The Role of Inositols in Endocrine and Neuroendocrine Tumors
Abstract
:1. Introduction
2. Materials and Methods
3. Results
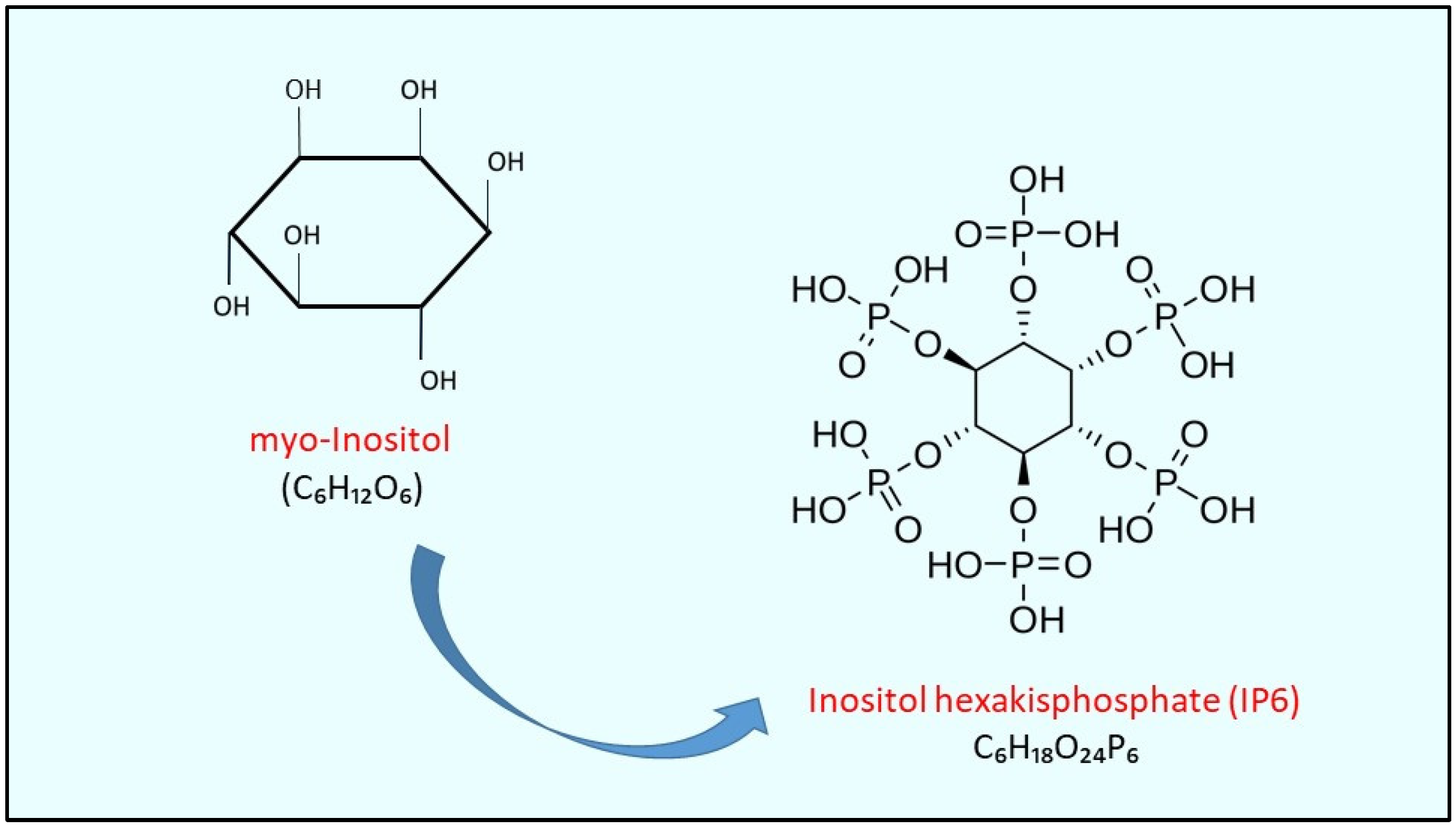
3.1. Molecular Mechanisms of the Role of Inositols in Cancer
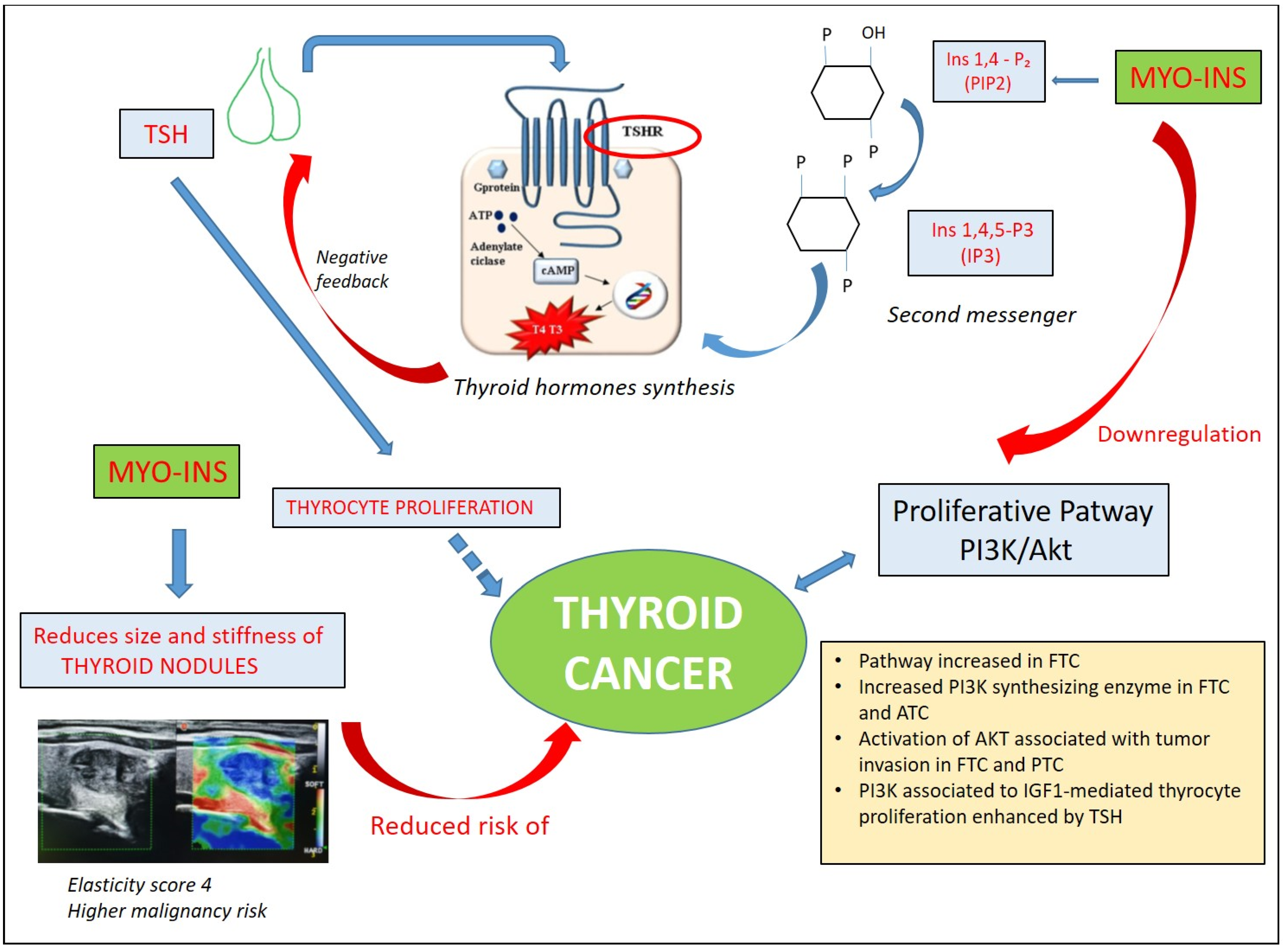
3.2. Inositols and Thyroid Cancer
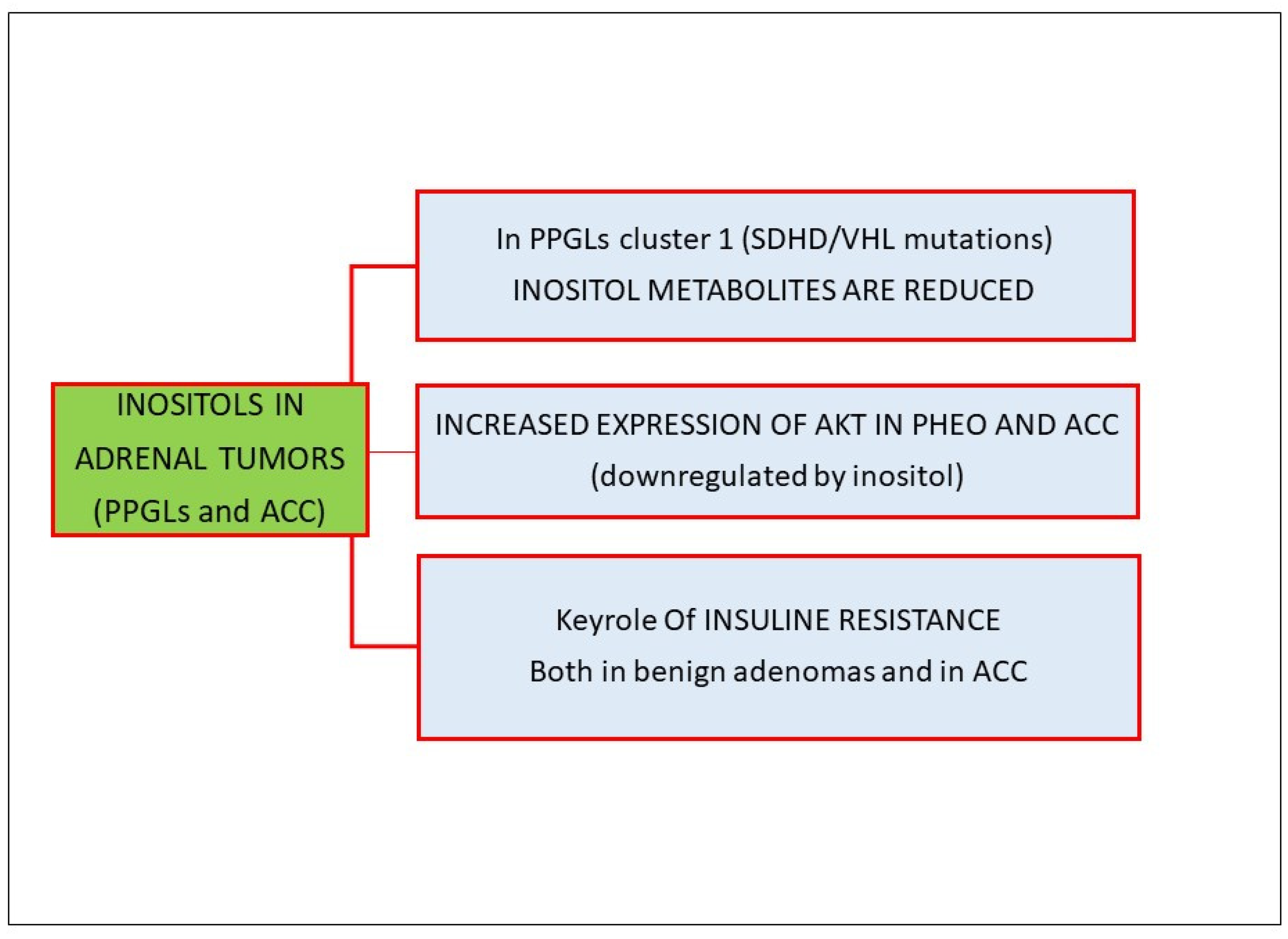
3.3. Inositols and Adrenal Tumors
3.4. Inositols and Pituitary Neuroendocrine Tumors (Pit-NETs)
3.5. Inositols and Neuroendocrine Neoplasms
4. Clinical Use of Inositols and New Therapeutic Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| myo-Ins | myo-inositol |
| IP6 | inositol hexakisphosphate |
| IP3 | inositol triphosphate |
| IGF-1 | insulin-growth factor 1 |
| IGFBP-1 | insulin-growth factor binding protein 1 |
| IGFBP-2 | insulin-growth factor binding protein 2 |
| MAP | mitogenic-activated protein |
| PI3K/Akt | phosphoinositide-3 kinase/Akt |
| EMT | epithelial–mesenchymal transition |
| pNETS | pancreatic neuroendocrine tumors |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| TSH | thyroid-stimulating hormone |
| PLC | phospholipase C |
| PTC | papillary thyroid carcinoma |
| FTC | follicular thyroid carcinoma |
| BTA | benign thyroid adenoma |
| FV-PTC | follicular variant of papillary thyroid carcinoma |
| pSTAT3 | phosphorylated-STAT3 |
| HGF | hepatocyte growth factor |
| MAPK | mitogen-activated protein kinase |
| PPGL | paragangliomas |
| mTOR | mammalian target of rapamycin |
| VEGF | vascular endothelial growth factor |
| Pit-NET | pituitary neuroendocrine tumors |
| NEN | neuroendocrine neoplasm |
| NET | neuroendocrine tumor |
| NEC | neuroendocrine carcinoma |
| GEP | gastro-entero-pancreatic system |
| PHEO | pheochromocytoma |
| ACC | adrenocortical carcinoma |
| PCOS | polycystic ovary syndrome |
| GDM | gestational diabetes mellitus |
References
- Shamsuddin, A.M. Metabolism and Cellular Functions of Ip6: A Review. Anticancer Res. 1999, 19, 3733–3736. [Google Scholar] [PubMed]
- Bizzarri, M.; Vucenik, I.; Appetecchia, M. Inositols as Adjuvant Treatments in Oncology. In A Clinical Guide to Inositols. Vittorio Unfer and Didier Dewailly Editors; Academic Press: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer Prevention and Therapy through the Modulation of Transcription Factors by Bioactive Natural Compounds. Semin. Cancer Biol. 2016, 40–41, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Irving, D.; Drasar, B.S. Fibre and Cancer of the Colon. Br. J. Cancer 1973, 28, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Graf, E.; Eaton, J.W. Dietary Suppression of Colonic Cancer. Fiber or Phytate? Cancer 1985, 56, 717–718. [Google Scholar] [CrossRef]
- Markiewicz, L.H.; Ogrodowczyk, A.M.; Wiczkowski, W.; Wroblewska, B. Phytate and Butyrate Differently Influence the Proliferation, Apoptosis and Survival Pathways in Human Cancer and Healthy Colonocytes. Nutrients 2021, 13, 1887. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agarwal, R. Prostate Cancer and Inositol Hexaphosphate: Efficacy and Mechanisms. Anticancer Res. 2005, 25, 2891–2903. [Google Scholar] [PubMed]
- Wawszczyk, J.; Kapral, M.; Lodowska, J.; Jesse, K.; Hollek, A.; Weglarz, L. Antiproliferative Effect of Inositol Hexaphosphate on Human Skin Melanoma Cells In Vitro. Acta Pol. Pharm. 2015, 72, 895–900. [Google Scholar]
- Liu, G.; Song, Y.; Cui, L.; Wen, Z.; Lu, X. Inositol Hexaphosphate Suppresses Growth and Induces Apoptosis in Ht-29 Colorectal Cancer Cells in Culture: Pi3k/Akt Pathway as a Potential Target. Int. J. Clin. Exp. Pathol. 2015, 8, 1402–1410. [Google Scholar] [PubMed]
- de Lima, E.M.; Kanunfre, C.C.; de Andrade, L.F.; Granato, D.; Rosso, N.D. Cytotoxic Effect of Inositol Hexaphosphate and Its Ni(Ii) Complex on Human Acute Leukemia Jurkat T Cells. Toxicol. In Vitro 2015, 29, 2081–2088. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. Sanra-a Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Agarwal, C.; Dhanalakshmi, S.; Singh, R.P.; Agarwal, R. Inositol Hexaphosphate Inhibits Growth and Induces G1 Arrest and Apoptotic Death of Androgen-Dependent Human Prostate Carcinoma Lncap Cells. Neoplasia 2004, 6, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Deliliers, G.L.; Servida, F.; Fracchiolla, N.S.; Ricci, C.; Borsotti, C.; Colombo, G.; Soligo, D. Effect of Inositol Hexaphosphate (Ip(6)) on Human Normal and Leukaemic Haematopoietic Cells. Br. J. Haematol. 2002, 117, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Weglarz, L.; Molin, I.; Orchel, A.; Parfiniewicz, B.; Dzierzewicz, Z. Quantitative Analysis of the Level of P53 and P21(Waf1) Mrna in Human Colon Cancer Ht-29 Cells Treated with Inositol Hexaphosphate. Acta Biochim. Pol. 2006, 53, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Fabrizi, G.; Masiello, M.G.; Proietti, S.; Palombo, A.; Minini, M.; Harrath, A.H.; Alwasel, S.H.; Ricci, G.; Catizone, A.; et al. Inositol Induces Mesenchymal-Epithelial Reversion in Breast Cancer Cells through Cytoskeleton Rearrangement. Exp. Cell Res. 2016, 345, 37–50. [Google Scholar] [CrossRef]
- Huang, C.; Ma, W.Y.; Hecht, S.S.; Dong, Z. Inositol Hexaphosphate Inhibits Cell Transformation and Activator Protein 1 Activation by Targeting Phosphatidylinositol-3’ Kinase. Cancer Res. 1997, 57, 2873–2878. [Google Scholar]
- Liao, J.; Seril, D.N.; Yang, A.L.; Lu, G.G.; Yang, G.Y. Inhibition of Chronic Ulcerative Colitis Associated Adenocarcinoma Development in Mice by Inositol Compounds. Carcinogenesis 2007, 28, 446–454. [Google Scholar] [CrossRef]
- Vucenik, I.; Shamsuddin, A.M. Protection against Cancer by Dietary Ip6 and Inositol. Nutr. Cancer 2006, 55, 109–125. [Google Scholar] [CrossRef]
- Vucenik, I.; Passaniti, A.; Vitolo, M.I.; Tantivejkul, K.; Eggleton, P.; Shamsuddin, A.M. Anti-Angiogenic Activity of Inositol Hexaphosphate (Ip6). Carcinogenesis 2004, 25, 2115–2123. [Google Scholar] [CrossRef]
- Graf, E.; Eaton, J.W. Antioxidant Functions of Phytic Acid. Free Radic. Biol. Med. 1990, 8, 61–69. [Google Scholar] [CrossRef]
- Baten, A.; Ullah, A.; Tomazic, V.J.; Shamsuddin, A.M. Inositol-Phosphate-Induced Enhancement of Natural Killer Cell Activity Correlates with Tumor Suppression. Carcinogenesis 1989, 10, 1595–1598. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Y.; Wang, X.L. Inositol Hexaphosphate-Induced Enhancement of Natural Killer Cell Activity Correlates with Suppression of Colon Carcinogenesis in Rats. World J. Gastroenterol. 2005, 11, 5044–5046. [Google Scholar] [CrossRef]
- Shamsuddin, A.M.; Ullah, A.; Chakravarthy, A.K. Inositol and Inositol Hexaphosphate Suppress Cell Proliferation and Tumor Formation in Cd-1 Mice. Carcinogenesis 1989, 10, 1461–1463. [Google Scholar] [CrossRef] [PubMed]
- Vucenik, I.; Sakamoto, K.; Bansal, M.; Shamsuddin, A.M. Inhibition of Rat Mammary Carcinogenesis by Inositol Hexaphosphate (Phytic Acid). A Pilot Study. Cancer Lett. 1993, 75, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Vucenik, I.; Tomazic, V.J.; Fabian, D.; Shamsuddin, A.M. Antitumor Activity of Phytic Acid (Inositol Hexaphosphate) in Murine Transplanted and Metastatic Fibrosarcoma, a Pilot Study. Cancer Lett. 1992, 65, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Unfer, V.; Facchinetti, F.; Soulage, C.O.; Greene, N.D.; Bizzarri, M.; Lagana, A.S.; Chan, S.Y.; Bevilacqua, A.; Pkhaladze, L.; et al. Inositols: From Established Knowledge to Novel Approaches. Int. J. Mol. Sci. 2021, 22, 105753. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Dinicola, S.; Cucina, A. Modulation of Both Insulin Resistance and Cancer Growth by Inositol. Curr. Pharm. Des. 2017, 23, 5200–5210. [Google Scholar] [CrossRef] [PubMed]
- Tsugane, S.; Inoue, M. Insulin Resistance and Cancer: Epidemiological Evidence. Cancer Sci. 2010, 101, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Thun, M.J. Obesity and Cancer. Oncogene 2004, 23, 6365–6378. [Google Scholar] [CrossRef]
- Vigneri, P.; Frasca, F.; Sciacca, L.; Pandini, G.; Vigneri, R. Diabetes and Cancer. Endocr. Relat. Cancer 2009, 16, 1103–1123. [Google Scholar] [CrossRef]
- Denley, A.; Wallace, J.C.; Cosgrove, L.J.; Forbes, B.E. The Insulin Receptor Isoform Exon 11- (Ir-a) in Cancer and Other Diseases: A Review. Horm. Metab. Res. 2003, 35, 778–785. [Google Scholar] [CrossRef]
- Fresno Vara, J.A.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; Gonzalez-Baron, M. Pi3k/Akt Signalling Pathway and Cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef]
- Lim, J.; Kim, J.H.; Paeng, J.Y.; Kim, M.J.; Hong, S.D.; Lee, J.I.; Hong, S.P. Prognostic Value of Activated Akt Expression in Oral Squamous Cell Carcinoma. J. Clin. Pathol. 2005, 58, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Daley, B.; Klubo-Gwiezdzinska, J. The Role of an Anti-Diabetic Drug Metformin in the Treatment of Endocrine Tumors. J. Mol. Endocrinol. 2019, 63, R17–R35. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Hegedus, L. Clinical Practice. The Thyroid Nodule. N. Engl. J. Med. 2004, 351, 1764–1771. [Google Scholar] [CrossRef]
- Antonelli, A.; Silvano, G.; Bianchi, F.; Gambuzza, C.; Tana, L.; Salvioni, G.; Baldi, V.; Gasperini, L.; Baschieri, L. Risk of Thyroid Nodules in Subjects Occupationally Exposed to Radiation: A Cross Sectional Study. Occup. Environ. Med. 1995, 52, 500–504. [Google Scholar] [CrossRef]
- Asteria, C.; Giovanardi, A.; Pizzocaro, A.; Cozzaglio, L.; Morabito, A.; Somalvico, F.; Zoppo, A. Us-Elastography in the Differential Diagnosis of Benign and Malignant Thyroid Nodules. Thyroid 2008, 18, 523–531. [Google Scholar] [CrossRef]
- Rago, T.; Scutari, M.; Santini, F.; Loiacono, V.; Piaggi, P.; Di Coscio, G.; Basolo, F.; Berti, P.; Pinchera, A.; Vitti, P. Real-Time Elastosonography: Useful Tool for Refining the Presurgical Diagnosis in Thyroid Nodules with Indeterminate or Nondiagnostic Cytology. J. Clin. Endocrinol. Metab. 2010, 95, 5274–5280. [Google Scholar] [CrossRef]
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedus, L.; Paschke, R.; Valcavi, R.; Vitti, P.; Aace Ace Ame Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules--2016 Update. Endocr. Pract. 2016, 22, 622–639. [Google Scholar] [CrossRef]
- Nordio, M.; Basciani, S. Evaluation of Thyroid Nodule Characteristics in Subclinical Hypothyroid Patients under a Myo-Inositol Plus Selenium Treatment. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Nordio, M.; Lagana, A.S.; Unfer, V. The Role of Inositol in Thyroid Physiology and in Subclinical Hypothyroidism Management. Front. Endocrinol. 2021, 12, 662582. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Watters, K.F.; Carpenter, A.D.; Ladenson, P.W.; Cooper, D.S.; Ding, E.L. Thyrotropin and Thyroid Cancer Diagnosis: A Systematic Review and Dose-Response Meta-Analysis. J. Clin. Endocrinol. Metab. 2012, 97, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Li, Z.M.; Liu, J.F.; Zhang, Z.Z.; Wang, L.S. An Overall and Dose-Response Meta-Analysis for Thyrotropin and Thyroid Cancer Risk by Histological Type. Oncotarget 2016, 7, 47750–47759. [Google Scholar] [CrossRef] [PubMed]
- de Alcantara-Jones, D.M.; de Alcantara-Nunes, T.F.; Rocha Bde, O.; de Oliveira, R.D.; Santana, A.C.; de Alcantara, F.T.; de Faria, T.M.; da Silva, I.C.; Araujo, L.M. Is There Any Association between Hashimoto’s Thyroiditis and Thyroid Cancer? A Retrospective Data Analysis. Radiol. Bras. 2015, 48, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Hsieh, A.T.; Lee, T.W.; Lee, T.I.; Chien, Y.M. The Association of Thyrotropin and Autoimmune Thyroid Disease in Developing Papillary Thyroid Cancer. Int. J. Endocrinol. 2017, 2017, 5940367. [Google Scholar] [CrossRef] [PubMed]
- Selek, A.; Cetinarslan, B.; Tarkun, I.; Canturk, Z.; Ustuner, B.; Akyay, Z. Thyroid Autoimmunity: Is Really Associated with Papillary Thyroid Carcinoma? Eur. Arch. Otorhinolaryngol. 2017, 274, 1677–1681. [Google Scholar] [CrossRef]
- Boi, F.; Pani, F.; Mariotti, S. Thyroid Autoimmunity and Thyroid Cancer: Review Focused on Cytological Studies. Eur. Thyroid J. 2017, 6, 178–186. [Google Scholar] [CrossRef]
- Nordio, M.; Basciani, S. Myo-Inositol Plus Selenium Supplementation Restores Euthyroid State in Hashimoto’s Patients with Subclinical Hypothyroidism. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 51–59. [Google Scholar]
- Payer, J.; Jackuliak, P.; Kuzma, M.; Dzupon, M.; Vanuga, P. Supplementation with Myo-Inositol and Selenium Improves the Clinical Conditions and Biochemical Features of Women with or at Risk for Subclinical Hypothyroidism. Front. Endocrinol. 2022, 13, 1067029. [Google Scholar] [CrossRef]
- Khatami, F.; Payab, M.; Sarvari, M.; Gilany, K.; Larijani, B.; Arjmand, B.; Tavangar, S.M. Oncometabolites as Biomarkers in Thyroid Cancer: A Systematic Review. Cancer Manag. Res. 2019, 11, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, L.; Shintu, L.; Nambiath Chandran, J.; Tintaru, A.; Ugolini, C.; Magalhaes, A.; Basolo, F.; Miccoli, P.; Caldarelli, S. Toward the Reliable Diagnosis of Indeterminate Thyroid Lesions: A Hrmas Nmr-Based Metabolomics Case of Study. J. Proteome Res. 2012, 11, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Ringel, M.D.; Hayre, N.; Saito, J.; Saunier, B.; Schuppert, F.; Burch, H.; Bernet, V.; Burman, K.D.; Kohn, L.D.; Saji, M. Overexpression and Overactivation of Akt in Thyroid Carcinoma. Cancer Res. 2001, 61, 6105–6111. [Google Scholar]
- Wu, G.; Mambo, E.; Guo, Z.; Hu, S.; Huang, X.; Gollin, S.M.; Trink, B.; Ladenson, P.W.; Sidransky, D.; Xing, M. Uncommon Mutation, but Common Amplifications, of the Pik3ca Gene in Thyroid Tumors. J. Clin. Endocrinol. Metab. 2005, 90, 4688–4693. [Google Scholar] [CrossRef]
- Garcia-Rostan, G.; Costa, A.M.; Pereira-Castro, I.; Salvatore, G.; Hernandez, R.; Hermsem, M.J.; Herrero, A.; Fusco, A.; Cameselle-Teijeiro, J.; Santoro, M. Mutation of the Pik3ca Gene in Anaplastic Thyroid Cancer. Cancer Res. 2005, 65, 10199–10207. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Liu, D.; Shan, Y.; Hu, S.; Studeman, K.; Condouris, S.; Wang, Y.; Trink, A.; El-Naggar, A.K.; Tallini, G.; et al. Genetic Alterations and Their Relationship in the Phosphatidylinositol 3-Kinase/Akt Pathway in Thyroid Cancer. Clin. Cancer Res. 2007, 13, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hou, P.; Ji, M.; Guan, H.; Studeman, K.; Jensen, K.; Vasko, V.; El-Naggar, A.K.; Xing, M. Highly Prevalent Genetic Alterations in Receptor Tyrosine Kinases and Phosphatidylinositol 3-Kinase/Akt and Mitogen-Activated Protein Kinase Pathways in Anaplastic and Follicular Thyroid Cancers. J. Clin. Endocrinol. Metab. 2008, 93, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Viciana, P.; Warne, P.H.; Dhand, R.; Vanhaesebroeck, B.; Gout, I.; Fry, M.J.; Waterfield, M.D.; Downward, J. Phosphatidylinositol-3-Oh Kinase as a Direct Target of Ras. Nature 1994, 370, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Segouffin-Cariou, C.; Billaud, M. Transforming Ability of Men2a-Ret Requires Activation of the Phosphatidylinositol 3-Kinase/Akt Signaling Pathway. J. Biol. Chem. 2000, 275, 3568–3576. [Google Scholar] [CrossRef]
- Zhu, Z.; Gandhi, M.; Nikiforova, M.N.; Fischer, A.H.; Nikiforov, Y.E. Molecular Profile and Clinical-Pathologic Features of the Follicular Variant of Papillary Thyroid Carcinoma. An Unusually High Prevalence of Ras Mutations. Am. J. Clin. Pathol. 2003, 120, 71–77. [Google Scholar] [CrossRef]
- Gupta, S.; Ramjaun, A.R.; Haiko, P.; Wang, Y.; Warne, P.H.; Nicke, B.; Nye, E.; Stamp, G.; Alitalo, K.; Downward, J. Binding of Ras to Phosphoinositide 3-Kinase P110alpha Is Required for Ras-Driven Tumorigenesis in Mice. An Unusually High Prevalence of Ras Mutations. Cell 2007, 129, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Vasko, V.V.; Kato, Y.; Kruhlak, M.; Saji, M.; Cheng, S.Y.; Ringel, M.D. Akt Activation Promotes Metastasis in a Mouse Model of Follicular Thyroid Carcinoma. Endocrinology 2005, 146, 4456–4463. [Google Scholar] [CrossRef] [PubMed]
- Vasko, V.; Saji, M.; Hardy, E.; Kruhlak, M.; Larin, A.; Savchenko, V.; Miyakawa, M.; Isozaki, O.; Murakami, H.; Tsushima, T.; et al. Akt Activation and Localisation Correlate with Tumour Invasion and Oncogene Expression in Thyroid Cancer. J. Med. Genet. 2004, 41, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Vitarelli, E.; Barresi, G.; Trimarchi, F.; Benvenga, S.; Trovato, M. Hgf/C-Met System Pathways in Benign and Malignant Histotypes of Thyroid Nodules: An Immunohistochemical Characterization. Histol. Histopathol. 2012, 27, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ciampolillo, A.; De Tullio, C.; Perlino, E.; Maiorano, E. The Igf-I Axis in Thyroid Carcinoma. Curr. Pharm. Des. 2007, 13, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Sarlis, N.J.; Benvenga, S. Molecular Signaling in Thyroid Cancer. Cancer Treat. Res. 2004, 122, 237–264. [Google Scholar] [CrossRef]
- Rezzonico, J.N.; Rezzonico, M.; Pusiol, E.; Pitoia, F.; Niepomniszcze, H. Increased Prevalence of Insulin Resistance in Patients with Differentiated Thyroid Carcinoma. Metab. Syndr. Relat. Disord. 2009, 7, 375–380. [Google Scholar] [CrossRef]
- Chen, G.; Xu, S.; Renko, K.; Derwahl, M. Metformin Inhibits Growth of Thyroid Carcinoma Cells, Suppresses Self-Renewal of Derived Cancer Stem Cells, and Potentiates the Effect of Chemotherapeutic Agents. J. Clin. Endocrinol. Metab. 2012, 97, E510–E520. [Google Scholar] [CrossRef]
- Lenders, J.W.; Duh, Q.Y.; Eisenhofer, G.; Gimenez-Roqueplo, A.P.; Grebe, S.K.; Murad, M.H.; Naruse, M.; Pacak, K.; Young, W.F., Jr.; Endocrine, S. Pheochromocytoma and Paraganglioma: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2014, 99, 1915–1942. [Google Scholar] [CrossRef] [PubMed]
- Dahia, P.L.; Ross, K.N.; Wright, M.E.; Hayashida, C.Y.; Santagata, S.; Barontini, M.; Kung, A.L.; Sanso, G.; Powers, J.F.; Tischler, A.S.; et al. A Hif1alpha Regulatory Loop Links Hypoxia and Mitochondrial Signals in Pheochromocytomas. PLoS Genet. 2005, 1, e8. [Google Scholar] [CrossRef]
- Flynn, A.; Dwight, T.; Harris, J.; Benn, D.; Zhou, L.; Hogg, A.; Catchpoole, D.; James, P.; Duncan, E.L.; Trainer, A.; et al. Pheo-Type: A Diagnostic Gene-Expression Assay for the Classification of Pheochromocytoma and Paraganglioma. J. Clin. Endocrinol. Metab. 2016, 101, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vega, L.J.; Buffet, A.; De Cubas, A.A.; Cascon, A.; Menara, M.; Khalifa, E.; Amar, L.; Azriel, S.; Bourdeau, I.; Chabre, O.; et al. Germline Mutations in Fh Confer Predisposition to Malignant Pheochromocytomas and Paragangliomas. Hum. Mol. Genet. 2014, 23, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Sun, N.; Greunke, C.; Feuchtinger, A.; Kircher, S.; Deutschbein, T.; Papathomas, T.; Bechmann, N.; William Wallace, P.; Peitzsch, M.; et al. Mass Spectrometry Imaging Identifies Metabolic Patterns Associated with Malignant Potential in Pheochromocytoma and Paraganglioma. Eur. J. Endocrinol. 2021, 185, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, M.A.; Thompson, G.B.; Duh, Q.Y.; Hamrahian, A.H.; Angelos, P.; Elaraj, D.; Fishman, E.; Kharlip, J.; American Association of Clinical, E.; American Association of Endocrine, S. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the Management of Adrenal Incidentalomas: Executive Summary of Recommendations. Endocr. Pract. 2009, 15, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Terzolo, M.; Pia, A.; Ali, A.; Osella, G.; Reimondo, G.; Bovio, S.; Daffara, F.; Procopio, M.; Paccotti, P.; Borretta, G.; et al. Adrenal Incidentaloma: A New Cause of the Metabolic Syndrome? J. Clin. Endocrinol. Metab. 2002, 87, 998–1003. [Google Scholar] [CrossRef]
- Wiesner, T.D.; Bluher, M.; Windgassen, M.; Paschke, R. Improvement of Insulin Sensitivity after Adrenalectomy in Patients with Pheochromocytoma. J. Clin. Endocrinol. Metab. 2003, 88, 3632–3636. [Google Scholar] [CrossRef]
- Fassnacht, M.; Weismann, D.; Ebert, S.; Adam, P.; Zink, M.; Beuschlein, F.; Hahner, S.; Allolio, B. Akt Is Highly Phosphorylated in Pheochromocytomas but Not in Benign Adrenocortical Tumors. J. Clin. Endocrinol. Metab. 2005, 90, 4366–4370. [Google Scholar] [CrossRef]
- Adler, J.T.; Hottinger, D.G.; Kunnimalaiyaan, M.; Chen, H. Inhibition of the Pi3k Pathway Suppresses Hormonal Secretion and Limits Growth in Pheochromocytoma Cells. World J. Surg. 2009, 33, 2452–2457. [Google Scholar] [CrossRef]
- De Martino, M.C.; van Koetsveld, P.M.; Pivonello, R.; Hofland, L.J. Role of the Mtor Pathway in Normal and Tumoral Adrenal Cells. Neuroendocrinology 2010, 92 (Suppl. S1), 28–34. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Sorice, G.P.; Prioletta, A.; Mezza, T.; Cipolla, C.; Salomone, E.; Giaccari, A.; Pontecorvi, A.; Della Casa, S. The Size of Adrenal Incidentalomas Correlates with Insulin Resistance. Is There a Cause-Effect Relationship? Clin. Endocrinol. 2011, 74, 300–305. [Google Scholar] [CrossRef]
- Altieri, B.; Tirabassi, G.; Della Casa, S.; Ronchi, C.L.; Balercia, G.; Orio, F.; Pontecorvi, A.; Colao, A.; Muscogiuri, G. Adrenocortical Tumors and Insulin Resistance: What Is the First Step? Int. J. Cancer 2016, 138, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.C.; Latronico, A.C. Insulin-Like Growth Factor System on Adrenocortical Tumorigenesis. Mol. Cell Endocrinol. 2012, 351, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.Q.; Fragoso, M.C.; Lotfi, C.F.; Santos, M.G.; Nishi, M.Y.; Costa, M.H.; Lerario, A.M.; Maciel, C.C.; Mattos, G.E.; Jorge, A.A.; et al. Expression of Insulin-Like Growth Factor-Ii and Its Receptor in Pediatric and Adult Adrenocortical Tumors. J. Clin. Endocrinol. Metab. 2008, 93, 3524–3531. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Frasca, F.; Pandini, G.; Sciacca, L.; Vigneri, R. Insulin Receptor Isoforms and Insulin Receptor/Insulin-Like Growth Factor Receptor Hybrids in Physiology and Disease. Endocr. Rev. 2009, 30, 586–623. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.H.; Kennedy, R.L.; Justice, S.K.; Price, A. Pituitary Adenomas with High and Low Basal Inositol Phospholipid Turnover; the Stimulatory Effect of Kinins and an Association with Interleukin-6 Secretion. Clin. Endocrinol. 1993, 39, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Pinzariu, O.; Georgescu, B.; Georgescu, C.E. Metabolomics-a Promising Approach to Pituitary Adenomas. Front. Endocrinol. 2018, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Faggi, L.; Giustina, A.; Tulipano, G. Effects of Metformin on Cell Growth and Ampk Activity in Pituitary Adenoma Cell Cultures, Focusing on the Interaction with Adenylyl Cyclase Activating Signals. Mol. Cell Endocrinol. 2018, 470, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Borrego, M.C.; Fuentes-Fayos, A.C.; Herrera-Martinez, A.D.; L-López, F.; Ibanez-Costa, A.; Moreno-Moreno, P.; Alhambra-Exposito, M.R.; Barrera-Martin, A.; Blanco-Acevedo, C.; Dios, E.; et al. Biguanides Exert Antitumoral Actions in Pituitary Tumor Cells through Ampk-Dependent and -Independent Mechanisms. J. Clin. Endocrinol. Metab. 2019, 104, 3501–3513. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Harris, C.; Baeg, K.J.; Aronson, A.; Wisnivesky, J.P.; Kim, M.K. Incidence Trends of Gastroenteropancreatic Neuroendocrine Tumors in the United States. Clin. Gastroenterol. Hepatol. 2019, 17, 2212–2217.e1. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 Who Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Vinik, A.I.; Chaya, C. Clinical Presentation and Diagnosis of Neuroendocrine Tumors. Hematol. Oncol. Clin. N. Am. 2016, 30, 21–48. [Google Scholar] [CrossRef] [PubMed]
- Briest, F.; Grabowski, P. Pi3k-Akt-Mtor-Signaling and Beyond: The Complex Network in Gastroenteropancreatic Neuroendocrine Neoplasms. Theranostics 2014, 4, 336–365. [Google Scholar] [CrossRef] [PubMed]
- Reidy-Lagunes, D.L.; Vakiani, E.; Segal, M.F.; Hollywood, E.M.; Tang, L.H.; Solit, D.B.; Pietanza, M.C.; Capanu, M.; Saltz, L.B. A Phase 2 Study of the Insulin-Like Growth Factor-1 Receptor Inhibitor Mk-0646 in Patients with Metastatic, Well-Differentiated Neuroendocrine Tumors. Cancer 2012, 118, 4795–4800. [Google Scholar] [CrossRef]
- Villaume, K.; Blanc, M.; Gouysse, G.; Walter, T.; Couderc, C.; Nejjari, M.; Vercherat, C.; Cordier-Bussat, M.; Roche, C.; Scoazec, J.Y. Vegf Secretion by Neuroendocrine Tumor Cells Is Inhibited by Octreotide and by Inhibitors of the Pi3k/Akt/Mtor Pathway. Neuroendocrinology 2010, 91, 268–278. [Google Scholar] [CrossRef] [PubMed]
- von Wichert, G.; Haeussler, U.; Greten, F.R.; Kliche, S.; Dralle, H.; Bohm, B.O.; Adler, G.; Seufferlein, T. Regulation of Cyclin D1 Expression by Autocrine Igf-I in Human Bon Neuroendocrine Tumour Cells. Oncogene 2005, 24, 1284–1289. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Cassidy, M.G.; Rychahou, P.; Starr, M.E.; Liu, J.; Li, X.; Epperly, G.; Weiss, H.L.; Townsend, C.M., Jr.; et al. Pi3k P110alpha/Akt Signaling Negatively Regulates Secretion of the Intestinal Peptide Neurotensin through Interference of Granule Transport. Mol. Endocrinol. 2012, 26, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- Shida, T.; Kishimoto, T.; Furuya, M.; Nikaido, T.; Koda, K.; Takano, S.; Kimura, F.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; et al. Expression of an Activated Mammalian Target of Rapamycin (Mtor) in Gastroenteropancreatic Neuroendocrine Tumors. Cancer Chemother. Pharmacol. 2010, 65, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, A.; Chang, D.K.; Nones, K.; Corbo, V.; Patch, A.M.; Bailey, P.; Lawlor, R.T.; Johns, A.L.; Miller, D.K.; Mafficini, A.; et al. Whole-Genome Landscape of Pancreatic Neuroendocrine Tumours. Nature 2017, 543, 65–71. [Google Scholar] [CrossRef]
- Catena, L.; Bajetta, E.; Milione, M.; Ducceschi, M.; Valente, M.; Dominoni, F.; Colonna, V. Mammalian Target of Rapamycin Expression in Poorly Differentiated Endocrine Carcinoma: Clinical and Therapeutic Future Challenges. Target. Oncol. 2011, 6, 65–68. [Google Scholar] [CrossRef]
- De Wilde, R.F.; Edil, B.H.; Hruban, R.H.; Maitra, A. Well-Differentiated Pancreatic Neuroendocrine Tumors: From Genetics to Therapy. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 199–208. [Google Scholar] [CrossRef]
- Missiaglia, E.; Dalai, I.; Barbi, S.; Beghelli, S.; Falconi, M.; della Peruta, M.; Piemonti, L.; Capurso, G.; Di Florio, A.; delle Fave, G.; et al. Pancreatic Endocrine Tumors: Expression Profiling Evidences a Role for Akt-Mtor Pathway. J. Clin. Oncol. 2010, 28, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, S.; Verzoni, E.; Prinzi, N.; Mennitto, A.; Femia, D.; Grassi, P.; Concas, L.; Vernieri, C.; Lo Russo, G.; Procopio, G. Everolimus Treatment for Neuroendocrine Tumors: Latest Results and Clinical Potential. Ther. Adv. Med. Oncol. 2017, 9, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Raffeld, M.; Mateo, C.; Sakamoto, A.; Moody, T.W.; Ito, T.; Venzon, D.J.; Serrano, J.; Jensen, R.T. Increased Expression of Insulin-Like Growth Factor I and/or Its Receptor in Gastrinomas Is Associated with Low Curability, Increased Growth, and Development of Metastases. Clin. Cancer Res. 2005, 11, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- Greff, D.; Juhasz, A.E.; Vancsa, S.; Varadi, A.; Sipos, Z.; Szinte, J.; Park, S.; Hegyi, P.; Nyirady, P.; Acs, N.; et al. Inositol Is an Effective and Safe Treatment in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Reprod. Biol. Endocrinol. 2023, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Bacic, I.; Druzijanic, N.; Karlo, R.; Skific, I.; Jagic, S. Efficacy of Ip6 + Inositol in the Treatment of Breast Cancer Patients Receiving Chemotherapy: Prospective, Randomized, Pilot Clinical Study. J. Exp. Clin. Cancer Res. 2010, 29, 12. [Google Scholar] [CrossRef]
- Druzijanic, N.J.; Juricic, J.; Perko, Z.; Kraljevic, D. IP-6 & Inositol: Adjuvant to chemo-therapy of colon cancer. A pilot clinical trial. Rev. Oncol. 2002, 4, 480. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mormando, M.; Puliani, G.; Bianchini, M.; Lauretta, R.; Appetecchia, M. The Role of Inositols in Endocrine and Neuroendocrine Tumors. Biomolecules 2024, 14, 1004. https://doi.org/10.3390/biom14081004
Mormando M, Puliani G, Bianchini M, Lauretta R, Appetecchia M. The Role of Inositols in Endocrine and Neuroendocrine Tumors. Biomolecules. 2024; 14(8):1004. https://doi.org/10.3390/biom14081004
Chicago/Turabian StyleMormando, Marilda, Giulia Puliani, Marta Bianchini, Rosa Lauretta, and Marialuisa Appetecchia. 2024. "The Role of Inositols in Endocrine and Neuroendocrine Tumors" Biomolecules 14, no. 8: 1004. https://doi.org/10.3390/biom14081004




