Hepatic Gene Expression and Metabolite Profiles of Androstenone and Skatole Relative to Plasma Estrone Sulfate Levels in Boars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Hepatocyte Isolation and Treatments
2.3. Assessment of Metabolite Production by HPLC
2.4. RNA Extraction and Evaluation of Gene Expression by Real-Time qPCR
2.5. Statistical Analysis
3. Results
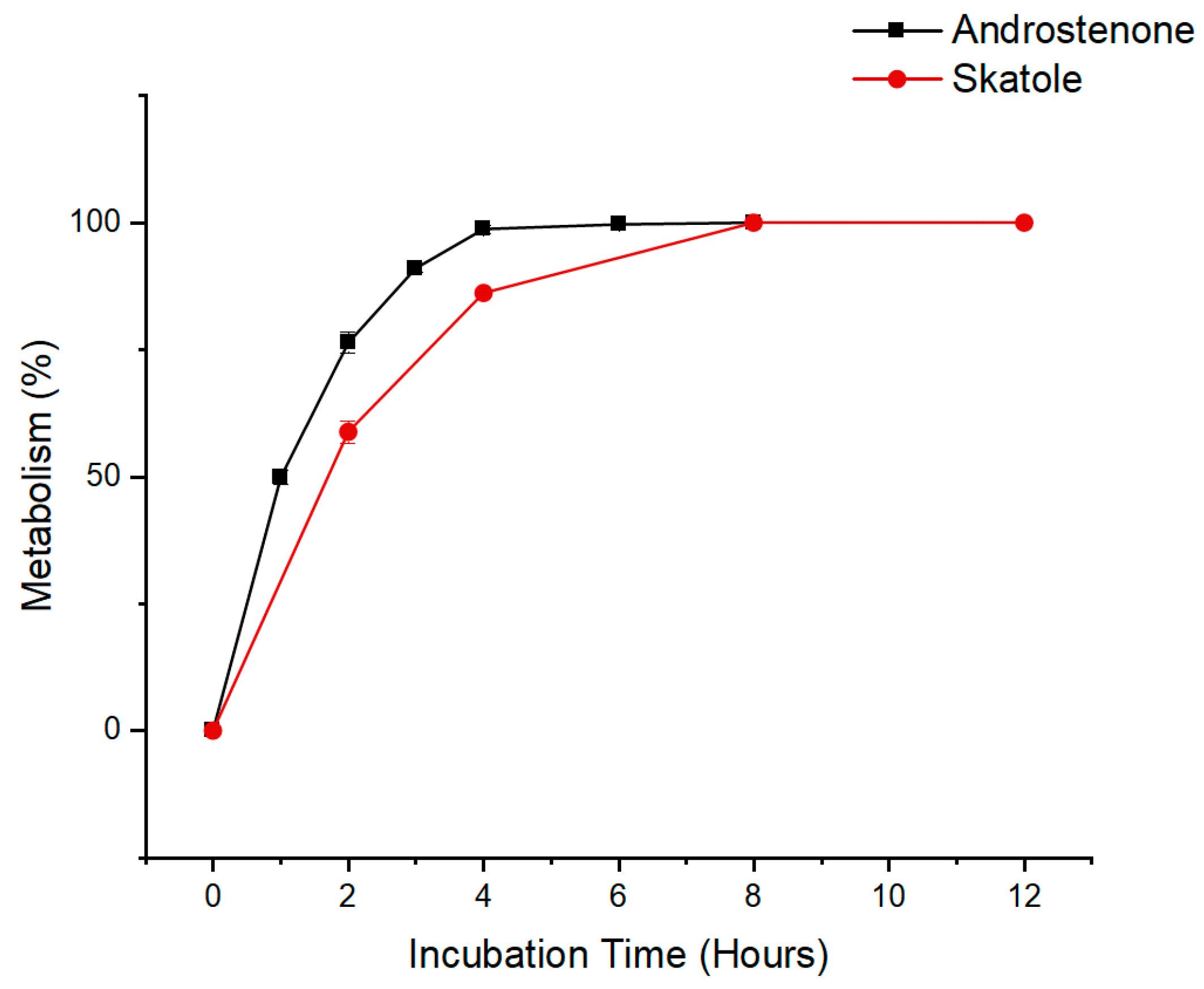
3.1. Time Course Analysis of Androstenone and Skatole Metabolism by Hepatocytes
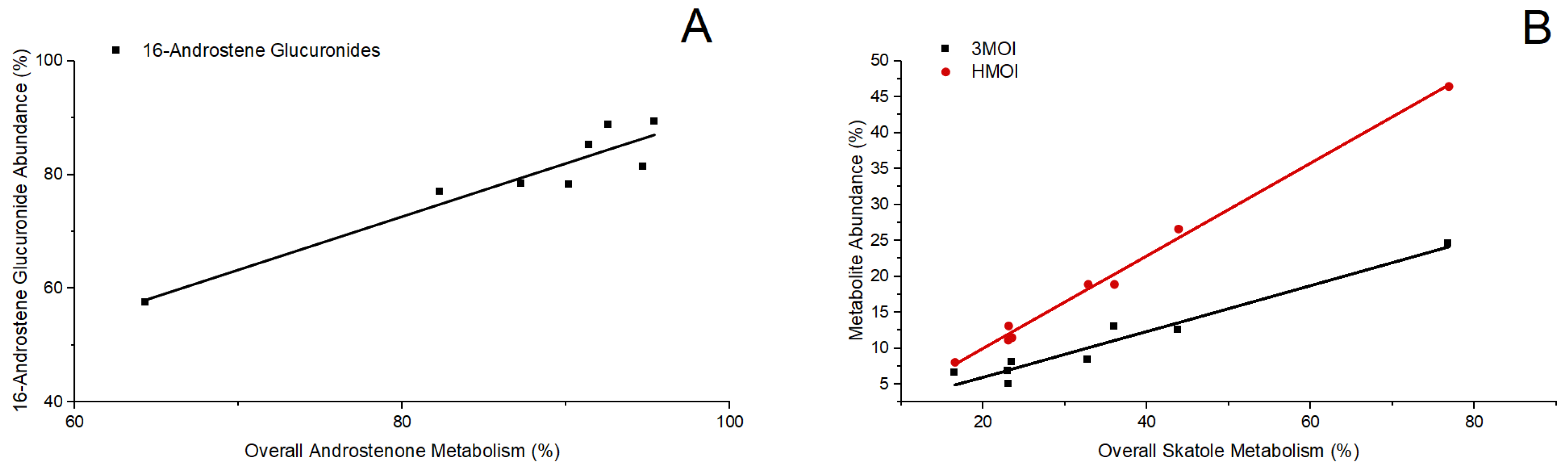
3.2. Assessment of Hepatic Gene Expression Levels and Metabolite Profiles of Androstenone and Skatole in Slaughter-Weight Boars
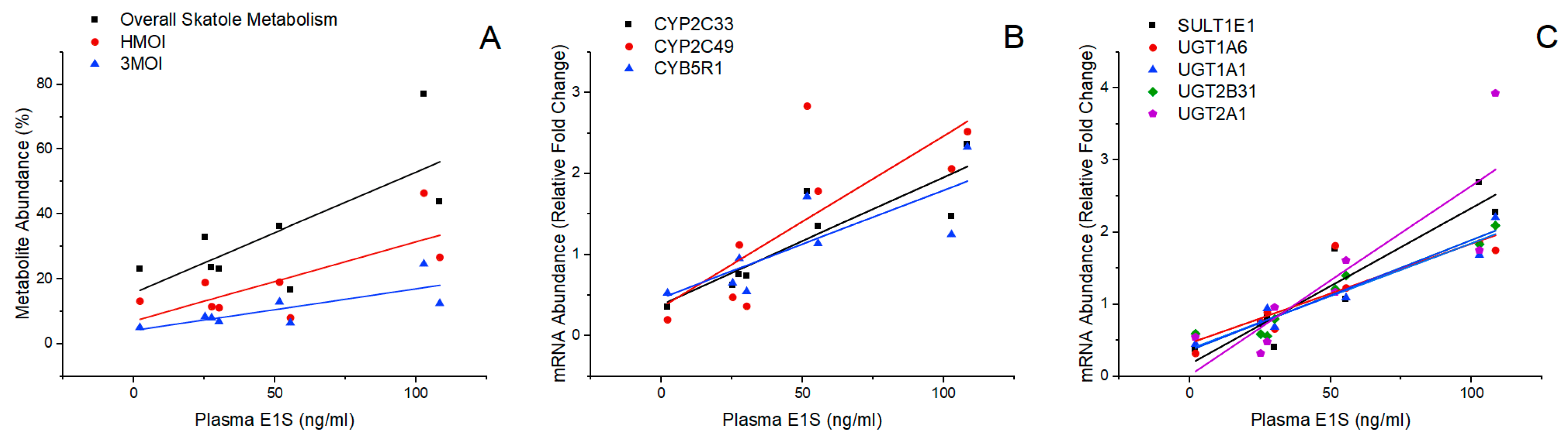
3.3. Relationship between Plasma E1S Levels and the Metabolism of Boar Taint Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gower, D.B. 16-Unsaturated C19 Steroids A Review of Their Chemistry, Biochemistry and Possible Physiological Role. J. Steroid Biochem. 1972, 3, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Morikawa, Y.; Matsunaga, T.; Hara, A.; Nishinaka, T. Porcine Aldo-Keto Reductase 1C Subfamily Members AKR1C1 and AKR1C4: Substrate Specificity, Inhibitor Sensitivity and Activators. J. Steroid Biochem. Mol. Biol. 2022, 221, 106113. [Google Scholar] [CrossRef] [PubMed]
- Bæk, C.; Hansen-Møller, J.; Friis, C.; Cornett, C.; Hansen, S.H. Identification of Selected Metabolites of Skatole in Plasma and Urine from Pigs. J. Agric. Food Chem. 1997, 45, 2332–2340. [Google Scholar] [CrossRef]
- Friis, C. Disposition of Skatole in Male and Female Pigs. In Proceedings of the Fifth European ISSX Meeting, Tours, France, 26–29 September 1993; p. 133. [Google Scholar]
- Wiercinska, P.; Lou, Y.; Squires, E.J. The Roles of Different Porcine Cytochrome P450 Enzymes and Cytochrome B5A in Skatole Metabolism. Animal 2012, 6, 834–845. [Google Scholar] [CrossRef]
- Friis, C. Is Boar Taint Related to Sex Differences or Polymorphism of Skatole Metabolism? In Proceedings of the European Association for Animal Production Working Group on Production and Utilization of Meat from Entire Male Pigs, Milton Keynes, UK, 27–29 September 1995. [Google Scholar]
- Matal, J.; Matuskova, Z.; Tunkova, A.; Anzenbacherova, E.; Anzenbacher, P. Porcine CYP2A19, CYP2E1 and CYP1A2 Forms Are Responsible for Skatole Biotransformation in the Reconstituted System. Neuroendocrinol. Lett. 2009, 30, 36–40. [Google Scholar] [PubMed]
- Burkina, V.; Rasmussen, M.K.; Oliinychenko, Y.; Zamaratskaia, G. Porcine Cytochrome 2A19 and 2E1. Basic Clin. Pharmacol. Toxicol. 2019, 124, 32–39. [Google Scholar] [CrossRef]
- Laderoute, H.; Bone, C.; Squires, E.J. The Sulfoconjugation of Androstenone and Dehydroepiandrosterone by Human and Porcine Sulfotransferase Enzymes. Steroids 2018, 136, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Diaz, G.J.; Squires, E.J. Phase II in Vitro Metabolism of 3-Methylindole Metabolites in Porcine Liver. Xenobiotica 2003, 33, 485–498. [Google Scholar] [CrossRef]
- Kojima, M.; Degawa, M. Sex Differences in the Constitutive Gene Expression of Sulfotransferases and UDP-Glucuronosyltransferases in the Pig Liver: Androgen-Mediated Regulation. Drug Metab. Pharmacokinet. 2014, 29, 192–197. [Google Scholar] [CrossRef]
- Kojima, M.; Degawa, M. Sex Differences in Constitutive mRNA Levels of CYP2B22, CYP2C33, CYP2C49, CYP3A22, CYP3A29 and CYP3A46 in the Pig Liver: Comparison between Meishan and Landrace Pigs. Drug Metab. Pharmacokinet. 2016, 31, 185–192. [Google Scholar] [CrossRef]
- Doran, E.; Whittington, F.W.; Wood, J.D.; McGivan, J.D. Cytochrome P450IIE1 (CYP2E1) Is Induced by Skatole and This Induction Is Blocked by Androstenone in Isolated Pig Hepatocytes. Chem. Biol. Interact. 2002, 140, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Nicolau-Solano, S.I.; Doran, O. Effect of Testosterone, Estrone Sulphate and Androstenone on 3β-Hydroxysteroid Dehydrogenase Protein Expression in Primary Cultured Hepatocytes. Livest. Sci. 2008, 114, 202–210. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Berger, T. Skatole Metabolism in the Pigs with Reduced Testicular Oestrogen Synthesis. Reprod. Domest. Anim. 2014, 49, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Zamaratskaia, G.; Ekstrand, B. Gender-Related Differences in Cytochrome P450 in Porcine Liver—Implication for Activity, Expression and Inhibition by Testicular Steroids. Reprod. Domest. Anim. 2011, 46, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Zamaratskaia, G.; Ekstrand, B. In Vitro Cytochrome P450 2E1 and 2A Activities in the Presence of Testicular Steroids. Reprod. Domest. Anim. 2011, 46, 149–154. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Zlabek, V.; Ropstad, E.; Andresen, O. In Vitro and In Vivo Association of Porcine Hepatic Cytochrome P450 3A and 2C Activities with Testicular Steroids. Reprod. Domest. Anim. 2012, 47, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberger, F.; Toole, G.S.; Christie, H.L.; Raeside, J.I. Plasma Levels of Several Androgens and Estrogens from Birth to Puberty in Male Domestic Pigs. Acta Endocrinol. 1993, 128, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, P.A.; Squires, E.J.; Raeside, J.I. Early Postnatal Plasma Concentrations of Testicular Steroid Hormones, Pubertal Development, and Carcass Leanness as Potential Indicators of Boar Taint in Market Weight Intact Male Pigs. J. Anim. Sci. 2001, 79, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Zamaratskaia, G.; Rydhmer, L.; Chen, G.; Madej, A.; Andersson, H.K.; Lundström, K. Boar Taint Is Related to Endocrine and Anatomical Changes at Puberty but Not to Aggressive Behaviour in Entire Male Pigs. Reprod. Domest. Anim. 2005, 40, 500–506. [Google Scholar] [CrossRef]
- Bone, C.; Squires, E.J. The Uptake and Deconjugation of Androstenone Sulfate in the Adipose Tissue of the Boar. Animals 2021, 11, 3158. [Google Scholar] [CrossRef]
- Wang, D.; Lu, R.; Rempala, G.; Sadee, W. Ligand-Free Estrogen Receptor α (ESR1) as Master Regulator for the Expression of CYP3A4 and Other Cytochrome P450 Enzymes in the Human Liver. Mol. Pharmacol. 2019, 96, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.M.; Wang, D. Co-Expression of Drug Metabolizing Cytochrome P450 Enzymes and Estrogen Receptor Alpha (ESR1) in Human Liver: Racial Differences and the Regulatory Role of ESR1. Drug Metab. Pers. Ther. 2021, 36, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Raeside, J.I.; Renaud, R.L. Estrogen and Androgen Production by Purified Leydig Cells of Mature Boars. Biol. Reprod. 1983, 28, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.A.; Squires, E.J. Effects of Nuclear Receptor Transactivation on Boar Taint Metabolism and Gene Expression in Porcine Hepatocytes. J. Steroid Biochem. Mol. Biol. 2013, 133, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.; Yuan, S.; Wood, K.; Katona, M.; Straub, A.C. Cytochrome B5 Reductases: Redox Regulators of Cell Homeostasis. J. Biol. Chem. 2022, 298, 102654. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.J.; Fashe, M.; Arakawa, S.; Moore, R.; Sueyoshi, T.; Negishi, M. Nuclear Receptor CAR-ERα Signaling Regulates the Estrogen Sulfotransferase Gene in the Liver. Sci. Rep. 2020, 10, 5001. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Ikenaka, Y.; Nakayama, S.M.M.; Kawai, Y.K.; Mizukawa, H.; Mitani, Y.; Nomiyama, K.; Tanabe, S.; Ishizuka, M. Uridine Diphosphate-Glucuronosyltransferase (UGT) 2B Subfamily Interspecies Differences in Carnivores. Toxicol. Sci. 2017, 158, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Hankele, A.K.; Bauersachs, S.; Ulbrich, S.E. Conjugated Estrogens in the Endometrium during the Estrous Cycle in Pigs. Reprod. Biol. 2018, 18, 336–343. [Google Scholar] [CrossRef]
- Sten, T.; Bichlmaier, I.; Kuuranne, T.; Leinonen, A.; Yli-Kauhaluoma, J.; Finel, M. UDP-Glucuronosyltransferases (UGTs) 2B7 and UGT2B17 Display Converse Specificity in Testosterone and Epitestosterone Glucuronidation, Whereas UGT2A1 Conjugates Both Androgens Similarly. Drug Metab. Dispos. 2009, 37, 417–423. [Google Scholar] [CrossRef]
- Itäaho, K.; Mackenzie, P.I.; Ikushiro, S.I.; Miners, J.O.; Finel, M. The Configuration of the 17-Hydroxy Group Variably Influences the Glucuronidation of β-Estradiol and Epiestradiol by Human UDP-Glucuronosyltransferases. Drug Metab. Dispos. 2008, 36, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Deming, S.L.; Zheng, W.; Xu, W.H.; Cai, Q.; Ruan, Z.; Xiang, Y.B.; Shu, X.O. UGT1A1 Genetic Polymorphisms, Endogenous Estrogen Exposure, Soy Food Intake, and Endometrial Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2008, 17, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Zamaratskaia, G. Regulation of Porcine Hepatic Cytochrome P450—Implication for Boar Taint. Comput. Struct. Biotechnol. J. 2014, 11, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, M. Factors Affecting the Level of Androstenone. Acta Vet. Scand. 2006, 48 (Suppl. S1), S7. [Google Scholar] [CrossRef]
- Lanthier, F.; Lou, Y.; Squires, E.J. Skatole Metabolism in the Intact Pre-Pubescent Male Pig: The Relationship between Hepatic Enzyme Activity and Skatole Concentrations in Plasma and Fat. Livest. Sci. 2007, 106, 145–153. [Google Scholar] [CrossRef]
- Kojima, M.; Degawa, M. Serum Androgen Level Is Determined by Autosomal Dominant Inheritance and Regulates Sex-Related CYP Genes in Pigs. Biochem. Biophys. Res. Commun. 2013, 430, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Moe, M.; Lien, S.; Bendixen, C.; Hedegaard, J.; Hornshøj, H.; Berget, I.; Meuwissen, T.H.; Grindflek, E. Gene Expression Profiles in Liver of Pigs with Extreme High and Low Levels of Androstenone. BMC Vet. Res. 2008, 4, 29. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Klausen, C.L.; Ekstrand, B. Regulation of Cytochrome P450 MRNA Expression in Primary Porcine Hepatocytes by Selected Secondary Plant Metabolites from Chicory (Cichorium intybus L.). Food Chem. 2014, 146, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Monshouwer, M.; Van’t Klooster, G.A.E.; Nijmeijer, S.M.; Witkamp, R.F.; Van Miert, A.S.J.P.A.M. Characterization of Cytochrome P450 Isoenzymes in Primary Cultures of Pig Hepatocytes. Toxicol. Vitr. 1998, 12, 715–723. [Google Scholar] [CrossRef]
- MacKenzie, P.I.; Hu, D.G.; Gardner-Stephen, D.A. The Regulation of UDP-Glucuronosyltransferase Genes by Tissue-Specific and Ligand-Activated Transcription Factors. Drug Metab. Rev. 2010, 42, 99–109. [Google Scholar] [CrossRef]
- Gerbal-Chaloin, S.; Briolotti, P.; Daujat-Chavanieu, M.; Rasmussen, M.K. Primary Hepatocytes Isolated from Human and Porcine Donors Display Similar Patterns of Cytochrome P450 Expression Following Exposure to Prototypical Activators of AhR, CAR and PXR. Curr. Res. Toxicol. 2021, 2, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Fashe, M.; Hashiguchi, T.; Yi, M.J.; Moore, R.; Negishi, M. Phenobarbital-Induced Phosphorylation Converts Nuclear Receptor RORα from a Repressor to an Activator of the Estrogen Sulfotransferase Gene Sult1e1 in Mouse Livers. FEBS Lett. 2018, 592, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, E.; Gervasi, P.G.; Marca, M.L.; Beffy, P.; Longo, V. Expression and Inducibility by Phenobarbital of CYP2C33, CYP2C42, CYP2C49, CYP2B22, and CYP3As in Porcine Liver, Kidney, Small Intestine, and Nasal Tissues. Xenobiotica 2010, 40, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Men, S.; Wang, H. Phenobarbital in Nuclear Receptor Activation: An Update. Drug Metab. Dispos. 2023, 51, 210–218. [Google Scholar] [CrossRef]
- Bone, C.; Squires, E.J. Nuclear Receptor Pathways Mediating the Development of Boar Taint. Metabolites 2022, 12, 785. [Google Scholar] [CrossRef]

|
Gene
Abbreviation | Gene Name | Function | Refseq ID |
|---|---|---|---|
| CYP2A19 | Cytochrome P450 2A19 | Phase I skatole metabolism [5,6,7,8] | NM_214417 |
| CYP2E1 | Cytochrome P450 2E1 | NM_214421 | |
| CYP2C33 | Cytochrome P450 2C33 | NM_214414 | |
| CYP2C49 | Cytochrome P450 2C49 | NM_214420 | |
| CYB5A | Cytochrome B5A | Phase I skatole metabolism and steroidogenesis [5,28] | XM_005666296 |
| CYBR1 | Cytochrome B5 reductase, type 1 | NM_001243918.1 | |
| CYBR3 | Cytochrome B5 reductase, type 3 | XM_003125982.4 | |
| AKR1C1 | Aldo-keto reductase 1C1 | Phase I androstenone metabolism [2] | NM_001044618 |
| SULT1A1 | Sulfotransferase 1A1 | Phase II sulfoconjugation of skatole [10] | NM_213765 |
| SULT2A1 | Sulfotransferase 2A1 | Phase II sulfoconjugation of androstenone [9] | NM_001037150 |
| SULT1E1 | Sulfotransferase 1E1 | Phase II sulfoconjugation of estrogens [29] | NM_213992.1 |
| UGT1A6 | UDP-glucuronosyltransferase 1A6 | Phase II glucuronidation of estrogens [30,31,32,33,34] | NM_001278750.1 |
| UGT1A1 | UDP-glucuronosyltransferase 1A1 | KJ922612.1 | |
| UGT2A1 | UDP-glucuronosyltransferase 2A1 | XM_003356958.4 | |
| UGT2B31 | UDP-glucuronosyltransferase 2B31 | NM_001244124.1 | |
| ACTB | β-Actin | - | XM_003357928 |
| Primer | Forward Sequence | Reverse Sequence |
|---|---|---|
| CYP2A19 | 5′-TGAACACGGAGCAGATGTACAAC-3′ | 5′-CTCCTTCACCGCGTCGTATC-3′ |
| CYP2E1 | 5′-TGCTCCACTACAAGAATGAGTTCTCT-3′ | 5′-GGGAGAACCGCCGAGTGT-3′ |
| CYP2C33 | 5′-TTGGATAAAGATGGCAGCTTCAG-3′ | 5′-AATGGTGGTGAAGAACAGGAAGA-3′ |
| CYP2C49 | 5′-TCCCCAACCCAGAGGTGTT-3′ | 5′-CCTTCTCCCACACAAATTCGTT-3′ |
| CYB5A | 5′-AGTCCGACAAAGCCGTGAA-3′ | 5′-CACCTCCAGCTTGTTCCCT-3′ |
| CYBR1 | 5′-ATTTCCTGAGGGAGGGAAGA-3′ | 5′-GGCTGAATGCTGAACTTTCC-3′ |
| CYBR3 | 5′-GTGATGACGACAAGGGCTTT-3′ | 5′-AAACTTTCCTTTGCCCTGGT-3′ |
| AKR1C1 | 5′-GGAGGACTTTTTCCCAAAGG-3′ | 5′-TCCCTCGTTCTTGCACTTCT-3′ |
| SULT1A1 | 5′-GAACAACGCCATGACCAACTAC-3′ | 5′-GGTTACAGCCTGCCATCTTC-3′ |
| SULT2A1 | 5′-ACACGAGAAGCGCCGTAGAG-3′ | 5′-TGGACATGTTGTTTTCTTTCATGA-3′ |
| SULT1E1 | 5′-GCATCAGATGAGCTTGTGGA-3′ | 5′-AGTCTCCTGCAATCCCCTTT-3′ |
| UGT1A6 | 5′-TGCTTTGGGCAAAATACCTC-3′ | 5′-CTTTGGGTGACCAAGCAGAT-3′ |
| UGT1A1 | 5′-ATAATTACCCGAGGCCCATC-3′ | 5′-CCCCAAAGAGAAAACCACAA-3′ |
| UGT2B31 | 5′-TTTGAGACAATGGGGAAAGC-3′ | 5′-AGGTAGGGGTTTTGCAGGTT-3′ |
| UGT2A1 | 5′-TGCACGTTACTGAAAATGCAAG-3′ | 5′-TTGTAAAAGCCAGAGCACATCA-3′ |
| ACTB | 5′-CGTGGACATCAGGAAGGAC-3′ | 5′-TCTGCTGGAAGGTGGACAG-3′ |
| (A) | ||
| Metabolite Abundance (%) | ||
| Metabolite | Mean ± Standard Error | Range |
| 16-Androstene Glucuronides | 79.45 ± 3.56 | 57.50–89.30 |
| Androstenols | 7.84 ± 1.17 | 3.90–13.30 |
| Overall Androstenone Metabolism | 87.28 ± 3.60 | 64.30–95.40 |
| Values shown represent the percentage of each metabolite quantified by high-performance liquid chromatography in the media from isolated hepatocytes following 3-h incubations with androstenone (n = 8). | ||
| (B) | ||
| Metabolite Abundance (%) | ||
| Metabolite | Mean ± Standard Error | Range |
| HMOI | 19.35 ± 4.40 | 8.06–46.45 |
| 3MOI | 10.61 ± 2.23 | 4.98–24.61 |
| 6-OH-3MI Glucuronide | 4.40 ± 0.43 | 1.84–5.61 |
| I3C | 0.12 ± 0.02 | 0.00–0.22 |
| Overall Skatole Metabolism | 34.46 ± 6.80 | 16.57–76.84 |
| Values shown represent the percentage of each metabolite quantified by high-performance liquid chromatography in the media from isolated hepatocytes following 3-h incubations with skatole (n = 8). | ||
| Gene Expression (Relative Fold Change) | ||
|---|---|---|
| Gene | Mean ± Standard Error | Range |
| CYP2A19 | 18.60 ± 11.63 | 0.01–89.11 |
| CYP2C33 | 1.18 ± 0.24 | 0.36–2.36 |
| CYP2E1 | 4.59 ± 2.08 | 0.01–15.86 |
| CYP2C49 | 1.42 ± 0.36 | 0.20–2.83 |
| AKR1C1 | 1.18 ± 0.23 | 0.28–2.33 |
| CYB5A | 1.25 ± 0.36 | 0.36–3.16 |
| CYB5R1 | 1.14 ± 0.22 | 0.53–2.32 |
| CYB5R3 | 1.32 ± 0.37 | 0.51–2.75 |
| SULT1A1 | 2.08 ± 0.87 | 0.30–6.58 |
| SULT2A1 | 1.18 ± 0.21 | 0.25–1.98 |
| SULT1E1 | 1.26 ± 0.31 | 0.40–2.69 |
| UGT1A6 | 1.16 ± 0.21 | 0.32–1.85 |
| UGT1A1 | 1.12 ± 0.20 | 0.44–2.20 |
| UGT2B31 | 1.13 ± 0.21 | 0.56–2.09 |
| UGT2A1 | 1.34 ± 0.41 | 0.31–3.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bone, C.; Squires, E.J. Hepatic Gene Expression and Metabolite Profiles of Androstenone and Skatole Relative to Plasma Estrone Sulfate Levels in Boars. Biomolecules 2024, 14, 850. https://doi.org/10.3390/biom14070850
Bone C, Squires EJ. Hepatic Gene Expression and Metabolite Profiles of Androstenone and Skatole Relative to Plasma Estrone Sulfate Levels in Boars. Biomolecules. 2024; 14(7):850. https://doi.org/10.3390/biom14070850
Chicago/Turabian StyleBone, Christine, and E. James Squires. 2024. "Hepatic Gene Expression and Metabolite Profiles of Androstenone and Skatole Relative to Plasma Estrone Sulfate Levels in Boars" Biomolecules 14, no. 7: 850. https://doi.org/10.3390/biom14070850
APA StyleBone, C., & Squires, E. J. (2024). Hepatic Gene Expression and Metabolite Profiles of Androstenone and Skatole Relative to Plasma Estrone Sulfate Levels in Boars. Biomolecules, 14(7), 850. https://doi.org/10.3390/biom14070850






