Preparation of Time-Sequential Functionalized ZnS-ZnO Film for Modulation of Interfacial Behavior of Metals in Biological Service Environments
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Fabrication of Superhydrophobic ZnS-ZnO@SA and DE@ZnS-ZnO@SA Composite Films
2.3. Characterization
2.4. Hydrogen Sulfide Release Profile at Different pH Values
2.5. Anti-Blood Adhesion Test
2.6. Antibacterial Property
2.7. Cell Compatibility Evaluation
2.8. Statistical Analysis
3. Results and Discussion
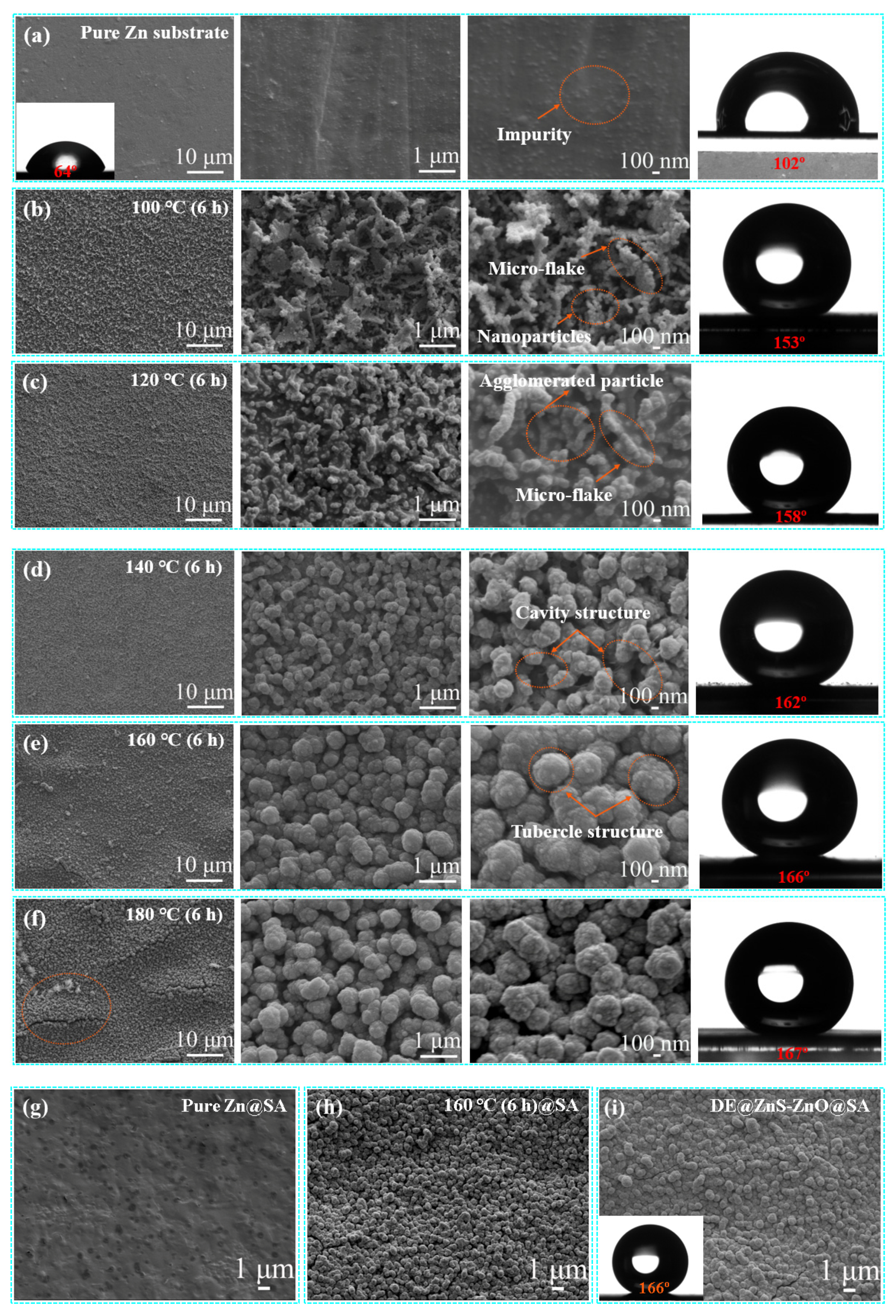
3.1. Morphology, Physical Properties, and Composition Characterization
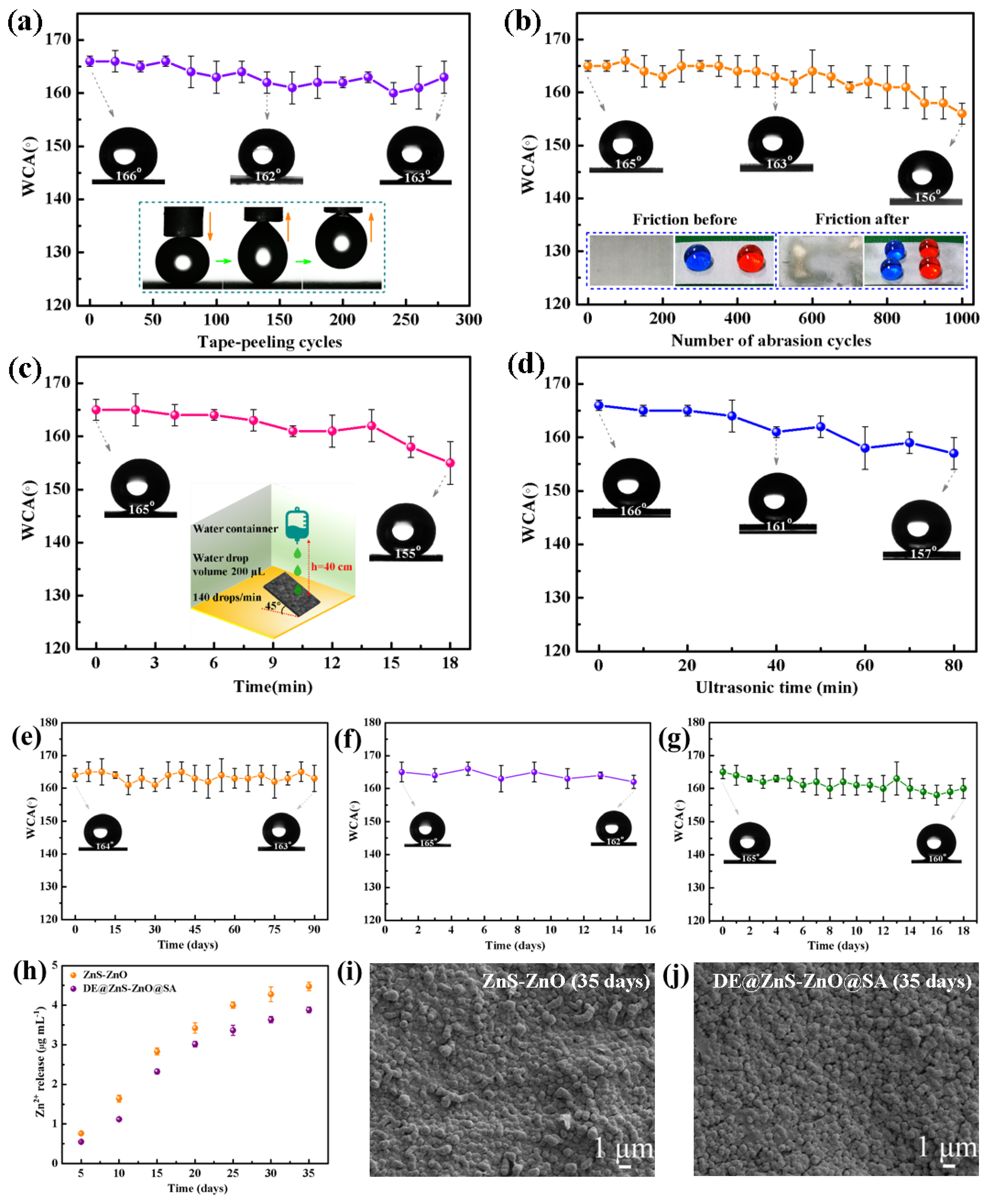
3.2. Mechanical Durability of the Superhydrophobic DE@ZnS-ZnO@SA Film Surface
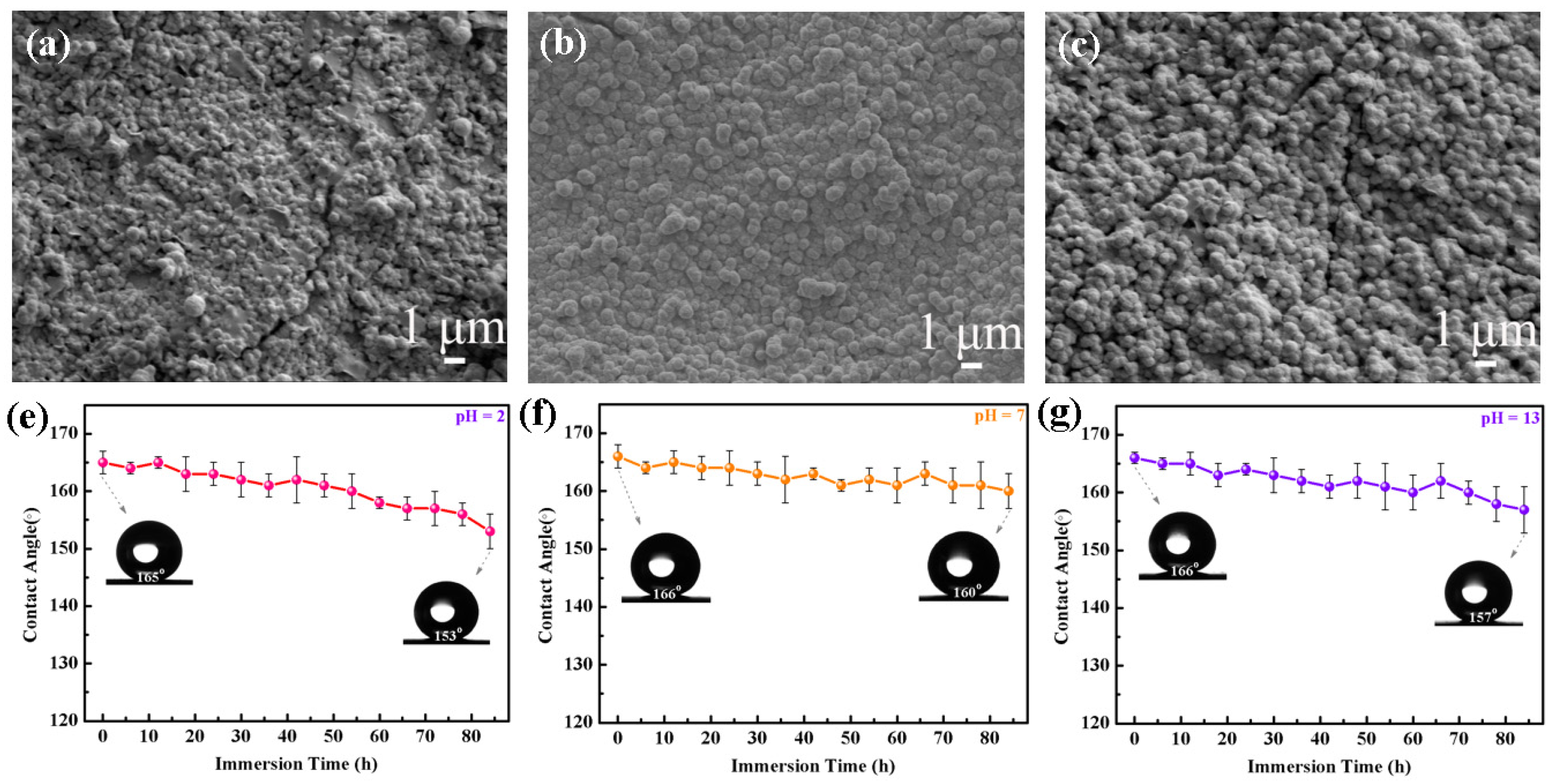
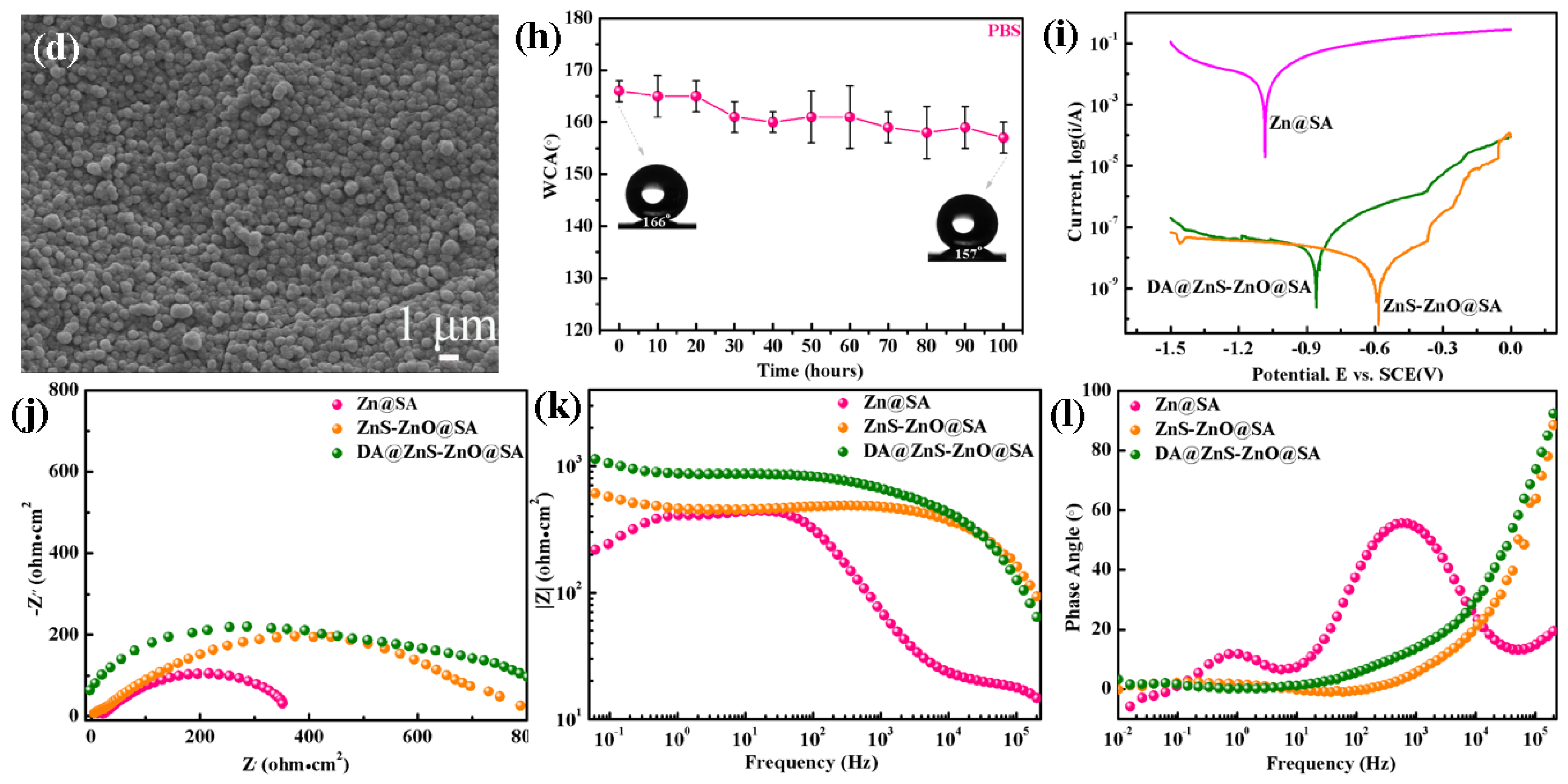
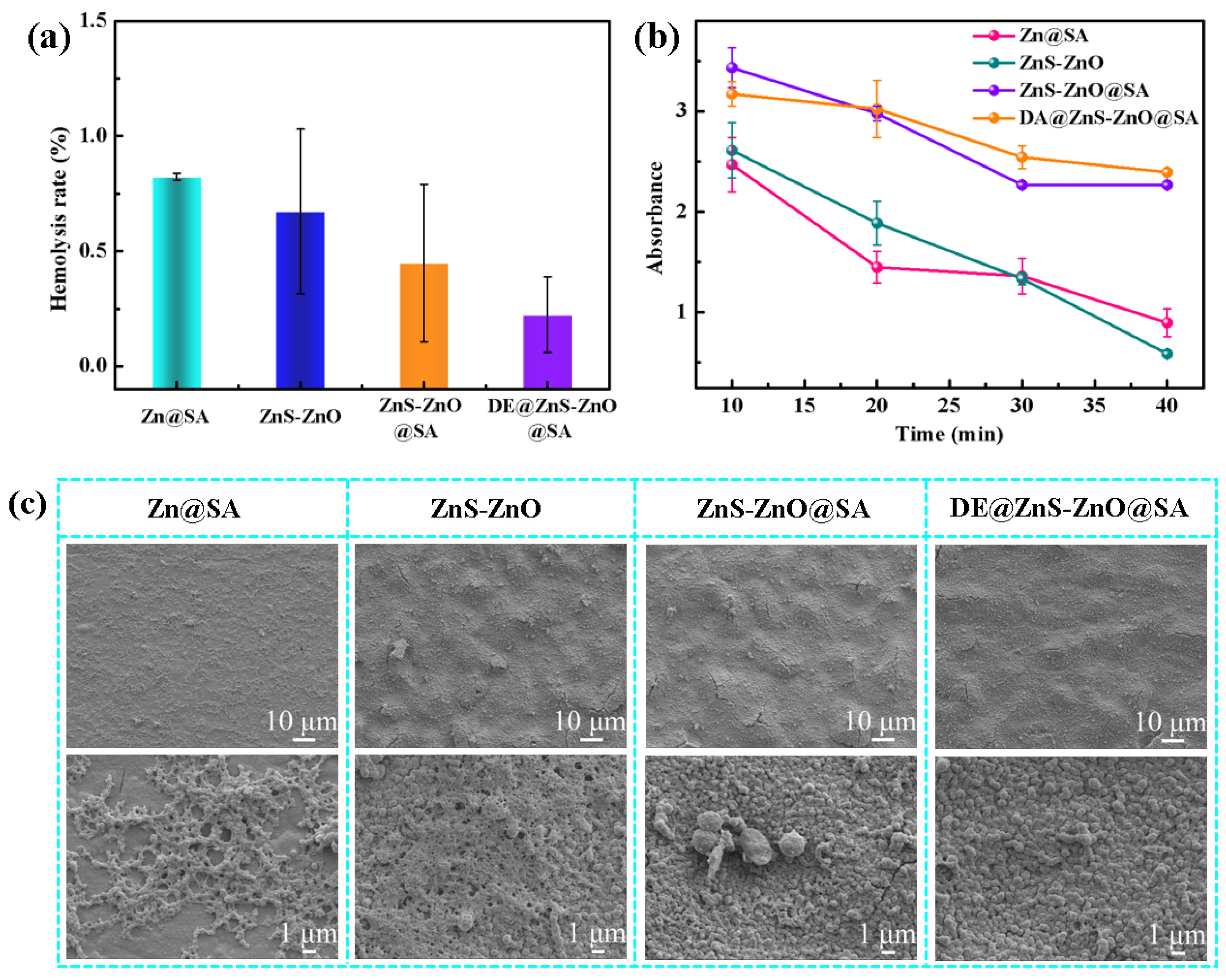
3.3. Chemical Durability and Corrosion Resistance of the DE@ZnS-ZnO@SA Film Surface
3.4. Wetting and Blood-Repelling Properties of the DE@ZnS-ZnO@SA Film Surface
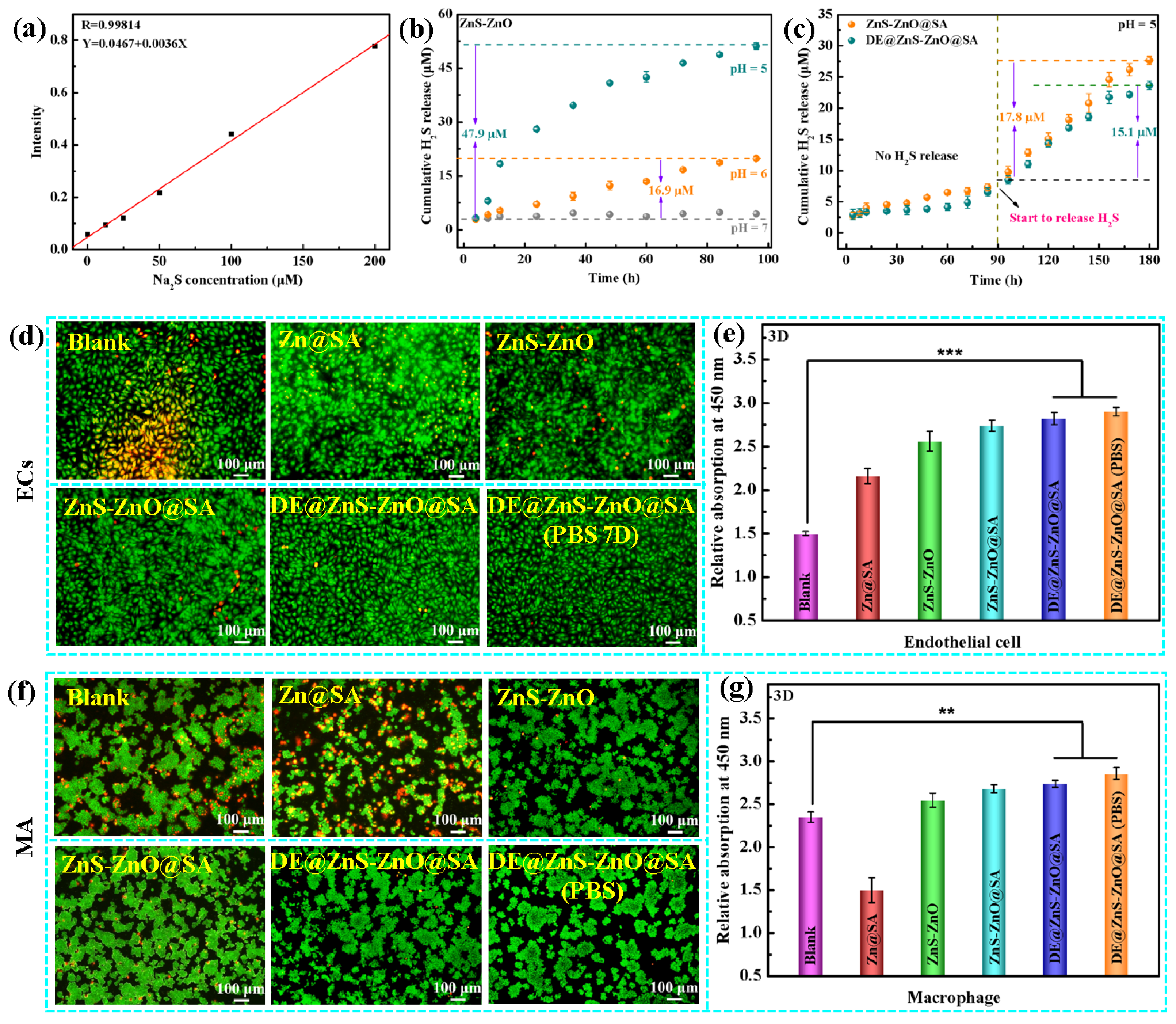
3.5. Hydrogen Sulfide Detection and Histocytocompatibility
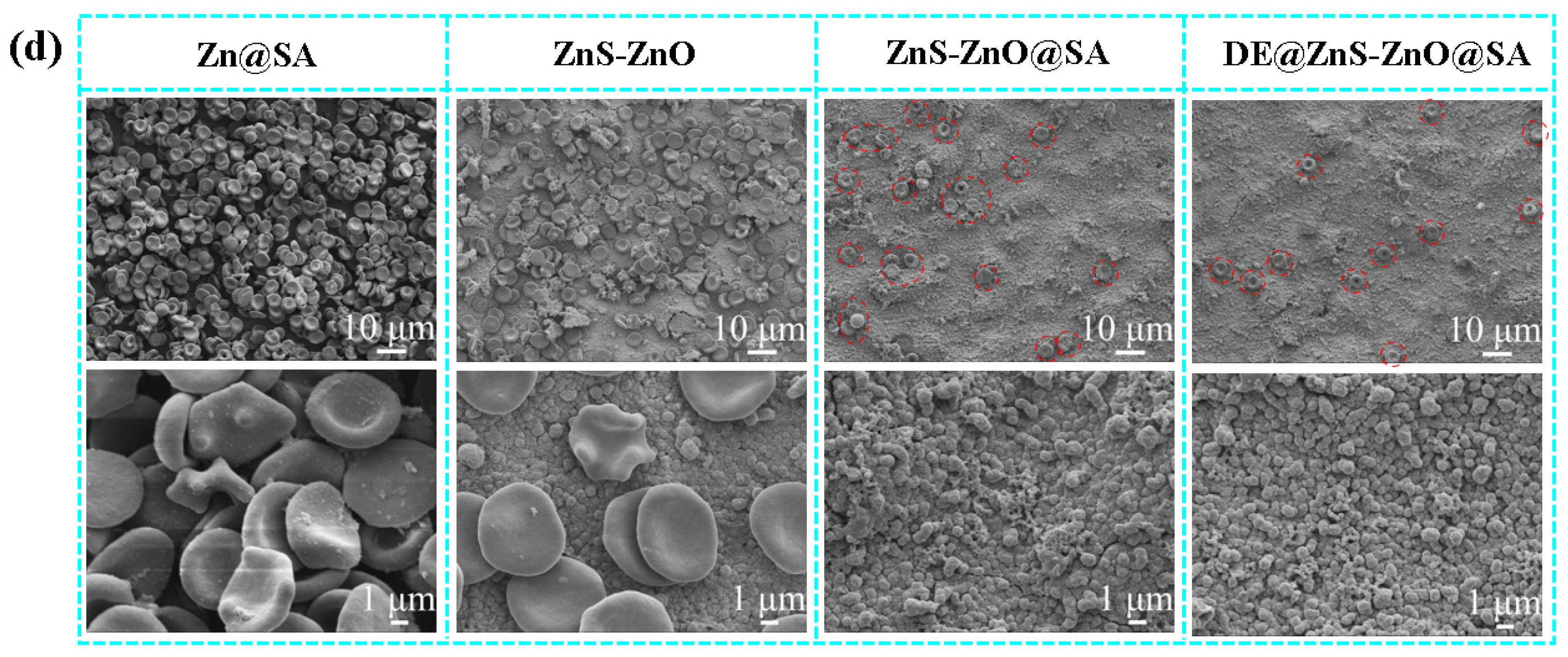
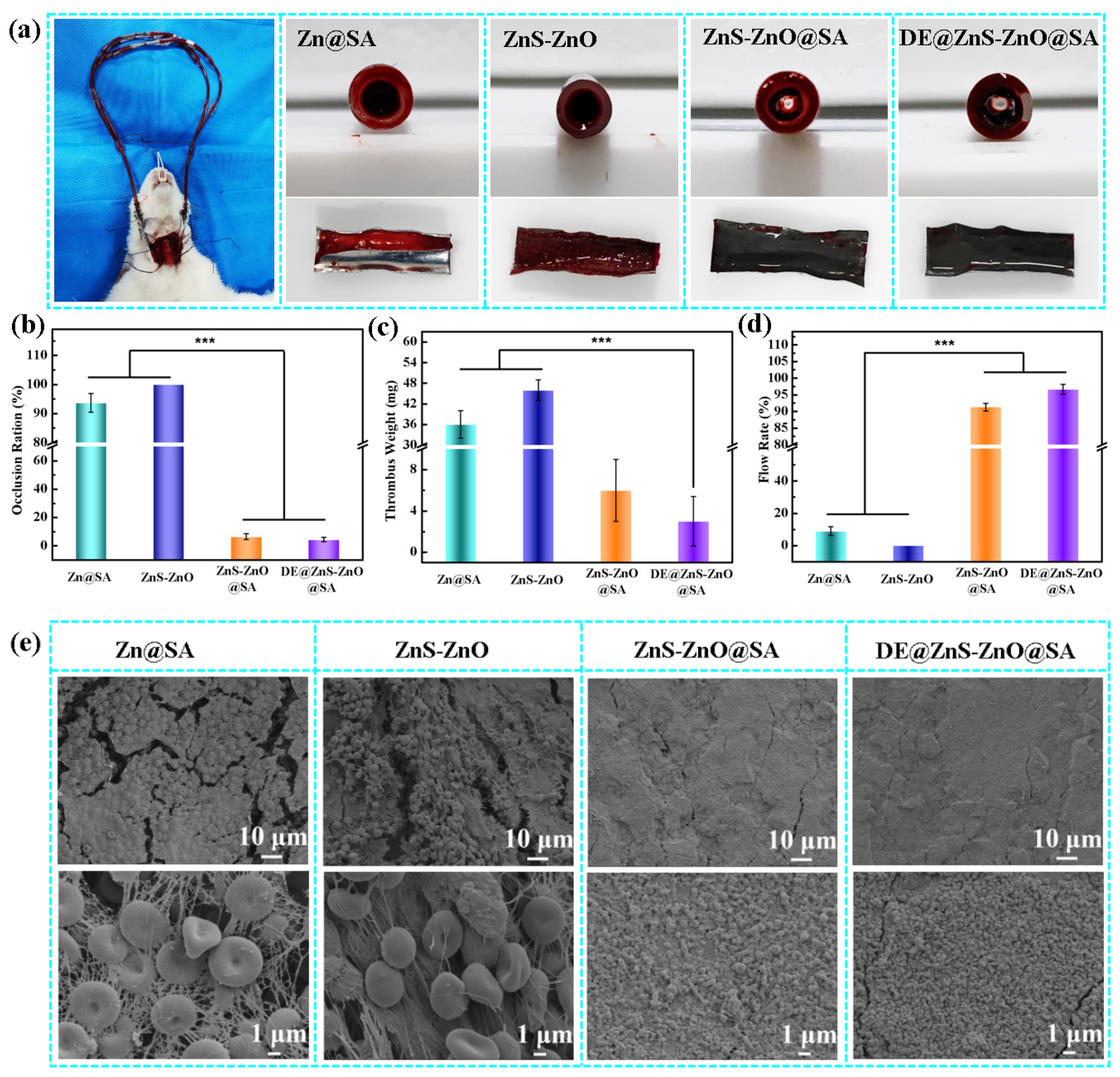
3.6. Anticoagulation
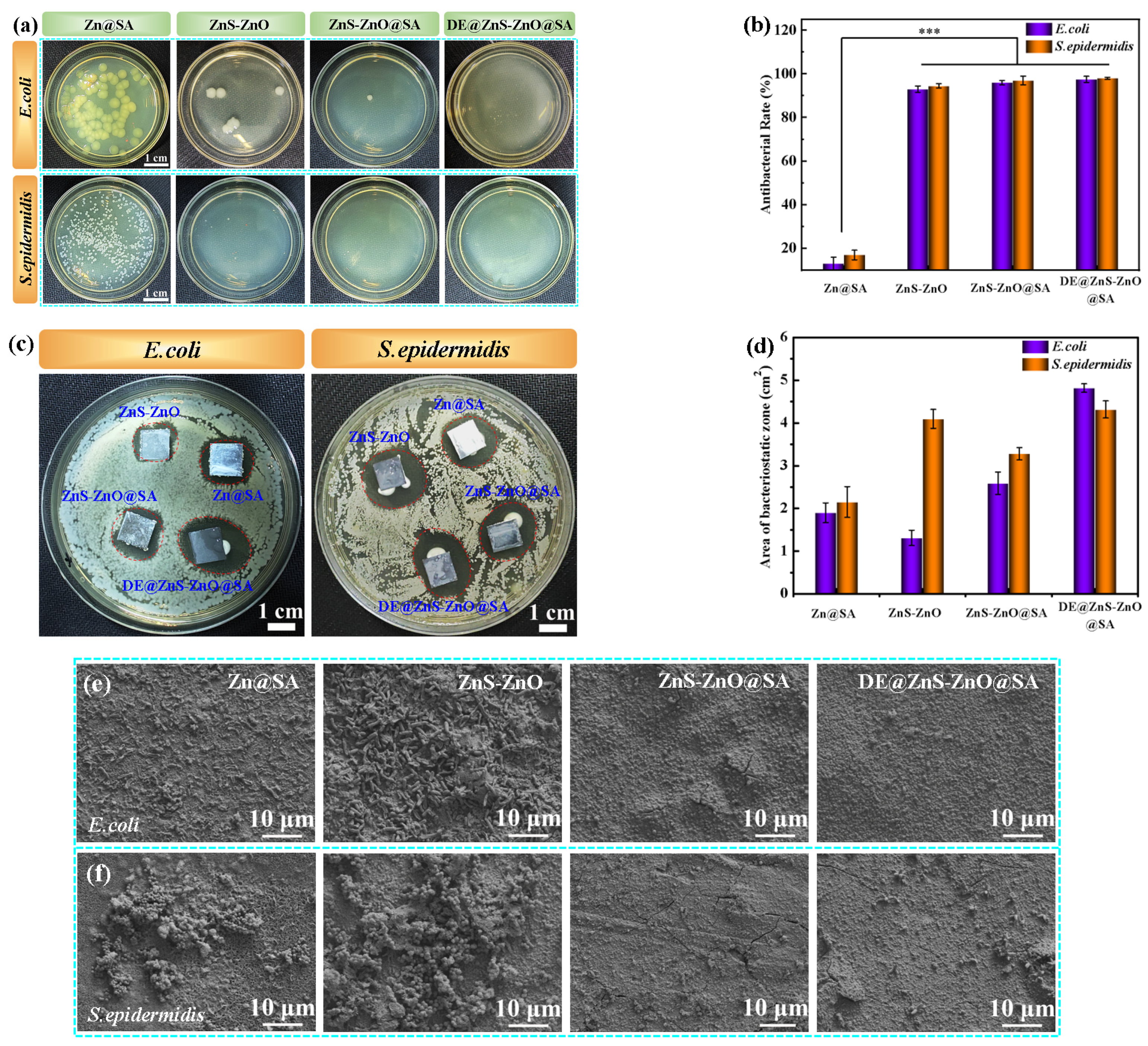
3.7. Antibacterial
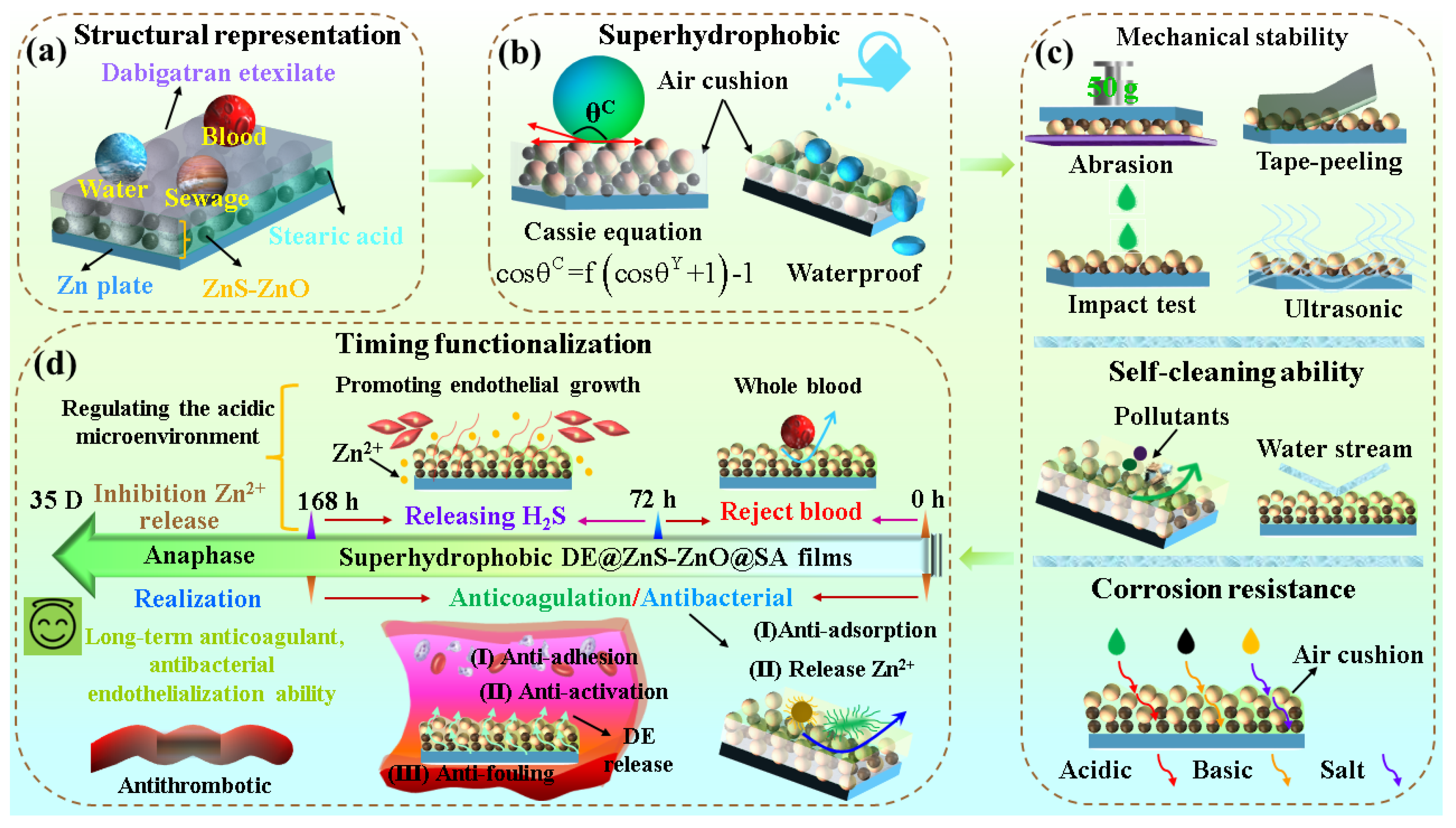
3.8. Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Feng, J.; Wang, J.H.; Wang, H.H.; Cao, X.Y.; Ma, X.L.; Rao, Y.; Pang, H.M.; Zhang, S.L.; Zhang, Y.H.; Wang, L.; et al. Multistage Anticoagulant Surfaces: A Synergistic Combination of Protein Resistance, Fibrinolysis, and Endothelialization. ACS Appl. Mater. Interfaces 2023, 15, 35860–35871. [Google Scholar] [CrossRef]
- Imani, S.M.; Maclachlan, R.; Chan, Y.T.; Shakeri, A.; Soleymani, L.; Didar, T.F. Hierarchical Structures, with Submillimeter Patterns, Micrometer Wrinkles, and Nanoscale Decorations, Suppress Biofouling and Enable Rapid Droplet Digitization. Small 2020, 16, 2004886. [Google Scholar] [CrossRef]
- Wang, J.; Pei, X.Y.; Zhang, J.W.; Liu, S.; Li, Y.; Wang, C.W. Reversible switch of wettability of ZnO@stearic acid nanoarray through alternative irradiation and heat-treatment. Ceram. Int. 2021, 47, 9164–9168. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.W.; Yin, Y.Y.; Jin, H.X.; Liu, S.; Li, Y.; Wang, C.W. The stable superhydrophobic ZnO@stearic acid nanocone array and its remarkable all-sided protective abilities in various extreme environments. J. Alloys Compd. 2019, 807, 151663. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Ding, L.; Tong, W.; Ma, C.; Yang, D.L.; Guan, X.; Xiao, Y.F.; Xu, K.L.; Li, Q.G.; Lv, C.J. Superhydrophobic blood-repellent tubes for clinical cardiac surgery. Mater. Des. 2023, 232, 112148. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, Y.M.; Yang, L.; Wu, Y.; Chen, N.Y.; Li, M.Y.; Li, Y.Y.; Wan, H.N.; Fu, D.H.; Luo, F.; et al. A Polyphenol-Network-Mediated CoatingModulates Inflammation and Vascular Healingon Vascular Stents. ACS Nano 2022, 16, 6585–6597. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, S.S.; Yang, L.; Qin, G.W.; Han, Y.; Zhang, E. Reduced inflammatory response of macrophages on nanostructured surface of Ti-Cu alloy. Mater. Lett. 2022, 319, 132298. [Google Scholar] [CrossRef]
- Zhao, D.W.; Du, C.M.; Zuo, K.Q.; Zhao, Y.X.; Xu, X.Q.; Li, Y.B.; Tian, S.; Yang, H.R.; Lu, Y.P.; Cheng, L.; et al. Calcium-Zinc Phosphate Chemical Conversion Coating Facilitates the Osteointegration of Biodegradable Zinc AlloyImplants by Orchestrating Macrophage Phenotype. Adv. Healthc. Mater. 2023, 12, 2202537. [Google Scholar] [CrossRef]
- Seo, D.K.; Cho, Y.H.; Kim, G.; Shin, H.; Lee, S.K.; Kim, J.E.; Chun, H.; Jung, J.S.; Choi, Y. Permanent Anticoagulation Blood-Vessel by Mezzo-Sized Double Re-Entrant Structure. Small 2023, 19, 2300564. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Duy, N.B.; Jeong, I.S.; Kim, J.F. Hemocompatibility challenge of membrane oxygenator for artificial lung technology. Acta Biomater. 2022, 152, 19–46. [Google Scholar] [CrossRef]
- Yu, H.; Qiu, H.; Ma, W.M.; Maitz, M.F.; Tu, Q.F.; Xiong, K.Q.; Chen, J.; Huang, N.; Yang, Z.L. Endothelium-Mimicking Surface Combats Thrombosis and Biofouling via Synergistic Long- and Short-Distance Defense Strategy. Small 2021, 17, 2100729. [Google Scholar] [CrossRef]
- Zou, D.; Yang, P.; Liu, J.N.; Dai, F.F.; Xiao, Y.Y.; Zhao, A.S.; Huang, N. Exosome-Loaded Pro-efferocytic Vascular Stent with Lp-PLA2-Triggered Release for Preventing In-Stent Restenosis. ACS Nano 2022, 16, 14925–14941. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, I.; See, C.W.; Young, M.L.; Wang, Y.; Zhu, D.H. Designing Better Cardiovascular Stent Materials: A Learning Curve. Adv. Funct. Mater. 2020, 31, 2005361. [Google Scholar] [CrossRef]
- Ciccone, V.; Genah, S.; Morbidelli, L. Endothelium as a Source and Target of H2S to Improve Its Trophism and Function. Antioxidants 2021, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Pung, E.; Lin, Y.; Wang, Q. Recent development of hydrogen sulfide-releasing biomaterials as novel therapies: A narrative review. Biomater. Transl. 2022, 3, 250–263. [Google Scholar] [PubMed]
- Rong, F.; Wang, T.; Zhou, Q.; Peng, H.; Yang, J.; Fan, Q.; Li, P. Intelligent polymeric hydrogen sulfide delivery systems for therapeutic applications. Bioact. Mater. 2023, 19, 198–216. [Google Scholar] [CrossRef]
- Zhang, J.W.; Pei, X.Y.; Weng, Y.J.; Chen, J.Y. Biological Influence and Clinical Applications of the unique flower shape of ZnO−ZnS@SA superhydrophobic composite films grown on nanorods. ACS Sustain. Chem. Eng. 2023, 11, 5559–5576. [Google Scholar] [CrossRef]
- Han, B.; Fang, W.H.; Zhao, S.Q.; Yang, Z.; Hoang, B.X. Zinc sulfide nanoparticles improve skin regeneration. Nanomedicine 2020, 29, 102263. [Google Scholar] [CrossRef]
- Wu, X.H.; Liew, Y.K.; Mai, C.W.; Then, Y.Y. Potential of Superhydrophobic Surface for Blood-Contacting Medical Devices. Int. J. Mol. Sci. 2021, 22, 3341. [Google Scholar] [CrossRef]
- Celik, N.; Sahin, F.; Ruzi, M.; Yay, M.; Unal, E.; Serdar Onses, M. Blood repellent superhydrophobic surfaces constructed from nanoparticle-free and biocompatible materials. Colloids Surf. B 2021, 205, 111864. [Google Scholar] [CrossRef]
- Wu, X.H.; Liew, Y.K.; Lim, W.M.; Mai, C.W.; Then, Y.Y. Blood compatible and noncytotoxic superhydrophobic graphene/titanium dioxide coating with antibacterial and antibiofilm properties. J. Appl. Polym. Sci. 2023, 140, 53629. [Google Scholar] [CrossRef]
- Zhang, J.W.; Pei, X.Y.; Liu, Y.H.; Ke, X.L.; Peng, Y.; Weng, Y.J.; Zhang, Q.Y.; Chen, J.Y. Combining Chitosan, Stearic Acid, and (Cu-, Zn-) MOFs to Prepare Robust Superhydrophobic Coatings with Biomedical Multifunctionalities. Adv. Healthc. Mater. 2023, 12, 2300746. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Nguyen, B.T.D.; Seo, J.H.; Hong, C.; Kim, D.; Ryu, S.; Lee, S.; Lee, G.; Cho, Y.H.; Kim, J.F.; et al. Superhydrophobic polydimethylsiloxane dip-coated polycaprolactone electrospun membrane for extracorporeal membrane oxygenation. J. Membr. Sci. 2023, 679, 121715. [Google Scholar] [CrossRef]
- Chen, Y.X.; Ao, J.; Zhang, J.T.; Gao, J.; Hao, L.W.; Jiang, R.J.; Zhang, Z.H.; Liu, Z.N.; Zhao, J. Luquan Ren Bioinspired superhydrophobic surfaces, inhibiting or promoting microbial contamination. Mater. Today 2023, 67, 468–494. [Google Scholar] [CrossRef]
- Zhang, J.W.; Pei, X.Y.; Huang, J.Q.; Ke, X.L.; Xu, C.; Zhao, W.; Li, L.; Weng, Y.J.; Chen, J.Y. Construction of Hierarchical Micro/Nanostructured ZnO/Cu−ZnMOFs@SA Super-hydrophobic Composite Coatings with Excellent Multifunctionality of Anticorrosion, Blood-Repelling, and Antimicrobial Properties. ACS Appl. Mater. Interfaces 2023, 15, 265–280. [Google Scholar] [CrossRef]
- Huang, N.M.; Wang, Y.; Zhang, Y.; Liu, L.; Yuan, N.Y.; Ding, J.N. Multifunctional coating on magnesium alloy: Superhydrophobic, self-healing, anti-corrosion and wear-resistant. Surf. Coat. Technol. 2023, 463, 129539. [Google Scholar] [CrossRef]
- Huang, H.; Qiang, L.; Fan, M.J.; Liu, Y.H.; Yang, A.C.; Chang, D.B.; Sheng, J.; Sun, T.; Wang, Y.W.; Guo, R.Y.; et al. 3D-printed tri-element-doped hydroxyapatite/polycaprolactone composite scaffolds with antibacterial potential for osteosarcoma therapy and bone regeneration. Bioact. Mater. 2024, 31, 18–37. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, Z.S.; Wang, J.P.; Wang, J.R.; Li, X.Z.; Kuang, C.T. Superhydrophobic coating constructed from rosin acid and TiO2 used as blood repellent dressing. Mater. Des. 2022, 222, 111072. [Google Scholar] [CrossRef]
- Ozkan, E.; Mondal, A.; Douglass, M.; Hopkins, S.P.; Garren, M.; Devine, R.; Pandey, R.; Manuel, J.; Singha, P.; Warnock, J.; et al. Bioinspired ultra-low fouling coatings on medical devices to prevent device-associated infections and thrombosis. J. Colloid Interface Sci. 2022, 608, 1015–1024. [Google Scholar] [CrossRef]
- Pei, X.Y.; Wang, J.; Zhang, J.W.; Liu, S.; Dai, X.G.; Li, Y.; Chen, J.B.; Wang, C.W. Facile preparation of superhydrophobic conductive textiles and the application of real-time sensor of joint motion sensor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127257. [Google Scholar] [CrossRef]
- Diener, H.C.; Easton, J.D.; Hart, R.G.; Kasner, S.; Kamel, H.; Ntaios, G. Review and update of the concept of embolic stroke of undetermined source. Nat. Rev. Neurol. 2022, 18, 455–465. [Google Scholar] [CrossRef]
- Li, C.L.; Chen, S.R.; Wang, Y.F.; Hou, Z.Y. ZnO/ZnS heterostructures grown on Zn foil substrate by hydrothermal method for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 25416–25427. [Google Scholar] [CrossRef]
- Pei, X.Y.; Wang, J.; Zhang, J.W.; Dai, X.G. Rapid response electrowetting on dielectric of Ag-ZnO@STA nanocomposite coatings with antibacterial properties and the application for diabetes detection. J. Alloys Compd. 2022, 905, 164273. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Huo, W.C.; Cao, T.; Liu, X.Y.; Ren, S.; Yang, J.; Ding, H.; Chen, K.; Dong, F.; Zhang, Y.X. Heterojunction interface of zinc oxide and zinc sulfide promoting reactive molecules activation and carrier separation toward efficient photocatalysis. J. Colloid Interface Sci. 2021, 588, 826–837. [Google Scholar] [CrossRef]
- Hitkari, G.; Singh, S.; Pandey, G. Photoluminescence behavior and visible light photocatalytic activity of ZnO, ZnO/ZnS and ZnO/ZnS/α-Fe2O3 nanocomposites. Trans. Nonferrous Met. Soc. China 2018, 28, 1386–1396. [Google Scholar] [CrossRef]
- Kole, A.K.; Biswas, S.; Tiwary, C.S.; Kumbhakar, P. A facile synthesis of graphene oxide–ZnS/ZnO nanocomposites and observations of thermal quenching of visible photoluminescence emission and nonlinear optical properties. J. Lumin. 2016, 179, 211–221. [Google Scholar] [CrossRef]
- Viswanatha, R.; Chakraborty, S.; Basu, S.; Sarma, D.D. Blue-emitting copper-doped zinc oxide nanocrystals. J. Phys. Chem. C 2006, 110, 22310. [Google Scholar] [CrossRef]
- Ma, W.J.; Ding, Y.C.; Zhang, M.J.; Gao, S.T.; Li, Y.S.; Huang, C.B.; Fu, G.D. Nature-inspired chemistry toward hierarchical superhydrophobic, antibacterial and biocompatible nanofibrous membranes for effective UV shielding, self-cleaning and oil-water separation. J. Hazard. Mater. 2020, 384, 121476. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Hu, J.; Cheng, B.; Yao, J.L.; Huang, Y.; Yang, H. Facile preparation of a robust, transparent superhydrophobic ZnO coating with self-cleaning, UV-blocking and bacterial anti-adhesion properties. Surf. Coat. Technol. 2024, 477, 130352. [Google Scholar] [CrossRef]
- Su, Y.C.; Wang, K.; Gao, J.L.; Yang, Y.; Qin, Y.X.; Zheng, Y.F.; Zhu, D.H. Enhanced cytocompatibility and antibacterial property of zinc phosphate coating on biodegradable zinc materials. Acta Biomater. 2019, 98, 174–185. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Li, B.A. Omniphobic membrane with nest-like re-entrant structure via electrospraying strategy for robust membrane distillation. J. Membr. Sci. 2021, 640, 119824. [Google Scholar] [CrossRef]
- Shao, Y.L.; Du, W.B.; Fan, Y.; Zhao, J.; Zhang, Z.H.; Ren, L.Q. Near-infrared light accurately controllable superhydrophobic surface from water sticking to repelling. Chem. Eng. J. 2022, 427, 131718. [Google Scholar] [CrossRef]
- Hou, C.M.; Fan, Z.; Yang, J.Q. Construction of super hydrophobic paper by modified nano-SiO2 hybrid medium fluoro-epoxy polymer and its properties. Prog. Org. Coat. 2024, 187, 108180. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; An, T.Z.; Xian, M.; Luckanagul, J.A.; Su, Z.H.; Lin, Y.; Wang, Q.A. Hydrogen sulfide-releasing alginate dressing for effective wound healing. Acta Biomater. 2020, 104, 85–94. [Google Scholar] [CrossRef]
- Wu, J.; Chen, A.Q.; Zhou, Y.J.; Zheng, S.; Yang, Y.; An, Y.; Xu, K.; He, H.C.; Kang, J.M.; Luckanagule, J.A.; et al. Novel H2S-Releasing hydrogel for wound repair via in situ polarization of M2 macrophages. Biomaterials 2019, 222, 119398. [Google Scholar] [CrossRef]
- Wang, Y.A.; Ma, B.X.; Liu, K.P.; Luo, R.F.; Wang, Y.B. A multi-in-one strategy with glucose-triggered long-term antithrombogenicity and sequentially enhanced endothelialization for biological valve leaflets. Biomaterials 2021, 275, 120981. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, W.J.; Cheng, S.J.; Li, J.H.; Zhang, H.Y. Surface-functionalized design of blood-contacting biomaterials for preventing coagulation and promoting hemostasis. Friction 2023, 11, 1371–1394. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Nguyen, S.H.; Guo, Y.C.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Wandiyanto, J.V.; Garvey, C.J.; Mahon, P.J.; Mainwaring, D.E.; et al. Bactericidal activity of self-assembled palmitic and stearic fatty acid crystals on highly ordered pyrolytic graphite. Acta Biomater. 2017, 59, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, C.; Castro, J.D.; Alves, C.F.A.; Fialho, L.; Carvalho, S. Tracking the chemically active Zn NPs to unravel the electrochemical activity evolution of Ta2O5 bone-like surface. Surf. Coat. Technol. 2023, 467, 129702. [Google Scholar] [CrossRef]
- He, Q.; Ma, Y.W.; Wang, X.S.; Jia, Y.Y.; Li, K.S.; Li, A.L. Superhydrophobic Flexible Silicone Rubber with Stable Performance, Anti-Icing, and Multilevel Rough Structure. ACS Appl. Polym. Mater. 2023, 5, 4729–4737. [Google Scholar] [CrossRef]
- Hu, C.; Luo, R.F.; Wang, Y.B. Heart Valves Cross-Linked with Erythrocyte Membrane Drug-Loaded Nanoparticles as a Biomimetic Strategy for Anti-coagulation, Anti-inflammation, Anti-calcification, and Endothelialization. ACS Appl. Mater. Interfaces 2020, 12, 41113–41126. [Google Scholar] [CrossRef]
- Paimard, G.; Gholivanda, M.B.; Shamsipura, M.; Ahmadi, E.; Shahlaei, M. Introduction of a thrombin sensor based on its interaction with dabigatran as an oral direct thrombin inhibitor. Mater. Sci. Eng. C 2021, 119, 111417. [Google Scholar] [CrossRef]
- Gorzelak-Pabis, P.; Broncel, M.; Pawlos, A.; Wojdan, K.; Gajewski, A.; Chałubinski, M.; Wo´zniak, E. Dabigatran: Its protective effect against endothelial cell damage by oxysterol. Biomed. Pharmacother. 2022, 147, 112679. [Google Scholar] [CrossRef]
- Huang, H.; Fan, M.J.; Yang, A.C.; Chang, D.B.; Li, J.S.; Yang, L.W.; Li, X.L.; Wang, M.Y.; Zheng, P.F.; Guo, T.L.; et al. Sodium alginate-polyethylene glycol paste loaded with zinc-doped tricalcium phosphate particles for the treatment of dentin hypersensitivity. Appl. Mater. Today 2024, 38, 102171. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, X.D.; Chen, Q.; Pan, Y.M.; Liu, C.T.; Shen, C.Y. A simple superhydrophobic/superhydrophilic Janus-paper with enhanced biocompatibility by PDMS and candle soot coating for actuator. Chem. Eng. J. 2021, 406, 126532. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Tang, Y.; Gao, X.; Pei, X.; Weng, Y.; Chen, J. Preparation of Time-Sequential Functionalized ZnS-ZnO Film for Modulation of Interfacial Behavior of Metals in Biological Service Environments. Biomolecules 2024, 14, 1041. https://doi.org/10.3390/biom14081041
Zhang J, Tang Y, Gao X, Pei X, Weng Y, Chen J. Preparation of Time-Sequential Functionalized ZnS-ZnO Film for Modulation of Interfacial Behavior of Metals in Biological Service Environments. Biomolecules. 2024; 14(8):1041. https://doi.org/10.3390/biom14081041
Chicago/Turabian StyleZhang, Jianwen, Yujie Tang, Xiaowa Gao, Xinyu Pei, Yajun Weng, and Junying Chen. 2024. "Preparation of Time-Sequential Functionalized ZnS-ZnO Film for Modulation of Interfacial Behavior of Metals in Biological Service Environments" Biomolecules 14, no. 8: 1041. https://doi.org/10.3390/biom14081041
APA StyleZhang, J., Tang, Y., Gao, X., Pei, X., Weng, Y., & Chen, J. (2024). Preparation of Time-Sequential Functionalized ZnS-ZnO Film for Modulation of Interfacial Behavior of Metals in Biological Service Environments. Biomolecules, 14(8), 1041. https://doi.org/10.3390/biom14081041



