The Immunological Profile of Adipose Mesenchymal Stromal/Stem Cells after Cell Expansion and Inflammatory Priming
Abstract
1. Introduction
2. Materials and Methods
2.1. AT-MSC Sample Collection and Culture
2.2. Inflammatory Priming
2.3. Flow Cytometry
2.4. Transcriptional Profiling
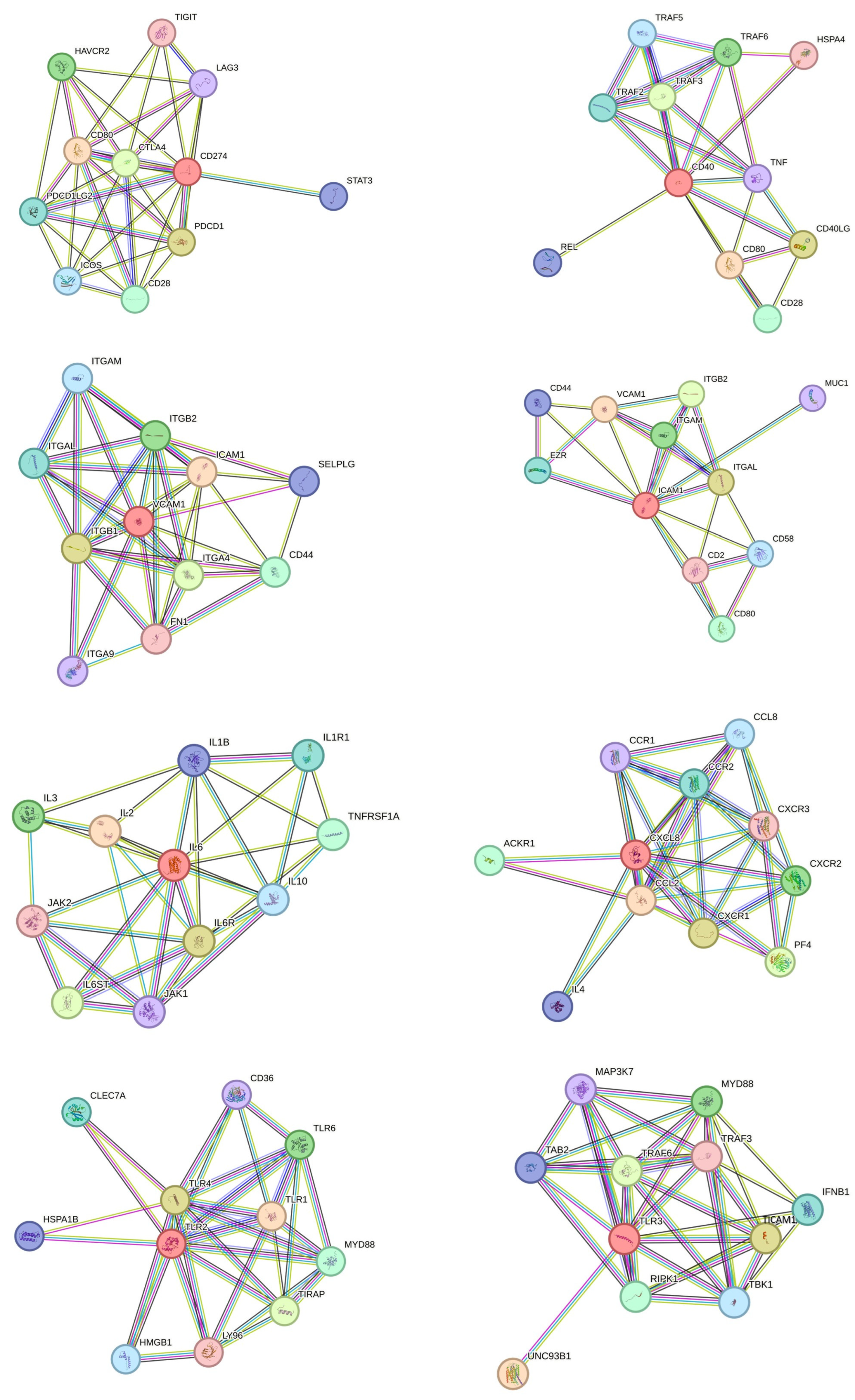
2.5. Protein–Protein Interaction Network
2.6. Statistical Analysis
3. Results
3.1. Morphology
3.2. Immune Comparative Screening of AT-MSCs
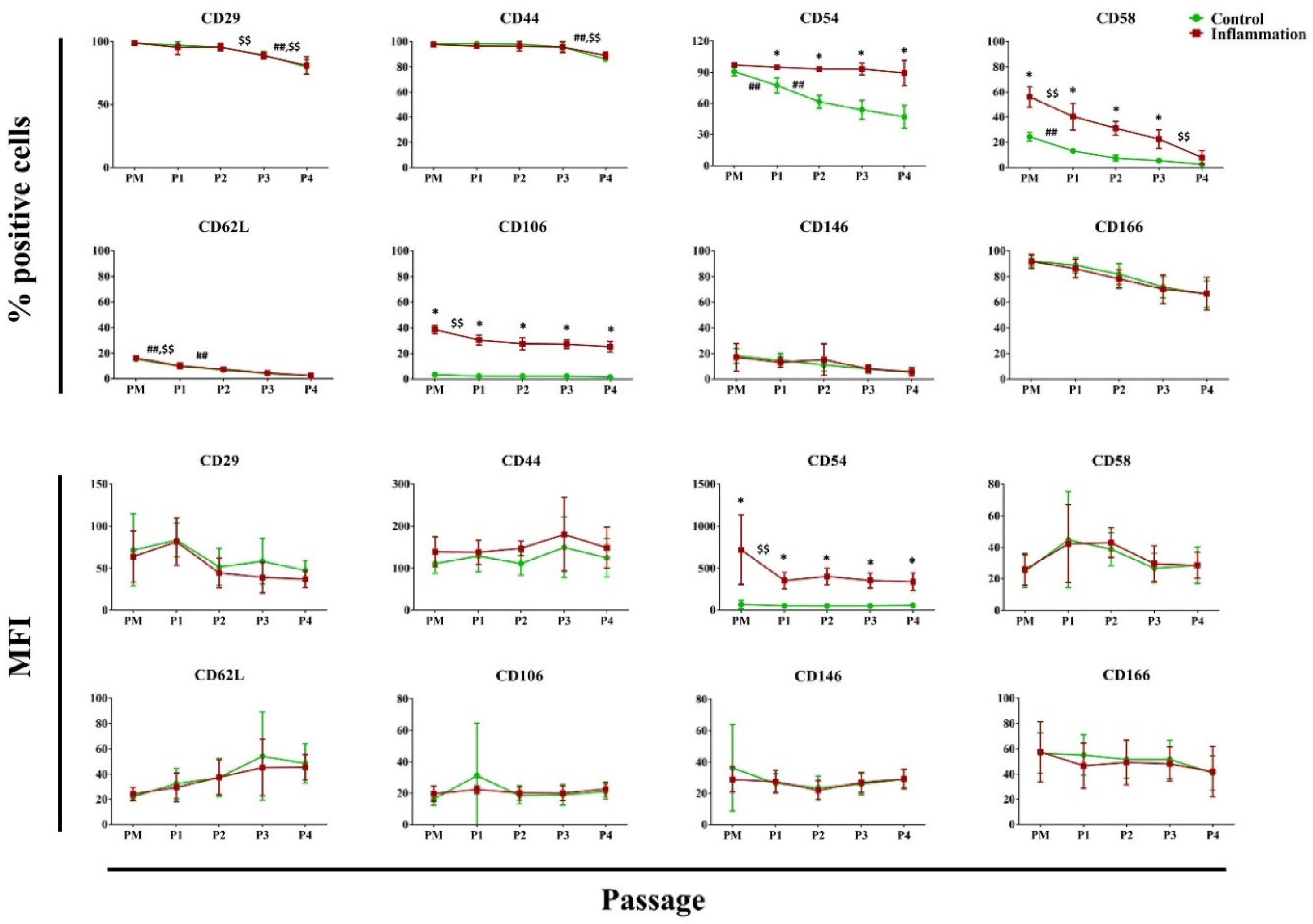
3.2.1. Hematopoietic and Stromal Markers
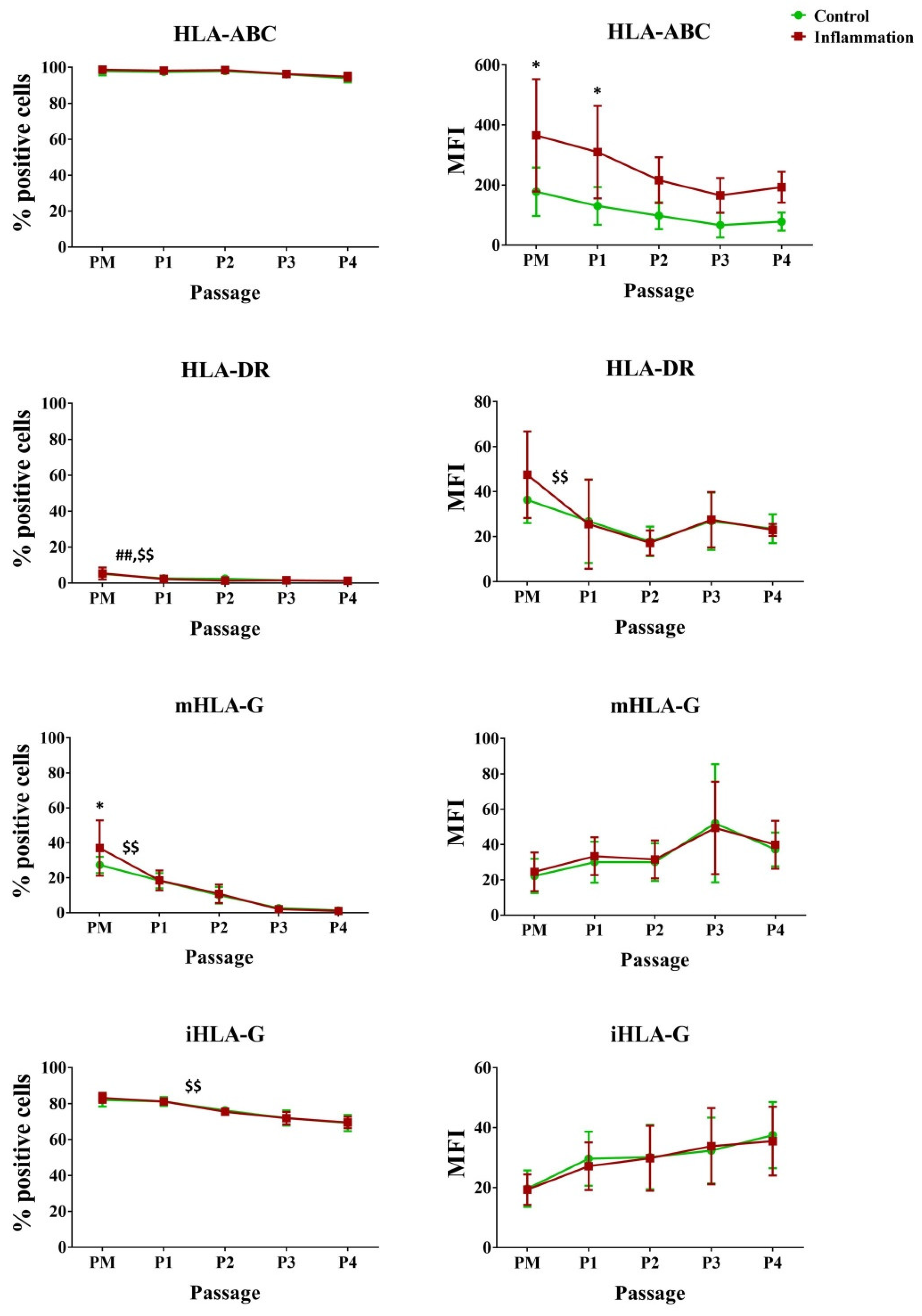
3.2.2. Human Leukocyte Antigens
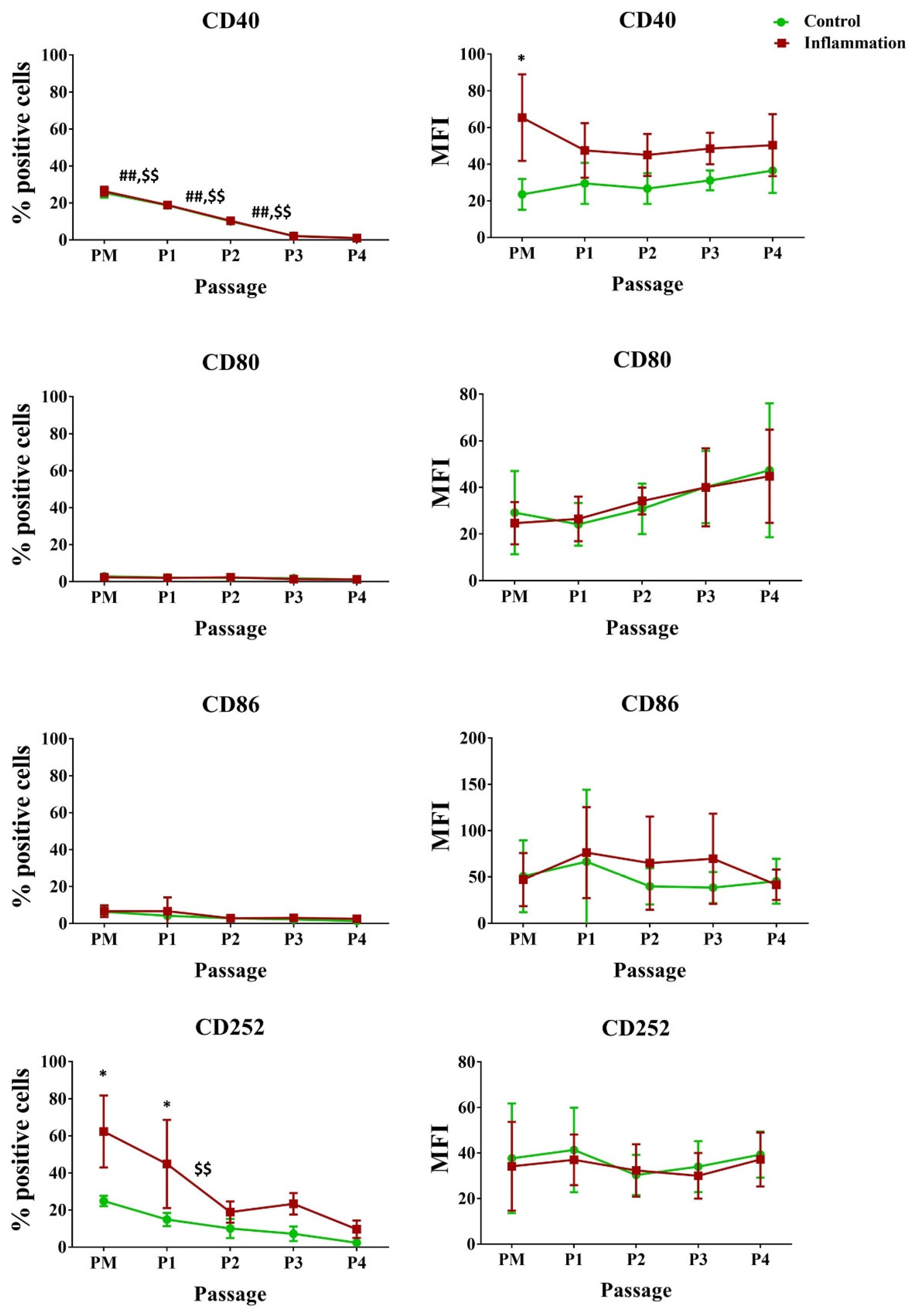
3.2.3. Co-Stimulatory Molecules
3.2.4. Cell Adhesion Molecules
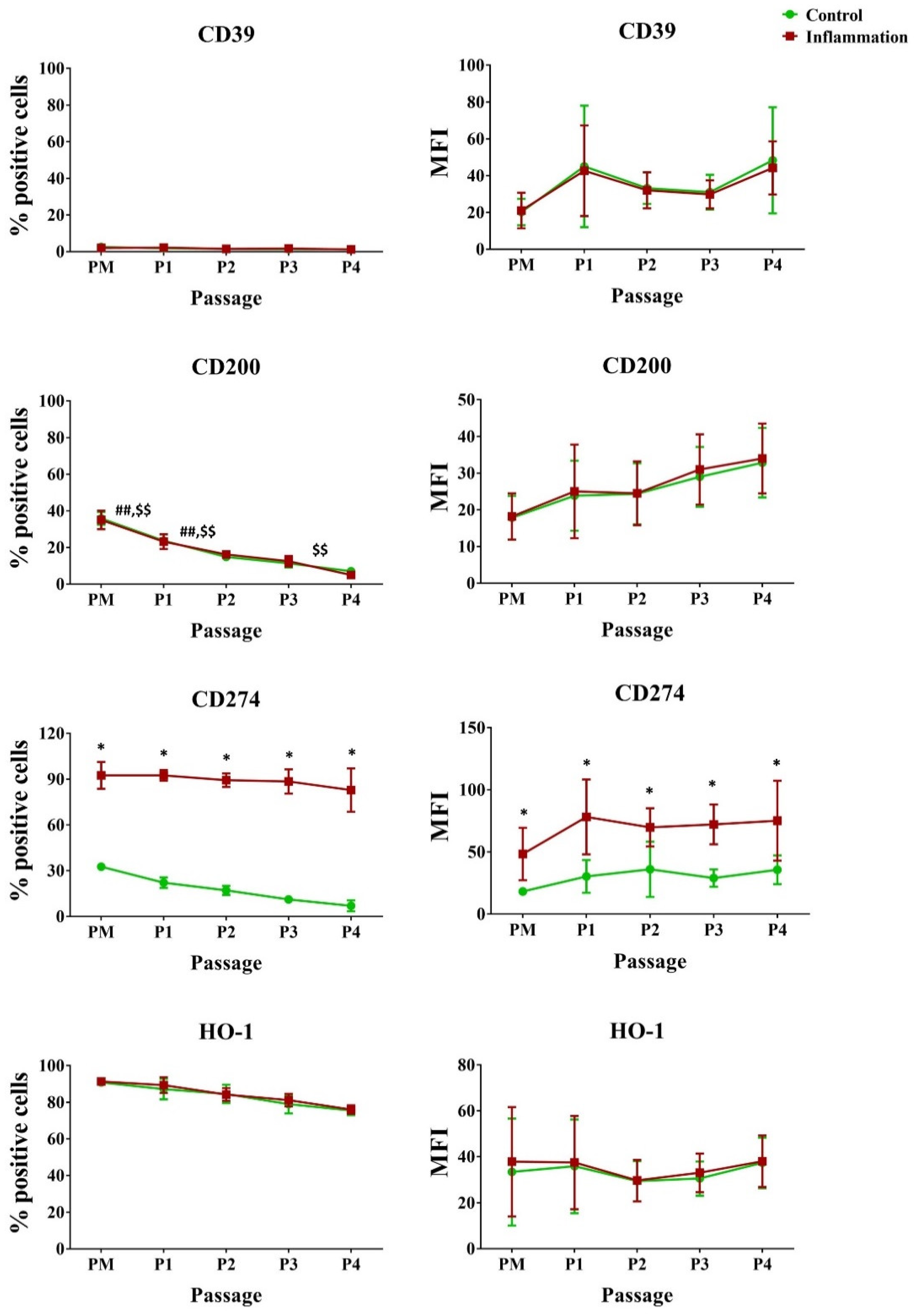
3.2.5. Immunoregulatory Mediators
3.2.6. Natural Killer Ligands
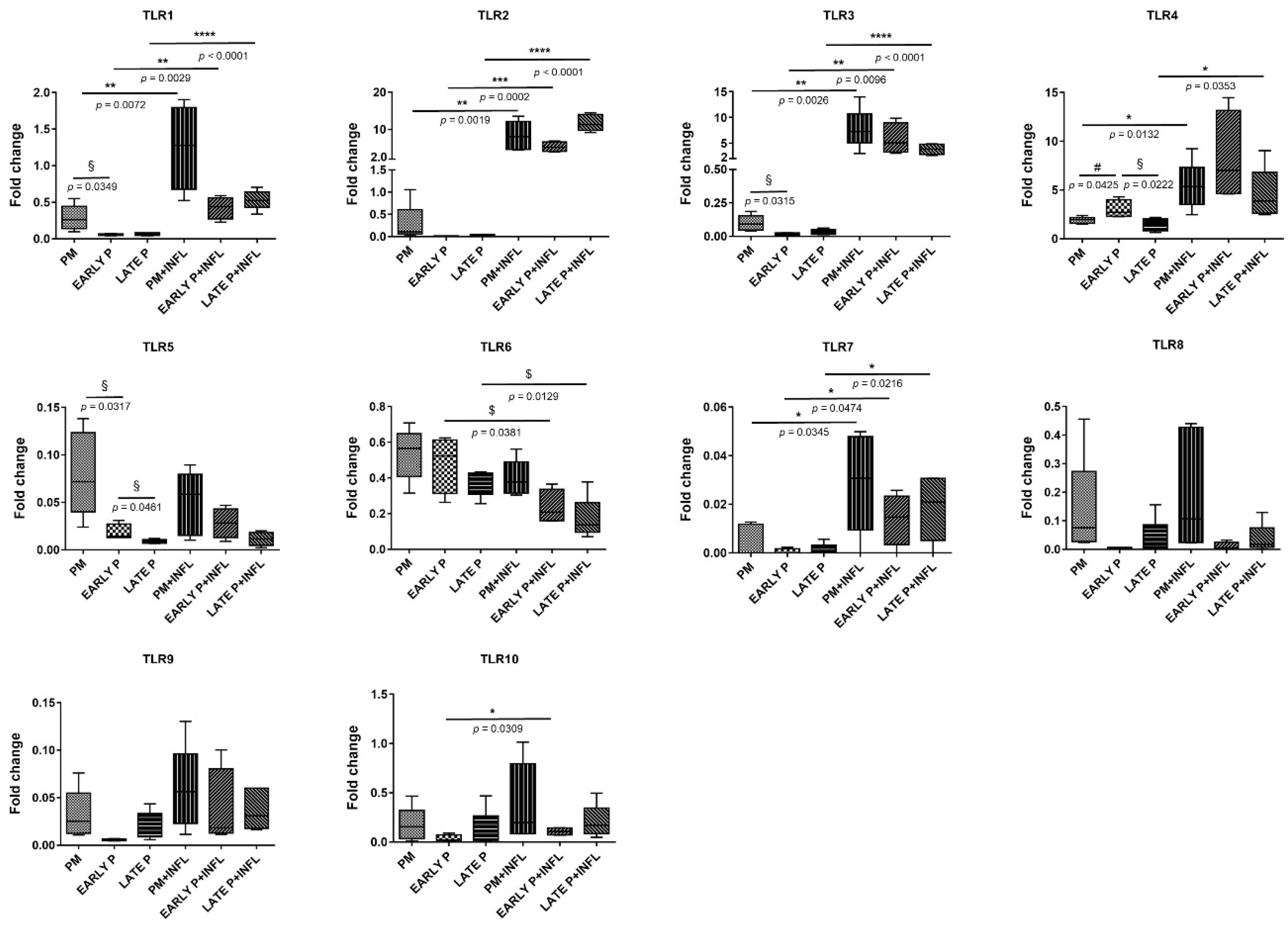
3.3. Analysis of the Transcriptional Profile of Cytokines/Chemokines and TLR
3.3.1. Cytokine/Chemokine Expression Pattern
3.3.2. TLR Expression Pattern
3.4. Protein Interaction Network and Gene Set Enrichment Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merimi, M.; El-Majzoub, R.; Lagneaux, L.; Moussa Agha, D.; Bouhtit, F.; Meuleman, N.; Fahmi, H.; Lewalle, P.; Fayyad-Kazan, M.; Najar, M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front. Cell Dev. Biol. 2021, 9, 661532. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Zocchi, M.R. Immunomodulatory Properties of Mesenchymal Stromal Cells: Still Unresolved “Yin and Yang”. Curr. Stem Cell Res. Ther. 2018, 14, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Prigione, I.; Benvenuto, F.; Bocca, P.; Battistini, L.; Uccelli, A.; Pistoia, V. Reciprocal Interactions Between Human Mesenchymal Stem Cells and γδ T Cells Or Invariant Natural Killer T Cells. Stem Cells 2009, 27, 693–702. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wen, D.G.; Gong, J.P.; Liu, Z.J. Research Status of Mesenchymal Stem Cells in Liver Transplantation. Cell Transplant. 2019, 28, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Krayem, M.; Merimi, M.; Burny, A.; Meuleman, N.; Bron, D.; Raicevic, G.; Lagneaux, L. Insights into inflammatory priming of mesenchymal stromal cells: Functional biological impacts. Inflamm. Res. 2018, 67, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Yong, D.; El-Jawhari, J.J.; Cuthbert, R.; Mcgonagle, D.; Win Naing, M.A.Y.; Jones, E. Identification of senescent cells in multipotent mesenchymal stromal cell cultures: Current methods and future directions. Cytotherapy 2019, 21, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Baer, P.C. Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J. Stem Cells 2014, 6, 256–265. [Google Scholar] [CrossRef]
- Kolaparthy, L.K.; Sanivarapu, S.; Moogla, S.; Kutcham, R.S. Adipose Tissue—Adequate, Accessible Regenerative Material. Int. J. Stem Cells 2015, 8, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Purandare, B.; Teklemariam, T.; Zhao, L.; Hantash, B.M. Temporal HLA profiling and immunomodulatory effects of human adult bone marrow- and adipose-derived mesenchymal stem cells. Regen. Med. 2014, 9, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, X.; Yin, Y.; Luo, B.; Liu, Y.; Yang, C. Inflammatory Microenvironment Accelerates Bone Marrow Mesenchymal Stem Cell Aging. Front. Bioeng. Biotechnol. 2022, 10, 692. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Rodrigues, R.M.R.M.R.M.; Buyl, K.; Branson, S.; Vanhaecke, T.; Lagneaux, L.; Rogiers, V.; De Kock, J. Proliferative and phenotypical characteristics of human adipose tissue-derived stem cells: Comparison of Ficoll gradient centrifugation and red blood cell lysis buffer treatment purification methods. Cytotherapy 2014, 16, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Merimi, M.; Buyl, K.; Daassi, D.; Rodrigues, R.M.; Melki, R.; Lewalle, P.; Vanhaecke, T.; Fahmi, H.; Rogiers, V.; Lagneaux, L.; et al. Transcriptional profile of cytokines, regulatory mediators and TLR in mesenchymal stromal cells after inflammatory signaling and cell-passaging. Int. J. Mol. Sci. 2021, 22, 7309. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Raicevic, G.; Id Boufker, H.; Stamatopoulos, B.; De Bruyn, C.; Meuleman, N.; Bron, D.; Toungouz, M.; Lagneaux, L. Modulated expression of adhesion molecules and galectin-1: Role during mesenchymal stromal cell immunoregulatory functions. Exp. Hematol. 2010, 38, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Adelipour, M.; Lubman, D.M.; Kim, J. Potential applications of mesenchymal stem cells and their derived exosomes in regenerative medicine. Expert Opin. Biol. Ther. 2023, 23, 491–507. [Google Scholar] [CrossRef]
- Qi, K.; Li, N.; Zhang, Z.; Melino, G. Tissue regeneration: The crosstalk between mesenchymal stem cells and immune response. Cell. Immunol. 2018, 326, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Raicevic, G.; Fayyad-Kazan, H.; Bron, D.; Toungouz, M.; Lagneaux, L. Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells. Cytotherapy 2016, 18, 160–171. [Google Scholar] [CrossRef]
- Najar, M.; Martel-Pelletier, J.; Pelletier, J.P.; Fahmi, H. Novel insights for improving the therapeutic safety and efficiency of mesenchymal stromal cells. World J. Stem Cells 2020, 12, 1474–1491. [Google Scholar] [CrossRef]
- Sharpe, M.E.; Morton, D.; Rossi, A. Nonclinical safety strategies for stem cell therapies. Toxicol. Appl. Pharmacol. 2012, 262, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, S.; Cossetti, C. How stem cells speak with host immune cells in inflammatory brain diseases. Glia 2013, 61, 1379–1401. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Davies, L.C. Mesenchymal stromal cells and the innate immune response. Immunol. Lett. 2015, 168, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Raicevic, G.; Fayyad-Kazan, H.; Kazan, H.F.; De Bruyn, C.; Bron, D.; Toungouz, M.; Lagneaux, L.; Lagneaux, L. Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: The expression and impact of inflammatory priming. Stem Cell Rev. 2012, 8, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Attia, J.V.D.; Dessens, C.E.; van de Water, R.; Houvast, R.D.; Kuppen, P.J.K.; Krijgsman, D. The molecular and functional characteristics of hla-g and the interaction with its receptors: Where to intervene for cancer immunotherapy? Int. J. Mol. Sci. 2020, 21, 8678. [Google Scholar] [CrossRef] [PubMed]
- De Kock, J.; Meuleman, P.; Raicevic, G.; Rodrigues, R.M.; Branson, S.; Meganathan, K.; De Boe, V.; Sachinidis, A.; Leroux-Roels, G.; Vanhaecke, T.; et al. Human skin-derived precursor cells are poorly immunogenic and modulate the allogeneic immune response. Stem Cells 2014, 32, 2215–2228. [Google Scholar] [CrossRef]
- Ayala-Cuellar, A.P.; Kang, J.H.; Jeung, E.B.; Choi, K.C. Roles of mesenchymal stem cells in tissue regeneration and immunomodulation. Biomol. Ther. 2019, 27, 25–33. [Google Scholar] [CrossRef]
- Al Battah, F.; De Kock, J.; Ramboer, E.; Heymans, A.; Vanhaecke, T.; Rogiers, V.; Snykers, S. Evaluation of the multipotent character of human adipose tissue-derived stem cells isolated by Ficoll gradient centrifugation and red blood cell lysis treatment. Toxicol. Vitr. 2011, 25, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Gangenahalli, G.U.; Singh, V.K.; Verma, Y.K.; Gupta, P.; Sharma, R.K.; Chandra, R.; Luthra, P.M. Hematopoietic stem cell antigen CD34: Role in adhesion or homing. Stem Cells Dev. 2006, 15, 305–313. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.S.; Deans, R.J.J.; Keating, A.; Prockop, D.J.J.; Horwitz, E.M.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Buyl, K.; Merimi, M.; Rodrigues, R.M.; Moussa Agha, D.; Melki, R.; Vanhaecke, T.; Bron, D.; Lewalle, P.; Meuleman, N.; Fahmi, H.; et al. The impact of cell-expansion and inflammation on the immune-biology of human adipose tissue-derived Mesenchymal stromal cells. J. Clin. Med. 2020, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Byrne, G.W. Does human leukocyte antigens sensitization matter for xenotransplantation? Xenotransplantation 2018, 25, e12411. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.; El-Feky, M.A.; El-Amir, M.I.; Hasan, A.S.; Tag-Adeen, M.; Urata, Y.; Goto, S.; Luo, L.; Yan, C.; Li, T.S. Immunomodulatory effect of mesenchymal stem cells: Cell origin and cell quality variations. Mol. Biol. Rep. 2019, 46, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Mun, C.H.; Kang, M.I.; Shin, Y.D.; Kim, Y.; Park, Y.B. The Expression of Immunomodulation-Related Cytokines and Genes of Adipose- and Bone Marrow-Derived Human Mesenchymal Stromal Cells from Early to Late Passages. Tissue Eng. Regen. Med. 2018, 15, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, R.; Benson, M.J.; De Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Croft, M. Control of Immunity by the TNFR-related molecule OX40 (CD134). Annu. Rev. Immunol. 2010, 28, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef] [PubMed]
- van Eeden, C.; Khan, L.; Osman, M.S.; Tervaert, J.W.C. Natural killer cell dysfunction and its role in COVID-19. Int. J. Mol. Sci. 2020, 21, 6351. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Castriconi, R.; Romagnani, P.; Spaggiari, G.M.; Marcenaro, S.; Dondero, A.; Lazzeri, E.; Lasagni, L.; Martini, S.; Rivera, P.; et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: Relevance for natural killer-dendritic cell interaction. Blood 2006, 107, 2030–2036. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Capobianco, A.; Becchetti, S.; Mingari, M.C.; Moretta, L. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006, 107, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Béland, S.; Désy, O.; Vallin, P.; Basoni, C.; De Serres, S.A. Innate immunity in solid organ transplantation: An update and therapeutic opportunities. Expert Rev. Clin. Immunol. 2015, 11, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Hosseini, S.A.; Rahim, F. Immunosuppressive therapy in allograft transplantation: From novel insights and strategies to tolerance and challenges. Cent. Eur. J. Immunol. 2014, 39, 400–409. [Google Scholar] [CrossRef]
- Li, P.; Li, S.-H.; Wu, J.; Zang, W.-F.; Dhingra, S.; Sun, L.; Weisel, R.D.; Li, R.-K. Interleukin-6 downregulation with mesenchymal stem cell differentiation results in loss of immunoprivilege. J. Cell. Mol. Med. 2013, 17, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.E.; Guabiraba, R.; Russo, R.C.; Teixeira, M.M. Targeting CCL5 in inflammation. Expert Opin. Ther. Targets 2013, 17, 1439–1460. [Google Scholar] [CrossRef] [PubMed]
- Delarosa, O.; Dalemans, W.; Lombardo, E. Toll-like receptors as modulators of mesenchymal stem cells. Front. Immunol. 2012, 3, 182. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, F.; de Jager, S.C.A.; Sluijter, J.P.G. Mesenchymal stem cell therapy for cardiac inflammation: Immunomodulatory properties and the influence of toll-like receptors. Mediat. Inflamm. 2013, 2013, 181020. [Google Scholar] [CrossRef]
- Raicevic, G.; Rouas, R.; Najar, M.; Stordeur, P.; Boufker, H.I.; Bron, D.; Martiat, P.; Goldman, M.; Nevessignsky, M.T.; Lagneaux, L.; et al. Inflammation modifies the pattern and the function of Toll-like receptors expressed by human mesenchymal stromal cells. Hum. Immunol. 2010, 71, 235–244. [Google Scholar] [CrossRef]
- Abarbanell, A.M.; Wang, Y.; Herrmann, J.L.; Weil, B.R.; Poynter, J.A.; Manukyan, M.C.; Meldrum, D.R. Toll-like receptor 2 mediates mesenchymal stem cell-associated myocardial recovery and VEGF production following acute ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1529-36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schreibelt, G.; Tel, J.; Sliepen, K.H.E.W.J.; Benitez-Ribas, D.; Figdor, C.G.; Adema, G.J.; de Vries, I.J.M. Toll-like receptor expression and function in human dendritic cell subsets: Implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol. Immunother. 2010, 59, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Shaw, A.C. Toll-like receptors in older adults. J. Am. Geriatr. Soc. 2007, 55, 1438–1444. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Planat-Benard, V.; Varin, A.; Casteilla, L. MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism. Front. Immunol. 2021, 12, 626755. [Google Scholar] [CrossRef] [PubMed]
- Lepperdinger, G. Inflammation and mesenchymal stem cell aging. Curr. Opin. Immunol. 2011, 23, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Cubedo, J. Adipose tissue depots and inflammation: Effects on plasticity and residentmesenchymal stem cell function. Cardiovasc. Res. 2017, 113, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Gong, X.; Fu, Z.; Song, X.; Ma, Q.; Miao, J.; Cai, R.; Yan, Z.; Wang, S.; Li, Q.; et al. The influence of inflammation on the characteristics of adipose-derived mesenchymal stem cells (ADMSCs) and tissue repair capability in a hepatic injury mouse model. Stem Cell Res. Ther. 2023, 14, 334. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]
- Liu, J.; Gao, J.; Liang, Z.; Gao, C.; Niu, Q.; Wu, F.; Zhang, L. Mesenchymal stem cells and their microenvironment. Stem Cell Res. Ther. 2022, 13, 429. [Google Scholar] [CrossRef]
- Noronha Nc, N.D.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buyl, K.; Merimi, M.; Rodrigues, R.M.; Rahmani, S.; Fayyad-Kazan, M.; Bouhtit, F.; Boukhatem, N.; Vanhaecke, T.; Fahmi, H.; De Kock, J.; et al. The Immunological Profile of Adipose Mesenchymal Stromal/Stem Cells after Cell Expansion and Inflammatory Priming. Biomolecules 2024, 14, 852. https://doi.org/10.3390/biom14070852
Buyl K, Merimi M, Rodrigues RM, Rahmani S, Fayyad-Kazan M, Bouhtit F, Boukhatem N, Vanhaecke T, Fahmi H, De Kock J, et al. The Immunological Profile of Adipose Mesenchymal Stromal/Stem Cells after Cell Expansion and Inflammatory Priming. Biomolecules. 2024; 14(7):852. https://doi.org/10.3390/biom14070852
Chicago/Turabian StyleBuyl, Karolien, Makram Merimi, Robim M. Rodrigues, Saida Rahmani, Mohammad Fayyad-Kazan, Fatima Bouhtit, Noureddine Boukhatem, Tamara Vanhaecke, Hassan Fahmi, Joery De Kock, and et al. 2024. "The Immunological Profile of Adipose Mesenchymal Stromal/Stem Cells after Cell Expansion and Inflammatory Priming" Biomolecules 14, no. 7: 852. https://doi.org/10.3390/biom14070852
APA StyleBuyl, K., Merimi, M., Rodrigues, R. M., Rahmani, S., Fayyad-Kazan, M., Bouhtit, F., Boukhatem, N., Vanhaecke, T., Fahmi, H., De Kock, J., & Najar, M. (2024). The Immunological Profile of Adipose Mesenchymal Stromal/Stem Cells after Cell Expansion and Inflammatory Priming. Biomolecules, 14(7), 852. https://doi.org/10.3390/biom14070852









