ramirez, M.A.; Sohn, Y.; Neininger, A.C.; Taneja, N.; McKinley, E.T.; et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat. Cell Biol. 2021, 23, 1240–1254. [Google Scholar] [CrossRef]
- D’Acunzo, P.; Pérez-González, R.; Kim, Y.; Hargash, T.; Miller, C.; Alldred, M.J.; Erdjument-Bromage, H.; Penikalapati, S.C.; Pawlik, M.; Saito, M.; et al. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci. Adv. 2021, 7, eabe5085. [Google Scholar] [CrossRef]
- Melentijevic, I.; Toth, M.L.; Arnold, M.L.; Guasp, R.J.; Harinath, G.; Nguyen, K.C.; Taub, D.; Parker, J.A.; Neri, C.; Gabel, C.V.; et al. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 2017, 542, 367–371. [Google Scholar] [CrossRef]
- Arnold, M.L.; Cooper, J.; Androwski, R.; Ardeshna, S.; Melentijevic, I.; Smart, J.; Guasp, R.J.; Nguyen, K.C.Q.; Bai, G.; Hall, D.H.; et al. Intermediate filaments associate with aggresome-like structures in proteostressed C. elegans neurons and influence large vesicle extrusions as exophers. Nat. Commun. 2023, 14, 4450. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef]
- Cotter, T.G.; Lennon, S.V.; Glynn, J.M.; Green, D.R. Microfilament-disrupting agents prevent the formation of apoptotic bodies in tumor cells undergoing apoptosis. Cancer Res. 1992, 52, 997–1005. [Google Scholar]
- Jackson, C.E.; Scruggs, B.S.; Schaffer, J.E.; Hanson, P.I. Effects of Inhibiting VPS4 Support a General Role for ESCRTs in Extracellular Vesicle Biogenesis. Biophys. J. 2017, 113, 1342–1352. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, R.; Wu, W.; Lei, L. miR-4454 Promotes Hepatic Carcinoma Progression by Targeting Vps4A and Rab27A. Oxid. Med. Cell. Longev. 2021, 2021, 9230435. [Google Scholar] [CrossRef]
- Han, Q.; Lv, L.; Wei, J.; Lei, X.; Lin, H.; Li, G.; Cao, J.; Xie, J.; Yang, W.; Wu, S.; et al. Vps4A mediates the localization and exosome release of β-catenin to inhibit epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2019, 457, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Tortola, L.; Egli, D.; Janjuha, S.; Rothgangl, T.; Marquart, K.F.; Ampenberger, F.; Kopf, M.; Schwank, G. Loss of Rnf31 and Vps4b sensitizes pancreatic cancer to T cell-mediated killing. Nat. Commun. 2022, 13, 1804. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Guo, C.; Zheng, H.; Zhao, K.; Luo, Y.; An, M.; Lin, Y.; Chen, J.; Li, Y.; Li, Y.; et al. SUMOylation-triggered ALIX activation modulates extracellular vesicles circTLCD4-RWDD3 to promote lymphatic metastasis of non-small cell lung cancer. Signal Transduct. Target. Ther. 2023, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.Y.; Tran, N.L.; Rusk, N.; Nakada, M.; Berens, M.E.; Symons, M. Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res. 2004, 64, 8271–8275. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, H.; Otani, T.; Sasaki, T.; Miura, Y.; Takai, Y.; Kogo, M. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells Devoted Mol. Cell. Mech. 2003, 8, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Zhang, T.; Chang, C.Y.; Wang, J.; Bazile, L.; Zhang, L.; Haffty, B.G.; Hu, W.; Feng, Z. Metabolic enzyme LDHA activates Rac1 GTPase as a noncanonical mechanism to promote cancer. Nat. Metab. 2022, 4, 1830–1846. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Chennakrishnaiah, S.; Audemard, E.; Montermini, L.; Meehan, B.; Rak, J. Oncogenic ras-driven cancer cell vesiculation leads to emission of double-stranded DNA capable of interacting with target cells. Biochem. Biophys. Res. Commun. 2014, 451, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.; Tran, P.T.; Gal, M.; Lee, S.J.; Na, S.H.; Hwangbo, C.; Lee, J.H. RAS-stimulated release of exosomal miR-494-3p promotes the osteolytic bone metastasis of breast cancer cells. Int. J. Mol. Med. 2023, 52, 84. [Google Scholar] [CrossRef] [PubMed]
- Montermini, L.; Meehan, B.; Garnier, D.; Lee, W.J.; Lee, T.H.; Guha, A.; Al-Nedawi, K.; Rak, J. Inhibition of oncogenic epidermal growth factor receptor kinase triggers release of exosome-like extracellular vesicles and impacts their phosphoprotein and DNA content. J. Biol. Chem. 2015, 290, 24534–24546. [Google Scholar] [CrossRef]
- Kang, Y.T.; Purcell, E.; Palacios-Rolston, C.; Lo, T.W.; Ramnath, N.; Jolly, S.; Nagrath, S. Isolation and Profiling of Circulating Tumor-Associated Exosomes Using Extracellular Vesicular Lipid-Protein Binding Affinity Based Microfluidic Device. Small 2019, 15, e1903600. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Dunlay, C.J.; Simeone, D.M.; Nagrath, S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 2014, 14, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Choi, M.; Lee, H.; Kim, Y.H.; Han, J.Y.; Lee, E.S.; Cho, Y. Direct isolation and characterization of circulating exosomes from biological samples using magnetic nanowires. J. Nanobiotechnol. 2019, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Garcia, V.; Rodriguez, M.; Compte, M.; Cisneros, E.; Veguillas, P.; Garcia, J.M.; Dominguez, G.; Campos-Martin, Y.; Cuevas, J.; et al. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer 2012, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Pan, S.; Gu, M.; Zhang, K.; Xia, T.; Zhang, S.; Chen, W.; Xie, H.; Fan, Y.; Yao, H.; et al. Extracellular vesicles rich in HAX1 promote angiogenesis by modulating ITGB6 translation. J. Extracell. Vesicles 2022, 11, e12221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mi, Y.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Xu, Y.; Cai, S.; Li, X.; Li, D. Highly-metastatic colorectal cancer cell released miR-181a-5p-rich extracellular vesicles promote liver metastasis by activating hepatic stellate cells and remodelling the tumour microenvironment. J. Extracell. Vesicles 2022, 11, e12186. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ge, X.; Shi, Z.; Yu, C.; Lu, C.; Wei, Y.; Zeng, A.; Wang, X.; Yan, W.; Zhang, J.; et al. Extracellular vesicles derived from hypoxic glioma stem-like cells confer temozolomide resistance on glioblastoma by delivering miR-30b-3p. Theranostics 2021, 11, 1763–1779. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.K.; Wong, S.W.K.; Chan, J.Y.T.; Mao, X.; Ng, T.H.; Yeung, C.L.S.; Leung, Z.; Fung, H.L.; Tang, A.H.N.; Wong, D.K.H.; et al. Patient pIgR-enriched extracellular vesicles drive cancer stemness, tumorigenesis and metastasis in hepatocellular carcinoma. J. Hepatol. 2022, 76, 883–895. [Google Scholar] [CrossRef] [PubMed]
- García-Silva, S.; Benito-Martín, A.; Nogués, L.; Hernández-Barranco, A.; Mazariegos, M.S.; Santos, V.; Hergueta-Redondo, M.; Ximénez-Embún, P.; Kataru, R.P.; Lopez, A.A.; et al. Melanoma-derived small extracellular vesicles induce lymphangiogenesis and metastasis through an NGFR-dependent mechanism. Nat. Cancer 2021, 2, 1387–1405. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Bardelli, A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef]
- Pang, B.; Zhu, Y.; Ni, J.; Thompson, J.; Malouf, D.; Bucci, J.; Graham, P.; Li, Y. Extracellular vesicles: The next generation of biomarkers for liquid biopsy-based prostate cancer diagnosis. Theranostics 2020, 10, 2309–2326. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Kosaka, N.; Konishi, Y.; Ohta, H.; Okamoto, H.; Sonoda, H.; Nonaka, R.; Yamamoto, H.; Ishii, H.; Mori, M.; et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat. Commun. 2014, 5, 3591. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Leong, S.M.; Kee, Z.; Caramat, P.V.; Teo, J.; Blanco, M.V.M.; Koay, E.S.C.; Cheong, W.K.; Soh, T.I.; Yong, W.P.; et al. Longitudinal monitoring reveals dynamic changes in circulating tumor cells (CTCs) and CTC-associated miRNAs in response to chemotherapy in metastatic colorectal cancer patients. Cancer Lett. 2018, 423, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra224. [Google Scholar] [CrossRef] [PubMed]
- Headley, M.B.; Bins, A.; Nip, A.; Roberts, E.W.; Looney, M.R.; Gerard, A.; Krummel, M.F. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature 2016, 531, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.J.; Tsui, D.W.; Murtaza, M.; Biggs, H.; Rueda, O.M.; Chin, S.F.; Dunning, M.J.; Gale, D.; Forshew, T.; Mahler-Araujo, B.; et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 2013, 368, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Tripathy, D.; Frenkel, E.P.; Shete, S.; Naftalis, E.Z.; Huth, J.F.; Beitsch, P.D.; Leitch, M.; Hoover, S.; Euhus, D.; et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 8152–8162. [Google Scholar] [CrossRef] [PubMed]
- Diehl, F.; Li, M.; Dressman, D.; He, Y.; Shen, D.; Szabo, S.; Diaz, L.A., Jr.; Goodman, S.N.; David, K.A.; Juhl, H.; et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 16368–16373. [Google Scholar] [CrossRef] [PubMed]
- Zill, O.A.; Banks, K.C.; Fairclough, S.R.; Mortimer, S.A.; Vowles, J.V.; Mokhtari, R.; Gandara, D.R.; Mack, P.C.; Odegaard, J.I.; Nagy, R.J.; et al. The Landscape of Actionable Genomic Alterations in Cell-Free Circulating Tumor DNA from 21,807 Advanced Cancer Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 3528–3538. [Google Scholar] [CrossRef]
- Locker, G.Y.; Hamilton, S.; Harris, J.; Jessup, J.M.; Kemeny, N.; Macdonald, J.S.; Somerfield, M.R.; Hayes, D.F.; Bast, R.C., Jr. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 5313–5327. [Google Scholar] [CrossRef]
- Kagohashi, K.; Satoh, H.; Ishikawa, H.; Ohtsuka, M.; Sekizawa, K. A re-evaluation of squamous cell carcinoma antigen (SCC) as a serum marker for non-small cell lung cancer. Med. Oncol. 2008, 25, 187–189. [Google Scholar] [CrossRef]
- De Luca, F.; Rotunno, G.; Salvianti, F.; Galardi, F.; Pestrin, M.; Gabellini, S.; Simi, L.; Mancini, I.; Vannucchi, A.M.; Pazzagli, M.; et al. Mutational analysis of single circulating tumor cells by next generation sequencing in metastatic breast cancer. Oncotarget 2016, 7, 26107–26119. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Han, L.; Yuan, J.; Sun, Y. Circulating tumor cell free DNA from plasma and urine in the clinical management of colorectal cancer. Cancer Biomark. Sect. A Dis. Markers 2020, 27, 29–37. [Google Scholar] [CrossRef]
- Dasgupta, S.K.; Abdel-Monem, H.; Niravath, P.; Le, A.; Bellera, R.V.; Langlois, K.; Nagata, S.; Rumbaut, R.E.; Thiagarajan, P. Lactadherin and clearance of platelet-derived microvesicles. Blood 2009, 113, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Munis, A.M.; Mattiuzzo, G.; Bentley, E.M.; Collins, M.K.; Eyles, J.E.; Takeuchi, Y. Use of Heterologous Vesiculovirus G Proteins Circumvents the Humoral Anti-envelope Immunity in Lentivector-Based In Vivo Gene Delivery. Mol. Ther. Nucleic Acids 2019, 17, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.; Jo, A.; Giedt, J.; Vinegoni, C.; Yang, K.S.; Peruzzi, P.; Chiocca, E.A.; Breakefield, X.O.; Lee, H.; Weissleder, R. Characterization of single microvesicles in plasma from glioblastoma patients. Neuro-Oncology 2019, 21, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, S.N.; Rider, M.A.; Bundy, J.L.; Liu, X.; Singh, R.K.; Meckes, D.G., Jr. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget 2016, 7, 86999–87015. [Google Scholar] [CrossRef] [PubMed]
- Li, S.R.; Man, Q.W.; Gao, X.; Lin, H.; Wang, J.; Su, F.C.; Wang, H.Q.; Bu, L.L.; Liu, B.; Chen, G. Tissue-derived extracellular vesicles in cancers and non-cancer diseases: Present and future. J. Extracell. Vesicles 2021, 10, e12175. [Google Scholar] [CrossRef] [PubMed]
- Lapitz, A.; Azkargorta, M.; Milkiewicz, P.; Olaizola, P.; Zhuravleva, E.; Grimsrud, M.M.; Schramm, C.; Arbelaiz, A.; O’Rourke, C.J.; La Casta, A.; et al. Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis and prognostication of cholangiocarcinoma. J. Hepatol. 2023, 79, 93–108. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.; Bai, T.; Xu, J.; Qian, Z.; Li, Y.; Guo, Z.; Zhu, Y. Detection of serum EphA2-EVs for pancreatic cancer diagnosis by light initiated chemiluminescent assay. Anal. Methods Adv. Methods Appl. 2022, 14, 1335–1341. [Google Scholar] [CrossRef]
- Moon, P.G.; Lee, J.E.; Cho, Y.E.; Lee, S.J.; Jung, J.H.; Chae, Y.S.; Bae, H.I.; Kim, Y.B.; Kim, I.S.; Park, H.Y.; et al. Identification of Developmental Endothelial Locus-1 on Circulating Extracellular Vesicles as a Novel Biomarker for Early Breast Cancer Detection. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1757–1766. [Google Scholar] [CrossRef]
- Kibria, G.; Ramos, E.K.; Lee, K.E.; Bedoyan, S.; Huang, S.; Samaeekia, R.; Athman, J.J.; Harding, C.V.; Lötvall, J.; Harris, L.; et al. A rapid, automated surface protein profiling of single circulating exosomes in human blood. Sci. Rep. 2016, 6, 36502. [Google Scholar] [CrossRef]
- Dash, S.; Wu, C.C.; Wu, C.C.; Chiang, S.F.; Lu, Y.T.; Yeh, C.Y.; You, J.F.; Chu, L.J.; Yeh, T.S.; Yu, J.S. Extracellular Vesicle Membrane Protein Profiling and Targeted Mass Spectrometry Unveil CD59 and Tetraspanin 9 as Novel Plasma Biomarkers for Detection of Colorectal Cancer. Cancers 2022, 15, 177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, Y.; Zeng, Y.; He, M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 2016, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Zhang, C.; Lee, Y.T.; Tran, B.V.; Wang, J.; Kim, H.; Lee, J.; Zhang, R.Y.; Wang, J.J.; Hu, J.; et al. HCC EV ECG score: An extracellular vesicle-based protein assay for detection of early-stage hepatocellular carcinoma. Hepatology 2023, 77, 774–788. [Google Scholar] [CrossRef]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, K.; Heidrich, I.; Kwiatkowski, M.; Banys-Paluchowski, M.; Andreas, A.; Wurlitzer, M.; Geffken, M.; Voß, H.; Zeller, T.; Blankenberg, S.; et al. Circulating Cellular Communication Network Factor 1 Protein as a Sensitive Liquid Biopsy Marker for Early Detection of Breast Cancer. Clin. Chem. 2022, 68, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; González-Prieto, R.; Zhang, M.; Geurink, P.P.; Kooij, R.; Iyengar, P.V.; van Dinther, M.; Bos, E.; Zhang, X.; Le Dévédec, S.E.; et al. Deubiquitinase Activity Profiling Identifies UCHL1 as a Candidate Oncoprotein That Promotes TGFβ-Induced Breast Cancer Metastasis. Clin. Cancer Res. 2020, 26, 1460–1473. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Wang, T.; Sun, X.; Zhang, P.; Chen, Y.; Jin, J.; Hua, D. UCH-L1-containing exosomes mediate chemotherapeutic resistance transfer in breast cancer. J. Surg. Oncol. 2017, 115, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Gibbs, L.D.; Maji, S.; Lewis, C.M.; Suzuki, S.; Vishwanatha, J.K. Serum exosomal-annexin A2 is associated with African-American triple-negative breast cancer and promotes angiogenesis. Breast Cancer Res. 2020, 22, 11. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Yang, S.; Pi, H.; Li, Z.; Yao, P.; Zhang, Q.; Wang, Q.; Shen, P.; Li, X.; et al. Proteomic Landscape of Exosomes Reveals the Functional Contributions of CD151 in Triple-Negative Breast Cancer. Mol. Cell. Proteom. 2021, 20, 100121. [Google Scholar] [CrossRef]
- Park, Y.H.; Shin, H.W.; Jung, A.R.; Kwon, O.S.; Choi, Y.J.; Park, J.; Lee, J.Y. Prostate-specific extracellular vesicles as a novel biomarker in human prostate cancer. Sci. Rep. 2016, 6, 30386. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Salmond, N.; Lynn, K.S.; Leong, H.S.; Williams, K.C. Clinical significance of STEAP1 extracellular vesicles in prostate cancer. Prostate Cancer Prostatic Dis. 2021, 24, 802–811. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, Y.; Ke, L.; Zhou, Q.; Chen, J.; Fan, M.; Wuethrich, A.; Trau, M.; Wang, J. Plasma extracellular vesicle phenotyping for the differentiation of early-stage lung cancer and benign lung diseases. Nanoscale Horiz. 2023, 8, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Faruque, H.A.; Kim, J.H.; Kim, K.J.; Choi, J.E.; Kim, B.A.; Kim, B.; Kim, Y.J.; Woo, M.H.; Park, J.Y.; et al. CD5L as an Extracellular Vesicle-Derived Biomarker for Liquid Biopsy of Lung Cancer. Diagnostics 2021, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [PubMed]
- San Lucas, F.A.; Allenson, K.; Bernard, V.; Castillo, J.; Kim, D.U.; Ellis, K.; Ehli, E.A.; Davies, G.E.; Petersen, J.L.; Li, D.; et al. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Maire, C.L.; Fuh, M.M.; Kaulich, K.; Fita, K.D.; Stevic, I.; Heiland, D.H.; Welsh, J.A.; Jones, J.C.; Görgens, A.; Ricklefs, T.; et al. Genome-wide methylation profiling of glioblastoma cell-derived extracellular vesicle DNA allows tumor classification. Neuro-Oncology 2021, 23, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Crescitelli, R.; Filges, S.; Karimi, N.; Urzì, O.; Alonso-Agudo, T.; Ståhlberg, A.; Lötvall, J.; Lässer, C.; Olofsson Bagge, R. Extracellular vesicle DNA from human melanoma tissues contains cancer-specific mutations. Front. Cell Dev. Biol. 2022, 10, 1028854. [Google Scholar] [CrossRef]
- Sun, L.; Du, M.; Kohli, M.; Huang, C.C.; Chen, X.; Xu, M.; Shen, H.; Wang, S.; Wang, L. An Improved Detection of Circulating Tumor DNA in Extracellular Vesicles-Depleted Plasma. Front. Oncol. 2021, 11, 691798. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, H.Y.; Hur, J.Y.; Kim, H.J.; Kim, I.A.; Kim, W.S.; Lee, K.Y. Genomic profiling of extracellular vesicle-derived DNA from bronchoalveolar lavage fluid of patients with lung adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 104–116. [Google Scholar] [CrossRef]
- Zavridou, M.; Strati, A.; Bournakis, E.; Smilkou, S.; Tserpeli, V.; Lianidou, E. Prognostic Significance of Gene Expression and DNA Methylation Markers in Circulating Tumor Cells and Paired Plasma Derived Exosomes in Metastatic Castration Resistant Prostate Cancer. Cancers 2021, 13, 780. [Google Scholar] [CrossRef] [PubMed]
- Ruhen, O.; Mirzai, B.; Clark, M.E.; Nguyen, B.; Salomon, C.; Erber, W.; Meehan, K. Comparison of Circulating Tumour DNA and Extracellular Vesicle DNA by Low-Pass Whole-Genome Sequencing Reveals Molecular Drivers of Disease in a Breast Cancer Patient. Biomedicines 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, J.; Xie, J.; Wang, Y.; Sun, J.; Liu, T.; Zhao, Y.; Zhao, X.; Wang, X.; Ma, Y.; et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020, 4, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, B.; Koch, R.; Radunski, U.; Corsham, S.; Cheong, N.; Inagaki, N.; Ban, N.; Wenzel, D.; Reinhardt, D.; Zapf, A.; et al. Intracellular ABC transporter A3 confers multidrug resistance in leukemia cells by lysosomal drug sequestration. Leukemia 2008, 22, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.T.; Kim, H.J.; Johnson-Camacho, K.C.; Kelley, T.; Newell, L.F.; Spellman, P.T.; Ngo, T.T.M. Diurnal stability of cell-free DNA and cell-free RNA in human plasma samples. Sci. Rep. 2020, 10, 16456. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, J.; Gong, Y.; Wu, Q.; Tan, S.; Sun, D.; Xu, X.; Zuo, Y.; Zhao, Y.; Wei, Y.Q.; et al. Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis. Mol. Cancer 2019, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Bryzgunova, O.E.; Zaripov, M.M.; Skvortsova, T.E.; Lekchnov, E.A.; Grigor’eva, A.E.; Zaporozhchenko, I.A.; Morozkin, E.S.; Ryabchikova, E.I.; Yurchenko, Y.B.; Voitsitskiy, V.E.; et al. Comparative Study of Extracellular Vesicles from the Urine of Healthy Individuals and Prostate Cancer Patients. PLoS ONE 2016, 11, e0157566. [Google Scholar] [CrossRef] [PubMed]
- Fettke, H.; Kwan, E.M.; Docanto, M.M.; Bukczynska, P.; Ng, N.; Graham, L.K.; Mahon, K.; Hauser, C.; Tan, W.; Wang, X.H.; et al. Combined Cell-free DNA and RNA Profiling of the Androgen Receptor: Clinical Utility of a Novel Multianalyte Liquid Biopsy Assay for Metastatic Prostate Cancer. Eur. Urol. 2020, 78, 173–180. [Google Scholar] [CrossRef]
- Zhou, G.; Lu, M.; Chen, J.; Li, C.; Zhang, J.; Chen, J.; Shi, X.; Wu, S. Identification of miR-199a-5p in serum as noninvasive biomarkers for detecting and monitoring osteosarcoma. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2015, 36, 8845–8852. [Google Scholar] [CrossRef]
- Zedan, A.H.; Hansen, T.F.; Assenholt, J.; Madsen, J.S.; Osther, P.J.S. Circulating miRNAs in localized/locally advanced prostate cancer patients after radical prostatectomy and radiotherapy. Prostate 2019, 79, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Mugoni, V.; Ciani, Y.; Nardella, C.; Demichelis, F. Circulating RNAs in prostate cancer patients. Cancer Lett. 2022, 524, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ding, X.; Bian, Y.; Wang, J.; Zhou, W.; Wang, X.; Li, P.; Shen, Y.; Wang, J.J.; Li, J.; et al. Discovery and validation of extracellular vesicle-associated miRNAs as noninvasive detection biomarkers for early-stage non-small-cell lung cancer. Mol. Oncol. 2021, 15, 2439–2452. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, W.; Wang, F.; Yang, S.; Hu, J.; Lu, B.; Pan, Z.; Ma, Y.; Zheng, M.; Zhou, L.; et al. Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol. Cancer 2021, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, D.; Wang, R.X.; Zhang, Y.T.; Zhang, S.Y.; Yang, C.; Pan, X.R.; Tong, J.H. Identification of potential biomarkers for digestive system cancers from serum-derived extracellular vesicle RNA. Clin. Chim. Acta Int. J. Clin. Chem. 2022, 531, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Ando, W.; Kikuchi, K.; Uematsu, T.; Yokomori, H.; Takaki, T.; Sogabe, M.; Kohgo, Y.; Otori, K.; Ishikawa, S.; Okazaki, I. Novel breast cancer screening: Combined expression of miR-21 and MMP-1 in urinary exosomes detects 95% of breast cancer without metastasis. Sci. Rep. 2019, 9, 13595. [Google Scholar] [CrossRef] [PubMed]
- Hinestrosa, J.P.; Kurzrock, R.; Lewis, J.M.; Schork, N.J.; Schroeder, G.; Kamat, A.M.; Lowy, A.M.; Eskander, R.N.; Perrera, O.; Searson, D.; et al. Early-stage multi-cancer detection using an extracellular vesicle protein-based blood test. Commun. Med. 2022, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, P.M.M.; Vieira, E.; Lemos, D.S.; Souza, I.L.M.; Zanata, S.M.; Pankievicz, V.C.; Tuleski, T.R.; Souza, E.M.; Wowk, P.F.; Urban, C.A.; et al. Identification of miRNAs Enriched in Extracellular Vesicles Derived from Serum Samples of Breast Cancer Patients. Biomolecules 2020, 10, 150. [Google Scholar] [CrossRef]
- Zou, X.; Li, M.; Huang, Z.; Zhou, X.; Liu, Q.; Xia, T.; Zhu, W. Circulating miR-532-502 cluster derived from chromosome X as biomarkers for diagnosis of breast cancer. Gene 2020, 722, 144104. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Xia, T.; Li, M.; Wang, T.; Liu, P.; Zhou, X.; Huang, Z.; Zhu, W. MicroRNA profiling in serum: Potential signatures for breast cancer diagnosis. Cancer Biomark. 2021, 30, 41–53. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, Y.; Zhang, X.; Yang, Y.; Zheng, X.; Li, X.; Liu, Y.; Zhang, Y. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol. Cancer 2018, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.M.; Ma, F.B.; Pan, S.R.; Liu, B.Z. Long noncoding RNA HOXA11-AS promotes gastric cancer cell proliferation and invasion via SRSF1 and functions as a biomarker in gastric cancer. World J. Gastroenterol. 2019, 25, 2763–2775. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, S.; Zhao, Y.; Wang, Y.; Piao, H.Y.; Wu, Y.; Zhang, J. Circulating long non-coding RNA PCGEM1 as a novel biomarker for gastric cancer diagnosis. Pathol. Res. Pract. 2019, 215, 152569. [Google Scholar] [CrossRef]
- Guo, X.; Lv, X.; Ru, Y.; Zhou, F.; Wang, N.; Xi, H.; Zhang, K.; Li, J.; Chang, R.; Xie, T.; et al. Circulating Exosomal Gastric Cancer-Associated Long Noncoding RNA1 as a Biomarker for Early Detection and Monitoring Progression of Gastric Cancer: A Multiphase Study. JAMA Surg. 2020, 155, 572–579. [Google Scholar] [CrossRef]
- Kahroba, H.; Samadi, N.; Mostafazadeh, M.; Hejazi, M.S.; Sadeghi, M.R.; Hashemzadeh, S.; Eftekhar Sadat, A.T.; Karimi, A. Evaluating the presence of deregulated tumoral onco-microRNAs in serum-derived exosomes of gastric cancer patients as noninvasive diagnostic biomarkers. Bioimpacts 2022, 12, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, Y.; Zhang, Q.; Zhang, H.; Du, J. Tumor-derived extracellular vesicles containing microRNA-1290 promote immune escape of cancer cells through the Grhl2/ZEB1/PD-L1 axis in gastric cancer. Transl. Res. 2021, 231, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cai, Y.; Chen, X.; Zhu, Y.; Cai, J. Circulating MiR-1290 as a potential diagnostic and disease monitoring biomarker of human gastrointestinal tumors. BMC Cancer 2021, 21, 989. [Google Scholar] [CrossRef]
- Varkalaite, G.; Vaitkeviciute, E.; Inciuraite, R.; Salteniene, V.; Juzenas, S.; Petkevicius, V.; Gudaityte, R.; Mickevicius, A.; Link, A.; Kupcinskas, L.; et al. Atrophic gastritis and gastric cancer tissue miRNome analysis reveals hsa-miR-129-1 and hsa-miR-196a as potential early diagnostic biomarkers. World J. Gastroenterol. 2022, 28, 653–663. [Google Scholar] [CrossRef]
- Chen, S.; Li, T.; Zhao, Q.; Xiao, B.; Guo, J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin. Chim. Acta 2017, 466, 167–171. [Google Scholar] [CrossRef]
- Roy, S.; Kanda, M.; Nomura, S.; Zhu, Z.; Toiyama, Y.; Taketomi, A.; Goldenring, J.; Baba, H.; Kodera, Y.; Goel, A. Diagnostic efficacy of circular RNAs as noninvasive, liquid biopsy biomarkers for early detection of gastric cancer. Mol. Cancer 2022, 21, 42. [Google Scholar] [CrossRef]
- Albino, D.; Falcione, M.; Uboldi, V.; Temilola, D.O.; Sandrini, G.; Merulla, J.; Civenni, G.; Kokanovic, A.; Stürchler, A.; Shinde, D.; et al. Circulating extracellular vesicles release oncogenic miR-424 in experimental models and patients with aggressive prostate cancer. Commun. Biol. 2021, 4, 119. [Google Scholar] [CrossRef]
- Guo, T.; Wang, Y.; Jia, J.; Mao, X.; Stankiewicz, E.; Scandura, G.; Burke, E.; Xu, L.; Marzec, J.; Davies, C.R.; et al. The Identification of Plasma Exosomal miR-423-3p as a Potential Predictive Biomarker for Prostate Cancer Castration-Resistance Development by Plasma Exosomal miRNA Sequencing. Front. Cell Dev. Biol. 2020, 8, 602493. [Google Scholar] [CrossRef]
- Jin, W.; Fei, X.; Wang, X.; Chen, F.; Song, Y. Circulating miRNAs as Biomarkers for Prostate Cancer Diagnosis in Subjects with Benign Prostatic Hyperplasia. J. Immunol. Res. 2020, 2020, 5873056. [Google Scholar] [CrossRef]
- Kryczka, J.; Migdalska-Sęk, M.; Kordiak, J.; Kiszałkiewicz, J.M.; Pastuszak-Lewandoska, D.; Antczak, A.; Brzeziańska-Lasota, E. Serum Extracellular Vesicle-Derived miRNAs in Patients with Non-Small Cell Lung Cancer-Search for Non-Invasive Diagnostic Biomarkers. Diagnostics 2021, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Xie, H.; Wei, T.; Zhu, D.; Zhang, C.; Yang, Y. Diagnostic potential of extracellular vesicle-associated microRNA-10b and tumor markers for lung adenocarcinoma. Oncol. Lett. 2021, 22, 614. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, J.; Liu, F.; Guo, J.; Gui, R. Diagnostic value of exosomal circMYC in radioresistant nasopharyngeal carcinoma. Head Neck 2020, 42, 3702–3711. [Google Scholar] [CrossRef]
- Casalone, E.; Birolo, G.; Pardini, B.; Allione, A.; Russo, A.; Catalano, C.; Mencoboni, M.; Ferrante, D.; Magnani, C.; Sculco, M.; et al. Serum Extracellular Vesicle-Derived microRNAs as Potential Biomarkers for Pleural Mesothelioma in a European Prospective Study. Cancers 2022, 15, 125. [Google Scholar] [CrossRef]
- Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; Bertier, L.; et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Campoy, I.; Lanau, L.; Altadill, T.; Sequeiros, T.; Cabrera, S.; Cubo-Abert, M.; Pérez-Benavente, A.; Garcia, A.; Borrós, S.; Santamaria, A.; et al. Exosome-like vesicles in uterine aspirates: A comparison of ultracentrifugation-based isolation protocols. J. Transl. Med. 2016, 14, 180. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef]
- Stranska, R.; Gysbrechts, L.; Wouters, J.; Vermeersch, P.; Bloch, K.; Dierickx, D.; Andrei, G.; Snoeck, R. Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma. J. Transl. Med. 2018, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lin, B.R.; Hsu, M.Y.; Cheng, C.M. Paper-based devices for isolation and characterization of extracellular vesicles. J. Vis. Exp. JoVE 2015, 98, e52722. [Google Scholar] [CrossRef]
- Greening, D.W.; Xu, R.; Ji, H.; Tauro, B.J.; Simpson, R.J. A protocol for exosome isolation and characterization: Evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol. Biol. 2015, 1295, 179–209. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, M.; Zhou, Y.; Tripathi, P.; Chandramohanadas, R.; Ai, Y. Exosome Purification and Analysis Using a Facile Microfluidic Hydrodynamic Trapping Device. Anal. Chem. 2020, 92, 10733–10742. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Xiang, X.; Ding, L.; Wong, A.L.; Zeng, Q.; Sethi, G.; Wang, L.; Lee, S.C.; Goh, B.C. Extracellular vesicles, the cornerstone of next-generation cancer diagnosis? Semin. Cancer Biol. 2021, 74, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, A.A.; Couch, Y.; Hadley, G.; Buchan, A.M. Neuroprotection in stroke: The importance of collaboration and reproducibility. Brain J. Neurol. 2017, 140, 2079–2092. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Reviews. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.L.; Herrmann, M.; Carter, D.R.F.; Turner, N.; Samuel, P.; Patel, B.A. Monitoring the electroactive cargo of extracellular vesicles can differentiate various cancer cell lines. Biosens. Bioelectron. 2024, 254, 116224. [Google Scholar] [CrossRef] [PubMed]
- Marleau, A.M.; Chen, C.S.; Joyce, J.A.; Tullis, R.H. Exosome removal as a therapeutic adjuvant in cancer. J. Transl. Med. 2012, 10, 134. [Google Scholar] [CrossRef]
- McKiernan, J.; Donovan, M.J.; Margolis, E.; Partin, A.; Carter, B.; Brown, G.; Torkler, P.; Noerholm, M.; Skog, J.; Shore, N.; et al. A Prospective Adaptive Utility Trial to Validate Performance of a Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer in Patients with Prostate-specific Antigen 2-10ng/ml at Initial Biopsy. Eur. Urol. 2018, 74, 731–738. [Google Scholar] [CrossRef]
- McKiernan, J.; Donovan, M.J.; O’Neill, V.; Bentink, S.; Noerholm, M.; Belzer, S.; Skog, J.; Kattan, M.W.; Partin, A.; Andriole, G.; et al. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol. 2016, 2, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Jin, X.; Wang, Y.; Zhang, Q.; Yang, L. High serum exosomal long non-coding RNA DANCR expression confers poor prognosis in patients with breast cancer. J. Clin. Lab. Anal. 2022, 36, e24186. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Zhu, Z.; Roy, S.; Jun, E.; Han, H.; Munoz, R.M.; Nishiwada, S.; Sharma, G.; Cridebring, D.; Zenhausern, F.; et al. An Exosome-based Transcriptomic Signature for Noninvasive, Early Detection of Patients with Pancreatic Ductal Adenocarcinoma: A Multicenter Cohort Study. Gastroenterology 2022, 163, 1252–1266.e2. [Google Scholar] [CrossRef] [PubMed]


| Cancer Type | Cargo | Sample | EV Source | The Assay | Results | Cite |
|---|---|---|---|---|---|---|
| Breast Cancer | CCN1 | 544 patients and 427 healthy controls | plasma | ELISA | Cancer detective sensitivity of 80% and specificity of 99% | [86] |
| Breast Cancer | UCHL1 | 10 patients and 25 healthy controls | serum | ELISA | UCHL1 levels in breast cancer patient serum samples higher than healthy donors, p < 0.01 | [87,88] |
| Breast Cancer | AnxA2 | 169 patients and 68 healthy controls | serum | ELISA | AnxA2 levels in breast cancer patient serum samples higher than healthy donors, p < 0.0001 | [89] |
| Breast Cancer | CD151 | 30 patients and 37 healthy controls | serum | Mass tag-based quantitative proteomics/Western Blot | CD151 expression was significantly increased in the TNBC patient-derived exosomes than healthy donors, p < 0.05 | [90] |
| Prostate cancer patients | PSMA | 82 cancer patients and 28 benign prostatic hyperplasia | plasma | ELISA | Cancer detective sensitivity of 91.7% and specificity of 83.3% | [91] |
| Prostate cancer patients | STEAP1 | 121 patients and 55 healthy controls | plasma | Western blot | Cancer detective sensitivity of 100% and specificity of 76.79% | [92] |
| Lung cancer | PTX3, THBS1, and CD63 | 28 early-stage lung cancer patients, 23 benign lung disease patients, and 26 healthy controls | plasma | Surface-enhanced Raman spectroscopy | Cancer detective sensitivity of 92.3% and specificity of 100% | [93] |
| Lung cancer Lung cancer | CD5L | 60 patients and 20 healthy controls | serum | Western blot | Cancer detective sensitivity of 92.9% and specificity of 94.1% | [94] |
| NFKBIA, NDUFB10, SLC7A7, ARPC5, SEPTIN9, HMGN1, H4C2, and lnc-PLA2G1B-2:3 | 64 early-stage lung cancer patients, 24 benign pulmonary nodule patients, and 22 healthy controls | plasma | Western blot | Cancer detective sensitivity of 95.8% and specificity of 91.7% | [13] |
| Cancer Type | Cargo | Sample | EV Source | The Assay | Results | Cite |
|---|---|---|---|---|---|---|
| Breast cancer | miR-142-5p, miR-320a, miR-4433b-5p | 31 patients with invasive ductal carcinoma, 16 healthy controls (CT) | serum | quantitative real-time PCR | Cancer detective sensitivity of 93.33% and a specificity of 68.75% | [118] |
| Breast cancer | miR-532-502 cluster | 354 patients with breast cancer and 404 healthy controls | plasma and serum | quantitative real-time PCR | The AUCs were 0.805 (95%CI: 0.702–0.908, D1) for the three-miRNA panel in plasma, and (95%CI: 0.821–0.969, D2) for the five-miRNA panel in serum. | [119] |
| Breast cancer | let-7b-5p, miR-106a-5p, miR-19a-3p, miR-19b-3p, miR-20a-5p, miR-223-3p, miR-25-3p, miR-425-5p, miR-451a, miR-92a-3p, miR-93-5p, and miR-16-5p | 216 patients and 214 healthy controls | serum | quantitative real-time PCR | Cancer detective sensitivity of 96.2% and a specificity of 94.9% | [120] |
| Gastric Cancer | long noncoding RNA HOTTIP | 126 patients and 120 healthy controls | serum | quantitative real-time PCR | Cancer detective sensitivity of 69.8 and specificity of 85.0% | [121] |
| Gastric Cancer | long noncoding RNA HOXA11-AS | 94 patients and 40 healthy controls | serum | quantitative real-time PCR | Cancer detective sensitivity of 78.7 and specificity of 97.8% | [122] |
| Gastric Cancer | long noncoding RNA PCGEM1 | 317 patients and 100 healthy controls | plasma | quantitative real-time PCR | Cancer detective sensitivity of 72.9 and specificity of 88.9% | [123] |
| Gastric Cancer | long noncoding RNA-GC1 | 607 patients and 219 healthy controls | plasma | quantitative real-time PCR | Cancer detective sensitivity of 88.24% and specificity of 82.29% | [124] |
| Gastric Cancer | onco-miRNA panel (miR-10a-5p, miR-19b-3p, miR-215-5p, and miR-18a-5p) | 43 patients and 43 healthy controls | serum | quantitative real-time PCR | Cancer detective sensitivity of 85%, 76.32%, 84.38%, 83.78%, 82.89% and specificity of 80.43%, 69.39%, 70.37%, 75.51%, 75.26%, respectively | [125] |
| Gastric Cancer | miR-1290 | 100 patients and 50 healthy controls | serum | quantitative real-time PCR | Cancer detective sensitivity of 26 and specificity of 90% | [126,127] |
| Gastric Cancer | miR-129-1 | 44 patients and 32 healthy controls | serum | quantitative real-time PCR | Cancer detective sensitivity of 84.2 and specificity of 78.9% | [128] |
| Gastric Cancer | miR-196a | 44 patients and 32 healthy controls | serum | quantitative real-time PCR | Cancer detective sensitivity of 89.5 and specificity of 94.7% | [128] |
| Gastric Cancer | hsa_circ_0000190 | 104 patients and 104 healthy controls | plasma | quantitative real-time PCR | Cancer detective sensitivity of 71.2 and specificity of 75% | [129] |
| Gastric Cancer | circRNA panel | 194 patients and 94 healthy controls | serum | quantitative real-time PCR | Cancer detective sensitivity of 78% and specificity of 78% | [130] |
| Prostate cancer patients | miR-424 | 58 cancer patients and 6 benign prostatic hyperplasia | plasma | quantitative real-time PCR | Metastatic castration-resistant-derived EVs had higher levels of miR-424 compared to normal and primary tumors | [131] |
| Prostate cancer patients | miR-423-3p | 58 cancer patients and 6 benign prostatic hyperplasia | plasma | quantitative real-time PCR | Cancer detective sensitivity of 80.95% and specificity of 82.41% | [132] |
| Prostate cancer patients | combinations of miR-141, miR-182, miR-200b, and miR-375 | 31 cancer patients and 31 benign prostatic hyperplasia | serum | quantitative real-time PCR | AUC = 0.923, 95% CI between 0.8620 and 0.9840 | [133] |
| Lung cancer | miR-23a, miR-361, miR-1228, and miR-let7i | 31 patients and 21 healthy controls | serum | quantitative real-time PCR | Cancer detective sensitivity of 52% and specificity of 83% | [134] |
| Lung cancer | microRNA-10b | 80 patients and 69 healthy controls | plasma | quantitative real-time PCR | Cancer detective sensitivity of 98.75% and specificity of 98.55% | [135] |
| Nasopharyngeal carcinoma | circMYC | 210 patients and 158 healthy controls | serum | quantitative real-time PCR | Cancer detective sensitivity of 90.24% and specificity of 94.51% | [136] |
| Pleural mesothelioma | miRNA panel (miR-11400, miR-148a-3p, miR-409-3p) | 82 patients and 82 healthy controls | serum | quantitative real-time PCR | Cancer detective sensitivity of 75% and specificity of 70% | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, D.; Cui, B.; Lv, H.; Lu, S.; Zhu, Y.; Cheng, Y.; Dang, L.; Zhang, H. Blood-Derived Extracellular Vesicles as a Promising Liquid Biopsy Diagnostic Tool for Early Cancer Detection. Biomolecules 2024, 14, 847. https://doi.org/10.3390/biom14070847
He D, Cui B, Lv H, Lu S, Zhu Y, Cheng Y, Dang L, Zhang H. Blood-Derived Extracellular Vesicles as a Promising Liquid Biopsy Diagnostic Tool for Early Cancer Detection. Biomolecules. 2024; 14(7):847. https://doi.org/10.3390/biom14070847
Chicago/Turabian StyleHe, Dan, Bozhou Cui, Hongkai Lv, Shuxian Lu, Yuan Zhu, Yuqiang Cheng, Lin Dang, and Hong Zhang. 2024. "Blood-Derived Extracellular Vesicles as a Promising Liquid Biopsy Diagnostic Tool for Early Cancer Detection" Biomolecules 14, no. 7: 847. https://doi.org/10.3390/biom14070847




