Neurosteroid Levels in GBA Mutated and Non-Mutated Parkinson’s Disease: A Possible Factor Influencing Clinical Phenotype?
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Clinical Assessment
4.3. Quantitative Analysis of NSs in Serum by Liquid Chromatography-Electrospray Tandem Mass Spectrometry (LC-MS/MS)
4.3.1. Chemicals and Reagents
4.3.2. Standard Solutions
4.3.3. Sample Preparation
4.3.4. LC-MS/MS Analysis
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet Lond Engl. 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Riboldi, G.M.; Di Fonzo, A.B. GBA, Gaucher Disease, and Parkinson’s Disease: From genetic to clinic to new therapeutic approaches. Cells 2019, 8, E364. [Google Scholar] [CrossRef]
- Cavallieri, F.; Cury, R.G.; Guimarães, T. Recent advances in the treatment of genetic forms of Parkinson’s Disease: Hype or hope? Cells 2023, 12, 764. [Google Scholar] [CrossRef]
- Billingsley, K.J.; Ding, J.; Alvarez Jerez, P. Genome-wide analysis of Structural Variants in Parkinson’s Disease. Ann. Neurol. Epub. 2023, 93, ana26608. [Google Scholar]
- Petrucci, S.; Ginevrino, M.; Trezzi, I. GBA-related Parkinson’s Disease: Dissection of genotype-phenotype correlates in a large Italian cohort. Mov. Disord. 2020, 35, 2106–2111. [Google Scholar] [CrossRef]
- Menozzi, E.; Schapira, A.H.V. Exploring the genotype-phenotype correlation in GBA-Parkinson Disease: Clinical aspects, biomarkers, and potential modifiers. Front. Neurol. 2021, 12, 694764. [Google Scholar] [CrossRef]
- Cilia, R.; Tunesi, S.; Marotta, G. Survival and dementia in GBA-associated Parkinson’s disease: The mutation matters. Ann. Neurol. 2016, 80, 662–673. [Google Scholar] [CrossRef]
- Yilmaz, C.; Karali, K.; Fodelianaki, G. Neurosteroids as regulators of neuroinflammation. Front. Neuroendocrinol. 2019, 55, 100788. [Google Scholar] [CrossRef]
- Di Michele, F.; Longone, P.; Romeo, E. Decreased plasma and cerebrospinal fluid content of neuroactive steroids in Parkinson’s disease. Neurol. Sci. 2003, 24, 172–173. [Google Scholar] [CrossRef] [PubMed]
- Di Michele, F.; Luchetti, S.; Bernardi, G.; Romeo, E.; Longone, P. Neurosteroid and neurotransmitter alterations in Parkinson’s disease. Front. Neuroendocrinol. 2013, 34, 132–142. [Google Scholar] [CrossRef]
- Luchetti, S.; Liere, P.; Pianos, A. Disease stage-dependent changes in brain levels and neuroprotective effects of neuroactive steroids in Parkinson’s disease. Neurobiol. Dis. 2023, 183, 106169. [Google Scholar] [CrossRef]
- Mullin, S.; Stokholm, M.G.; Hughes, D. Brain microglial activation increased in glucocerebrosidase (GBA) mutation carriers without Parkinson’s disease. Mov. Disord. 2021, 36, 774–779. [Google Scholar] [CrossRef]
- Compagnone, N.A.; Mellon, S.H. Neurosteroids: Biosynthesis and function of these novel neuromodulators. Front. Neuroendocrinol. 2000, 21, 1–56. [Google Scholar] [CrossRef]
- Mellon, S.H.; Griffin, L.D. Neurosteroids: Biochemistry and clinical significance. Trends Endocrinol. Metab. 2002, 13, 35–43. [Google Scholar] [CrossRef]
- Frau, R.; Abbiati, F.; Bini, V. Targeting neurosteroid synthesis as a therapy for schizophrenia-related alterations induced by early psychosocial stress. Schizophr. Res. 2015, 168, 640–648. [Google Scholar] [CrossRef]
- Rupprecht, R.; Wetzel, C.H.; Dorostkar, M. Translocator protein (18kDa) TSPO: A new diagnostic or therapeutic target for stress-related disorders? Mol. Psychiatry 2022, 27, 2918–2926. [Google Scholar] [CrossRef]
- Walton, N.L.; Antonoudiou, P.; Maguire, J.L. Neurosteroid influence on affective tone. Neurosci. Biobehav. Rev. 2023, 152, 105327. [Google Scholar] [CrossRef]
- Frau, R.; Traccis, F.; Concas, L. Prefrontal allopregnanolone synergizes with D1 receptor activation to disrupt sensorimotor gating in male Sprague-Dawley rats. Psychopharmacology 2023, 240, 1359–1372. [Google Scholar] [CrossRef]
- Zoetmulder, M.; Biernat, H.B.; Nikolic, M.; Korbo, L.; Friberg, L.; Jennum, P.J. Prepulse inhibition is associated with attention, processing speed, and 123I-FP-CIT SPECT in Parkinson’s Disease. J. Park Dis. 2014, 4, 77–87. [Google Scholar] [CrossRef]
- Lipari, N.; Centner, A.; Glinski, J.; Cohen, S.; Manfredsson, F.P.; Bishop, C. Characterizing the relationship between L-DOPA-induced-dyskinesia and psychosis-like behaviors in a bilateral rat model of Parkinson’s disease. Neurobiol. Dis. 2023, 176, 105965. [Google Scholar] [CrossRef]
- Brunialti, E.; Villa, A.; Mekhaeil, M. Inhibition of microglial β-glucocerebrosidase hampers the microglia-mediated antioxidant and protective response in neurons. J. Neuroinflammation 2021, 18, 220. [Google Scholar] [CrossRef]
- Genazzani, A.R.; Petraglia, F.; Bernardi, F. Circulating levels of allopregnanolone in humans: Gender, age, and endocrine influences. J. Clin. Endocrinol. Metab. 1998, 83, 2099–2103. [Google Scholar] [CrossRef]
- Bali, A.; Jaggi, A.S. Multifunctional aspects of allopregnanolone in stress and related disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 64–78. [Google Scholar] [CrossRef]
- Mosher, L.J.; Cadeddu, R.; Yen, S. Allopregnanolone is required for prepulse inhibition deficits induced by D1 dopamine receptor activation. Psychoneuroendocrinology 2019, 108, 53–61. [Google Scholar] [CrossRef]
- Darbra, S.; Mòdol, L.; Pallarès, M. Allopregnanolone infused into the dorsal (CA1) hippocampus increases prepulse inhibition of startle response in Wistar rats. Psychoneuroendocrinology 2012, 37, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Zetsen, S.P.G.; Schellekens, A.F.A.; Paling, E.P.; Kan, C.C.; Kessels, R.P.C. Cognitive functioning in long-term benzodiazepine users. Eur. Addict. Res. 2022, 28, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.J.H.; Pine, D.S.; Holmes, E.A.; Reif, A. Benzodiazepines for the long-term treatment of anxiety disorders?—Authors’ reply. Lancet 2021, 398, 120. [Google Scholar] [CrossRef] [PubMed]
- Billioti De Gage, S.; Moride, Y.; Ducruet, T. Benzodiazepine use and risk of Alzheimer’s disease: Case-control study. BMJ 2014, 349, g5205. [Google Scholar] [CrossRef]
- Leng, Y.; Stone, K.L.; Yaffe, K. Race differences in the association between sleep medication use and risk of dementia. J. Alzheimers Dis. 2023, 91, 1133–1139. [Google Scholar] [CrossRef]
- Bäckström, T.; Turkmen, S.; Das, R.; Doverskog, M.; Blackburn, T.P. The GABA system, a new target for medications against cognitive impairment—Associated with neuroactive steroids. J. Intern. Med. 2023, 294, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Del Ser, T.; Zea, M.-A.; Valentí, M. Effects of commonly prescribed drugs on cognition and mild cognitive impairment in healthy elderly people. J. Psychopharmacol. 2019, 33, 965–974. [Google Scholar] [CrossRef]
- Skrahina, V.; Gaber, H.; Vollstedt, E. The Rostock International Parkinson’s Disease (ROPAD) study: Protocol and initial findings. Mov. Disord. 2021, 36, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Fraternali, A.; Cavallieri, F. Quantitative dopamine transporter imaging assessment in Parkinson’s disease patients carrying GBA gene mutations compared with idiopathic PD patients: A case-control study. Brain Behav. 2023, 13, e3060. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Ferri, L.; Cavallieri, F. Increased stroke risk in patients with Parkinson’s Disease with LRRK2 mutations. Mov. Disord. 2022, 37, 1117–1118. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Poewe, W.; Rascol, O. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Jost, S.T.; Kaldenbach, M.; Antonini, A. Levodopa Dose Equivalency in Parkinson’s Disease: Updated systematic review and proposals. Mov. Disord. 2023, 38, 1236–1252. [Google Scholar] [CrossRef]
- Trivisano, M.; Lucchi, C.; Rustichelli, C. Reduced steroidogenesis in patients with PCDH 19-female limited epilepsy. Epilepsia 2017, 58, e91–e95. [Google Scholar] [CrossRef]
- Meletti, S.; Lucchi, C.; Monti, G. Decreased allopregnanolone levels in cerebrospinal fluid obtained during status epilepticus. Epilepsia 2017, 58, e16–e20. [Google Scholar] [CrossRef]
- Meletti, S.; Lucchi, C.; Monti, G. Low levels of progesterone and derivatives in cerebrospinal fluid of patients affected by status epilepticus. J. Neurochem. 2018, 147, 275–284. [Google Scholar] [CrossRef]

| Variable | GBA-PD (n = 22) | NM-PD (n = 22) | GBA-HC (n = 14) | NM-HC (n = 15) |
|---|---|---|---|---|
| No. (%); Mean [SD]; Median {Range} | ||||
| Age | 63.68 [8.43]; 63.50 {46.00–79.00} | 63.05 [8.59]; 62.50 {46.00–79.00} | 49.36 [12.58]; 47.50 {30.00–73.00} *°§ | 60.60 [11.77]; 62.00 {41.00–78.00} |
| Sex, male | 11/22 (50%) | 11/22 (50%) | 8/14 (57.14%) | 4/15 (26.66%) |
| LEDD | 704.32 [546.05]; 740.00 {0.00–2297.00} | 693.68 [318.02]; 655.00 {157.00–1250.00} | NA | NA |
| H&Y | 2.34 [0.49]; 2.50 {1.00–3.00} | 2.45 [0.50]; 2.50 {2.00–4.00} | NA | NA |
| Disease duration | 7.77 [4.38]; 6.50 {2.00–17.00} | 7.41 [3.31]; 7.50 {3.00–15.00} | NA | NA |
| MDS-UPDRS part-I score | 13.36 [7.42]; 13.00 {3.00–26.00} | 10.23 [6.04]; 9.50 {0.00–22.00} | 2.21 [3.01]; 1.00 {0.00–9.00} *° | 3.60 [2.29]; 4.00 {0.00–8.00} *° |
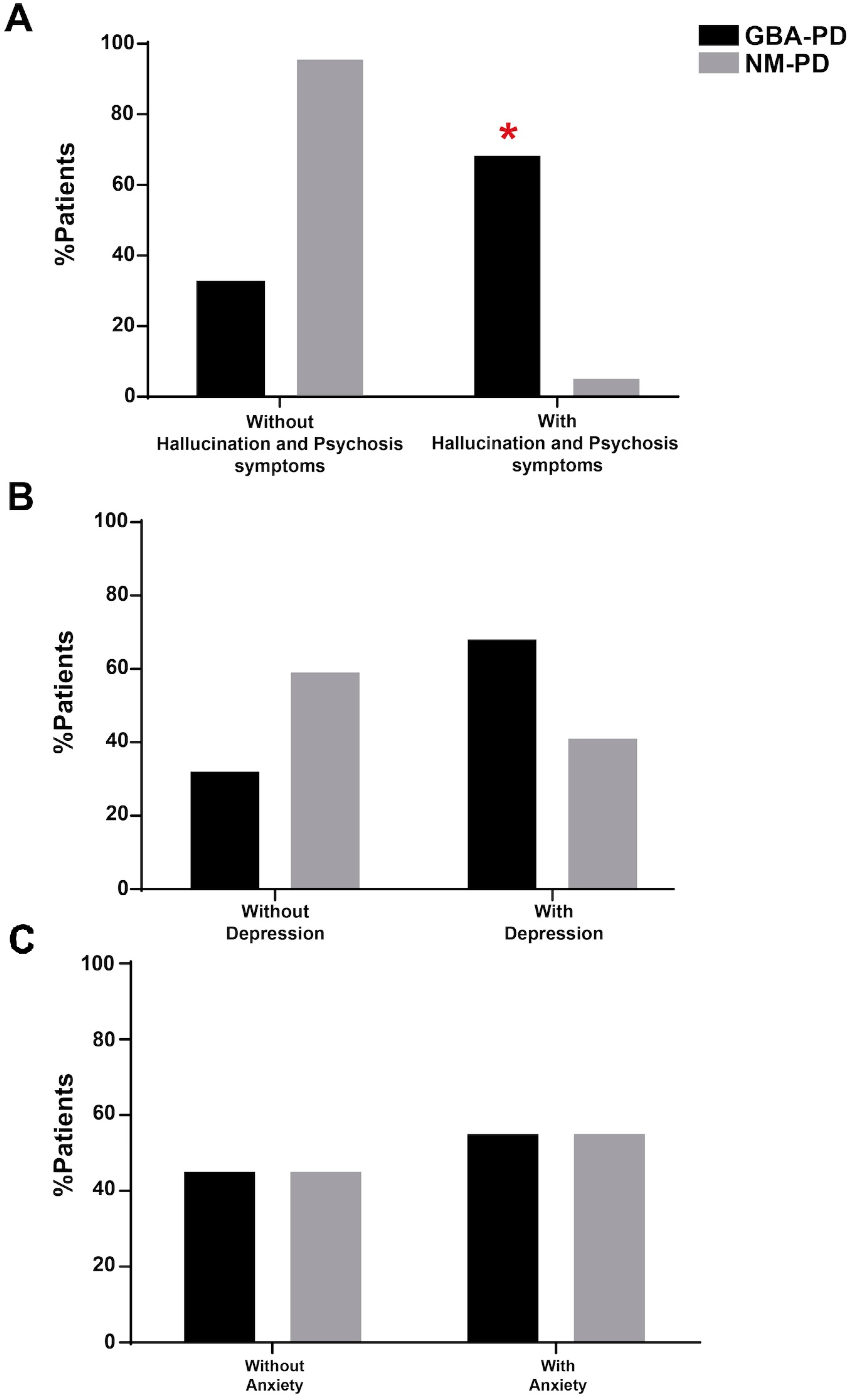
| MDS-UPDRS item 1.2 (hallucinations and psychosis) Present Not present | 15/22 (68.18%) 7/22 (31.81%) ç | 1/22 (4.54%) 21/22 (95.45%) | 0/14 (0.00%) 14/14 (100.00%) | 0/15 (0.00%) 15/15 (100.00%) |
| MDS-UPDRS item 1.3 (depressed mood) Present Not present | 15/22 (68.18%) 7/22 (31.81%) | 9/22 (40.90%) 13/22 (59.09%) | 3/14 (21.42%) 11/14 (78.57%) | 0/15 (0.00%) 15/15 (100.00%) |
| MDS-UPDRS item 1.4 (anxious mood) Present Not present | 12/22 (54.54%) 10/22 (45.45%) | 12/22 (54.54%) 10/22 (45.45%) | 3/14 (21.42%) 11/14 (78.57%) | 0/15 (0.00%) 15/15 (100.00%) |
| MDS-UPDRS part-II score | 17.00 [10.52]; 15.00 {2.00–39.00} | 10.50 [5.68]; 11.50 {0.00–21.00} ^ | 0.29 [0.469]; 0.00 {0.00–1.00} *° | 0.80 [1.424]; 0.00 {0.00–5.00} *° |
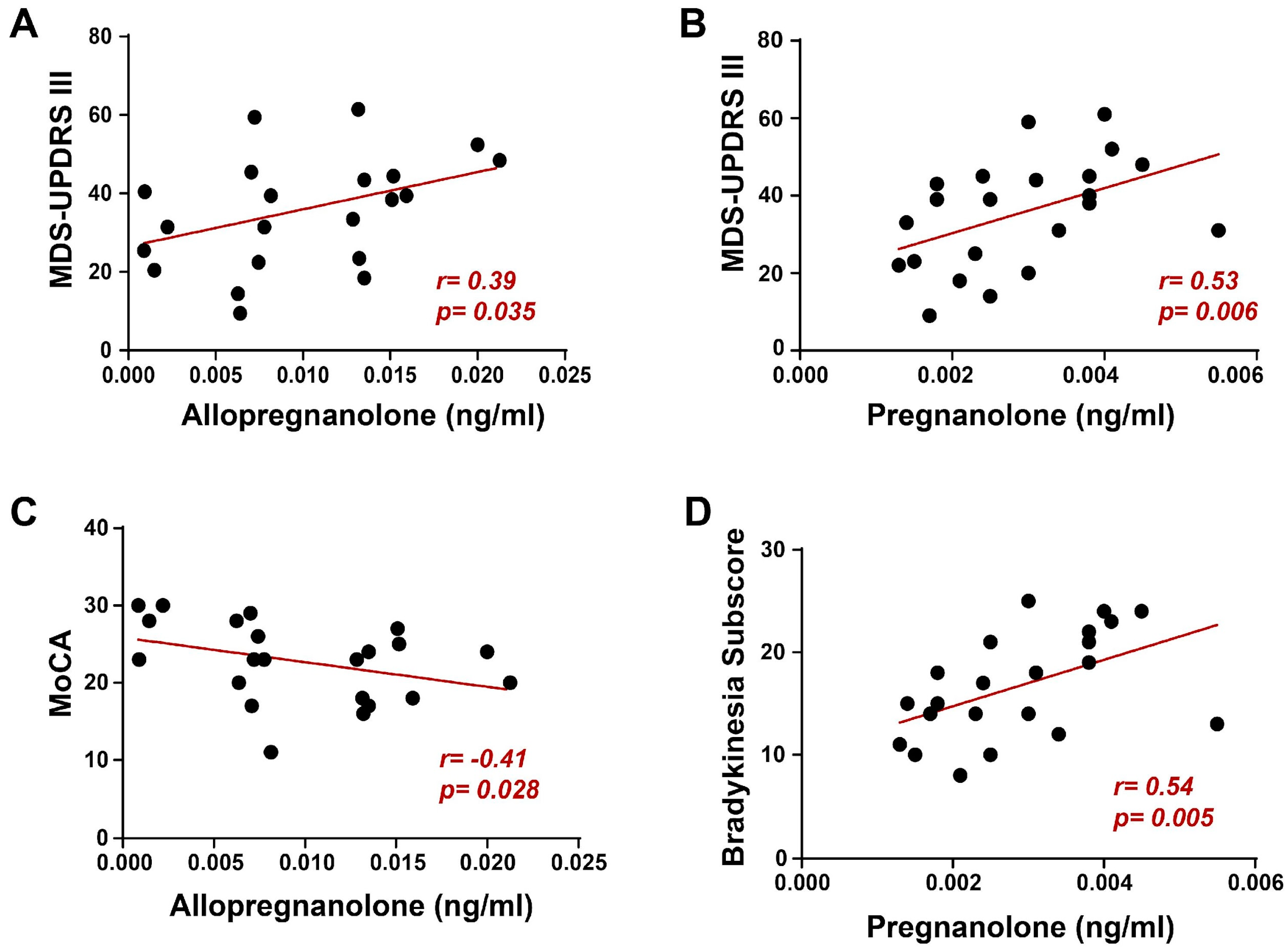
| MDS-UPDRS part-III score | 36.00 [13.22]; 38.50 {14.00–61.00} | 29.68 [11.77]; 28.00 {13.00–59.00} | 0.00 [0.00]; 0.00 {0.00–0.00} *° | 0.33 [0.724]; 0.00 {0.00–2.00} *° |
| MDS-UPDRS part-IV score | 4.95 [4.89]; 3.00 {0.00–14.00} | 3.36 [3.69]; 3.00 {0.00–12.00} | 0.00 [0.00]; 0.00 {0.00–0.00} *° | 0.00 [0.00]; 0.00 {0.00–0.00} *° |
| Tremor Subscore | 4.05 [3.34]; 3.50 {0.00–11.00} | 3.27 [3.74]; 2.00 {0.00–16.00} | 0.00 [0.00]; 0.00 {0.00–0.00} *° | 0.27 [0.704]; 0.00 {0.00–2.00} *° |
| Bradykinesia subscore | 17.86 [5.52]; 17.00 {8.00–26.00} | 14.95 [5.54]; 14.00 {6.00–22.00} | 0.00 [0.00]; 0.00 {0.00–0.00} *° | 0.00 [0.00]; 0.00 {0.00–0.00} *° |
| Rigidity subscore | 5.27 [2.89]; 5.00 {1.00–12.00} | 4.64 [2.88]; 4.00 {0.00–11.00} | 0.00 [0.00]; 0.00 {0.00–0.00} *° | 0.00 [0.00]; 0.00 {0.00–0.00} *° |
| PIGD subscore | 7.14 [4.70]; 7.00 {0.00–19.00} | 4.68 [3.34]; 4.00 {0.00–15.00} | 0.00 [0.00]; 0.00 {0.00–0.00} *° | 0.00 [0.00]; 0.00 {0.00–0.00} *° |
| Gait subscore | 2.59 [1.81]; 2.00 {0.00–7.00} | 1.73 [1.42]; 1.00 {0.00–6.00} | 0.00 [0.00]; 0.00 {0.00–0.00} *° | 0.07 [0.258]; 0.00 {0.00–0.00} *° |
| Fluctuations subscore | 3.55 [3.40]; 3.00 {0.00–10.00} | 2.68 [2.80]; 2.50 {0.00–8.00} | 0.00 [0.00]; 0.00 {0.00–0.00} *° | 0.00 [0.00]; 0.00 {0.00–0.00} *° |
| Dyskinesia subscore | 0.62 [1.79]; 0.00 {0.00–4.00} | 0.68 [1.32]; 0.00 {0.00–4.00} | 0.00 [0.00]; 0.00 {0.00–0.00} *° | 0.00 [0.00]; 0.00 {0.00–0.00} *° |
| MoCA | 22.73 [5.10]; 23.00 {11.00–30.00} | 21.82 [5.43]; 24.00 {12.00–28.00} | 29.79 [0.80]; 30.00 {27.00–30.00} *° | 29.40 [0.98]; 30.00 {27.00–30.00} *° |
| Motor phenotype Akineto-rigid Tremor | 13/22 (59.09%) 9/22 (40.90%) | 9/22 (40.90%) 13/22 (59.09%) | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallieri, F.; Lucchi, C.; Grisanti, S.; Monfrini, E.; Fioravanti, V.; Toschi, G.; Di Rauso, G.; Rossi, J.; Di Fonzo, A.; Biagini, G.; et al. Neurosteroid Levels in GBA Mutated and Non-Mutated Parkinson’s Disease: A Possible Factor Influencing Clinical Phenotype? Biomolecules 2024, 14, 1022. https://doi.org/10.3390/biom14081022
Cavallieri F, Lucchi C, Grisanti S, Monfrini E, Fioravanti V, Toschi G, Di Rauso G, Rossi J, Di Fonzo A, Biagini G, et al. Neurosteroid Levels in GBA Mutated and Non-Mutated Parkinson’s Disease: A Possible Factor Influencing Clinical Phenotype? Biomolecules. 2024; 14(8):1022. https://doi.org/10.3390/biom14081022
Chicago/Turabian StyleCavallieri, Francesco, Chiara Lucchi, Sara Grisanti, Edoardo Monfrini, Valentina Fioravanti, Giulia Toschi, Giulia Di Rauso, Jessica Rossi, Alessio Di Fonzo, Giuseppe Biagini, and et al. 2024. "Neurosteroid Levels in GBA Mutated and Non-Mutated Parkinson’s Disease: A Possible Factor Influencing Clinical Phenotype?" Biomolecules 14, no. 8: 1022. https://doi.org/10.3390/biom14081022







