Novel Insights into the Antimicrobial and Antibiofilm Activity of Pyrroloquinoline Quinone (PQQ); In Vitro, In Silico, and Shotgun Proteomic Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Microbial Strains
2.3. Antimicrobial Activity Screening
2.4. MIC Determination
2.5. MBC Determination
2.6. MFC Assessment
2.7. TEM (Transmission Electron Microscopy) Analysis
2.8. Antibiofilm Inhibition Activity
2.9. Shotgun Proteomics Analysis
2.9.1. Treatments and Lysate Sample Preparation
2.9.2. Protein Tryptic Digest
2.9.3. Nano-LC MS/MS Examination
2.9.4. Processing of Proteomics Data Analysis
2.9.5. Statistical Analysis
2.10. In Silico Molecular Docking Studies
3. Results
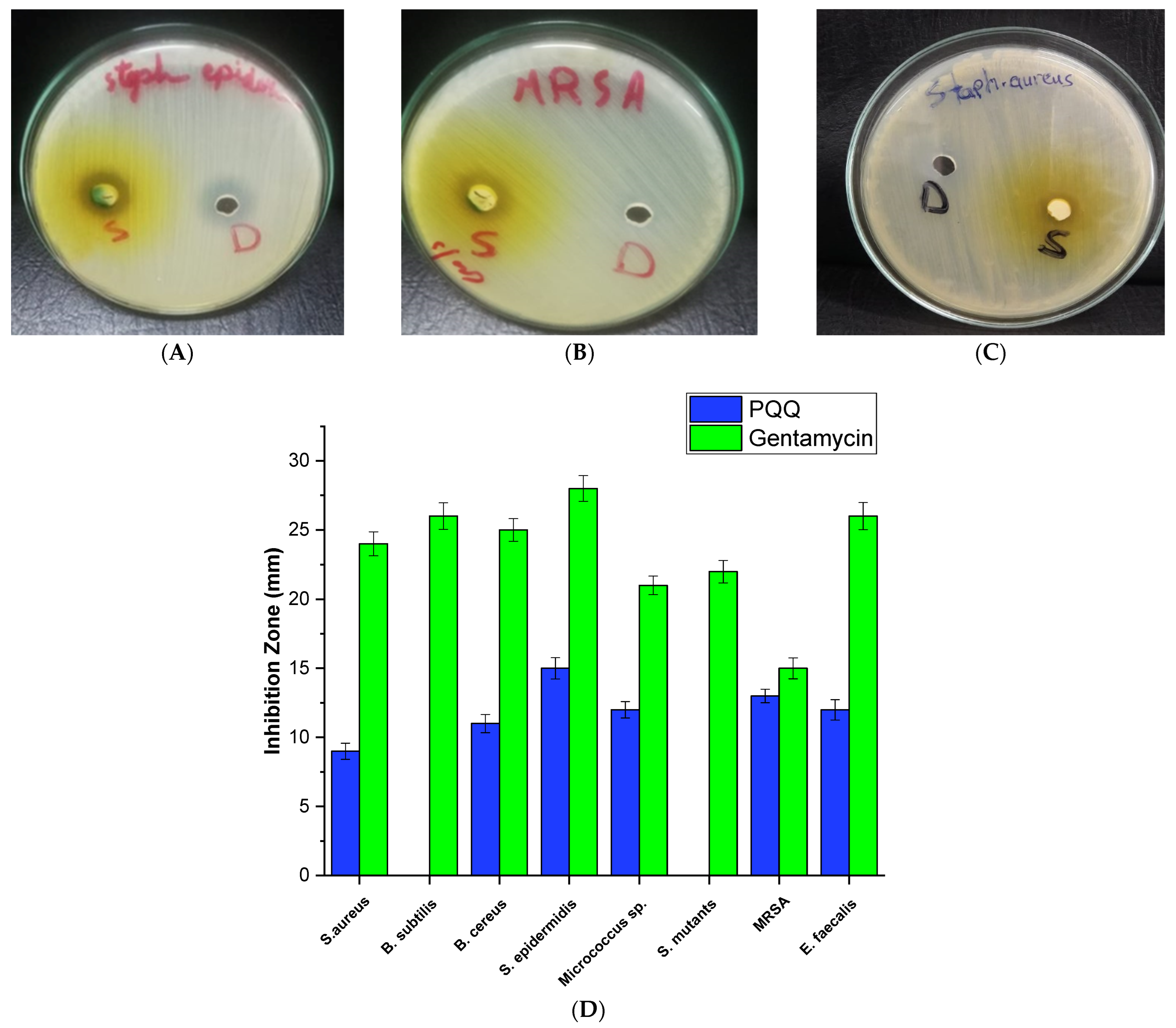
3.1. Assessment of Antimicrobial Potential
3.1.1. Antifungal Activity
3.1.2. Antibacterial Activity against Gram-Positive Bacteria
3.1.3. Antibacterial Activity against Gram-Negative Bacteria
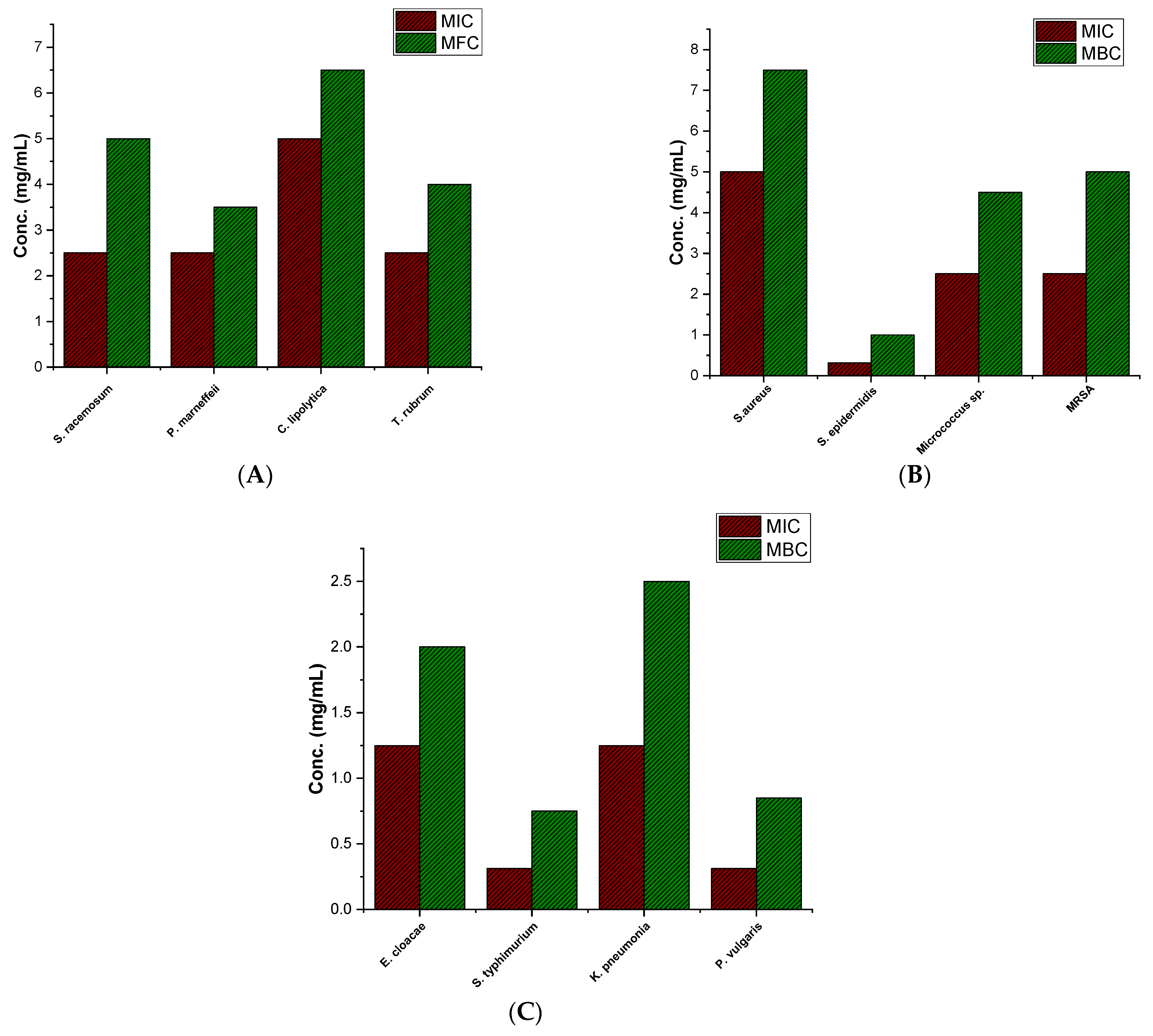
3.2. Determination of MIC against Microbial Strains
3.2.1. Evaluation of Antifungal Activity
3.2.2. Evaluation of Antibacterial Activity against Gram-Positive Bacteria
3.2.3. Assessment of Antibacterial Activity against Gram-Negative Bacteria
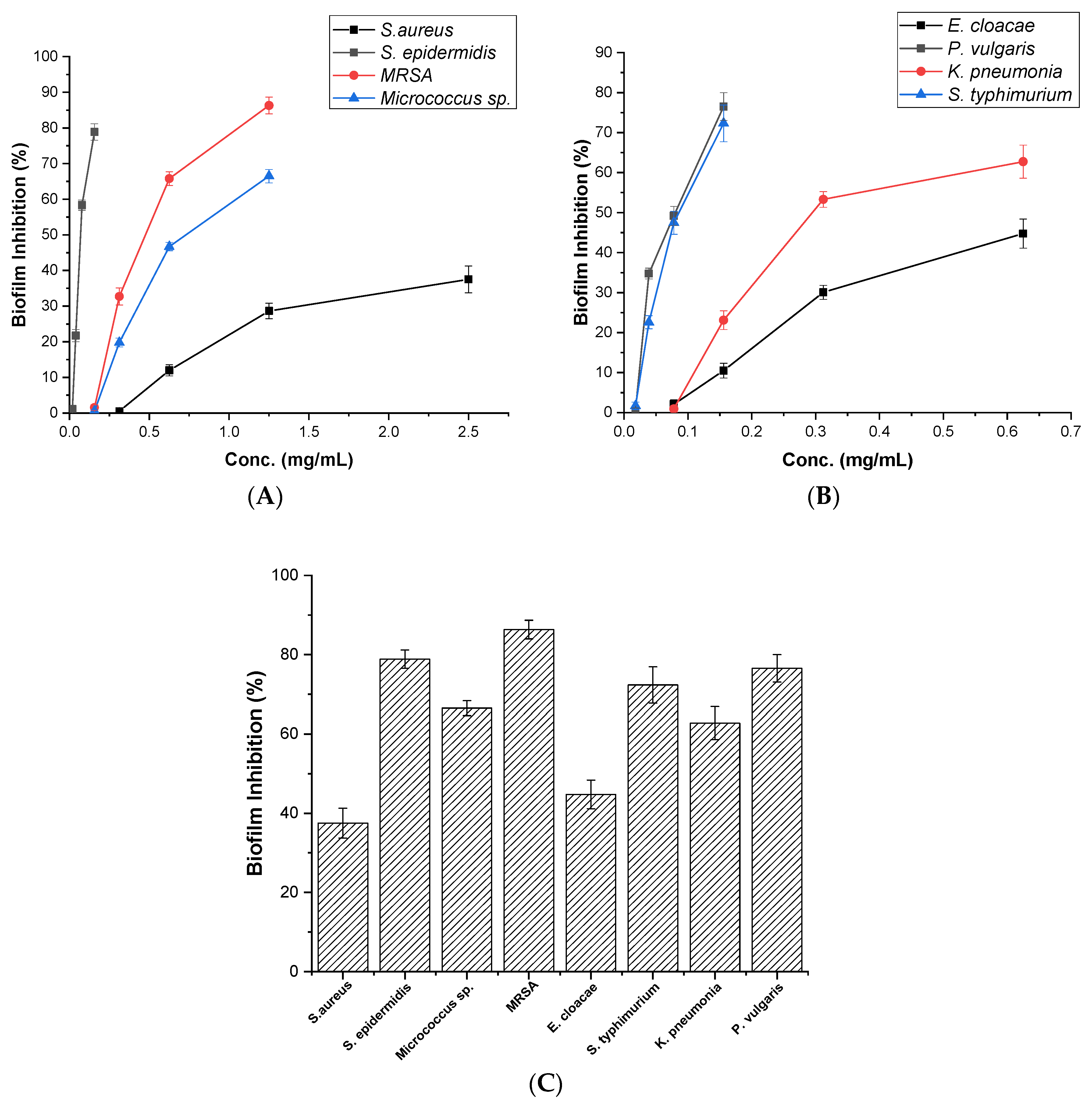
3.3. Assessment of Antibiofilm Activity
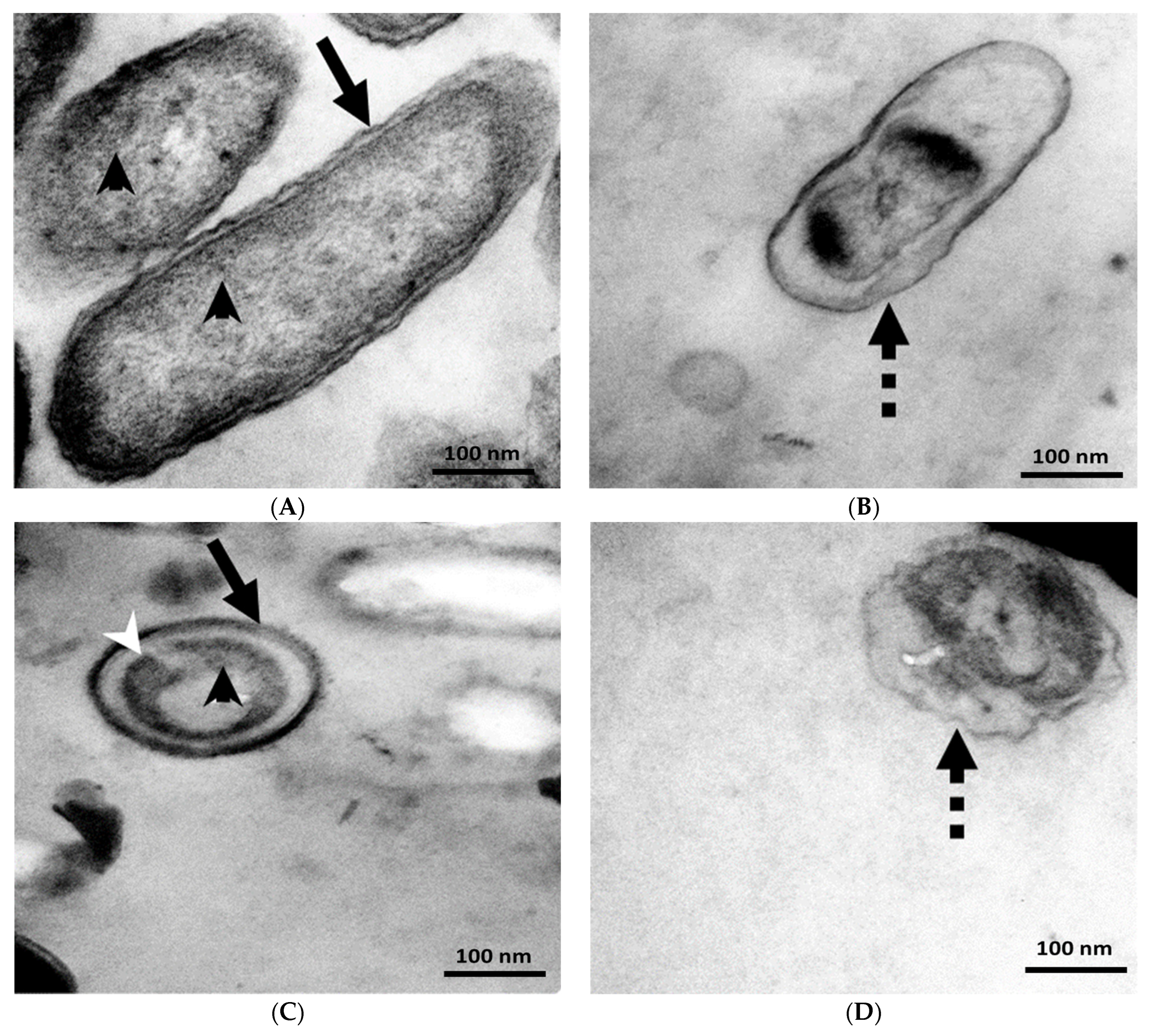
3.4. TEM Analysis
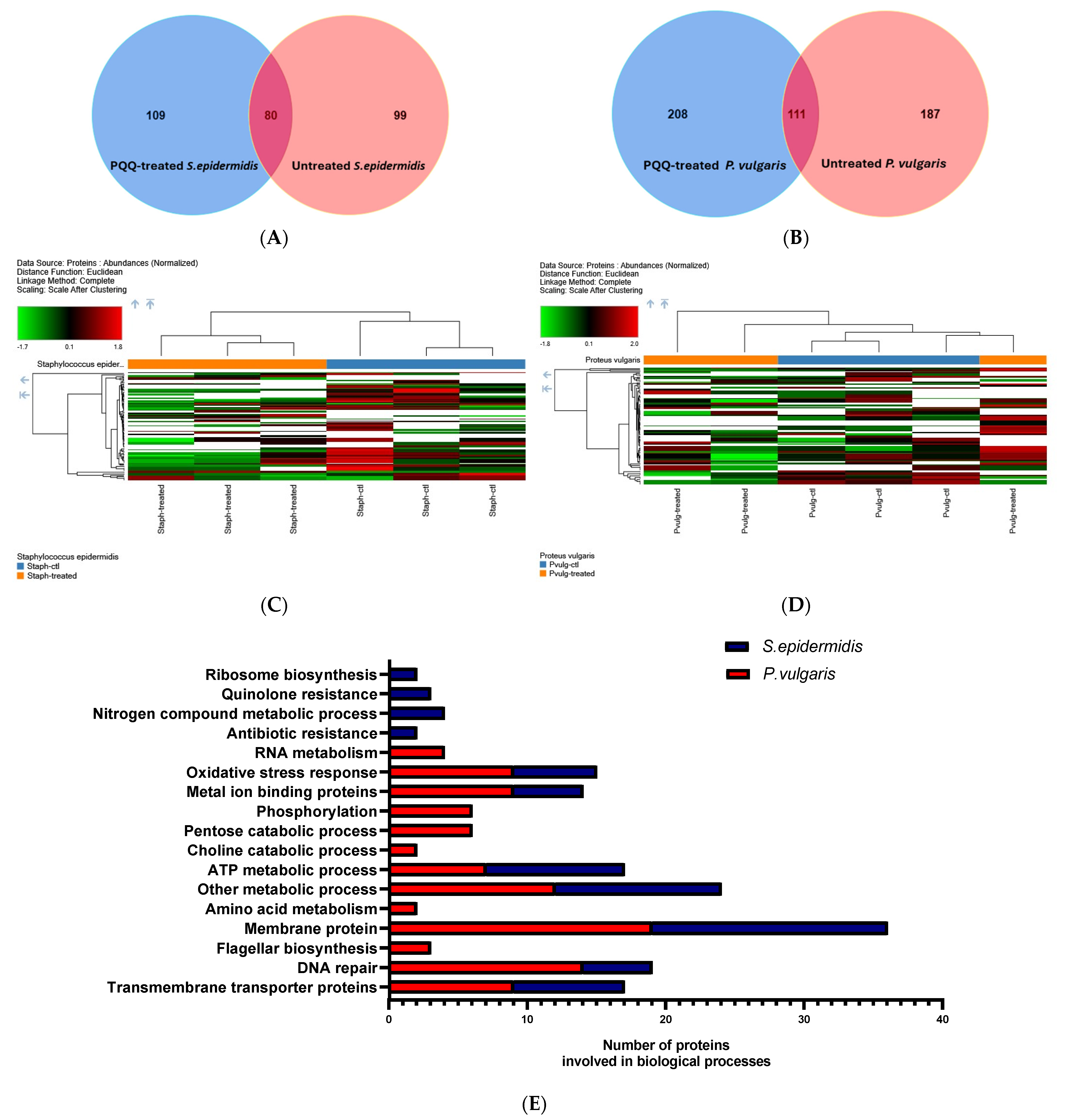
3.5. Shotgun Proteomic Analysis
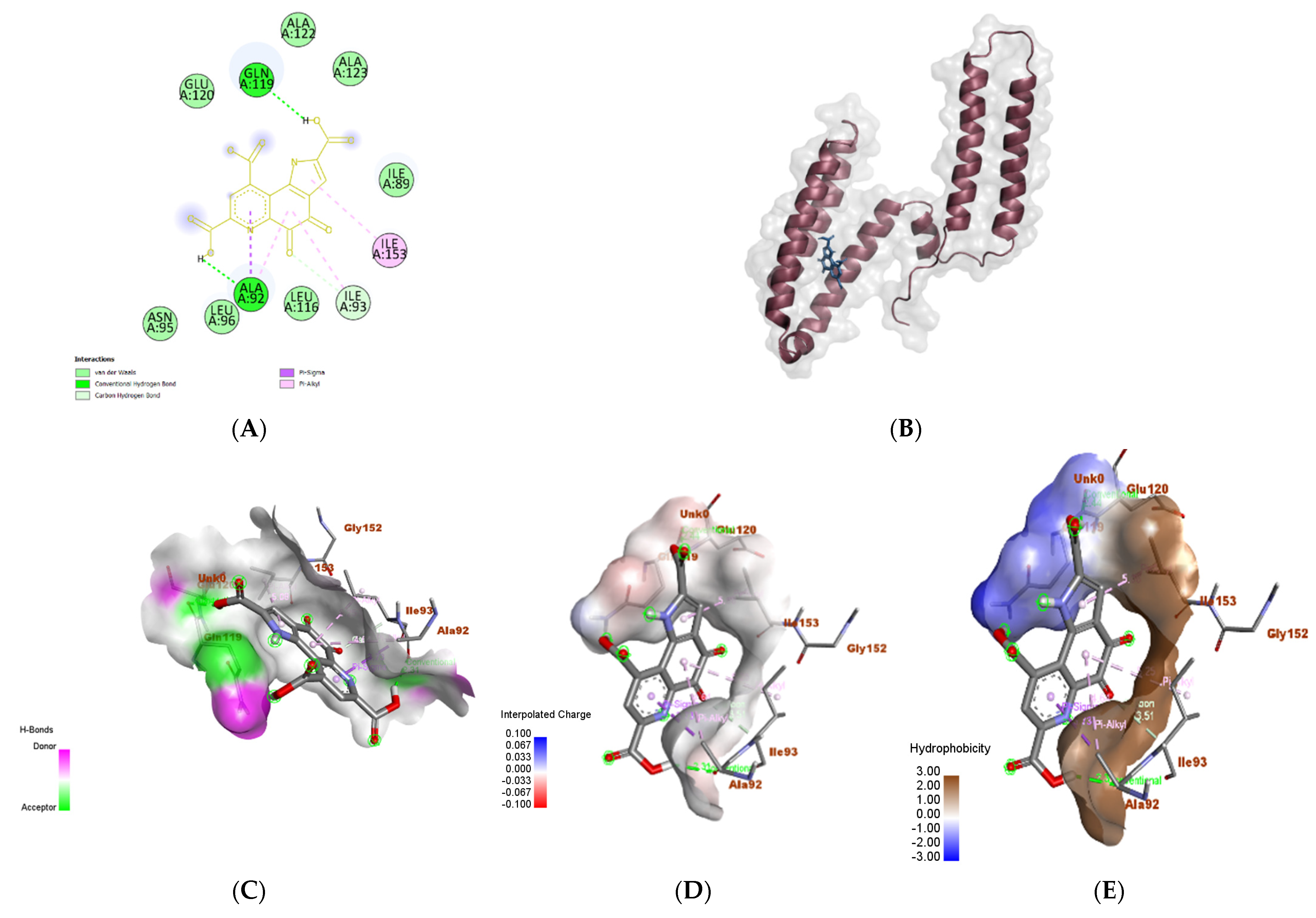
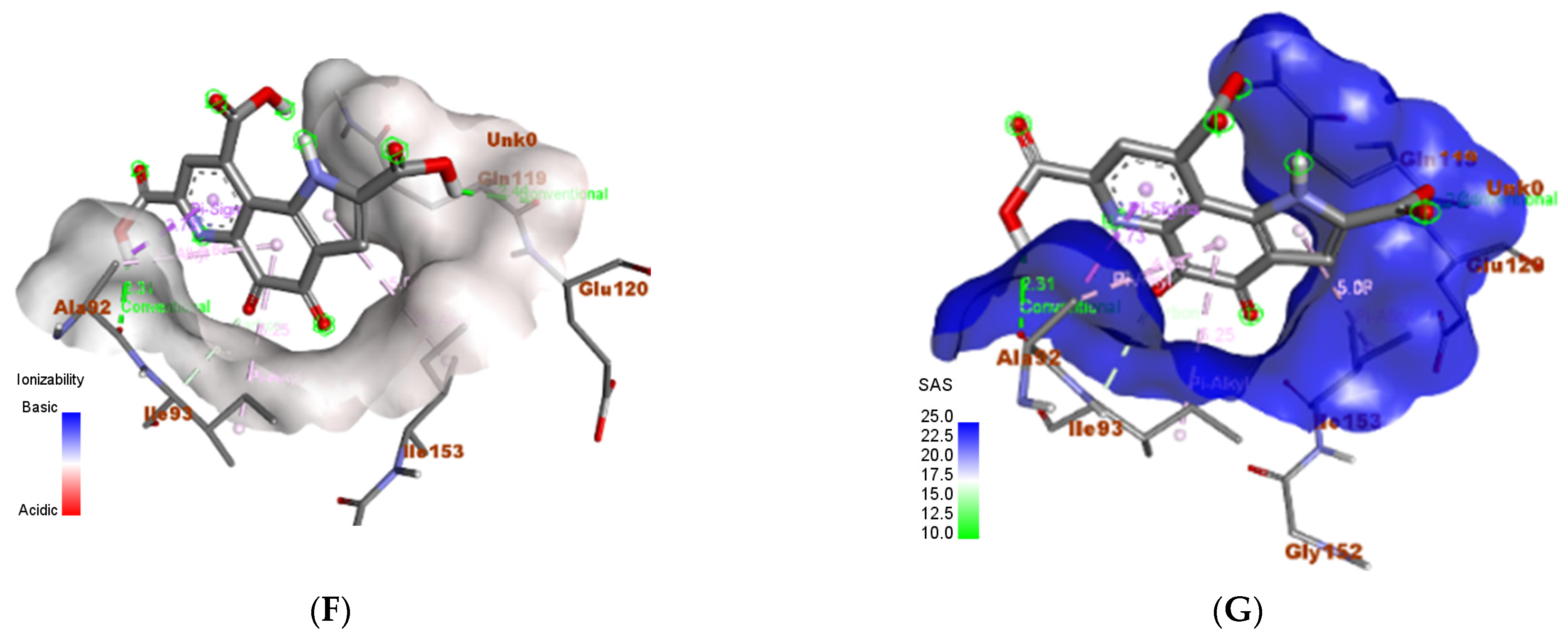
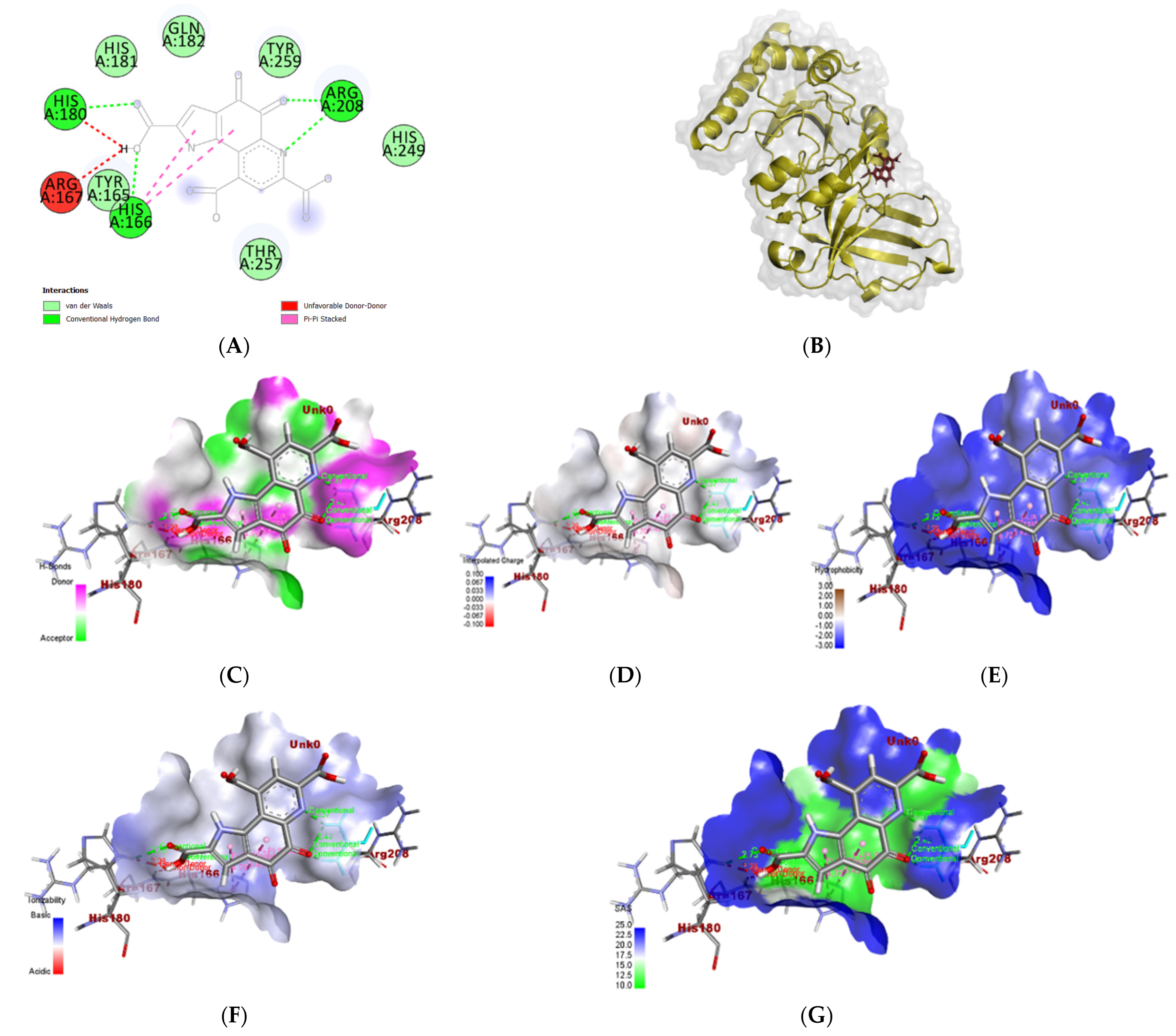
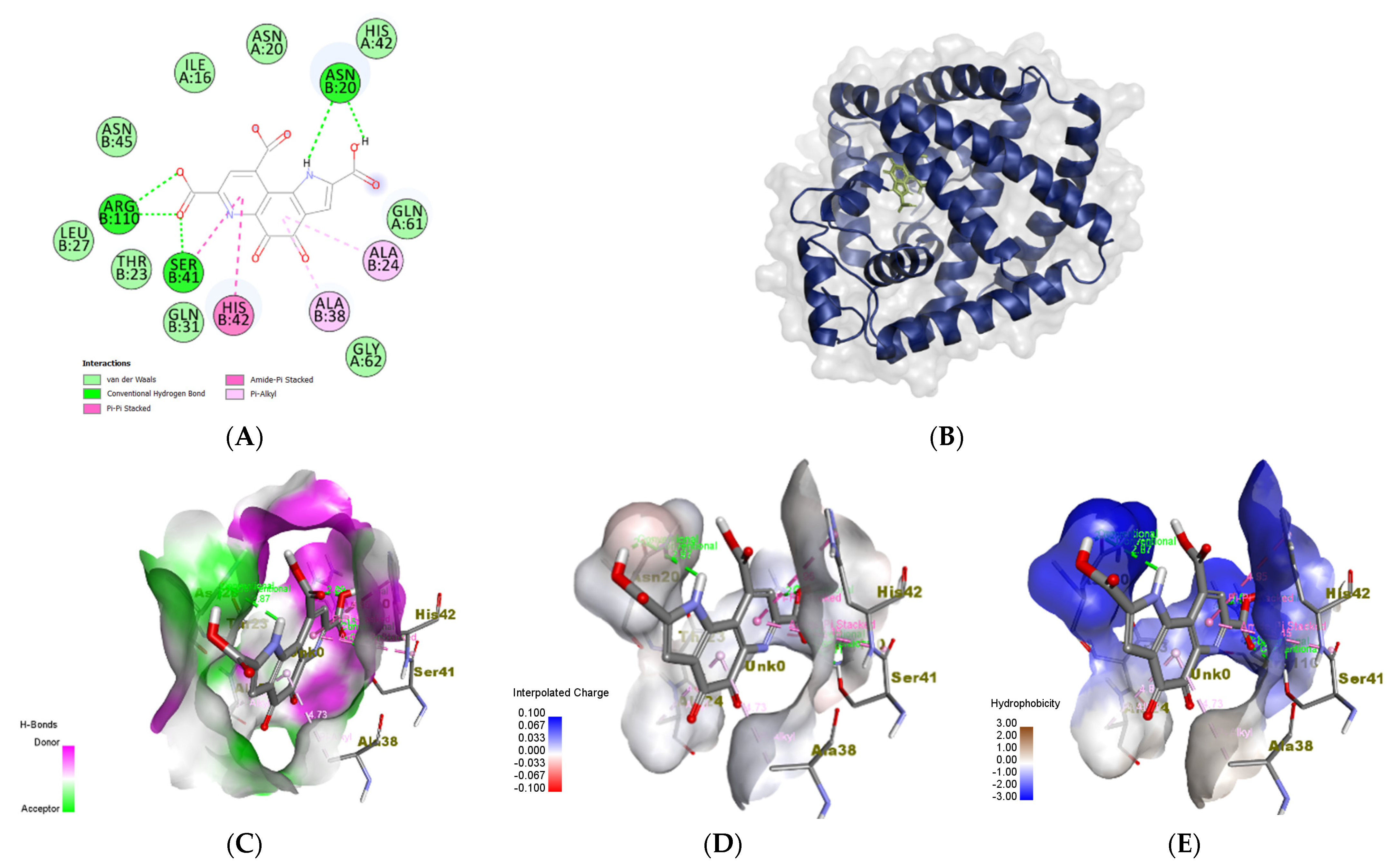
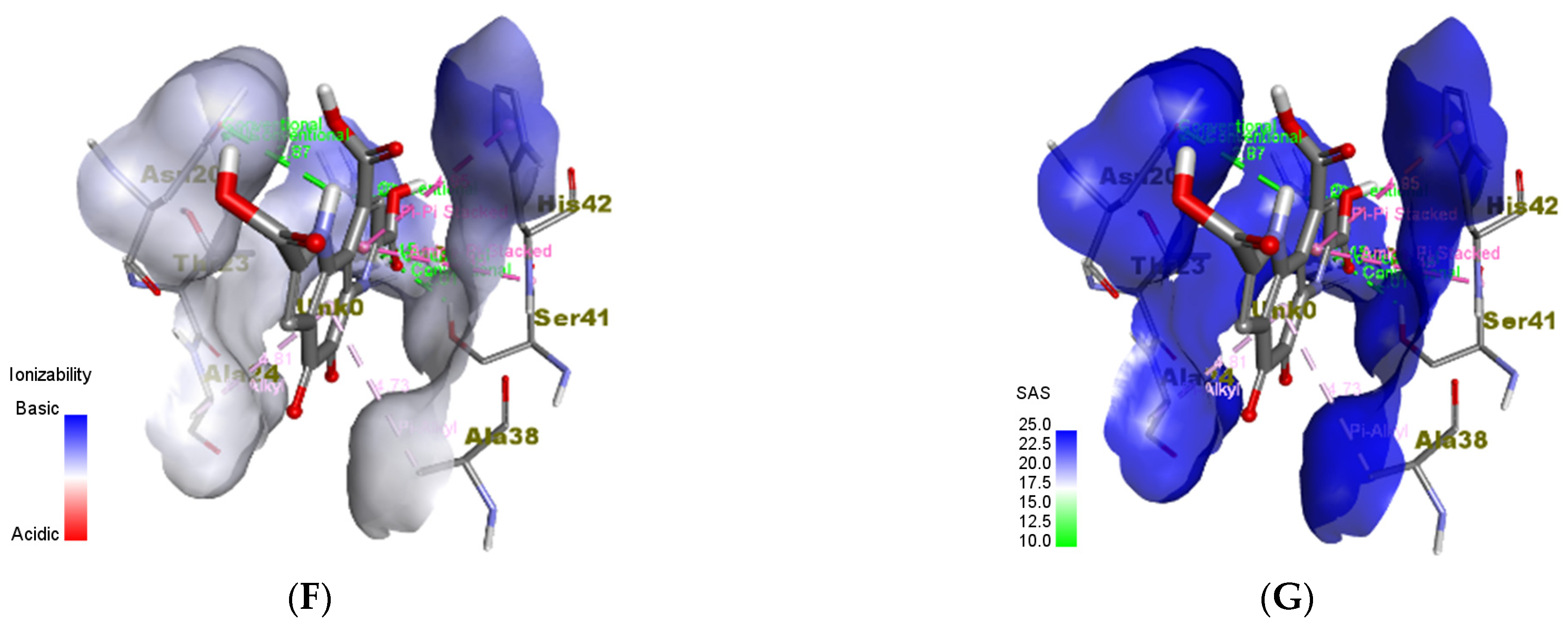
3.6. In Silico Molecular Docking Analyses
3.6.1. Talaromyces marneffei
3.6.2. Proteus vulgaris
3.6.3. Staphylococcus epidermidis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGregor, J.C.; Quach, Y.; Bearden, D.T.; Smith, D.H.; Sharp, S.E.; Guzman-Cottrill, J.A. Variation in antibiotic susceptibility of uropathogens by age among ambulatory pediatric patients. J. Pediatr. Nurs. 2014, 29, 152–157. [Google Scholar] [CrossRef]
- Adrizain, R.; Suryaningrat, F.; Alam, A.; Setiabudi, D. Incidence of multidrug-resistant, extensively drug-resistant and pan-drug-resistant bacteria in children hospitalized at Dr. Hasan Sadikin general hospital Bandung Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 125, 012077. [Google Scholar] [CrossRef]
- Thompson, T. The staggering death toll of drug-resistant bacteria. Nature 2022. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645. [Google Scholar] [CrossRef]
- Saleem, M.; Deters, B.; de la Bastide, A.; Korzen, M. Antibiotics overuse and bacterial resistance. Ann. Microbiol. Res. 2019, 3, 93. [Google Scholar]
- D’Andrea, M.M.; Fraziano, M.; Thaller, M.C.; Rossolini, G.M. The urgent need for novel antimicrobial agents and strategies to fight antibiotic resistance. Antibiotics 2019, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Antimicrobials 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M.M. Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef] [PubMed]
- Alangaden, G.J. Nosocomial fungal infections: Epidemiology, infection control, and prevention. Infect. Dis. Clin. 2011, 25, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Mallah, S.I.; Ghorab, O.K.; Al-Salmi, S.; Abdellatif, O.S.; Tharmaratnam, T.; Iskandar, M.A.; Sefen, J.A.N.; Sidhu, P.; Atallah, B.; El-Lababidi, R.; et al. COVID-19: Breaking down a global health crisis. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Saied, E.M.; El-Maradny, Y.A.; Osman, A.A.; Darwish, A.M.G.; Nahas, H.H.A.; Niedbała, G.; Piekutowska, M.; Abdel-Rahman, M.A.; Balbool, B.A.; Abdel-Azeem, A.M. A comprehensive review about the molecular structure of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Insights into natural products against COVID-19. Pharmaceutics 2021, 13, 1759. [Google Scholar] [CrossRef] [PubMed]
- Krysan, D.J. The unmet clinical need of novel antifungal drugs. Virulence 2017, 8, 135–137. [Google Scholar] [CrossRef]
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 2020, 18, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Elshaer, S.E.; Hamad, G.M.; Sobhy, S.E.; Darwish, A.M.G.; Baghdadi, H.H.; Abo Nahas, H.; El-Demerdash, F.M.; Kabeil, S.S.A.; Altamimi, A.S.; Al-Olayan, E.; et al. Supplementation of Saussurea costus root alleviates sodium nitrite-induced hepatorenal toxicity by modulating metabolic profile, inflammation, and apoptosis. Front. Pharmacol. 2024, 15, 1378249. [Google Scholar] [CrossRef] [PubMed]
- Jonscher, K.R.; Rucker, R.B. Pyrroloquinoline quinone: Its profile, effects on the liver and implications for health and disease prevention. In Dietary Interventions in Liver Disease; Academic Press: Cambridge, MA, USA, 2019; pp. 157–173. [Google Scholar]
- Zhu, W.; Martins, A.M.; Klinman, J.P. Chapter Fourteen-Methods for Expression, Purification, and Characterization of PqqE, a Radical SAM Enzyme in the PQQ Biosynthetic Pathway. In Methods in Enzymology; Bandarian, V., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 606, pp. 389–420. [Google Scholar]
- Kumazawa, T.; Sato, K.; Seno, H.; Ishii, A.; Suzuki, O. Levels of pyrroloquinoline quinone in various foods. Biochem. J. 1995, 307, 331–333. [Google Scholar] [CrossRef]
- Akagawa, M.; Minematsu, K.; Shibata, T.; Kondo, T.; Ishii, T.; Uchida, K. Identification of lactate dehydrogenase as a mammalian pyrroloquinoline quinone (PQQ)-binding protein. Sci. Rep. 2016, 6, 26723. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gong, N.; Liu, M.; Pan, X.; Sang, S.; Sun, X.; Yu, Z.; Fang, Q.; Zhao, N.; Fei, G. Beneficial synergistic effects of microdose lithium with pyrroloquinoline quinone in an Alzheimer’s disease mouse model. Neurobiol. Aging 2014, 35, 2736–2745. [Google Scholar] [CrossRef]
- Jonscher, K.R.; Chowanadisai, W.; Rucker, R.B. Pyrroloquinoline-Quinone Is More Than an Antioxidant: A Vitamin-like Accessory Factor Important in Health and Disease Prevention. Biomolecules 2021, 11, 1441. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Yao, Z.-W.; Peng, Y.; Mao, S.-S.; Xu, D.; Qin, X.-F.; Zhang, R.-J. PQQ ameliorates D-galactose induced cognitive impairments by reducing glutamate neurotoxicity via the GSK-3β/Akt signaling pathway in mouse. Sci. Rep. 2018, 8, 8894. [Google Scholar] [CrossRef]
- Qin, X.; Yang, M.; Cai, H.; Liu, Y.; Gorris, L.; Aslam, M.Z.; Jia, K.; Sun, T.; Wang, X.; Dong, Q. Antibiotic Resistance of Salmonella Typhimurium Monophasic Variant 1, 4,[5], 12: i:-in China: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Hindler, J.A.; Howard, B.J.; Keiser, J.F. Antimicrobial agents and antimicrobial susceptibility testing. In Howard BJ. Clinical and Pathogenic Microbiology, 2nd ed.; Mosby: St. Louis, MI, USA, 1994. [Google Scholar]
- Yousef, M.M.; Zohri, A.N.A.; Darwish, A.M.G.; Shamseldin, A.; Kabeil, S.A.; Abdelkhalek, A.; Binsuwaidan, R.; Jaremko, M.; Alshwyeh, H.A.; Hafez, E.E.; et al. Exploring the antibacterial potential of plant extracts and essential oils against Bacillus thermophilus in beet sugar for enhanced sucrose retention: A comparative assessment and implications. Front. Microbiol. 2023, 14, 1219823. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bajko, E.; Matejczyk, M.; Kaczyński, P.; Łozowicka, B.; Lewandowski, W. The Study of Anti-/Pro-Oxidant, Lipophilic, Microbial and Spectroscopic Properties of New Alkali Metal Salts of 5-O-Caffeoylquinic Acid. Int. J. Mol. Sci. 2018, 19, 463. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J. Clin. Microbiol. 2001, 39, 954–958. [Google Scholar] [CrossRef]
- Amin, B. Isolation and characterization of antiprotozoal and antimicrobial metabolite from Penicillium roqueforti. Afr. J. Mycol. Biotech. 2016, 21, 13–26. [Google Scholar]
- Amin, B.H.; Abou-Dobara, M.I.; Diab, M.A.; Gomaa, E.A.; El-Mogazy, M.A.; El-Sonbati, A.Z.; El-Ghareib, M.S.; Hussien, M.A.; Salama, H.M. Synthesis, characterization, and biological investigation of new mixed-ligand complexes. Appl. Organomet. Chem. 2020, 34, e5689. [Google Scholar] [CrossRef]
- Amin, B.H.; Amer, A.; Azzam, M.; Abd El-Sattar, N.E.A.; Mahmoud, D.; Al-Ashaal, S.; Al-Khalaf, A.A.; Hozzein, W.N. Antimicrobial and anticancer activities of Periplaneta americana tissue lysate: An in vitro study. J. King Saud. Univ.-Sci. 2022, 34, 102095. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.A.; FAbd El-Kareem, H.; Alzamami, A.; Fahmy, C.A.; Elesawy, B.H.; Mostafa Mahmoud, M.; Ghareeb, A.; El Askary, A.; Abo Nahas, H.H.; Attallah, N.G.M.; et al. Novel Exopolysaccharide from Marine Bacillus subtilis with Broad Potential Biological Activities: Insights into Antioxidant, Anti-Inflammatory, Cytotoxicity, and Anti-Alzheimer Activity. Metabolites 2022, 12, 715. [Google Scholar] [CrossRef] [PubMed]
- Magdeldin, S.; Moresco, J.J.; Yamamoto, T.; Yates, J.R., III. Off-line multidimensional liquid chromatography and auto sampling result in sample loss in LC/LC–MS/MS. J. Proteome Res. 2014, 13, 3826–3836. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.R.; Hosny, N.; Mohamed, D.I.; Abo Nahas, H.H.; Albogami, A.; Al-Hazani, T.M.I.; Ibrahim, I.A.A.; Falemban, A.H.; Bamagous, G.A.; Saied, E.M. Unveiling the multifaceted antiproliferative efficacy of Cichorium endivia root extract by dual modulation of apoptotic and inflammatory genes, inducing cell cycle arrest, and targeting COX-2. RSC Adv. 2024, 14, 19400–19427. [Google Scholar] [CrossRef]
- Ahmed, E.A.; El-Derany, M.O.; Anwar, A.M.; Saied, E.M.; Magdeldin, S. Metabolomics and Lipidomics Screening Reveal Reprogrammed Signaling Pathways toward Cancer Development in Non-Alcoholic Steatohepatitis. Int. J. Mol. Sci. 2023, 24, 210. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Lin, Y.-C.-D.; Li, J.; Huang, K.-Y.; Shrestha, S.; Hong, H.-C.; Tang, Y.; Chen, Y.-G.; Jin, C.-N.; Yu, Y. miRTarBase 2020: Updates to the experimentally validated microRNA–target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.M.; Amin, M.K.; Alzohairy, A.M.; Elashtokhy, M.M.A.; Samir, O.; Saleh, I.; Arif, I.A.; Osman, G.H.; Hassanein, S.E. In silico Targeting, inhibition and analysis of polyketide synthase enzyme in Aspergillus ssp. Saudi J. Biol. Sci. 2020, 27, 3187–3198. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Hetényi, C.; van der Spoel, D. Efficient docking of peptides to proteins without prior knowledge of the binding site. Protein Sci. A Publ. Protein Soc. 2002, 11, 1729–1737. [Google Scholar] [CrossRef]
- Khirallah, S.M.; Ramadan, H.M.M.; Aladl, H.A.A.; Ayaz, N.O.; Kurdi, L.A.F.; Jaremko, M.; Alshawwa, S.Z.; Saied, E.M. Antidiabetic Potential of Novel 1,3,5-Trisubstituted-2-Thioxoimidazloidin-4-One Analogues: Insights into α-Glucosidase, α-Amylase, and Antioxidant Activities. Pharmaceuticals 2022, 15, 1576. [Google Scholar] [CrossRef]
- Khirallah, S.M.; Ramadan, H.M.M.; Shawky, A.; Qahl, S.H.; Baty, R.S.; Alqadri, N.; Alsuhaibani, A.M.; Jaremko, M.; Emwas, A.H.; Saied, E.M. Development of Novel 1,3-Disubstituted-2-Thiohydantoin Analogues with Potent Anti-Inflammatory Activity; In Vitro and In Silico Assessments. Molecules 2022, 27, 6271. [Google Scholar] [CrossRef]
- Salem, M.G.; El-Maaty, D.M.A.; El-Deen, Y.I.M.; Elesawy, B.H.; Askary, A.E.; Saleh, A.; Saied, E.M.; Behery, M.E. Novel 1,3-Thiazole Analogues with Potent Activity against Breast Cancer: A Design, Synthesis, In Vitro, and In Silico Study. Molecules 2022, 27, 4898. [Google Scholar] [CrossRef]
- Healey, R.D.; Saied, E.M.; Cong, X.; Karsai, G.; Gabellier, L.; Saint-Paul, J.; Del Nero, E.; Jeannot, S.; Drapeau, M.; Fontanel, S.; et al. Discovery and Mechanism of Action of Small Molecule Inhibitors of Ceramidases. Angew. Chem.-Int. Ed. 2022, 61, e202109967. [Google Scholar] [CrossRef]
- El Azab, I.H.; Saied, E.M.; Osman, A.A.; Mehana, A.E.; Saad, H.A.; Elkanzi, N.A.A. Novel N-bridged pyrazole-1-carbothioamides with potential antiproliferative activity: Design, synthesis, in vitro and in silico studies. Future Med. Chem. 2021, 13, 1743–1766. [Google Scholar] [CrossRef]
- Samaha, D.; Hamdo, H.H.; Cong, X.; Schumacher, F.; Banhart, S.; Aglar, Ö.; Möller, H.M.; Heuer, D.; Kleuser, B.; Saied, E.M.; et al. Liposomal FRET Assay Identifies Potent Drug-Like Inhibitors of the Ceramide Transport Protein (CERT). Chem.-A Eur. J. 2020, 26, 16616–16621. [Google Scholar] [CrossRef]
- Mohamed, D.I.; Ezzat, S.F.; Elayat, W.M.; El-Kharashi, O.A.; Abd El-Kareem, H.F.; Abo Nahas, H.H.; Abdel-Wahab, B.A.; Alshawwa, S.Z.; Saleh, A.; Helmy, Y.A.; et al. Hepatoprotective Role of Carvedilol against Ischemic Hepatitis Associated with Acute Heart Failure via Targeting miRNA-17 and Mitochondrial Dynamics-Related Proteins: An In Vivo and In Silico Study. Pharmaceuticals 2022, 15, 832. [Google Scholar] [CrossRef]
- Mohamed, D.I.; El-Waseef, D.A.E.D.A.; Nabih, E.S.; El-Kharashi, O.A.; El-Kareem, H.F.A.; Nahas, H.H.A.; Abdel-Wahab, B.A.; Helmy, Y.A.; Alshawwa, S.Z.; Saied, E.M. Acetylsalicylic Acid Suppresses Alcoholism-Induced Cognitive Impairment Associated with Atorvastatin Intake by Targeting Cerebral miRNA155 and NLRP3: In Vivo, and In Silico Study. Pharmaceutics 2022, 14, 529. [Google Scholar] [CrossRef]
- Liao, S.; Tung, E.T.; Zheng, W.; Chong, K.; Xu, Y.; Dai, P.; Guo, Y.; Bartlam, M.; Yuen, K.Y.; Rao, Z. Crystal structure of the Mp1p ligand binding domain 2 reveals its function as a fatty acid-binding protein. J. Biol. Chem. 2010, 285, 9211–9220. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Lau, C.C.; Tung, E.T.; Chong, K.T.; Yang, F.; Zhang, H.; Lo, R.K.; Cai, J.P.; Au-Yeung, R.K.; et al. Mp1p Is a Virulence Factor in Talaromyces (Penicillium) marneffei. PLoS Negl. Trop. Dis. 2016, 10, e0004907. [Google Scholar] [CrossRef]
- Cao, L.; Chan, C.M.; Lee, C.; Wong, S.S.; Yuen, K.Y. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect. Immun. 1998, 66, 966–973. [Google Scholar] [CrossRef]
- Al-Karmalawy, A.A.; Dahab, M.A.; Metwaly, A.M.; Elhady, S.S.; Elkaeed, E.B.; Eissa, I.H.; Darwish, K.M. Molecular Docking and Dynamics Simulation Revealed the Potential Inhibitory Activity of ACEIs Against SARS-CoV-2 Targeting the hACE2 Receptor. Front. Chem. 2021, 9, 661230. [Google Scholar] [CrossRef] [PubMed]
- Vemula, D.; Maddi, D.R.; Bhandari, V. Homology modeling, virtual screening, molecular docking, and dynamics studies for discovering Staphylococcus epidermidis FtsZ inhibitors. Front. Mol. Biosci. 2023, 10, 1087676. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. The Inhibitory Potential of Ferulic Acid Derivatives against the SARS-CoV-2 Main Protease: Molecular Docking, Molecular Dynamics, and ADMET Evaluation. Biomedicines 2022, 10, 1787. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Das, S.; Stanley, A.; Yadav, L.; Sudhakar, A.; Varma, A. Optimized Hydrophobic Interactions and Hydrogen Bonding at the Target-Ligand Interface Leads the Pathways of Drug-Designing. PLoS ONE 2010, 5, e12029. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.M.; Zhou, C.; Lindgren, J.K.; Galac, M.R.; Corey, B.; Endres, J.E.; Olson, M.E.; Fey, P.D. Transcriptional Regulation of icaADBC by both IcaR and TcaR in Staphylococcus epidermidis. J. Bacteriol. 2019, 201, e00524-18. [Google Scholar] [CrossRef]
- Mahmood Janlou, M.A.; Sahebjamee, H.; Yazdani, M.; Fozouni, L. Structure-based virtual screening and molecular dynamics approaches to identify new inhibitors of Staphylococcus aureus sortase A. J. Biomol. Struct. Dyn. 2023, 42, 1157–1169. [Google Scholar] [CrossRef]
- Pietruś, W.; Kafel, R.; Bojarski, A.J.; Kurczab, R. Hydrogen Bonds with Fluorine in Ligand-Protein Complexes-the PDB Analysis and Energy Calculations. Molecules 2022, 27, 1005. [Google Scholar] [CrossRef]
- Puehringer, S.; Metlitzky, M.; Schwarzenbacher, R. The pyrroloquinoline quinone biosynthesis pathway revisited: A structural approach. BMC Biochem. 2008, 9, 8. [Google Scholar] [CrossRef]
- Nakano, M.; Murayama, Y.; Hu, L.; Ikemoto, K.; Uetake, T.; Sakatani, K. Effects of antioxidant supplements (BioPQQ™) on cerebral blood flow and oxygen metabolism in the prefrontal cortex. In Oxygen Transport to Tissue XXXVIII; Springer: Berlin/Heidelberg, Germany, 2016; pp. 215–222. [Google Scholar]
- Kumar, N.; Kar, A. Pyrroloquinoline quinone (PQQ) has potential to ameliorate streptozotocin-induced diabetes mellitus and oxidative stress in mice: A histopathological and biochemical study. Chem.-Biol. Interact. 2015, 240, 278–290. [Google Scholar] [CrossRef]
- Wang, Z.; Han, N.; Zhao, K.; Li, Y.; Chi, Y.; Wang, B. Protective effects of pyrroloquinoline quinone against oxidative stress-induced cellular senescence and inflammation in human renal tubular epithelial cells via Keap1/Nrf2 signaling pathway. Int. Immunopharmacol. 2019, 72, 445–453. [Google Scholar] [CrossRef]
- Lin, X.; Yang, F.; Huang, J.; Jiang, S.; Tang, Y.; Li, J. Ameliorate effect of pyrroloquinoline quinone against cyclophosphamide-induced nephrotoxicity by activating the Nrf2 pathway and inhibiting the NLRP3 pathway. Life Sci. 2020, 256, 117901. [Google Scholar] [CrossRef]
- Zhu, B.-q.; Simonis, U.; Cecchini, G.; Zhou, H.-z.; Li, L.; Teerlink, J.R.; Karliner, J.S. Comparison of pyrroloquinoline quinone and/or metoprolol on myocardial infarct size and mitochondrial damage in a rat model of ischemia/reperfusion injury. J. Cardiovasc. Pharmacol. Ther. 2006, 11, 119–128. [Google Scholar] [CrossRef]
- Bauerly, K.; Harris, C.; Chowanadisai, W.; Graham, J.; Havel, P.J.; Tchaparian, E.; Satre, M.; Karliner, J.S.; Rucker, R.B. Altering pyrroloquinoline quinone nutritional status modulates mitochondrial, lipid, and energy metabolism in rats. PLoS ONE 2011, 6, e21779. [Google Scholar] [CrossRef]
- Shiojima, Y.; Takahashi, M.; Takahashi, R.; Moriyama, H.; Bagchi, D.; Bagchi, M.; Akanuma, M. Effect of dietary pyrroloquinoline quinone disodium salt on cognitive function in healthy volunteers: A randomized, double-blind, placebo-controlled, parallel-group study. J. Am. Coll. Nutr. 2021, 41, 796–809. [Google Scholar] [CrossRef]
- Wen, H.; He, Y.; Zhang, K.; Yang, X.; Hao, D.; Jiang, Y.; He, B. Mini-review: Functions and action mechanisms of PQQ in osteoporosis and neuro injury. Curr. Stem Cell Res. Ther. 2020, 15, 32–36. [Google Scholar]
- Narayanasamy, S.; Dougherty, J.; van Doorn, H.R.; Le, T. Pulmonary talaromycosis: A window into the immunopathogenesis of an endemic mycosis. Mycopathologia 2021, 186, 707–715. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Lo, G.C.S.; Lam, C.S.K.; Chow, W.-N.; Ngan, A.H.Y.; Wu, A.K.L.; Tsang, D.N.C.; Tse, C.W.S.; Que, T.-L.; Tang, B.S.F. In vitro activity of posaconazole against Talaromyces marneffei by broth microdilution and Etest methods and comparison to itraconazole, voriconazole, and anidulafungin. Antimicrob. Agents Chemother. 2017, 61, e01480-16. [Google Scholar] [CrossRef]
- Lei, H.L.; Li, L.H.; Chen, W.S.; Song, W.N.; He, Y.; Hu, F.Y.; Chen, X.J.; Cai, W.P.; Tang, X.P. Susceptibility profile of echinocandins, azoles and amphotericin B against yeast phase of Talaromyces marneffei isolated from HIV-infected patients in Guangdong, China. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1099–1102. [Google Scholar] [CrossRef]
- Benitez, L.L.; Carver, P.L. Adverse effects associated with long-term administration of azole antifungal agents. Drugs 2019, 79, 833–853. [Google Scholar] [CrossRef]
- Luo, H.; Pan, K.-S.; Luo, X.-L.; Zheng, D.-Y.; Andrianopoulos, A.; Wen, L.-M.; Zheng, Y.-Q.; Guo, J.; Huang, C.-Y.; Li, X.-Y. In vitro susceptibility of berberine combined with antifungal agents against the yeast form of Talaromyces marneffei. Mycopathologia 2019, 184, 295–301. [Google Scholar] [CrossRef]
- Byrd, A.L.; Deming, C.; Cassidy, S.K.B.; Harrison, O.J.; Ng, W.-I.; Conlan, S.; Program, N.C.S.; Belkaid, Y.; Segre, J.A.; Kong, H.H. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 2017, 9, eaal4651. [Google Scholar] [CrossRef]
- Woo, Y.R.; Lee, S.H.; Cho, S.H.; Lee, J.D.; Kim, H.S. Characterization and analysis of the skin microbiota in rosacea: Impact of systemic antibiotics. J. Clin. Med. 2020, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 2022, 21, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.H.; Monk, I.R.; Gonçalves da Silva, A.; Seemann, T.; Chua, K.Y.L.; Kearns, A.; Hill, R.; Woodford, N.; Bartels, M.D.; Strommenger, B. Global spread of three multidrug-resistant lineages of Staphylococcus epidermidis. Nat. Microbiol. 2018, 3, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Cave, R.; Chen, L.; Yangkyi, T.; Liu, Y.; Li, K.; Meng, G.; Niu, K.; Zhang, W.; Tang, N. Antibiotic resistance and molecular characteristics of methicillin-resistant Staphylococcus epidermidis recovered from hospital personnel in China. J. Glob. Antimicrob. Resist. 2020, 22, 195–201. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Rutherford, J.C. The emerging role of urease as a general microbial virulence factor. PLoS Pathog. 2014, 10, e1004062. [Google Scholar] [CrossRef]
- Chandra, H.; Singh, C.; Kumari, P.; Yadav, S.; Mishra, A.P.; Laishevtcev, A.; Brisc, C.; Brisc, M.C.; Munteanu, M.A.; Bungau, S. Promising roles of alternative medicine and plant-based nanotechnology as remedies for urinary tract infections. Molecules 2020, 25, 5593. [Google Scholar] [CrossRef]
- Wang, X.; Andersson, R.; Soltesz, V.; Bengmark, S. Bacterial translocation after major hepatectomy in patients and rats. Arch. Surg. 1992, 127, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Mondot, S.; Kang, S.; Furet, J.P.; Aguirre de Cárcer, D.; McSweeney, C.; Morrison, M.; Marteau, P.; Dore, J.; Leclerc, M. Highlighting new phylogenetic specificities of Crohn’s disease microbiota. Inflamm. Bowel Dis. 2011, 17, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Anam, S.; Mahmood, T.; Abdullah, R.M.; Nisar, S.; Kalsoom, F.; Luqman, M.; Anjum, F.R. Antimicrobial profiling and molecular characterization of antibiotic resistant genes of Proteus vulgaris isolated from tertiary care hospital, Islamabad, Pakistan. Pak. J. Pharm. Sci. 2019, 32, 2887–2891. [Google Scholar] [PubMed]
- Zhang, H.; Chang, M.; Zhang, X.; Cai, P.; Dai, Y.; Song, T.; Wu, Z.; Xu, H.; Qiao, M. Functional identification and evolutionary analysis of two novel plasmids mediating quinolone resistance in Proteus vulgaris. Microorganisms 2020, 8, 1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, T.; Qin, C.; Xu, H.; Qiao, M. A Novel Non-Coding RNA CsiR Regulates the Ciprofloxacin Resistance in Proteus vulgaris by Interacting with emrB mRNA. Int. J. Mol. Sci. 2021, 22, 10627. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.M.P.; Ferrari, R.G.; Conte-Junior, C.A. Virulence factors in Salmonella Typhimurium: The sagacity of a bacterium. Curr. Microbiol. 2019, 76, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Stecher, B.; Robbiani, R.; Walker, A.W.; Westendorf, A.M.; Barthel, M.; Kremer, M.; Chaffron, S.; Macpherson, A.J.; Buer, J.; Parkhill, J. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007, 5, e244. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.D.; Mohakud, N.K.; Panda, R.K.; Sahu, B.R.; Suar, M. Prevalence and multidrug resistance in Salmonella enterica Typhimurium: An overview in South East Asia. World J. Microbiol. Biotechnol. 2021, 37, 185. [Google Scholar] [CrossRef] [PubMed]
- Villar, R.G.; Macek, M.D.; Simons, S.; Hayes, P.S.; Goldoft, M.J.; Lewis, J.H.; Rowan, L.L.; Hursh, D.; Patnode, M.; Mead, P.S. Investigation of multidrug-resistant Salmonella serotype Typhimurium DT104 infections linked to raw-milk cheese in Washington State. JAMA 1999, 281, 1811–1816. [Google Scholar] [CrossRef]
- Wang, X.; Biswas, S.; Paudyal, N.; Pan, H.; Li, X.; Fang, W.; Yue, M. Antibiotic resistance in Salmonella Typhimurium isolates recovered from the food chain through national antimicrobial resistance monitoring system between 1996 and 2016. Front. Microbiol. 2019, 10, 985. [Google Scholar] [CrossRef]
- Penesyan, A.; Paulsen, I.T.; Kjelleberg, S.; Gillings, M.R. Three faces of biofilms: A microbial lifestyle, a nascent multicellular organism, and an incubator for diversity. NPJ Biofilms Microbiomes 2021, 7, 80. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Preda, V.G.; Săndulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef]
- Zhu, W.; Klinman, J.P. Biogenesis of the peptide-derived redox cofactor pyrroloquinoline quinone. Curr. Opin. Chem. Biol. 2020, 59, 93–103. [Google Scholar] [CrossRef]
- Yadavalli, S.S.; Yuan, J. Bacterial Small Membrane Proteins: The Swiss Army Knife of Regulators at the Lipid Bilayer. J. Bacteriol. 2022, 204, e0034421. [Google Scholar] [CrossRef]
- Khodadadi, E.; Zeinalzadeh, E.; Taghizadeh, S.; Mehramouz, B.; Kamounah, F.S.; Khodadadi, E.; Ganbarov, K.; Yousefi, B.; Bastami, M.; Kafil, H.S. Proteomic Applications in Antimicrobial Resistance and Clinical Microbiology Studies. Infect. Drug Resist. 2020, 13, 1785–1806. [Google Scholar] [CrossRef]
- Torres-Sangiao, E.; Giddey, A.D.; Leal Rodriguez, C.; Tang, Z.; Liu, X.; Soares, N.C. Proteomic Approaches to Unravel Mechanisms of Antibiotic Resistance and Immune Evasion of Bacterial Pathogens. Front. Med. 2022, 9, 850374. [Google Scholar] [CrossRef]
- Nathansen, J.; Meyer, F.; Müller, L.; Schmitz, M.; Borgmann, K.; Dubrovska, A. Beyond the Double-Strand Breaks: The Role of DNA Repair Proteins in Cancer Stem-Cell Regulation. Cancers 2021, 13, 4818. [Google Scholar] [CrossRef]
- Memar, M.Y.; Yekani, M.; Celenza, G.; Poortahmasebi, V.; Naghili, B.; Bellio, P.; Baghi, H.B. The central role of the SOS DNA repair system in antibiotics resistance: A new target for a new infectious treatment strategy. Life Sci. 2020, 262, 118562. [Google Scholar] [CrossRef]
- Rajpurohit, Y.S.; Desai, S.S.; Misra, H.S. Pyrroloquinoline quinone and a quinoprotein kinase support γ-radiation resistance in Deinococcus radiodurans and regulate gene expression. J. Basic. Microbiol. 2013, 53, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.S. Antimicrobial-induced DNA damage and genomic instability in microbial pathogens. PLoS Pathog. 2015, 11, e1004678. [Google Scholar] [CrossRef] [PubMed]
- Maslowska, K.H.; Makiela-Dzbenska, K.; Fijalkowska, I.J. The SOS system: A complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 2019, 60, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Podlesek, Z.; Žgur Bertok, D. The DNA Damage Inducible SOS Response Is a Key Player in the Generation of Bacterial Persister Cells and Population Wide Tolerance. Front. Microbiol. 2020, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.T.; Kang, H.Q.; Ma, P.; Li, P.P.; Huang, L.Y.; Gu, B. SOS response and its regulation on the fluoroquinolone resistance. Ann. Transl. Med. 2015, 3, 358. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zhao, H. Quinolone Antibiotics: Resistance and Therapy. Infect. Drug Resist. 2023, 16, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Ohno, Y.; Tsuneda, S. ldhA-induced persister in Escherichia coli is formed through accidental SOS response via intracellular metabolic perturbation. Microbiol. Immunol. 2022, 66, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Bald, D.; Koul, A. Respiratory ATP synthesis: The new generation of mycobacterial drug targets? FEMS Microbiol. Lett. 2010, 308, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fraunfelter, V.M.; Pugh, B.A.; Williams, A.P.L.; Ward, K.T.; Jackson, D.O.; Austin, M.; Ciprich, J.F.; Dippy, L.; Dunford, J.; Edwards, G.N.; et al. Quinoline Compounds Targeting the c-Ring of ATP Synthase Inhibit Drug-Resistant Pseudomonas aeruginosa. ACS Infect. Dis. 2023, 9, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Bald, D.; Villellas, C.; Lu, P.; Koul, A. Targeting Energy Metabolism in Mycobacterium tuberculosis, a New Paradigm in Antimycobacterial Drug Discovery. mBio 2017, 8, e00272-17. [Google Scholar] [CrossRef] [PubMed]
- Balemans, W.; Vranckx, L.; Lounis, N.; Pop, O.; Guillemont, J.; Vergauwen, K.; Mol, S.; Gilissen, R.; Motte, M.; Lançois, D.; et al. Novel antibiotics targeting respiratory ATP synthesis in Gram-positive pathogenic bacteria. Antimicrob. Agents Chemother. 2012, 56, 4131–4139. [Google Scholar] [CrossRef]
- Balaji, S.; Neupane, R.; Malla, S.; Khupse, R.; Amawi, H.; Kumari, S.; Tukaramrao, D.B.; Chattopadhyay, S.; Ashby, C.R., Jr.; Boddu, S.H.S.; et al. IND-2, a Quinoline Derivative, Inhibits the Proliferation of Prostate Cancer Cells by Inducing Oxidative Stress, Apoptosis and Inhibiting Topoisomerase II. Life 2022, 12, 1879. [Google Scholar] [CrossRef] [PubMed]
- Ben Yaakov, D.; Shadkchan, Y.; Albert, N.; Kontoyiannis, D.P.; Osherov, N. The quinoline bromoquinol exhibits broad-spectrum antifungal activity and induces oxidative stress and apoptosis in Aspergillus fumigatus. J. Antimicrob. Chemother. 2017, 72, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Q.; Cao, Y.-J.; Cui, L.-F.; Zhang, C.-Y.; Zhang, B.-L.; Ma, Q.-Y. Pyrroloquinoline quinone promotes growth and intestinal health and alleviates tris(1,3-dichloro-2-propyl) phosphate-induced hepatic oxidative stress in zebrafish. Aquaculture 2023, 573, 739618. [Google Scholar] [CrossRef]
- Massier, S.; Robin, B.; Mégroz, M.; Wright, A.; Harper, M.; Hayes, B.; Cosette, P.; Broutin, I.; Boyce, J.D.; Dé, E.; et al. Phosphorylation of Extracellular Proteins in Acinetobacter baumannii in Sessile Mode of Growth. Front. Microbiol. 2021, 12, 738780. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.B.; Rajamohan, G. Protein phosphorylation mechanisms: A novel paradigm of antimicrobial resistance in ‘critical threat’ pathogens. Future Microbiol. 2020, 15, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Lee, W.; Noh, G.M.; Jeong, B.H.; Park, I.; Lee, S.J. Fluoroquinolone resistance of Staphylococcus epidermidis isolated from healthy conjunctiva and analysis of their mutations in quinolone-resistance determining region. Antimicrob. Resist. Infect. Control 2020, 9, 177. [Google Scholar] [CrossRef]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Kumazawa, T.; Hiwasa, T.; Takiguchi, M.; Suzuki, O.; Sato, K. Activation of Ras signaling pathways by pyrroloquinoline quinone in NIH3T3 mouse fibroblasts. Int. J. Mol. Med. 2007, 19, 765–770. [Google Scholar] [CrossRef]
- Qiu, K.; Zhao, Q.; Wang, J.; Qi, G.-H.; Wu, S.-G.; Zhang, H.-J. Effects of Pyrroloquinoline Quinone on Lipid Metabolism and Anti-Oxidative Capacity in a High-Fat-Diet Metabolic Dysfunction-Associated Fatty Liver Disease Chick Model. Int. J. Mol. Sci. 2021, 22, 1458. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, R.; Elias, M. Quorum quenching enzymes and their effects on virulence, biofilm, and microbiomes: A review of recent advances. Expert. Rev. Anti Infect. Ther. 2020, 18, 1221–1233. [Google Scholar] [CrossRef]
- Gao, Y.; Kamogashira, T.; Fujimoto, C.; Iwasaki, S.; Yamasoba, T. Pyrroloquinoline quinone (PQQ) protects mitochondrial function of HEI-OC1 cells under premature senescence. NPJ Aging 2022, 8, 3. [Google Scholar] [CrossRef]



| Receptor (Organism) | Ligand | Binding Affinity (kcal/mol) |
|---|---|---|
| Mp1p receptor (T. marneffei) | Palmitic Acid | −4.9 |
| PQQ | −5.8 | |
| Endonuclease PvuRTs1I (P. vulgaris) | EPE | −5.2 |
| PQQ | −7 | |
| TcaR (S. epidermidis) | Methicillin (A) | −7.5 |
| Methicillin (B) | −7.6 | |
| PQQ | −8.8 |
| Protein | Ligand | Hydrophobic Residues | Distance | H-Bond Residues | Distance |
|---|---|---|---|---|---|
| Mp1p receptor (T. marneffei) | Palmitic Acid | VAL82 LEU85 LEU145 ILE149 | 4.6958 4.58823 4.64399 5.25498 | - | - |
| PQQ | ALA92:CB ALA92 ILE93 ILE153 | 3.72868 4.64191 5.24657 5.082 | ALA92 GLN119 ILE93 | 2.30691 2.44378 3.51331 | |
| Endonuclease PvuRTs1I (P. vulgaris) | EPE | - | - | ARG208 THR257 TYR259 HIS181 | 2.19337 2.92358 2.20303 3.77114 |
| PQQ | A:HIS166 A:HIS166 | 5.42052 4.70423 | HIS166 HIS180 ARG208 ARG208 ARG208 | 2.30924 2.75025 1.89771 2.36602 2.49164 | |
| TcaR (S. epidermidis) | Methicillin (A) | HIS42:HD2 HIS42 ALA24 ALA38 VAL63 HIS42 HIS42 | 2.89919 5.13187 4.14757 4.03449 5.03687 4.38379 4.62078 | ASN20 HIS42 ASN20 ASN45 ARG110 | 2.92935 2.27991 2.76373 3.05598 4.19579 |
| Methicillin (B) | HIS42 VAL63 HIS42 | 3.94929 4.54622 5.12969 | - | - | |
| PQQ | HIS42 SER41:C,O;HIS42:N ALA24 ALA38 | 4.95108 5.44787 4.81024 4.72874 | SER41 ARG110 ARG110 ASN20 ASN20 | 2.01432 2.15174 2.28594 2.87169 2.01751 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labib, M.M.; Alqahtani, A.M.; Abo Nahas, H.H.; Aldossari, R.M.; Almiman, B.F.; Ayman Alnumaani, S.; El-Nablaway, M.; Al-Olayan, E.; Alsunbul, M.; Saied, E.M. Novel Insights into the Antimicrobial and Antibiofilm Activity of Pyrroloquinoline Quinone (PQQ); In Vitro, In Silico, and Shotgun Proteomic Studies. Biomolecules 2024, 14, 1018. https://doi.org/10.3390/biom14081018
Labib MM, Alqahtani AM, Abo Nahas HH, Aldossari RM, Almiman BF, Ayman Alnumaani S, El-Nablaway M, Al-Olayan E, Alsunbul M, Saied EM. Novel Insights into the Antimicrobial and Antibiofilm Activity of Pyrroloquinoline Quinone (PQQ); In Vitro, In Silico, and Shotgun Proteomic Studies. Biomolecules. 2024; 14(8):1018. https://doi.org/10.3390/biom14081018
Chicago/Turabian StyleLabib, Mai M., Alaa M. Alqahtani, Hebatallah H. Abo Nahas, Rana M. Aldossari, Bandar Fahad Almiman, Sarah Ayman Alnumaani, Mohammad El-Nablaway, Ebtesam Al-Olayan, Maha Alsunbul, and Essa M. Saied. 2024. "Novel Insights into the Antimicrobial and Antibiofilm Activity of Pyrroloquinoline Quinone (PQQ); In Vitro, In Silico, and Shotgun Proteomic Studies" Biomolecules 14, no. 8: 1018. https://doi.org/10.3390/biom14081018
APA StyleLabib, M. M., Alqahtani, A. M., Abo Nahas, H. H., Aldossari, R. M., Almiman, B. F., Ayman Alnumaani, S., El-Nablaway, M., Al-Olayan, E., Alsunbul, M., & Saied, E. M. (2024). Novel Insights into the Antimicrobial and Antibiofilm Activity of Pyrroloquinoline Quinone (PQQ); In Vitro, In Silico, and Shotgun Proteomic Studies. Biomolecules, 14(8), 1018. https://doi.org/10.3390/biom14081018







