Pathological Defects in a Drosophila Model of Alzheimer’s Disease and Beneficial Effects of the Natural Product Lisosan G
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
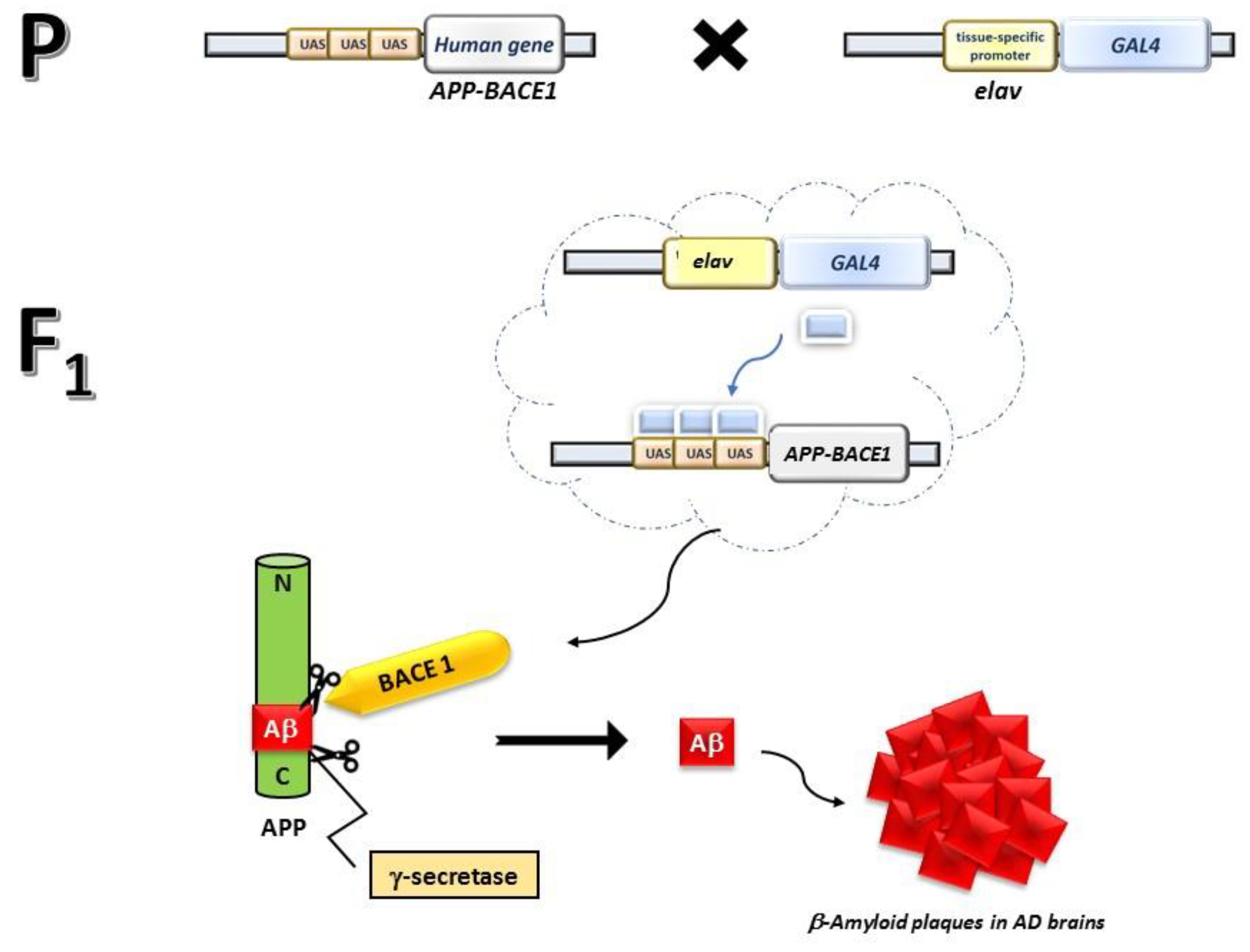
2.2. Fly Strains and Crosses
2.3. Standard and Integrated Diet, Treatments
2.4. Tissue Preparation
2.5. Aβ Immunohistochemistry—Congo-Red Staining
2.6. Imaging and Quantification of Aβ Plaques
2.7. Confocal Immunostaining
2.8. Protein Extraction and Western Blotting Analysis
2.9. Determination of Reactive Oxygen Species (ROS)
2.10. 9 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
2.11. RNA Analysis and Gene Expression Assay
2.12. Statistical Analysis
3. Results
3.1. Lisosan G Diet Reduces the Number of Amyloid β Plaques in AD Brains
3.2. Lisosan G Diet Reduces the Apoptosis Levels in AD Brains
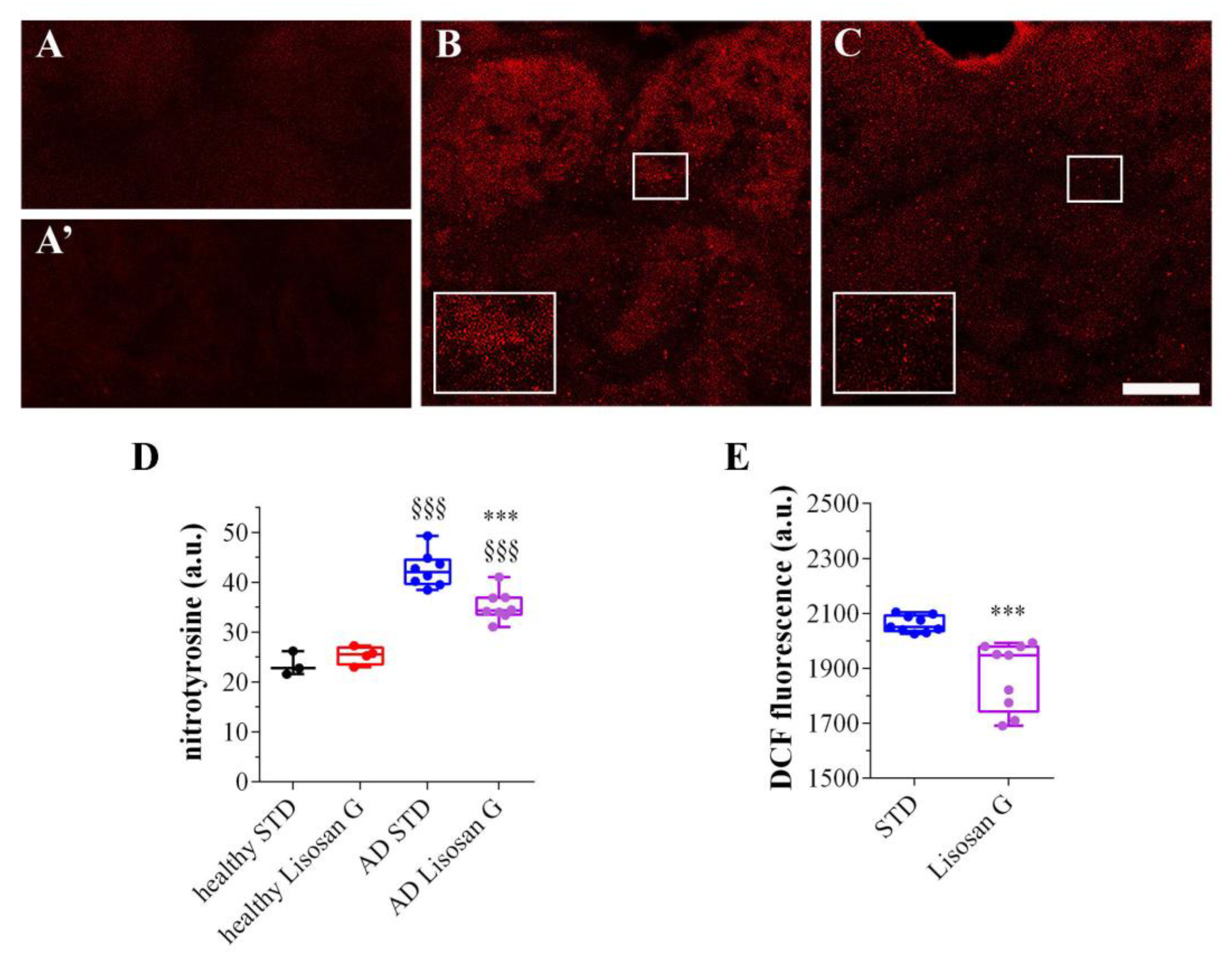
3.3. Lisosan G Diet Reduces the Oxidation Levels of AD Brains
3.4. Lisosan G Diet Increases Autophagy Turnover of AD Brains
3.5. Effect of Lisosan G on Nucleolar Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- De Strooper, B.; Annaert, W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J. Cell Sci. 2000, 113 Pt 11, 1857–1870. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Burdick, D.; Soreghan, B.; Kwon, M.; Kosmoski, J.; Knauer, M.; Henschen, A.; Yates, J.; Cotman, C.; Glabe, C. Assembly and aggregation properties of synthetic Alzheimer’s A4/beta amyloid peptide analogs. J. Biol. Chem. 1992, 267, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Chow, V.W.; Mattson, M.P.; Wong, P.C.; Gleichmann, M. An overview of APP processing enzymes and products. Neuromol. Med. 2010, 12, 1–12. [Google Scholar] [CrossRef]
- Bier, E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 2005, 6, 9–23. [Google Scholar] [CrossRef]
- Lenz, S.; Karsten, P.; Schulz, J.B.; Voigt, A. Drosophila as a screening tool to study human neurodegenerative diseases. J. Neurochem. 2013, 127, 453–460. [Google Scholar] [CrossRef]
- Bellen, H.J.; Tong, C.; Tsuda, H. 100 years of Drosophila research and its impact on vertebrate neuroscience: A history lesson for the future. Nat. Rev. Neurosci. 2010, 11, 514–522. [Google Scholar] [CrossRef] [PubMed]
- McGurk, L.; Berson, A.; Bonini, N.M. Drosophila as an In Vivo Model for Human Neurodegenerative Disease. Genetics 2015, 201, 377–402. [Google Scholar] [CrossRef]
- Periz, G.; Fortini, M.E. Proteolysis in Alzheimer’s disease. Can plasmin tip the balance? EMBO Rep. 2000, 1, 477–478. [Google Scholar] [CrossRef]
- Luo, L.Q.; Martin-Morris, L.E.; White, K. Identification, secretion, and neural expression of APPL, a Drosophila protein similar to human amyloid protein precursor. J. Neurosci. 1990, 10, 3849–3861. [Google Scholar] [CrossRef] [PubMed]
- Greeve, I.; Kretzschmar, D.; Tschäpe, J.A.; Beyn, A.; Brellinger, C.; Schweizer, M.; Nitsch, R.M.; Reifegerste, R. Age-dependent neurodegeneration and Alzheimer-amyloid plaque formation in transgenic Drosophila. J. Neurosci. 2004, 24, 3899–3906. [Google Scholar] [CrossRef] [PubMed]
- Carmine-Simmen, K.; Proctor, T.; Tschäpe, J.; Poeck, B.; Triphan, T.; Strauss, R.; Kretzschmar, D. Neurotoxic effects induced by the Drosophila amyloid-beta peptide suggest a conserved toxic function. Neurobiol. Dis. 2009, 33, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Stempfle, D.; Kanwar, R.; Loewer, A.; Fortini, M.E.; Merdes, G. In vivo reconstitution of gamma-secretase in Drosophila results in substrate specificity. Mol. Cell. Biol. 2010, 30, 3165–3175. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Vepuri, V.; Mhatre, S.D.; Paddock, B.E.; Miller, S.; Michelson, S.J.; Delvadia, R.; Desai, A.; Vinokur, M.; Melicharek, D.J.; et al. Characterization of a Drosophila Alzheimer’s disease model: Pharmacological rescue of cognitive defects. PLoS ONE 2011, 6, e20799. [Google Scholar] [CrossRef] [PubMed]
- Coulson, E.J.; Paliga, K.; Beyreuther, K.; Masters, C.L. What the evolution of the amyloid protein precursor supergene family tells us about its function. Neurochem. Int. 2000, 36, 175–184. [Google Scholar] [CrossRef]
- Bilen, J.; Bonini, N.M. Drosophila as a model for human neurodegenerative disease. Annu. Rev. Genet. 2005, 39, 153–171. [Google Scholar] [CrossRef]
- Link, C.D. Invertebrate models of Alzheimer’s disease. Genes Brain Behav. 2005, 4, 147–156. [Google Scholar] [CrossRef]
- Phelps, C.B.; Brand, A.H. Ectopic gene expression in Drosophila using GAL4 system. Methods 1998, 14, 367–379. [Google Scholar] [CrossRef]
- Finelli, A.; Kelkar, A.; Song, H.J.; Yang, H.; Konsolaki, M. A model for studying Alzheimer’s Abeta42-induced toxicity in Drosophila melanogaster. Mol. Cell. Neurosci. 2004, 26, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Liu, H.P.; Chiang, A.S.; Hearn, S.A.; Konsolaki, M.; Zhong, Y. Dissecting the pathological effects of human Abeta40 and Abeta42 in Drosophila: A potential model for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 6623–6628. [Google Scholar] [CrossRef] [PubMed]
- Crowther, D.C.; Kinghorn, K.J.; Miranda, E.; Page, R.; Curry, J.A.; Duthie, F.A.; Gubb, D.C.; Lomas, D.A. Intraneuronal Abeta, non-amyloid aggregates and neurodegeneration in a Drosophila model of Alzheimer’s disease. Neuroscience 2005, 132, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Tsintzas, E.; Niccoli, T. Using Drosophila amyloid toxicity models to study Alzheimer’s disease. Ann. Hum. Genet. 2024, 1–15. [Google Scholar] [CrossRef]
- Fernández-Sanz, P.; Ruiz-Gabarre, D.; García-Escudero, V. Modulating Effect of Diet on Alzheimer’s Disease. Diseases 2019, 7, 12. [Google Scholar] [CrossRef]
- Bolus, H.; Crocker, K.; Boekhoff-Falk, G.; Chtarbanova, S. Modeling Neurodegenerative Disorders in Drosophila melanogaster. Int. J. Mol. Sci. 2020, 21, 3055. [Google Scholar] [CrossRef]
- Xiu, M.; Wang, Y.; Yang, D.; Zhang, X.; Dai, Y.; Liu, Y.; Lin, X.; Li, B.; He, J. Using Drosophila melanogaster as a suitable platform for drug discovery from natural products in inflammatory bowel disease. Front. Pharmacol. 2022, 13, 1072715. [Google Scholar]
- Su, T.T. Drug screening in Drosophila; why, when, and when not? Wiley Interdiscip. Rev. Dev. Biol. 2019, 8, e346. [Google Scholar] [CrossRef]
- Freires, I.A.; Morelo, D.F.C.; Soares, L.F.F.; Costa, I.S.; de Araujo, L.P.; Breseghello, I.; Abdalla, H.B.; Lazarini, J.G.; Rosalen, P.L.; Pigossi, S.C.; et al. Progress and promise of alternative animal and non-animal methods in biomedical research. Arch. Toxicol. 2023, 97, 2329–2342. [Google Scholar] [CrossRef] [PubMed]
- Koon, A.C.; Chan, H.Y. As a Model Organism to Study RNA Toxicity of Repeat Expansion-Associated Neurodegenerative and Neuromuscular Diseases. Front. Cell. Neurosci. 2017, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Göethel, G.; Augsten, L.V.; das Neves, G.M.; Gonçalves, I.L.; de Souza, J.P.S.; Garcia, S.C.; Eifler-Lima, V.L. The Role of Alternative Toxicological Trials in Drug Discovery Programs. The Case of Caenorhabditis elegans and Other Methods. Curr. Med. Chem. 2022, 29, 5270–5288. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; More, V. Recent Advances in Drug Discovery Toxicology. Int. J. Toxicol. 2023, 42, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Xu, W.; Fan, Y.; Wang, H.X. Drosophila as an emerging model organism for studies of food-derived antioxidants. Food Res. Int. 2021, 143, 110307. [Google Scholar] [CrossRef] [PubMed]
- Rivas-García, L.; Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Esteban-Muñoz, A.; Giampieri, F.; Sumalla-Cano, S.; Battino, M.; Quiles, J.L.; Llopis, J.; Sánchez-González, C. Unravelling potential biomedical applications of the edible flower Tulbaghia violacea. Food Chem. 2022, 381, 132096. [Google Scholar] [CrossRef] [PubMed]
- Rivas-García, L.; Quiles, J.L.; Roma-Rodrigues, C.; Raposo, L.R.; Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Esteban-Muñoz, A.; Varela-López, A.; García, L.C.; Cianciosi, D.; et al. Rosa x hybrida extracts with dual actions: Antiproliferative effects against tumour cells and inhibitor of Alzheimer disease. Food Chem. Toxicol. 2021, 149, 112018. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Chirulli, V.; Gervasi, P.G.; Nencioni, S.; Pellegrini, M. Lisosan G, a powder of grain, does not interfere with the drug metabolizing enzymes and has a protective role on carbon tetrachloride-induced hepatotoxicity. Biotechnol. Lett. 2007, 29, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Rossino, M.G.; Cammalleri, M.; Timperio, A.M.; Fanelli, G.; Dal Monte, M.; Pucci, L.; Casini, G. The Potential of Lisosan G as a Possible Treatment for Glaucoma. Front. Pharmacol. 2021, 12, 719951. [Google Scholar] [CrossRef]
- Giusti, L.; Gabriele, M.; Penno, G.; Garofolo, M.; Longo, V.; Del Prato, S.; Lucchesi, D.; Pucci, L. A Fermented Whole Grain Prevents Lipopolysaccharides-Induced Dysfunction in Human Endothelial Progenitor Cells. Oxidative Med. Cell. Longev. 2017, 2017, 1026268. [Google Scholar] [CrossRef]
- La Marca, M.; Beffy, P.; Pugliese, A.; Longo, V. Fermented wheat powder induces the antioxidant and detoxifying system in primary rat hepatocytes. PLoS ONE 2013, 8, e83538. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Pucci, L.; Arvay, J.; Longo, V. Anti-inflammatory and antioxidant effect of fermented whole wheat on TNF alpha-stimulated HT-29 and NF-kappa B signaling pathway activation. J. Funct. Foods 2018, 45, 392–400. [Google Scholar] [CrossRef]
- Frassinetti, S.; Della Croce, C.M.; Caltavuturo, L.; Longo, V. Antimutagenic and antioxidant activity of Lisosan G in Saccharomyces cerevisiae. Food Chem. 2012, 135, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, D.; Russo, R.; Gabriele, M.; Longo, V.; Del Prato, S.; Penno, G.; Pucci, L. Grain and bean lysates improve function of endothelial progenitor cells from human peripheral blood: Involvement of the endogenous antioxidant defenses. PLoS ONE 2014, 9, e109298. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Rossino, M.G.; Cammalleri, M.; Locri, F.; Pucci, L.; Dal Monte, M.; Casini, G. Lisosan G Protects the Retina from Neurovascular Damage in Experimental Diabetic Retinopathy. Nutrients 2018, 10, 1932. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Fanelli, G.; Silvestri, F.; Cherubini, A.; Del Quondam, S.; Bongiorni, S.; Taddei, A.R.; Ceci, M.; De Palma, C.; Perrotta, C.; et al. Nutraceutical Strategy to Counteract Eye Neurodegeneration and Oxidative Stress in Drosophila melanogaster Fed with High-Sugar Diet. Antioxidants 2021, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Buonanno, F.; Lupidi, G.; Bongiorni, S.; Belardi, R.; Zecchini, S.; Giovarelli, M.; Coazzoli, M.; De Palma, C.; Perrotta, C.; et al. The Natural Compound Climacostol as a Prodrug Strategy Based on pH Activation for Efficient Delivery of Cytotoxic Small Agents. Front. Chem. 2019, 7, 463. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Bongiorni, S.; Taddei, A.R.; Mezzetti, M.; Silvestri, F.; Coazzoli, M.; Zecchini, S.; Giovarelli, M.; Perrotta, C.; De Palma, C.; et al. Defects of full-length dystrophin trigger retinal neuron damage and synapse alterations by disrupting functional autophagy. Cell. Mol. Life Sci. 2021, 78, 1615–1636. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Silvestri, F.; Bongiorni, S.; Taddei, A.R.; Fanelli, G.; Rinalducci, S.; De Palma, C.; Perrotta, C.; Prantera, G.; Cervia, D. Retinal damage in a new model of hyperglycemia induced by high-sucrose diets. Pharmacol. Res. 2021, 166, 105488. [Google Scholar] [CrossRef]
- Catalani, E.; Zecchini, S.; Giovarelli, M.; Cherubini, A.; Del Quondam, S.; Brunetti, K.; Silvestri, F.; Roux-Biejat, P.; Napoli, A.; Casati, S.R.; et al. RACK1 is evolutionary conserved in satellite stem cell activation and adult skeletal muscle regeneration. Cell Death Discov. 2022, 8, 459. [Google Scholar] [CrossRef]
- Cammalleri, M.; Locri, F.; Catalani, E.; Filippi, L.; Cervia, D.; Dal Monte, M.; Bagnoli, P. The Beta Adrenergic Receptor Blocker Propranolol Counteracts Retinal Dysfunction in a Mouse Model of Oxygen Induced Retinopathy: Restoring the Balance between Apoptosis and Autophagy. Front. Cell. Neurosci. 2017, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Del Quondam, S.; Brunetti, K.; Cherubini, A.; Bongiorni, S.; Taddei, A.R.; Zecchini, S.; Giovarelli, M.; De Palma, C.; Perrotta, C.; et al. Neuroprotective role of plumbagin on eye damage induced by high-sucrose diet in adult fruit fly Drosophila melanogaster. Biomed. Pharmacother. 2023, 166, 115298. [Google Scholar] [CrossRef] [PubMed]
- Cervia, D.; Catalani, E.; Dal Monte, M.; Casini, G. Vascular endothelial growth factor in the ischemic retina and its regulation by somatostatin. J. Neurochem. 2012, 120, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Cervia, D.; Nunn, C.; Fehlmann, D.; Langenegger, D.; Schuepbach, E.; Hoyer, D. Pharmacological characterisation of native somatostatin receptors in AtT-20 mouse tumour corticotrophs. Br. J. Pharmacol. 2003, 139, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, C.; Buonanno, F.; Zecchini, S.; Giavazzi, A.; Proietti Serafini, F.; Catalani, E.; Guerra, L.; Belardinelli, M.C.; Picchietti, S.; Fausto, A.M.; et al. Climacostol reduces tumour progression in a mouse model of melanoma via the p53-dependent intrinsic apoptotic programme. Sci. Rep. 2016, 6, 27281. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Brunetti, K.; Del Quondam, S.; Bongiorni, S.; Picchietti, S.; Fausto, A.M.; Lupidi, G.; Marcantoni, E.; Perrotta, C.; Achille, G.; et al. Exposure to the Natural Compound Climacostol Induces Cell Damage and Oxidative Stress in the Fruit Fly Drosophila melanogaster. Toxics 2024, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Ecker, A.; Gonzaga, T.K.S.D.; Seeger, R.L.; Santos, M.M.D.; Loreto, J.S.; Boligon, A.A.; Meinerz, D.F.; Lugokenski, T.H.; Rocha, J.B.T.D.; Barbosa, N.V. High-sucrose diet induces diabetic-like phenotypes and oxidative stress in Drosophila melanogaster: Protective role of Syzygium cumini and Bauhinia forficata. Biomed. Pharmacother. 2017, 89, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Reshaping data with the reshape package. J. Stat. Soft. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Klunk, W.E.; Pettegrew, J.W.; Abraham, D.J. Quantitative evaluation of congo red binding to amyloid-like proteins with a beta-pleated sheet conformation. J. Histochem. Cytochem. 1989, 37, 1273–1281. [Google Scholar] [CrossRef]
- Molino, S.; Dossena, M.; Buonocore, D.; Ferrari, F.; Venturini, L.; Ricevuti, G.; Verri, M. Polyphenols in dementia: From molecular basis to clinical trials. Life Sci. 2016, 161, 69–77. [Google Scholar] [CrossRef]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hwang, Y.J.; Ryu, H.; Kowall, N.W. Nucleolar dysfunction in Huntington’s disease. Biochim. Biophys. Acta 2014, 1842, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Neuropathological diagnosis of Alzheimer’s disease: A perspective from longitudinal clinicopathological studies. Neurobiol. Aging 1997, 18, S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Verri, M.; Pastoris, O.; Dossena, M.; Aquilani, R.; Guerriero, F.; Cuzzoni, G.; Venturini, L.; Ricevuti, G.; Bongiorno, A.I. Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer’s disease. Int. J. Immunopathol. Pharmacol. 2012, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Pedersen, W.A. Effects of amyloid precursor protein derivatives and oxidative stress on basal forebrain cholinergic systems in Alzheimer’s disease. Int. J. Dev. Neurosci. 1998, 16, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Emerging neuroprotective strategies for Alzheimer’s disease: Dietary restriction, telomerase activation, and stem cell therapy. Exp. Gerontol. 2000, 35, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Colurso, G.J.; Nilson, J.E.; Vervoort, L.G. Quantitative assessment of DNA fragmentation and beta-amyloid deposition in insular cortex and midfrontal gyrus from patients with Alzheimer’s disease. Life Sci. 2003, 73, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Takuma, K.; Yan, S.S.; Stern, D.M.; Yamada, K. Mitochondrial dysfunction, endoplasmic reticulum stress, and apoptosis in Alzheimer’s disease. J. Pharmacol. Sci. 2005, 97, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, R.; Rasheed, M.Z.; Rawat, M.; Alam, M.M.; Tabassum, H.; Parvez, S. Effect of melatonin on Aβ42 induced changes in the mitochondrial function related to Alzheimer’s disease in Drosophila melanogaster. Neurosci. Lett. 2019, 711, 134376. [Google Scholar] [CrossRef]
- Wang, X.; Davis, R.L. Early Mitochondrial Fragmentation and Dysfunction in a Drosophila Model for Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 143–155. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Aliev, G.; Nunomura, A.; Fujioka, H.; Russell, R.L.; Atwood, C.S.; Johnson, A.B.; Kress, Y.; Vinters, H.V.; Tabaton, M.; et al. Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci. 2001, 21, 3017–3023. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Ischiropoulos, H.; al-Mehdi, A.B. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 1995, 364, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Bandookwala, M.; Sengupta, P. 3-Nitrotyrosine: A versatile oxidative stress biomarker for major neurodegenerative diseases. Int. J. Neurosci. 2020, 130, 1047–1062. [Google Scholar] [CrossRef]
- Xie, L.; Helmerhorst, E.; Taddei, K.; Plewright, B.; Van Bronswijk, W.; Martins, R. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J. Neurosci. 2002, 22, Rc221. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Youssef, P.; Chami, B.; Lim, J.; Middleton, T.; Sutherland, G.T.; Witting, P.K. Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer’s disease. Sci. Rep. 2018, 8, 11553. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, T.; Deore, S.L.; Kide, A.A.; Shende, B.A.; Sharma, R.; Dadarao Chakole, R.; Nemade, L.S.; Kishor Kale, N.; Borah, S.; Shrikant Deokar, S.; et al. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease, and Parkinson’s disease, Huntington’s disease and Amyotrophic Lateral Sclerosis -An updated review. Mitochondrion 2023, 71, 83–92. [Google Scholar] [CrossRef]
- Catalani, E.; Brunetti, K.; Del Quondam, S.; Cervia, D. Targeting Mitochondrial Dysfunction and Oxidative Stress to Prevent the Neurodegeneration of Retinal Ganglion Cells. Antioxidants 2023, 12, 2011. [Google Scholar] [CrossRef]
- Casati, S.R.; Cervia, D.; Roux-Biejat, P.; Moscheni, C.; Perrotta, C.; De Palma, C. Mitochondria and Reactive Oxygen Species: The Therapeutic Balance of Powers for Duchenne Muscular Dystrophy. Cells 2024, 13, 574. [Google Scholar] [CrossRef]
- Talebi, M.; Mohammadi Vadoud, S.A.; Haratian, A.; Talebi, M.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The interplay between oxidative stress and autophagy: Focus on the development of neurological diseases. Behav. Brain. Funct. 2022, 18, 3. [Google Scholar] [CrossRef]
- Pestov, D.G.; Strezoska, Z.; Lau, L.F. Evidence of p53-dependent cross-talk between ribosome biogenesis and the cell cycle: Effects of nucleolar protein Bop1 on G(1)/S transition. Mol. Cell. Biol. 2001, 21, 4246–4255. [Google Scholar] [CrossRef]
- Yang, K.; Yang, J.; Yi, J. Nucleolar Stress: Hallmarks, sensing mechanism and diseases. Cell Stress 2018, 2, 125–140. [Google Scholar] [CrossRef]
- Parlato, R.; Kreiner, G. Nucleolar activity in neurodegenerative diseases: A missing piece of the puzzle? J. Mol. Med. 2013, 91, 541–547. [Google Scholar] [CrossRef]
- Payão, S.L.; Smith, M.A.; Winter, L.M.; Bertolucci, P.H. Ribosomal RNA in Alzheimer’s disease and aging. Mech. Ageing Dev. 1998, 105, 265–272. [Google Scholar] [CrossRef]
- Pietrzak, M.; Rempala, G.; Nelson, P.T.; Zheng, J.J.; Hetman, M. Epigenetic silencing of nucleolar rRNA genes in Alzheimer’s disease. PLoS ONE 2011, 6, e22585. [Google Scholar] [CrossRef]
- Ding, Q.; Markesbery, W.R.; Chen, Q.; Li, F.; Keller, J.N. Ribosome dysfunction is an early event in Alzheimer’s disease. J. Neurosci. 2005, 25, 9171–9175. [Google Scholar] [CrossRef]
- Baral, S.S.; Lieux, M.E.; DiMario, P.J. Nucleolar stress in Drosophila neuroblasts, a model for human ribosomopathies. Biol. Open 2020, 9, bio046565. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Smith, M.A.; Zhu, X.; Baus, D.; Merrick, W.C.; Tartakoff, A.M.; Hattier, T.; Harris, P.L.; Siedlak, S.L.; Fujioka, H.; et al. Ribosomal RNA in Alzheimer disease is oxidized by bound redox-active iron. J. Biol. Chem. 2005, 280, 20978–20986. [Google Scholar] [CrossRef] [PubMed]
- Pfister, A.S. Emerging Role of the Nucleolar Stress Response in Autophagy. Front. Cell. Neurosci. 2019, 13, 156. [Google Scholar] [CrossRef]
- Miguel-Aliaga, I.; Jasper, H.; Lemaitre, B. Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster. Genetics 2018, 210, 357–396. [Google Scholar] [CrossRef]
- Chan, H.Y.; Bonini, N.M. Drosophila models of human neurodegenerative disease. Cell Death Differ. 2000, 7, 1075–1080. [Google Scholar] [CrossRef]
- Marsh, J.L.; Thompson, L.M. Drosophila in the study of neurodegenerative disease. Neuron 2006, 52, 169–178. [Google Scholar] [CrossRef]





| Gene Name | FlyBase ID | Primer Sequence * | Amplicon Size |
|---|---|---|---|
| sod1 | FBgn0003462 | F: 5′-ACCGACTCCAAGATTACGCTC-3′ R: 5′-CAGTGGCCGACATCGGAATA-3′ | 197 bp |
| sod2 | FBgn0010213 | F: 5′-AATCTAAATGCCGCCGAGGA-3′ R: 5′-CTCTTCCACTGCGACTCGAT-3′ | 197 bp |
| cat | FBgn0000261 | F: 5′-CTATGGCTCGCACACCTTCA-3′ R: 5′-TCGTCCAACTGGGGAACTTG-3′ | 194 bp |
| rpl32 | FBgn0002626 | F: 5′-GACCATCCGCCCAGCATAC-3′ R: 5′-CGGCGACGCACTCTGTT-3′ | 138 bp |
| Treatment | Mean Area (nm2) |
|---|---|
| STD | 19.65 ± 1.83 |
| Lisosan G-supplemented food | 11.32 ± 2.03 *** |
| Healthy | AD | AD + Lisosan G | |
|---|---|---|---|
| Aβ plaques | − | + | − |
| Apoptosis | − | + | − |
| Oxidative stress | − | + | − |
| Autophagy | ● | ‡‡ | ● |
| 18S and 28S rDNA stability | + | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bongiorni, S.; Catalani, E.; Arisi, I.; Lazzarini, F.; Del Quondam, S.; Brunetti, K.; Cervia, D.; Prantera, G. Pathological Defects in a Drosophila Model of Alzheimer’s Disease and Beneficial Effects of the Natural Product Lisosan G. Biomolecules 2024, 14, 855. https://doi.org/10.3390/biom14070855
Bongiorni S, Catalani E, Arisi I, Lazzarini F, Del Quondam S, Brunetti K, Cervia D, Prantera G. Pathological Defects in a Drosophila Model of Alzheimer’s Disease and Beneficial Effects of the Natural Product Lisosan G. Biomolecules. 2024; 14(7):855. https://doi.org/10.3390/biom14070855
Chicago/Turabian StyleBongiorni, Silvia, Elisabetta Catalani, Ivan Arisi, Francesca Lazzarini, Simona Del Quondam, Kashi Brunetti, Davide Cervia, and Giorgio Prantera. 2024. "Pathological Defects in a Drosophila Model of Alzheimer’s Disease and Beneficial Effects of the Natural Product Lisosan G" Biomolecules 14, no. 7: 855. https://doi.org/10.3390/biom14070855





