Aerosol Inhalation of Gene Delivery Therapy for Pulmonary Diseases
Abstract
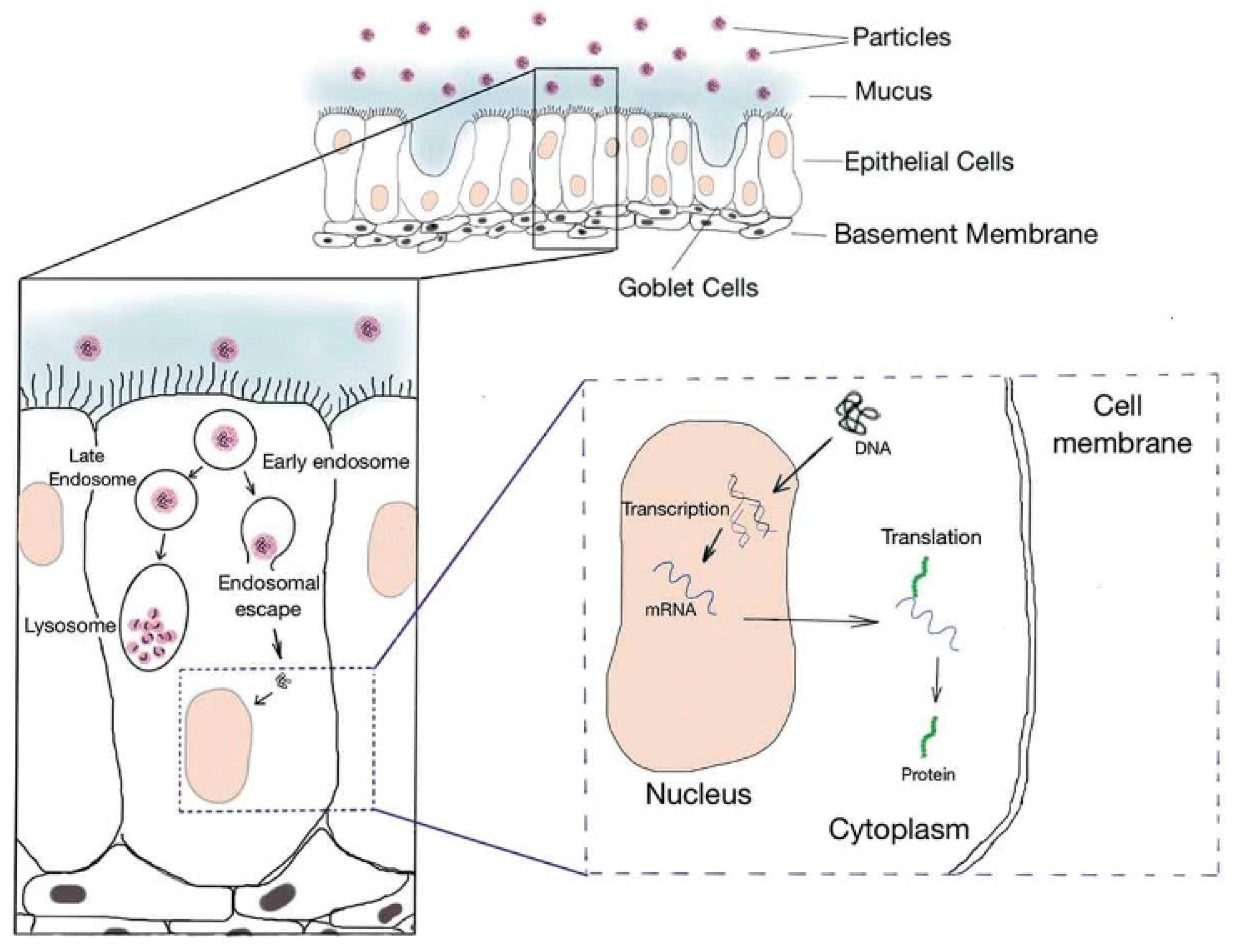
:1. Introduction
2. Basic Characteristics of Inhalation Delivery
3. Viral Vector-Based Carriers
3.1. Lentivirus
3.2. Adeno-Associated Virus Vector (AAV)
3.3. Adenovirus
4. Non-Viral Vectors
4.1. Liposomes
4.2. Polymers
4.2.1. Poly-Ethylenimine (PEI)
4.2.2. Other Polyplexes
4.2.3. Synthetic Esters
4.3. Peptide-Based Nanoparticles
4.4. Natural Biocompatible Components
4.4.1. Chitosan
4.4.2. Exosome-Based Vectors
5. Different Types of Genes for Aerosol Inhalation
5.1. DNA
5.2. mRNA
5.3. Other Types of RNA
5.4. Clustered Regulatory Interspaced Short Palindromic Repeats (CRISPR)/CRISPR-Associated Protein 9 (CRISPR/Cas9)
6. Application of Aerosol Inhalation of Gene Delivery in Pulmonary Diseases
6.1. Vaccines
6.2. Cystic Fibrosis and Other Chronic Diseases
6.3. Lung Cancer
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Forbes, B.; O’Lone, R.; Allen, P.P.; Cahn, A.; Clarke, C.; Collinge, M.; Dailey, L.A.; Donnelly, L.E.; Dybowski, J.; Hassall, D.; et al. Challenges for inhaled drug discovery and development: Induced alveolar macrophage responses. Adv. Drug Deliv. Rev. 2014, 71, 15–33. [Google Scholar] [CrossRef]
- Sahin, U.; Kariko, K.; Tureci, O. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Rowe, S.; Zuckerman, J.; Dorgan, D.; Lascano, J.; McCoy, K.; Jain, M.; Schechter, M.; Lommatzsch, S.; Indihar, V.; Lechtzin, N. Inhaled mRNA therapy for treatment of cystic fibrosis: Interim results of a randomized, double-blind, placebo-controlled phase 1/2 clinical study. J. Cyst. Fibros. 2023, 22, 656–664. [Google Scholar] [CrossRef]
- Sudhakar, V.; Richardson, R.M. Gene Therapy for Neurodegenerative Diseases. Neurotherapeutics 2019, 16, 166–175. [Google Scholar] [CrossRef]
- Figueiredo, A.C.M.d. Real-World Effectiveness of Gene Therapy Zolgensma®(Onasemnogene Abeparvovec-Xioi) for the Treatment of Spinal Muscular Atrophy (SMA). Ph.D. Thesis, Universidade de Lisboa, Lisboa, Portugal, 2021. [Google Scholar]
- Giamas, G. Cancer Gene Therapy: Vision and strategy for the new decade. Cancer Gene Ther. 2020, 27, 115. [Google Scholar] [CrossRef]
- Garbuzenko, O.; Kuzmov, A.; Taratula, O.; Pine, S.R.; Minko, T. Strategy to enhance lung cancer treatment by five essential elements: Inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics 2019, 9, 8362–8376. [Google Scholar] [CrossRef]
- Liu, M.; Hu, S.; Yan, N.; Popowski, K.D.; Cheng, K. Inhalable extracellular vesicle delivery of IL-12 mRNA to treat lung cancer and promote systemic immunity. Nat. Nanotechnol. 2024, 19, 565–575. [Google Scholar] [CrossRef]
- Yannaki, E.; Psatha, N.; Papadopoulou, A.; Athanasopoulos, T.; Gravanis, A.; Roubelakis, M.G.; Tsirigotis, P.; Anagnostopoulos, A.; Anagnou, N.P.; Vassilopoulos, G. Success Stories and Challenges Ahead in Hematopoietic Stem Cell Gene Therapy: Hemoglobinopathies as Disease Models. Hum. Gene Ther. 2021, 32, 1120–1137. [Google Scholar] [CrossRef]
- Chakradhar, S. Treatments that made headlines in 2018. Nat. Med. 2018, 24, 1785–1787. [Google Scholar] [CrossRef] [PubMed]
- Chow, V.A.; Shadman, M.; Gopal, A.K. The Journal of the American Society of Hematology. Translating anti-CD19 CAR T-cell therapy into clinical practice for relapsed/refractory diffuse large B-cell lymphoma. Blood 2018, 132, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hodi, F.S. Talimogene laherparepvec for the treatment of advanced melanoma. Clin. Cancer Res. 2016, 22, 3127–3131. [Google Scholar] [CrossRef]
- Anguela, X.M.; High, K.A. Entering the Modern Era of Gene Therapy. Ann. Rev. Med. 2019, 70, 273–288. [Google Scholar] [CrossRef]
- Stamatatos, L.; Czartoski, J.L.; Wan, Y.-H.; Homad, L.J.; Rubin, V.; Glantz, H.; Neradilek, M.B.; Seydoux, E.; Jennewein, M.F.; MacCamy, A.J.; et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 2021, 372, 1413–1418. [Google Scholar] [CrossRef]
- Belda, F.J.; Mora, O.; López-Martínez, M.; Torres, N.; Vivanco, A.B.N.; Marfil, S.; Pradenas, E.; Massanella, M.; Blanco, J.; Christie, R.; et al. Demonstration of antibodies against SARS-CoV-2, neutralizing or binding, in seroconversion panels after mRNA-1273, BNT-162b2 and Ad26.COV2.S vaccine administration. Plasmatology 2023, 17, 26348535231202681. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, Z.-P.; Tian, H.; Chen, X. Enhancers in polymeric nonviral gene delivery systems. View 2020, 2, 20200072. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Jin, X.; Shou, Z.-X. Surface-engineered smart nanocarrier-based inhalation formulations for targeted lung cancer chemotherapy: A review of current practices. Drug Deliv. 2021, 28, 1995–2010. [Google Scholar] [CrossRef]
- Iwasaki, A.; Foxman, E.F.; Molony, R.D. Early local immune defences in the respiratory tract. Nat. Rev. Immunol. 2016, 17, 7–20. [Google Scholar] [CrossRef]
- Youngren-Ortiz, S.R.; Gandhi, N.S.; Espana-Serrano, L.; Chougule, M.B. Aerosol Delivery of siRNA to the Lungs. Part 1: Rationale for Gene Delivery Systems. Kona 2016, 33, 63–85. [Google Scholar] [CrossRef]
- Ari, A. Jet, Ultrasonic, and Mesh Nebulizers: An Evaluation of Nebulizers for Better Clinical Outcomes. Eurasian J. Pulmonol. 2014, 16, 1–7. [Google Scholar] [CrossRef]
- Varghese Vadakkan, M.; Kumar, G.S.V. Advancements in Devices and Particle Engineering in Dry Powder Inhalation Technology. Curr. Top. Med. Chem. 2016, 16, 1990–2008. [Google Scholar] [CrossRef]
- Alton, E.; Armstrong, D.K.; Ashby, D.; Bayfield, K.J.; Bilton, D.; Bloomfield, E.V.; Boyd, A.C.; Brand, J.; Buchan, R.; Calcedo, R.; et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2015, 3, 684–691. [Google Scholar] [CrossRef]
- Brugarolas, J.; Beckermann, K.; Rini, B.I.; Vogelzang, N.J.; Lam, E.T.; Hamilton, J.C.; Schluep, T.; Yi, M.; Wong, S.; Gamelin, E.; et al. Initial results from the phase 1 study of ARO-HIF2 to silence HIF2-alpha in patients with advanced ccRCC (AROHIF21001). J. Clin. Oncol. 2022, 40, 339. [Google Scholar] [CrossRef]
- Drevinek, P.; Pressler, T.; Cipolli, M.; De Boeck, K.; Schwarz, C.; Bouisset, F.; Boff, M.; Henig, N.; Paquette-Lamontagne, N.; Montgomery, S.; et al. Antisense oligonucleotide eluforsen is safe and improves respiratory symptoms in F508DEL cystic fibrosis. J. Cyst. Fibros. 2020, 19, 99–107. [Google Scholar] [CrossRef]
- Zhang, Y.; Satterlee, A.; Huang, L. In vivo gene delivery by nonviral vectors: Overcoming hurdles? Mol. Ther. 2012, 20, 1298–1304. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M. Image-based analysis of lipid nanoparticle–mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Merkel, O.M.; Beyerle, A.; Beckmann, B.M.; Zheng, M.; Hartmann, R.K.; Stöger, T.; Kissel, T.H. Polymer-related off-target effects in non-viral siRNA delivery. Biomaterials 2011, 32, 2388–2398. [Google Scholar] [CrossRef]
- Tao, Y.-K.; Hou, X.; Zuo, F.; Li, X.; Pang, Y.; Jiang, G. Application of Nanoparticle-Based siRNA and CRISPR/Cas9 Delivery Systems in Gene-Targeted Therapy. Nanomedicine 2019, 14, 511–514. [Google Scholar] [CrossRef]
- Loughrey, D.; Dahlman, J.E. Non-liver mRNA Delivery. Acc. Chem. Res. 2022, 55, 13–23. [Google Scholar] [CrossRef]
- Abdelaziz, H.M.; Gaber, M.; Abd-Elwakil, M.M.; Mabrouk, M.T.; Elgohary, M.M.; Kamel, N.M.; Kabary, D.M.; Freag, M.S.; Samaha, M.W.; Mortada, S.M.; et al. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J. Control. Release 2018, 269, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Okamoto, H. Present situation and future progress of inhaled lung cancer therapy: Necessity of inhaled formulations with drug delivery functions. Chem. Pharm. Bull. 2020, 68, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.S.; Brain, J.D.; Davies, L.A.; Fiegel, J.; Gumbleton, M.; Kim, K.-J.; Sakagami, M.; Vanbever, R.; Ehrhardt, C. The particle has landed—Characterizing the fate of inhaled pharmaceuticals. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23 (Suppl. 2), S71–S87. [Google Scholar] [CrossRef] [PubMed]
- Gomes dos Reis, L.G.; Svolos, M.; Hartwig, B.; Windhab, N.; Young, P.M.; Traini, D. Inhaled gene delivery: A formulation and delivery approach. Expert Opin. Drug Deliv. 2017, 14, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Cho, M.-H.; Kim, S. Recent advances in aerosol gene delivery systems using non-viral vectors for lung cancer therapy. Expert Opin. Drug Deliv. 2019, 16, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.B.; Vasquez, P.A.; Mellnik, J.; McKinley, S.A.; Vose, A.; Mu, F.W.; Henderson, A.G.; Donaldson, S.H.; Alexis, N.E.; Boucher, R.C.; et al. A Biophysical Basis for Mucus Solids Concentration as a Candidate Biomarker for Airways Disease. PLoS ONE 2014, 9, e87681. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Cai, L.; Xu, C.; Sun, S.; Liu, Y.; Rosenecker, J.; Guan, S. Nanotechnologies in Delivery of DNA and mRNA Vaccines to the Nasal and Pulmonary Mucosa. Nanomaterials 2022, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, X.; Ren, X.; Sun, H.; Wu, L.; Wang, C.; Ye, X.; York, P.; Gao, Z.; Jiang, H.; et al. Multiscale Co-reconstruction of Lung Architectures and Inhalable Materials Spatial Distribution. Adv. Sci. 2021, 8, 2003941. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-H.; Park, S.-J.; Lee, S.; Cho, C.S.; Cho, M.-H. Aerosol gene delivery using viral vectors and cationic carriers for in vivo lung cancer therapy. Expert Opin. Drug Deliv. 2015, 12, 977–991. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Anselmo, A.C.; Mitragotri, S. Viral vector-based gene therapies in the clinic. Bioeng. Transl. Med. 2022, 7, e10258. [Google Scholar] [CrossRef]
- Ahamadi, M.; Kast, J.; Chen, P.; Huang, X.; Dutta, S.; Upreti, V. Oncolytic Viral Kinetics Mechanistic Modeling of Talimogene Laherparepvec (IMLYGIC®) a First-in-Class Oncolytic Viral Therapy in patients with Advanced Melanoma. CPT Pharmacomet. Syst. Pharmacol. 2022, 12, 250–260. [Google Scholar] [CrossRef]
- Hwang, S.-K.; Lim, H.-T.; Minai-Tehrani, A.; Lee, E.-S.; Park, J.; Park, S.B.; Beck, G.R., Jr.; Cho, M.-H. Repeated aerosol delivery of carboxyl-terminal modulator protein suppresses tumor in the lungs of K-rasLA1 mice. Am. J. Respir. Crit. Care Med. 2009, 179, 1131–1140. [Google Scholar] [CrossRef]
- Cooney, A.L.; Abou Alaiwa, M.H.; Shah, V.S.; Bouzek, D.C.; Stroik, M.R.; Powers, L.S.; Gansemer, N.D.; Meyerholz, D.K.; Welsh, M.J.; Stoltz, D.A.; et al. Lentiviral-mediated phenotypic correction of cystic fibrosis pigs. JCI Insight 2016, 1, e88730. [Google Scholar] [CrossRef]
- Donnelley, M.; Cmielewski, P.; Knight, E.; Carpentieri, C.; McCarron, A.; Rout-Pitt, N.; Parsons, D.; Farrow, N. Repeat or single-dose lentiviral vector administration to mouse lungs? It’s all about the timing. Gene Ther. 2023, 30, 698–705. [Google Scholar] [CrossRef]
- Rothe, M.; Modlich, U.; Schambach, A. Biosafety Challenges for Use of Lentiviral Vectors in Gene Therapy. Curr. Gene Ther. 2013, 13, 453–468. [Google Scholar] [CrossRef]
- Aguero, J.; Ishikawa, K.; Hadri, L.; Santos-Gallego, C.G.; Fish, K.M.; Kohlbrenner, E.; Hammoudi, N.; Kho, C.; Lee, A.; Ibáñez, B.; et al. Intratracheal Gene Delivery of SERCA2a Ameliorates Chronic Post-Capillary Pulmonary Hypertension. J. Am. Coll Cardiol. 2016, 67, 2032–2046. [Google Scholar] [CrossRef]
- Moss, R.B.; Rodman, D.; Spencer, L.T.; Aitken, M.L.; Zeitlin, P.L.; Waltz, D.; Milla, C.; Brody, A.S.; Clancy, J.P.; Ramsey, B.; et al. Heald, Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: A multicenter, double-blind, placebo-controlled trial. Chest 2004, 125, 509–521. [Google Scholar] [CrossRef]
- MacLoughlin, R.J.; Higgins, B.D.; Devaney, J.; O'Toole, D.; Laffey, J.G.; O'Brien, T. Aerosol-mediated delivery of AAV2/6-IkappaBalpha attenuates lipopolysaccharide-induced acute lung injury in rats. Hum. Gene Ther. 2015, 26, 36–46. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, S.; Cui, T.; Li, J.; Zhu, F.; Zhong, N.; Huang, W.; Zhao, Z.; Wang, Z. Heterologous booster with inhaled adenovirus vector COVID-19 vaccine generated more neutralizing antibodies against different SARS-CoV-2 variants. Emerg. Microbes Infect. 2022, 11, 2689–2697. [Google Scholar] [CrossRef]
- Nidetz, N.F.; McGee, M.C.; Tse, L.V.; Li, C.; Cong, L.; Li, Y.; Huang, W. Adeno-associated viral vector-mediated immune responses: Understanding barriers to gene delivery. Pharmacol. Ther. 2020, 207, 107453. [Google Scholar] [CrossRef]
- Böttger, R.; Pauli, G.; Chao, P.-H.; AL Fayez, N.; Hohenwarter, L.; Li, S.-D. Lipid-based nanoparticle technologies for liver targeting. Adv. Drug Deliv. Rev. 2020, 154–155, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, M.; Wang, T.J.J.o.C.R. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control. Release 2017, 303, 130–150. [Google Scholar] [CrossRef] [PubMed]
- Leong, E.W.X.; Ge, R. Lipid Nanoparticles as Delivery Vehicles for Inhaled Therapeutics. Biomedicines 2022, 10, 2179. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Babapoor, E.; Dezfulian, M. Amikacin-loaded niosome nanoparticles improve amikacin activity against antibiotic-resistant Klebsiella pneumoniae strains. World J. Microbiol. Biotechnol. 2022, 38, 230. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Christopher, M.E.; Nagata, L.P.; Zabielski, M.A.; Li, H.; Wong, J.P.; Samuel, J. Intranasal immunization with liposome-encapsulated plasmid DNA encoding influenza virus hemagglutinin elicits mucosal, cellular and humoral immune responses. J. Clin. Virol. 2004, 31 (Suppl. 1), S99–S106. [Google Scholar] [CrossRef]
- Gulyuz, S.; Bayram, D.; Ozkose, U.U.; Bolat, Z.B.; Koçak, P.; Saka, O.M.; Devrim, B.; Parlak Khalily, M.; Telci, D.; Şahin, F.; et al. Synthesis, biocompatibility and gene encapsulation of poly(2-Ethyl 2-Oxazoline)-dioleoyl phosphatidylethanolamine (PE-tOx-DOPE) and post-modifications with peptides and fluorescent dye coumarin. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 981–993. [Google Scholar]
- Stribling, R.; Brunette, E.; Liggitt, D.; Gaensler, K.; Debs, R. Aerosol gene delivery in vivo. Proc. Natl. Acad. Sci. USA 1992, 89, 11277–11281. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.A.; Nunez-Alonso, G.A.; McLachlan, G.; Hyde, S.C.; Gill, D.R. Aerosol delivery of DNA/liposomes to the lung for cystic fibrosis gene therapy. Hum. Gene Ther. Clin. Dev. 2014, 25, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Rosada, R.S.; de la Torre, L.G.; Frantz, F.G.; Trombone, A.P.; Zárate-Bladés, C.R.; Fonseca, D.M.; Souza, P.R.; Brandão, I.T.; Masson, A.P.; Soares, G.; et al. Protection against tuberculosis by a single intranasal administration of DNA-hsp65 vaccine complexed with cationic liposomes. BMC Immunol. 2008, 9, 38. [Google Scholar] [CrossRef]
- Allon, N.; Saxena, A.; Chambers, C.; Doctor, B.P. A new liposome-based gene delivery system targeting lung epithelial cells using endothelin antagonist. J. Control. Release 2012, 160, 217–224. [Google Scholar] [CrossRef]
- Lokugamage, M.P.; Vanover, D.; Beyersdorf, J.; Hatit, M.Z.C.; Rotolo, L.; Echeverri, E.S.; Peck, H.E.; Ni, H.; Yoon, J.-K.; Kim, Y.; et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng. 2021, 5, 1059–1068. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Chan, H.-K. Lipid nanoparticles for the inhalation of mRNA. Nat. Biomed. Eng. 2021, 5, 949–950. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jozic, A.; Lin, Y.; Eygeris, Y.; Bloom, E.; Tan, X.; Acosta, C.; MacDonald, K.D.; Welsher, K.D.; Sahay, G. Engineering Lipid Nanoparticles for Enhanced Intracellular Delivery of mRNA through Inhalation. ACS Nano 2022, 16, 14792–14806. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Manan, R.S.; Liang, S.-Q.; Gordon, A.; Jiang, A.; Varley, A.; Gao, G.; Langer, R.; Xue, W.; Anderson, D. Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing. Nat. Biotechnol. 2023, 41, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhu, Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 1041–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Chen, Y.; Zhang, Y.-M.; Yang, Y.; Chen, J.-T.; Liu, Y. Recycling gene carrier with high efficiency and low toxicity mediated by L-cystine-bridged bis(beta-cyclodextrin)s. Sci. Rep. 2014, 4, 7471. [Google Scholar] [CrossRef] [PubMed]
- Shim, B.-S.; Park, S.-M.; Quan, J.-S.; Jere, D.; Chu, H.; Song, M.K.; Kim, D.W.; Jang, Y.-S.; Yang, M.-S.; Han, S.H. Intranasal immunization with plasmid DNA encoding spike protein of SARS-coronavirus/polyethylenimine nanoparticles elicits anti-gen-specific humoral and cellular immune responses. BMC Immunol. 2010, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.-F.; Worth, L.L.; Densmore, C.L.; Xu, B.; Duan, X.; Kleinerman, E.S. Aerosol gene therapy with PEI: IL-12 eradicates osteosarcoma lung metastases. Clin. Cancer Res. 2003, 9, 3462–3468. [Google Scholar]
- Cortez-Jugo, C.; Masoumi, S.; Chan, P.P.Y.; Friend, J.; Yeo, L. Nebulization of siRNA for inhalation therapy based on a microfluidic surface acoustic wave platform. Ultrason. Sonochemistry 2022, 88, 106088. [Google Scholar] [CrossRef]
- Dailey, L.A.; Kleemann, E.; Merdan, T.; Petersen, H.; Schmehl, T.; Gessler, T.; Hänze, J.; Seeger, W.; Kissel, T. Modified polyethylenimines as non viral gene delivery systems for aerosol therapy: Effects of nebulization on cellular uptake and transfection efficiency. J. Control. Release 2004, 100, 425–436. [Google Scholar] [CrossRef]
- Zamora-Avila, D.E.; Zapata-Benavides, P.; Franco-Molina, M.A.; Saavedra-Alonso, S.; Trejo-Avila, L.M.; Resendez-Perez, D.; Mendez-Vazquez, J.L.; Isaias-Badillo, J.; Rodriguez-Padilla, C. WT1 gene silencing by aerosol delivery of PEI–RNAi complexes inhibits B16-F10 lung metastases growth. Cancer Gene Ther. 2009, 16, 892–899. [Google Scholar] [CrossRef]
- Oskuee, R.K.; Dabbaghi, M.; Gholami, L.; Taheri-Bojd, S.; Balali-Mood, M.; Mousavi, S.H.; Malaekeh-Nikouei, B. Investigating the influence of polyplex size on toxicity properties of polyethylenimine mediated gene delivery. Life Sci. 2018, 197, 101–108. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Kleemann, E.; Dailey, L.A.; Abdelhady, H.G.; Gessler, T.; Schmehl, T.; Roberts, C.; Davies, M.C.; Seeger, W.; Kissel, T.H. Modified polyethylenimines as non-viral gene delivery systems for aerosol gene therapy: Investigations of the complex structure and stability during air-jet and ultrasonic nebulization. J. Control. Release 2004, 100, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Gothwal, A.; Iyer, A.K.; Jain, K.; Chourasia, M.K.; Gupta, U. Dendrimer nanohybrid carrier systems: An expanding horizon for targeted drug and gene delivery. Drug Discov. Today 2017, 23, 300–314. [Google Scholar] [CrossRef]
- Han, Y.; Yang, Y.; Sun, Q.; Li, B.; Yue, C.; Liu, Y.; de la Fuente, J.M.; Cui, D. Dual-targeted lung cancer therapy via inhalation delivery of UCNP-siRNA-AS1411 nanocages. Cancer Biol. Med. 2021, 19, 1047–1060. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Bai, X.; Zhao, G.; Chen, Q.; Li, Z.; Gao, M.; Ho, W.; Xu, X.; Zhang, X.-Q. Inhaled siRNA nanoparticles targeting IL11 inhibit lung fibrosis and improve pulmonary function post-bleomycin challenge. Sci. Adv. 2022, 8, eabn7162. [Google Scholar] [CrossRef]
- Suberi, A.; Grun, M.K.; Mao, T.; Israelow, B.; Reschke, M.; Grundler, J.; Akhtar, L.; Lee, T.; Shin, K.; Piotrowski-Daspit, A.S.; et al. Inhalable polymer nanoparticles for versatile mRNA delivery and mucosal vaccination. bioRxiv 2022. [Google Scholar] [CrossRef]
- Ma, Z.; Wong, S.W.; Forgham, H.; Esser, L.; Lai, M.; Leiske, M.N.; Kempe, K.; Sharbeen, G.; Youkhana, J.; Mansfeld, F.; et al. Aerosol delivery of star polymer-siRNA nanoparticles as a therapeutic strategy to inhibit lung tumor growth. Biomaterials 2022, 285, 121539. [Google Scholar] [CrossRef]
- Patel, A.K.; Kaczmarek, J.C.; Bose, S.; Kauffman, K.J.; Mir, F.; Heartlein, M.W.; DeRosa, F.; Langer, R.; Anderson, D.G. Inhaled Nanoformulated mRNA Polyplexes for Protein Production in Lung Epithelium. Adv. Mater. 2019, 31, e1805116. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, L.; Vanover, D.; Bruno, N.C.; Peck, H.E.; Zurla, C.; Murray, J.; Noel, R.K.; O’farrell, L.; Araínga, M.; Orr-Burks, N.; et al. Species-agnostic polymeric formulations for inhalable messenger RNA delivery to the lung. Nat. Mater. 2023, 22, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Meng, Q.; Liu, K. Peptide-based gene delivery vectors. J. Mater. Chem. B 2019, 7, 1824–1841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, J.; Xu, D. Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J. Control. Release 2016, 229, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, A.; Baoum, A.; Ohta, N.; Jacquez, S.; Seo, G.-M.; Berkland, C.; Tamura, M. Intratracheal administration of a nanoparticle-based therapy with the angiotensin II type 2 receptor gene attenuates lung cancer growth. Cancer Res. 2012, 72, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, S.; Alhakamy, N.A.; Uppalapati, D.; Delzeit, J.; Berkland, C.J.; Tamura, M. Combined Local Pulmonary and Systemic Delivery of AT2R Gene by Modified TAT Peptide Nanoparticles Attenuates Both Murine and Human Lung Carcinoma Xenografts in Mice. J. Pharm. Sci. 2017, 106, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Munder, A.; Hedtfeld, S.; Braubach, P.; Glage, S.; Zhang, L.; Lienenklaus, S.; Schultze, A.; Hasenpusch, G.; Garrels, W.; et al. Self-assembled peptide–poloxamine nanoparticles enable in vitro and in vivo genome restoration for cystic fibrosis. Nat. Nanotechnol. 2019, 14, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhou, Y.-F.; Xu, B.; Mai, K.-J.; Deng, Y.-B.; Zhang, L.M. Dendronized Chitosan Derivative as a Biocompatible Gene Delivery Carrier. Biomacromolecules 2011, 12, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y. Recent advances of chitosan-based nanoparticles for biomedical and biotechnological applications. Int. J. Biol. Macromol. 2022, 203, 379–388. [Google Scholar] [CrossRef]
- Collado-González, M.; Esteban, M.A. Chitosan-nanoparticles effects on mucosal immunity: A systematic review. Fish Shellfish. Immunol. 2022, 130, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Raghuwanshi, D.; Mishra, V.; Das, D.; Kaur, K.; Suresh, M.R. Dendritic Cell Targeted Chitosan Nanoparticles for Nasal DNA Immunization against SARS CoV Nucleocapsid Protein. Mol. Pharm. 2012, 9, 946–956. [Google Scholar] [CrossRef]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Overcoming cisplatin resistance in non-small cell lung cancer with Mad2 silencing siRNA delivered systemically using EGFR-targeted chitosan nanoparticles. Acta Biomater. 2017, 47, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, D.; Cheng, X.; Zou, Z.; Chen, X.; He, C. Mucoadhesive, Antibacterial, and Reductive Nanogels as a Mucolytic Agent for Efficient Nebulized Therapy to Combat Allergic Asthma. ACS Nano 2022, 16, 11161–11173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bahamondez-Canas, T.F.; Zhang, Y.; Leal, J.; Smyth, H.D.C. PEGylated Chitosan for Nonviral Aerosol and Mucosal Delivery of the CRISPR/Cas9 System in Vitro. Mol. Pharm. 2018, 15, 4814–4826. [Google Scholar] [CrossRef] [PubMed]
- Pofali, P.; Mondal, A.; Londhe, V.Y. Exosome as a Natural Gene Delivery Vector for Cancer Treatment. Curr. Cancer Drug Targets 2020, 20, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Popowski, K.D.; Lopez de Juan Abad, B.; George, A.; Silkstone, D.; Belcher, E.; Chung, J.; Ghodsi, A.; Lutz, H.; Davenport, J.; Flanagan, M.; et al. Inhalable exosomes outperform liposomes as mRNA and protein drug carriers to the lung. Extracell. Vesicle 2022, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Dinh, P.-U.C.; Cores, J.; Hensley, M.T.; Vandergriff, A.C.; Tang, J.; Allen, T.A.; Caranasos, T.G.; Adler, K.B.; Lobo, L.J.; Cheng, K. Derivation of therapeutic lung spheroid cells from minimally invasive transbronchial pulmonary biopsies. Respir. Res. 2017, 18, 132. [Google Scholar] [CrossRef]
- Wang, Z.; Popowski, K.D.; Zhu, D.; de Juan Abad, B.L.; Wang, X.; Liu, M.; Lutz, H.; De Naeyer, N.; DeMarco, C.T.; Denny, T.N.; et al. Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat. Biomed. Eng. 2022, 6, 791–805. [Google Scholar] [CrossRef]
- Dinh, P.-U.C.; Paudel, D.; Brochu, H.; Popowski, K.D.; Gracieux, M.C.; Cores, J.; Huang, K.; Hensley, M.T.; Harrell, E.; Vandergriff, A.C.; et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat. Commun. 2020, 11, 1064. [Google Scholar] [CrossRef]
- Wang, W.; Fu, F.; Huang, Z.; Wang, W.; Chen, M.; Yue, X.; Fu, J.; Feng, X.; Huang, Y.; Wu, C.; et al. Inhalable Biomimetic Protein Corona-Mediated Nanoreactor for Self-Amplified Lung Adenocarcinoma Ferroptosis Therapy. ACS Nano 2022, 16, 8370–8387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jing, W.; Chen, C.; Zhang, S.; Abdalla, M.; Sun, P.; Wang, G.; You, W.; Yang, Z.; Zhang, J.; et al. Inhaled mRNA Nanoformulation with Biogenic Ribosomal Protein Reverses Established Pulmonary Fibrosis in a Bleomycin-Induced Murine Model. Adv. Mater. 2022, 34, e2107506. [Google Scholar] [CrossRef]
- Perricone, M.A.; Morris, J.E.; Pavelka, K.; Plog, M.S.; O’Sullivan, B.P.; Joseph, P.M.; Dorkin, H.; Lapey, A.; Balfour, R.; Meeker, D.P.; et al. Aerosol and lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. II. Transfection efficiency in airway epithelium. Hum. Gene Ther. 2001, 12, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Densmore, C.L.; Kleinerman, E.S.; Gautam, A.; Jia, S.-F.; Xu, B.; Worth, L.L.; Waldrep, J.C.; Fung, Y.-K.; T’Ang, A.; Knight, V. Growth suppression of established human osteosarcoma lung metastases in mice by aerosol gene therapy with PEI– p53 complexes. Cancer Gene Ther. 2001, 8, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Warminski, M.; Mamot, A.; Depaix, A.; Kowalska, J.; Jemielity, J. Chemical modifications of mRNA ends for thera-peutic applications. Acc. Chem. Res. 2023, 56, 2814–2826. [Google Scholar] [CrossRef]
- Haque, A.K.M.A.; Dewerth, A.; Antony, J.S.; Riethmüller, J.; Schweizer, G.R.; Weinmann, P.; Latifi, N.; Yasar, H.; Pedemonte, N.; Sondo, E.; et al. Chemically modified hCFTR mRNAs recuperate lung function in a mouse model of cystic fibrosis. Sci. Rep. 2018, 8, 16776. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, J.; Zhang, Z.; Wu, J.; Zhang, J.; Hu, H.; Zhu, T.; Zhang, J.; Luo, L.; Fan, P.; et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: Preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis. 2021, 21, 1654–1664. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.Y.; Qiu, Y.; Lam, J.K. Inhaled RNA Therapy: From Promise to Reality. Trends Pharmacol. Sci. 2020, 41, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Xu, C.; Wang, P.; Zhang, J.; Tian, H.; Park, K.; Chen, X. Pulmonary Codelivery of Doxorubicin and siRNA by pH-Sensitive Nanoparticles for Therapy of Metastatic Lung Cancer. Small 2015, 11, 4321–4333. [Google Scholar] [CrossRef]
- Xu, C.; Tian, H.; Sun, H.; Jiao, Z.; Zhang, Y.; Chen, X. A pH sensitive co-delivery system of siRNA and doxorubicin for pulmonary administration to B16F10 metastatic lung cancer. RSC Adv. 2015, 5, 103380–103385. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Chow, M.Y.T.; Chang, R.Y.K.; Chan, H.-K. Inhalation delivery technology for genome-editing of respiratory diseases. Adv. Drug Deliv. Rev. 2021, 168, 217–228. [Google Scholar] [CrossRef]
- Lino, C.A.; Harper, J.C.; Carney, J.P.; Timlin, J.A. Delivering CRISPR: A review of the challenges and approaches. Drug Deliv. 2018, 25, 1234–1257. [Google Scholar] [CrossRef]
- Wan, T.; Ping, Y. Delivery of genome-editing biomacromolecules for treatment of lung genetic disorders. Adv. Drug Deliv. Rev. 2021, 168, 196–216. [Google Scholar] [CrossRef]
- Loo, C.-Y.; Lee, W.-H.; Zhou, Q.T. Recent Advances in Inhaled Nanoformulations of Vaccines and Therapeutics Targeting Respiratory Viral Infections. Pharm. Res. 2023, 40, 1015–1036. [Google Scholar] [CrossRef]
- Sarangi, M.K.; Padhi, S.; Rath, G.; Nanda, S.S.; Yi, D.K. Success of nano-vaccines against COVID-19: A transformation in nanomedicine. Expert Rev. Vaccines 2022, 21, 1739–1761. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Jiao, Z.; Li, X.; He, Z.; Li, Y.; Yang, F.; Zhao, X.; Wang, Y.; Huang, W.; Qin, M.J. Inhaled SARS-CoV-2 vaccine for single-dose dry powder aerosol immunization. Nature 2023, 624, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.V.; Fernandez de Castro, J.; Valdespino-Gomez, J.L.; Garcia-Garcia Mde, L.; Islas-Romero, R.; Echaniz-Aviles, G.; Jimenez-Corona, A.; Sepulveda-Amor, J. Aerosolized measles and measles-rubella vaccines induce better measles antibody booster responses than injected vaccines: Randomized trials in Mexican schoolchildren. Bull. World Health Organ. 2002, 80, 806–812. [Google Scholar]
- Carter, N.J.; Curran, M.P. Live attenuated influenza vaccine (FluMist®; fluenz™) a review of its use in the prevention of seasonal influenza in children and adults. Drugs 2011, 71, 1591–1622. [Google Scholar] [CrossRef]
- Lorenzi, J.C.; Trombone, A.P.; Rocha, C.D.; Almeida, L.P.; Lousada, R.L.; Malardo, T.; Fontoura, I.C.; Rossetti, R.A.; Gembre, A.F.; Silva, A.M.; et al. Intranasal vaccination with messenger RNA as a new approach in gene therapy: Use against tuberculosis. BMC Biotechnol. 2010, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Kurono, Y. The mucosal immune system of the upper respiratory tract and recent progress in mucosal vaccines. Auris Nasus Larynx 2022, 49, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Marasini, N.; Haque, S.; Kaminskas, L.M. Polymer-drug conjugates as inhalable drug delivery systems: A review. Curr. Opin. Colloid Interface Sci. 2017, 31, 18–29. [Google Scholar] [CrossRef]
- D'Angelo, I.; Conte, C.; La Rotonda, M.I.; Miro, A.; Quaglia, F.; Ungaro, F. Improving the efficacy of inhaled drugs in cystic fibrosis: Challenges and emerging drug delivery strategies. Adv. Drug Deliv. Rev. 2014, 75, 92–111. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, L.; Li, Z.; Chen, G.; Sun, M.; Oupicky, D. Treatment of acute lung injury and early- and late-stage pulmonary fibrosis with combination emulsion siRNA polyplexes. J. Control. Release 2019, 314, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.-L.; Barbera, J.A.; Beghetti, M.; Corris, P.; Gaine, S.; Gibbs, J.S.; et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2009, 30, 2493–2537. [Google Scholar] [CrossRef]
- Wu, X.; Yu, Y.; Wang, M.; Dai, D.; Yin, J.; Liu, W.; Kong, D.; Tang, S.; Meng, M.; Gao, T.; et al. AAV-delivered muscone-induced transgene system for treating chronic diseases in mice via inhalation. Nat. Commun. 2024, 15, 1122. [Google Scholar] [CrossRef]
- De Koning, H.J.; Van Der Aalst, C.M.; De Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Cho, W.-Y.; Hong, S.-H.; Singh, B.; Islam, M.A.; Lee, S.; Lee, A.Y.; Gankhuyag, N.; Kim, J.-E.; Yu, K.-N.; Kim, K.-H.; et al. Suppression of tumor growth in lung cancer xenograft model mice by poly(sorbitol-co-PEI)-mediated delivery of osteopontin siRNA. Eur. J. Pharm. Biopharm. 2015, 94, 450–462. [Google Scholar] [CrossRef]
- Hitzman, C.J. Respiratory Delivery of 5-fluorouracil for the Treatment of Lung Cancer; University of Minnesota: Minneapolis, MN, USA, 2005. [Google Scholar]
- Song, X.; Liu, C.; Wang, N.; Huang, H.; He, S.; Gong, C.; Wei, Y. Delivery of CRISPR/Cas systems for cancer gene therapy and immunotherapy. Adv. Drug Deliv. Rev. 2021, 168, 158–180. [Google Scholar] [CrossRef]
- Kotterman, M.A.; Chalberg, T.W.; Schaffer, D.V. Viral Vectors for Gene Therapy: Translational and Clinical Outlook. Annu. Rev. Biomed. Eng. 2015, 17, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bruggeman, K.F.; Franks, S.J.; Gautam, V.; Hodgetts, S.I.; Harvey, A.R.; Williams, R.J.; Nisbet, D.R. Is Viral Vector Gene Delivery More Effective Using Biomaterials? Adv. Heal. Mater. 2020, 10, e2001238. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Cortez-Jugo, C.; Chen, J.; Wang, T.Y.; Mitchell, A.J.; Tsantikos, E.; Bertleff-Zieschang, N.; Lin, Y.W.; Song, J.; Cheng, Y.; et al. Engineering of Nebulized Metal–Phenolic Capsules for Controlled Pulmonary Deposition. Adv. Sci. 2020, 7, 1902650. [Google Scholar] [CrossRef]
- Ichikawa, M.; Muramatsu, N.; Matsunaga, W.; Ishikawa, T.; Okuda, T.; Okamoto, H.; Gotoh, A. Effects of inhalable gene transfection as a novel gene therapy for non-small cell lung cancer and malignant pleural mesothelioma. Sci. Rep. 2022, 12, 8634. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Scagliotti, G.V.; Selvaggi, G.; Novello, S.; Hirsch, F.R. The biology of epidermal growth factor receptor in lung cancer. Clin. Cancer Res. 2004, 10, 4227s–4232s. [Google Scholar] [CrossRef]



| Viral vectors | Lentivirus |
| Adeno-associated virus vector (AAV) | |
| Adenovirus | |
| Non-viral vectors | Liposomes |
| Polymers (poly-ethylenimine (PEI), other polyplexes, synthetic esters) | |
| Peptide-based nanoparticles | |
| Natural biocompatible components (chitosan or exosome-based vectors) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Zhang, J.; Wang, X.; Jing, H.; Li, H. Aerosol Inhalation of Gene Delivery Therapy for Pulmonary Diseases. Biomolecules 2024, 14, 904. https://doi.org/10.3390/biom14080904
Huang Y, Zhang J, Wang X, Jing H, Li H. Aerosol Inhalation of Gene Delivery Therapy for Pulmonary Diseases. Biomolecules. 2024; 14(8):904. https://doi.org/10.3390/biom14080904
Chicago/Turabian StyleHuang, Yiheng, Jiahao Zhang, Xiaofeng Wang, Hui Jing, and Hecheng Li. 2024. "Aerosol Inhalation of Gene Delivery Therapy for Pulmonary Diseases" Biomolecules 14, no. 8: 904. https://doi.org/10.3390/biom14080904





