Markers of Mitochondrial Function and DNA Repair Associated with Physical Function in Centenarians
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Physical Function Assessment
2.3. Cognitive Ability Assessment
2.4. Blood Sampling, PBMCs and Plasma Isolation
2.5. Analysis of Mitochondrial Function in PBMCs
2.6. mtDNA Copy Number from PBMCs
2.7. Whole Cell Extract (WCE) from PBMCs
2.8. Radiolabelling of Oligonucleotides
2.9. APE1 Endonuclease Activity Assay of WCE from PBMCs
2.10. Protein Carbonylation of Circulating Plasma Proteins
2.11. ELISA Analysis of Circulating Plasma Samples
2.12. Statistical Analyses
3. Results
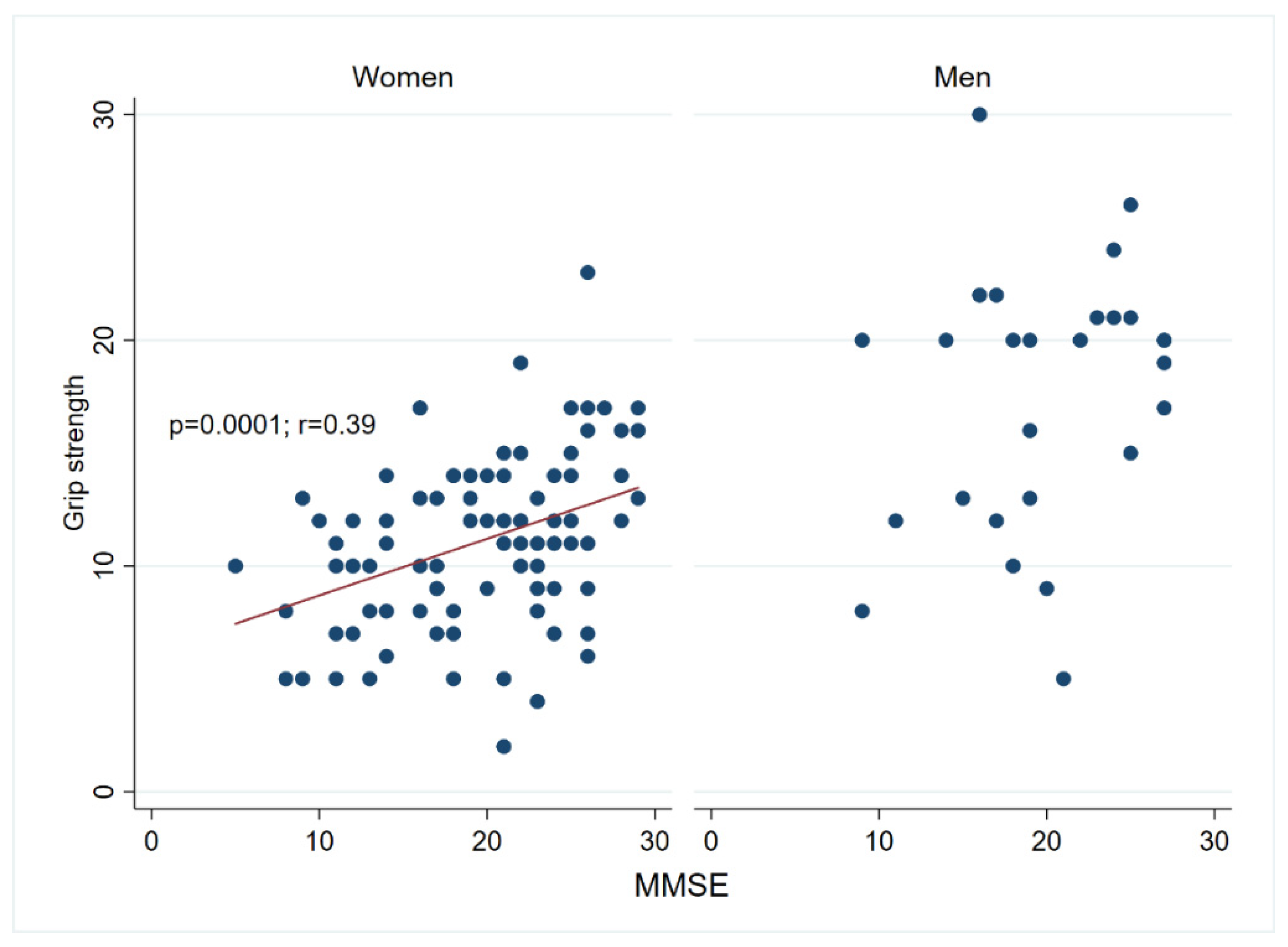
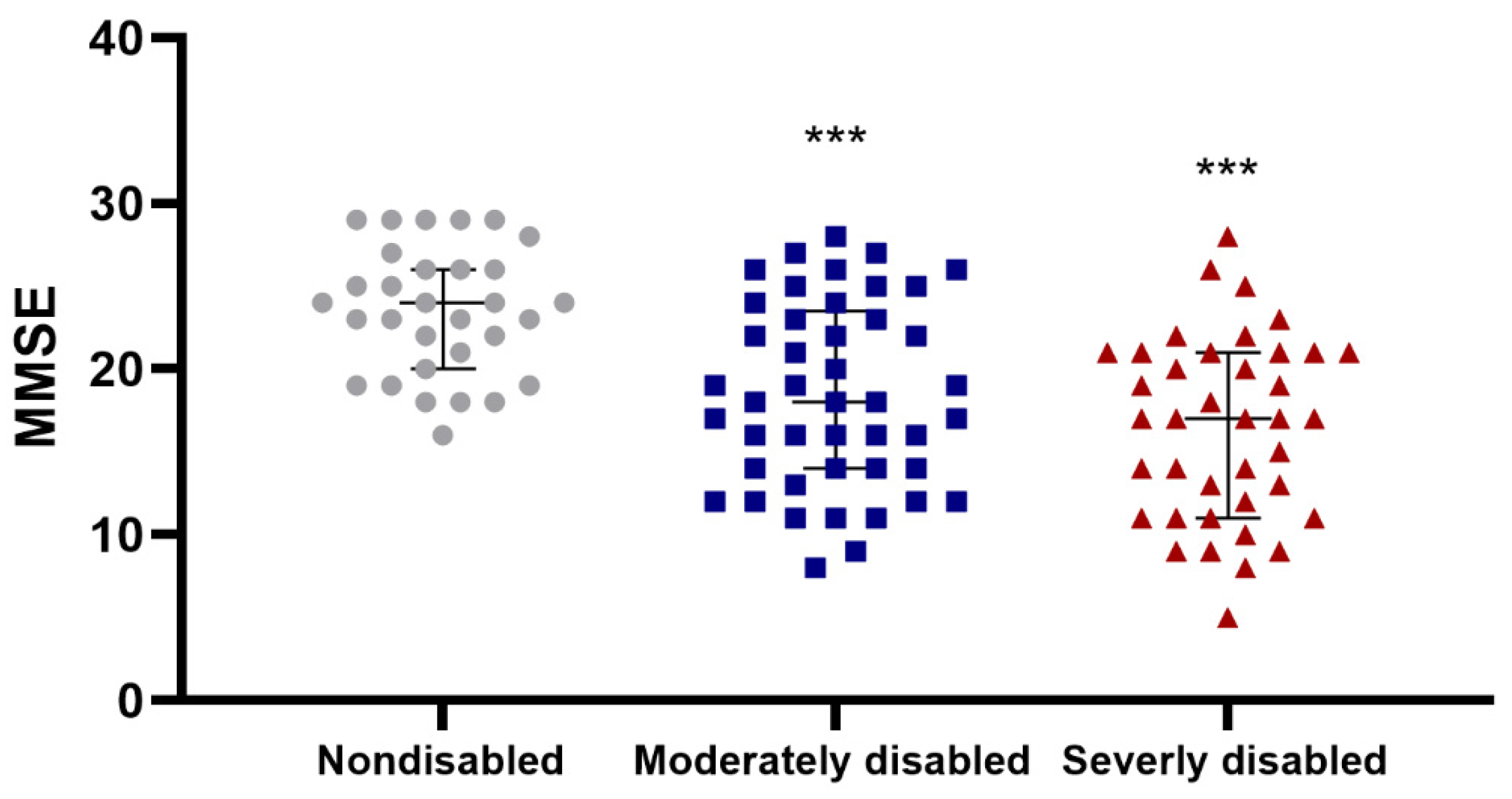
3.1. Association between Cognitive Measurements and Physical Performance in Centenarians
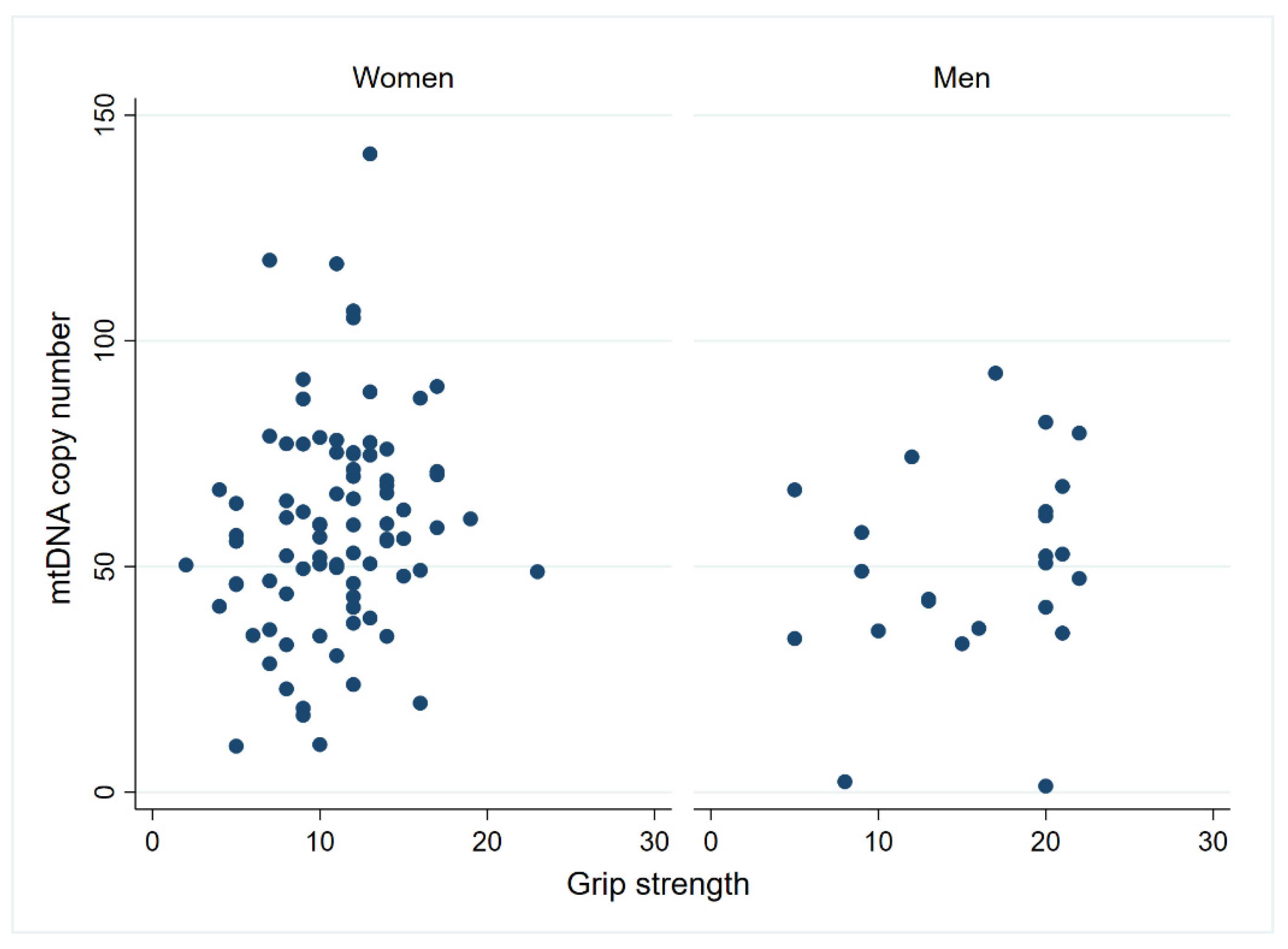
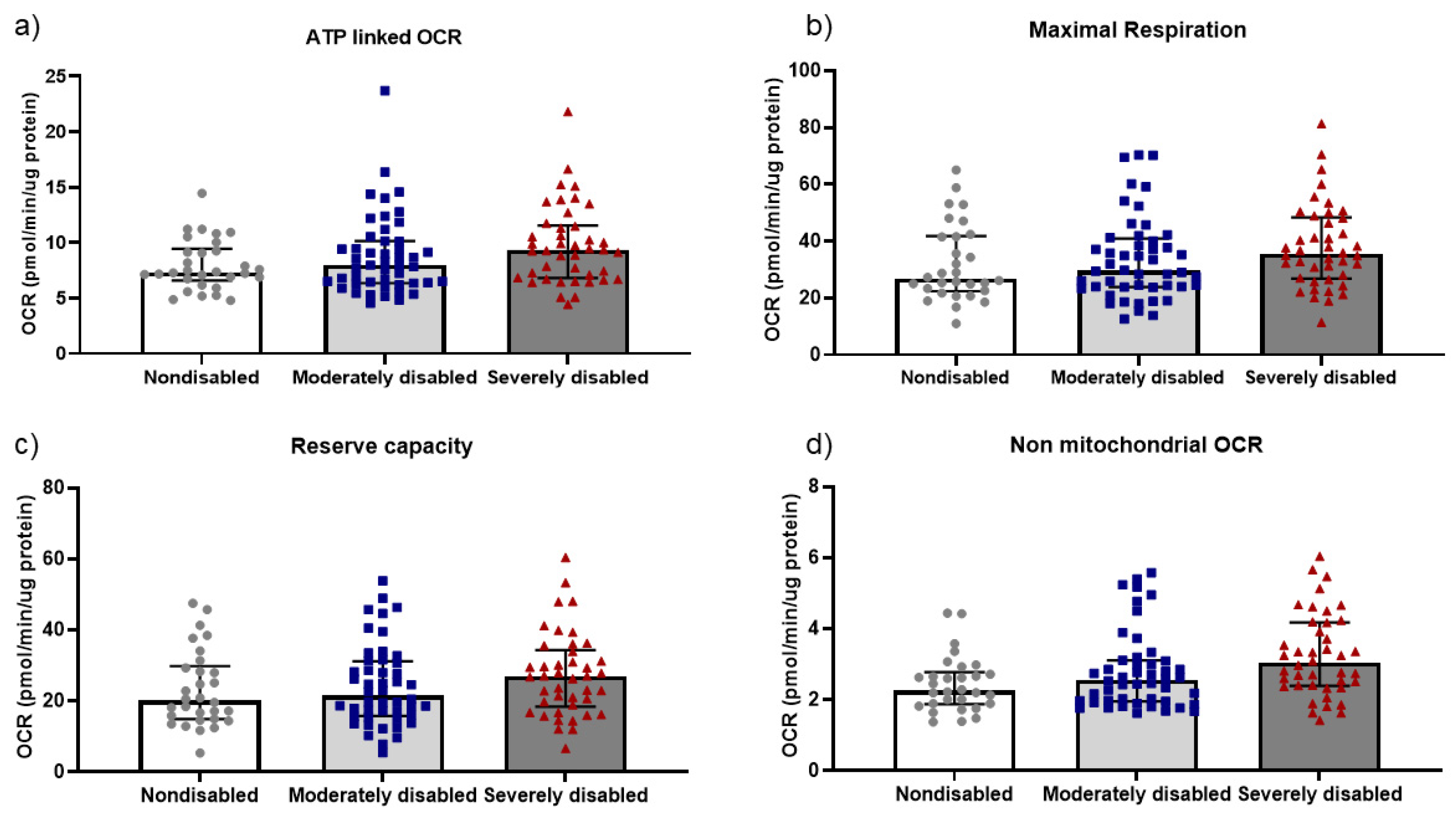
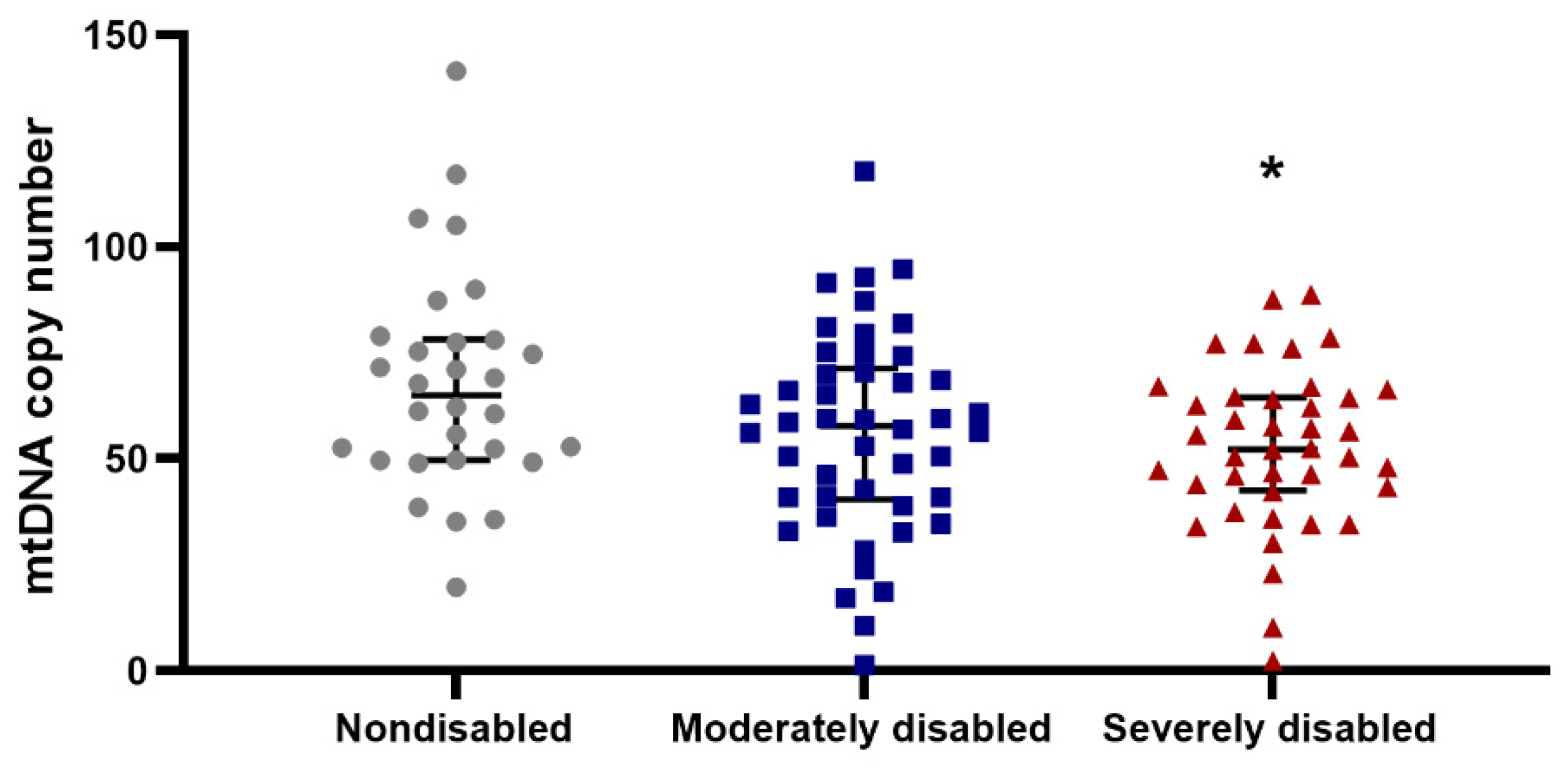
3.2. Mitochondrial Function in PBMCs and Physical Performance in Centenarians
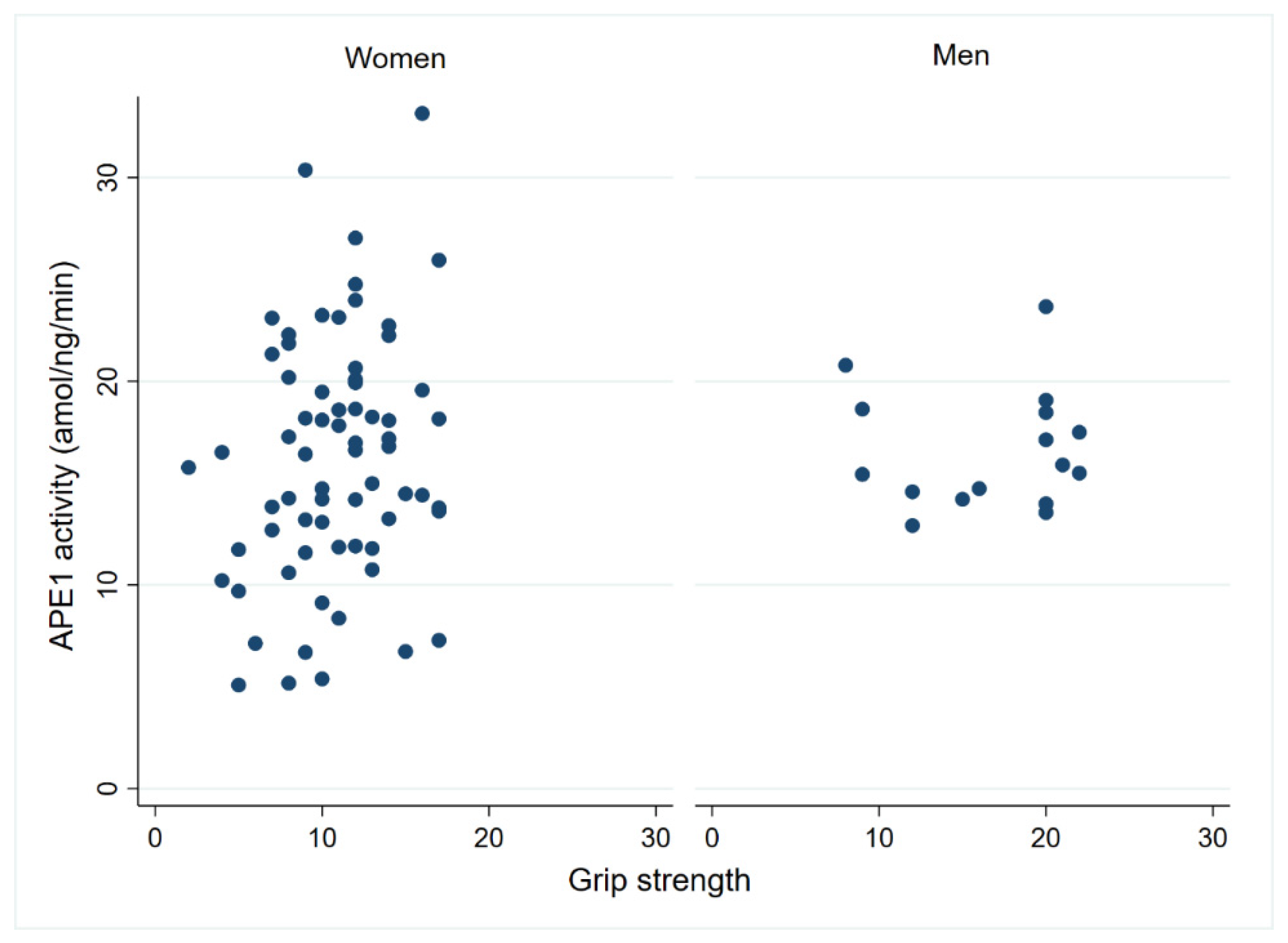
3.3. APE1 Endonuclease Activity in Centenarian PBMCs Is Associated with Physical Performance
3.4. Circulating NAD+/NADH Levels’ Association with Physical Performance
3.5. Physical Abilities and Oxidative Stress Markers in Centenarians
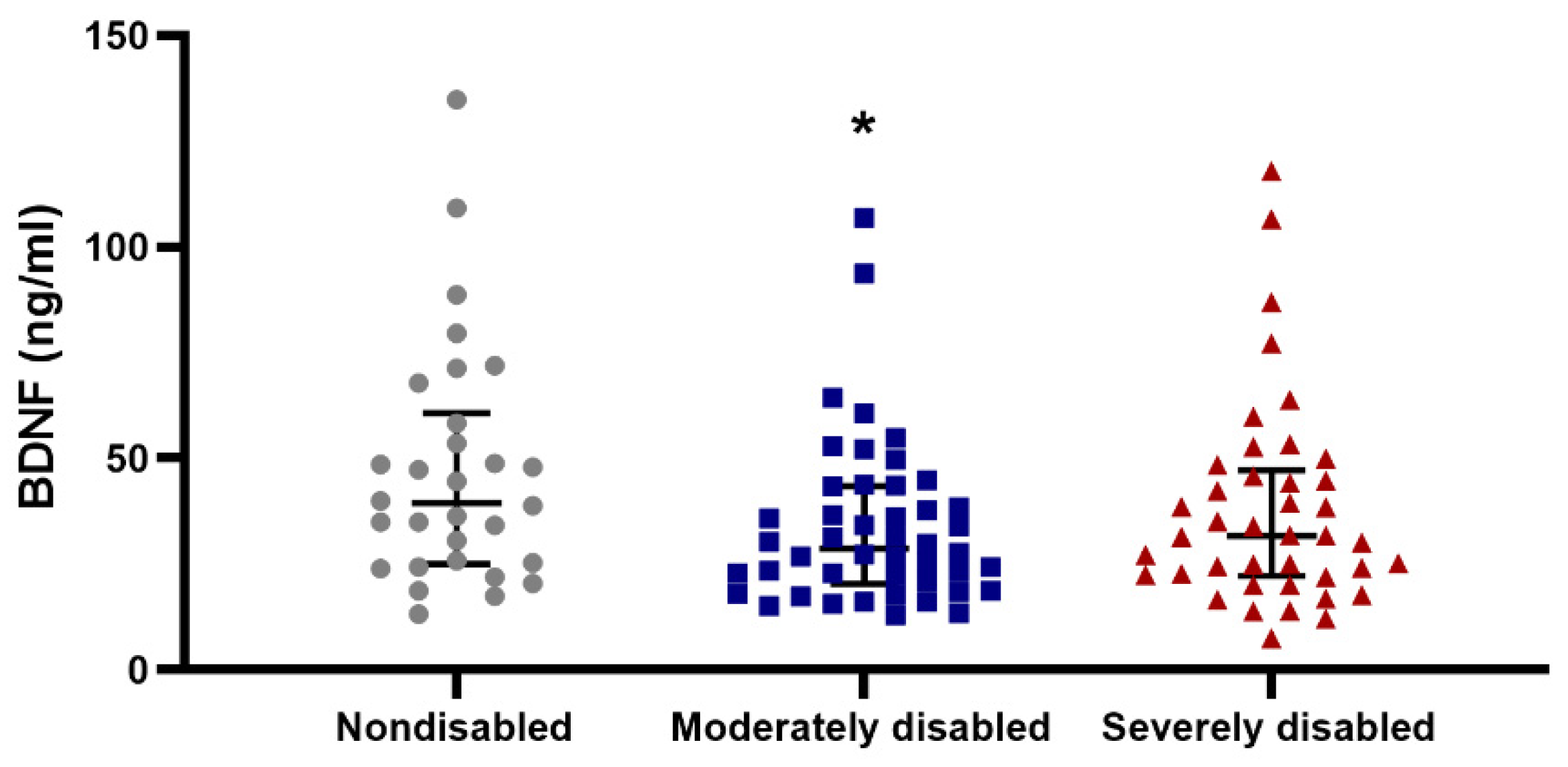
3.6. BDNF Association with Physical Abilities in Centenarians
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barth, E.; Sieber, P.; Stark, H.; Schuster, S. Robustness during Aging—Molecular Biological and Physiological Aspects. Cells 2020, 9, 1862. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Patto, M.; Bueno, B.; Ribeiro, Ó.; Teixeira, L.; Afonso, R.M. Association between handgrip strength, walking, age-related illnesses and cognitive status in a sample of Portuguese centenarians. Eur. Rev. Aging Phys. Act. 2017, 14, 9. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995, 332, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Gow, A.J.; Pattie, A.; Deary, I.J. Lifecourse Activity Participation From Early, Mid, and Later Adulthood as Determinants of Cognitive Aging: The Lothian Birth Cohort 1921. J. Gerontol. B Psychol. Sci. Soc. Sci. 2017, 72, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Frederiksen, H.; Vaupel, J.W.; McGue, M. Age trajectories of genetic variance in physical functioning: A longitudinal study of Danish twins aged 70 years and older. Behav. Genet. 2003, 33, 125–136. [Google Scholar] [CrossRef]
- Robitaille, A.; Muniz, G.; Lindwall, M.; Piccinin, A.M.; Hoffman, L.; Johansson, B.; Hofer, S.M. Physical activity and cognitive functioning in the oldest old: Within- and between-person cognitive activity and psychosocial mediators. Eur. J. Ageing 2014, 11, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Roman, I.; Ferrando, B.; Holst, C.M.; Mengel-From, J.; Rasmussen, S.H.; Thinggaard, M.; Bohr, V.A.; Christensen, K.; Stevnsner, T. Molecular markers of DNA repair and brain metabolism correlate with cognition in centenarians. GeroScience 2022, 44, 103–125. [Google Scholar] [CrossRef]
- Stevnsner, T.; Sanchez-Roman, I. Molecular markers associated with cognitive impairment in centenarians. Aging 2022, 14, 4191–4192. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- López-Gil, L.; Pascual-Ahuir, A.; Proft, M. Genomic Instability and Epigenetic Changes during Aging. Int. J. Mol. Sci. 2023, 24, 14279. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Davin, A.; Ferrari, R.R.; Pansarasa, O. Mitochondria: Between aging, frailty and sarcopenia. Aging 2023, 15, 7863. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.S.; Dunham-Snary, K.J. Blood-based bioenergetics: A liquid biopsy of mitochondrial dysfunction in disease. Trends Endocrinol. Metab. 2023, 34, 554–570. [Google Scholar] [CrossRef]
- Alonso, M.; Zabala, C.; Mansilla, S.; De Brun, L.; Martínez, J.; Garau, M.; Rivas, G.; Acosta, C.; Lens, D.; Cerisola, A.; et al. Blood cell respiration rates and mtDNA copy number: A promising tool for the diagnosis of mitochondrial disease. Mitochondrion 2021, 61, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, D.J.; Bharadwaj, M.S.; Van Horn, C.G.; Kritchevsky, S.B.; Nicklas, B.J.; Molina, A.J.A. Respirometric Profiling of Muscle Mitochondria and Blood Cells Are Associated with Differences in Gait Speed Among Community-Dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Filograna, R.; Mennuni, M.; Alsina, D.; Larsson, N.G. Mitochondrial DNA copy number in human disease: The more the better? FEBS Lett. 2021, 595, 976–1002. [Google Scholar] [CrossRef] [PubMed]
- Mengel-From, J.; Thinggaard, M.; Dalgård, C.; Kyvik, K.O.; Christensen, K.; Christiansen, L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum. Genet. 2014, 133, 1149–1159. [Google Scholar] [CrossRef]
- Ashar, F.N.; Moes, A.; Moore, A.Z.; Grove, M.L.; Chaves, P.H.M.; Coresh, J.; Newman, A.B.; Matteini, A.M.; Bandeen-Roche, K.; Boerwinkle, E.; et al. Association of mitochondrial DNA levels with frailty and all-cause mortality. J. Mol. Med. 2015, 93, 177–186. [Google Scholar] [CrossRef]
- Li, M.; Wilson, D.M., 3rd. Human apurinic/apyrimidinic endonuclease 1. Antioxid. Redox Signal. 2014, 20, 678–707. [Google Scholar] [CrossRef] [PubMed]
- Sykora, P.; Wilson, D.M., 3rd; Bohr, V.A. Base excision repair in the mammalian brain: Implication for age related neurodegeneration. Mech. Ageing Dev. 2013, 134, 440–448. [Google Scholar] [CrossRef]
- Kravvariti, E.; A Ntouros, P.; Vlachogiannis, N.I.; Pappa, M.; Souliotis, V.L.; Sfikakis, P.P. Geriatric Frailty Is Associated With Oxidative Stress, Accumulation, and Defective Repair of DNA Double-Strand Breaks Independently of Age and Comorbidities. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 603–610. [Google Scholar] [CrossRef]
- Xue, Z.; Demple, B. Knockout and Inhibition of Ape1: Roles of Ape1 in Base Excision DNA Repair and Modulation of Gene Expression. Antioxidants 2022, 11, 1817. [Google Scholar] [CrossRef] [PubMed]
- Lillenes, M.S.; Espeseth, T.; Støen, M.; Lundervold, A.J.; Frye, S.A.; Rootwelt, H.; Reinvang, I.; Tønjum, T. DNA base excision repair gene polymorphisms modulate human cognitive performance and decline during normal life span. Mech. Ageing Dev. 2011, 132, 449–458. [Google Scholar] [CrossRef]
- Yang, J.-L.; Lin, Y.-T.; Chuang, P.-C.; Bohr, V.A.; Mattson, M.P. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. NeuroMol. Med. 2014, 16, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Lommatzsch, M.; Zingler, D.; Schuhbaeck, K.; Schloetcke, K.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging 2005, 26, 115–123. [Google Scholar] [CrossRef]
- Wang, R.; Holsinger, R.M.D. Exercise-induced brain-derived neurotrophic factor expression: Therapeutic implications for Alzheimer’s dementia. Ageing Res. Rev. 2018, 48, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Nakano, I.; Kinugawa, S.; Hori, H.; Fukushima, A.; Yokota, T.; Takada, S.; Kakutani, N.; Obata, Y.; Yamanashi, K.; Anzai, T. Serum Brain-Derived Neurotrophic Factor Levels Are Associated with Skeletal Muscle Function but Not with Muscle Mass in Patients with Heart Failure. Int. Heart J. 2020, 61, 96–102. [Google Scholar] [CrossRef]
- Roh, E.; Hwang, S.Y.; Song, E.; Park, M.J.; Yoo, H.J.; Baik, S.H.; Kim, M.; Won, C.W.; Choi, K.M. Association of plasma brain-derived neurotrophic factor levels and frailty in community-dwelling older adults. Sci. Rep. 2022, 12, 18605. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wei, Y.; Lautrup, S.; Yang, B.; Wang, Y.; Cordonnier, S.; Bohr, V.A. NAD+ supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS–STING. Proc. Natl. Acad. Sci. USA 2021, 118, e2011226118. [Google Scholar] [CrossRef]
- Rasmussen, S.H.; Andersen-Ranberg, K.; Thinggaard, M.; Jeune, B.; Skytthe, A.; Christiansen, L.; Vaupel, J.W.; McGue, M.; Christensen, K. Cohort Profile: The 1895, 1905, 1910 and 1915 Danish Birth Cohort Studies—Secular trends in the health and functioning of the very old. Int. J. Epidemiol. 2017, 46, 1746–1746j. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Hjelmborg, J.; Mortensen, J.; Mcgue, M.; Vaupel, J.W.; Christensen, K. Age trajectories of grip strength: Cross-sectional and longitudinal data among 8342 danes aged 46 to 102. Ann. Epidemiol. 2006, 16, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.; Keijzers, G.; Gram, M.; Desler, C.; Bendix, L.; Budtz-Jørgensen, E.; Molbo, D.; Croteau, D.L.; Osler, M.; Stevnsner, T.; et al. Relationships between human vitality and mitochondrial respiratory parameters, reactive oxygen species production and dNTP levels in peripheral blood mononuclear cells. Aging 2013, 5, 850–864. [Google Scholar] [CrossRef] [PubMed]
- Ferrick, D.A.; Neilson, A.; Beeson, C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov. Today 2008, 13, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, D.J.; Bharadwaj, M.S.; Jorgensen, M.J.; Register, T.C.; Molina, A.J. Blood cell respirometry is associated with skeletal and cardiac muscle bioenergetics: Implications for a minimally invasive biomarker of mitochondrial health. Redox Biol. 2016, 10, 65–77. [Google Scholar] [CrossRef]
- Ruas, J.S.; Siqueira-Santos, E.S.; Amigo, I.; Rodrigues-Silva, E.; Kowaltowski, A.J.; Castilho, R.F. Underestimation of the Maximal Capacity of the Mitochondrial Electron Transport System in Oligomycin-Treated Cells. PLoS ONE 2016, 11, e0150967. [Google Scholar] [CrossRef] [PubMed]
- Paz-Elizur, T. DNA repair activity for oxidative damage and risk of lung cancer. J. Natl. Cancer Inst. 2003, 95, 1312–1319. [Google Scholar] [CrossRef]
- Paz-Elizur, T.; Elinger, D.; Leitner-Dagan, Y.; Blumenstein, S.; Krupsky, M.; Berrebi, A.; Schechtman, E.; Livneh, Z. Development of an enzymatic DNA repair assay for molecular epidemiology studies: Distribution of OGG activity in healthy individuals. DNA Repair 2007, 6, 45–60. [Google Scholar] [CrossRef]
- Sevilya, Z.; Leitner-Dagan, Y.; Pinchev, M.; Kremer, R.; Elinger, D.; Lejbkowicz, F.; Rennert, H.S.; Freedman, L.S.; Rennert, G.; Paz-Elizur, T.; et al. Development of APE1 enzymatic DNA repair assays: Low APE1 activity is associated with increase lung cancer risk. Carcinogenesis 2015, 36, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Tangen, G.G.; Robinson, H.S. Measuring physical performance in highly active older adults: Associations with age and gender? Aging Clin. Exp. Res. 2020, 32, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Portegijs, E.; Karavirta, L.; Saajanaho, M.; Rantalainen, T.; Rantanen, T. Assessing physical performance and physical activity in large population-based aging studies: Home-based assessments or visits to the research center? BMC Public Health 2019, 19, 1570. [Google Scholar] [CrossRef]
- Rasmussen, S.H.; Thinggaard, M.; Højgaard, M.B.; Jeune, B.; Christensen, K.; Andersen-Ranberg, K. Improvement in Activities of Daily Living Among Danish Centenarians?—A Comparative Study of Two Centenarian Cohorts Born 20 Years Apart. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, M.; Nygaard, M.; Tan, Q.; Jeune, B.; Semkovska, M.; Christensen, K.; Thinggaard, M.; Mengel-From, J. Cognitively high-performing oldest old individuals are physically active and have strong motor skills—A study of the Danish 1905 and 1915 birth cohorts. Arch. Gerontol. Geriatr. 2024, 122, 105398. [Google Scholar] [CrossRef] [PubMed]
- Sgarbi, G.; Matarrese, P.; Pinti, M.; Lanzarini, C.; Ascione, B.; Gibellini, L.; Dika, E.; Patrizi, A.; Tommasino, C.; Capri, M.; et al. Mitochondria hyperfusion and elevated autophagic activity are key mechanisms for cellular bioenergetic preservation in centenarians. Aging (Albany NY) 2014, 6, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Villani, G.; Mazzucchelli, F.; Bresolin, N.; Papa, S.; Attardi, G. Marked aging-related decline in efficiency of oxidative phosphorylation in human skin fibroblasts. FASEB J. 2003, 17, 1706–1708. [Google Scholar] [CrossRef]
- Grevendonk, L.; Connell, N.J.; McCrum, C.; Fealy, C.E.; Bilet, L.; Bruls, Y.M.H.; Mevenkamp, J.; Schrauwen-Hinderling, V.B.; Jörgensen, J.A.; Moonen-Kornips, E.; et al. Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function. Nat. Commun. 2021, 12, 4773. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Huang, J.; Chu, X.; Qian, D.; Wang, Z.; Sun, X.; Chen, F.; Xu, J.; Li, S.; et al. Blood biomarkers and functional disability among extremely longevous individuals: A population-based study. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 623–627. [Google Scholar] [CrossRef]
- Rajan, K.B.; Hebert, L.E.; Scherr, P.A.; de Leon, C.F.M.; Evans, D.A. Disability in basic and instrumental activities of daily living is associated with faster rate of decline in cognitive function of older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 624–630. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, S.; Liu, Y.; Gang, X.; Wang, G. Grip Strength and the Risk of Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis of Longitudinal Cohort Studies. Front. Aging Neurosci. 2021, 13, 625551. [Google Scholar] [CrossRef] [PubMed]
- Creavin, S.T.; Wisniewski, S.; Noel-Storr, A.H.; Trevelyan, C.M.; Hampton, T.; Rayment, D.; Thom, V.M.; Nash, K.J.; Elhamoui, H.; Milligan, R.; et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst. Rev. 2016, 13, CD011145. [Google Scholar] [CrossRef] [PubMed]
- McGue, M.; Christensen, K. The heritability of cognitive functioning in very old adults: Evidence from Danish twins aged 75 years and older. Psychol. Aging 2001, 16, 272. [Google Scholar] [CrossRef] [PubMed]
- Loesch, D.Z.; Annesley, S.J.; Trost, N.; Bui, M.Q.; Lay, S.T.; Storey, E.; Fisher, P.R. Novel Blood Biomarkers Are Associated with White Matter Lesions in Fragile X-Associated Tremor/Ataxia Syndrome. Neurodegener. Dis. 2016, 17, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Scheibye-Knudsen, M.; Ramamoorthy, M.; Sykora, P.; Maynard, S.; Lin, P.-C.; Minor, R.K.; Wilson, D.M., 3rd; Cooper, M.; Spencer, R.; de Cabo, R.; et al. Cockayne syndrome group B protein prevents the accumulation of damaged mitochondria by promoting mitochondrial autophagy. J. Exp. Med. 2012, 209, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Tavallaie, M.; Voshtani, R.; Deng, X.; Qiao, Y.; Jiang, F.; Collman, J.P.; Fu, L. Moderation of mitochondrial respiration mitigates metabolic syndrome of aging. Proc. Natl. Acad. Sci. USA 2020, 117, 9840–9850. [Google Scholar] [CrossRef] [PubMed]
- Herpich, C.; Franz, K.; Klaus, S.; Müller-Werdan, U.; Ost, M.; Norman, K. Age-related fatigue is associated with reduced mitochondrial function in peripheral blood mononuclear cells. Exp. Gerontol. 2021, 144, 111177. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, P.Y.; Jenkins, G.D.; Welkie, B.P.; McDonnell, S.K.; Evans, J.M.; Cerhan, J.R.; E Olson, J.; Thibodeau, S.N.; Cicek, M.S.; Ryu, E. Association of mitochondrial DNA copy number with self-rated health status. Appl. Clin. Genet. 2018, 11, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Silaidos, C.; Pilatus, U.; Grewal, R.; Matura, S.; Lienerth, B.; Pantel, J.; Eckert, G.P. Sex-associated differences in mitochondrial function in human peripheral blood mononuclear cells (PBMCs) and brain. Biol. Sex Differ. 2018, 9, 34. [Google Scholar] [CrossRef]
- Junker, A.; Wang, J.; Gouspillou, G.; Ehinger, J.K.; Elmér, E.; Sjövall, F.; Fisher-Wellman, K.H.; Neufer, P.D.; Molina, A.J.A.; Ferrucci, L.; et al. Human studies of mitochondrial biology demonstrate an overall lack of binary sex differences: A multivariate meta-analysis. FASEB J. 2022, 36, e22146. [Google Scholar] [CrossRef]
- Tell, G.; Quadrifoglio, F.; Tiribelli, C.; Kelley, M.R. The many functions of APE1/Ref-1: Not only a DNA repair enzyme. Antioxid. Redox Signal. 2009, 11, 601–619. [Google Scholar] [CrossRef]
- Howard, C.; Ferrucci, L.; Sun, K.; Fried, L.P.; Walston, J.; Varadhan, R.; Guralnik, J.M.; Semba, R.D. Oxidative protein damage is associated with poor grip strength among older women living in the community. J. Appl. Physiol. (1985) 2007, 103, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Tsuji, M.; Oguchi, T.; Kasuga, K.; Kimura, A.; Futamura, A.; Ono, K. Serum BDNF as a Potential Biomarker of Alzheimer’s Disease: Verification through Assessment of Serum, Cerebrospinal Fluid, and Medial Temporal Lobe Atrophy. Front. Neurol. 2021, 12, 653267. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef]
- Nakahashi, T.; Fujimura, H.; Altar, C.; Li, J.; Kambayashi, J.-I.; Tandon, N.N.; Sun, B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000, 470, 113–117. [Google Scholar] [CrossRef]
- Fujimura, H.; Chen, R.; Nakamura, T.; Nakahashi, T.; Kambayashi, J.-I.; Sun, B.; Altar, C.; Tandon, N.N. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 2002, 87, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Iino, N.; Koda, R.; Narita, I.; Kaneko, Y. Brain-derived neurotrophic factor is associated with sarcopenia and frailty in Japanese hemodialysis patients. Geriatr. Gerontol. Int. 2021, 21, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lautrup, S.; Holst, C.M.; Yde, A.; Asmussen, S.; Thinggaard, V.; Larsen, K.; Laursen, L.S.; Richner, M.; Vægter, C.B.; Prieto, G.A.; et al. The role of aging and brain-derived neurotrophic factor signaling in expression of base excision repair genes in the human brain. Aging Cell 2023, 22, e13905. [Google Scholar] [CrossRef]
- Janssens, G.E.; Grevendonk, L.; Perez, R.Z.; Schomakers, B.V.; Bosch, J.d.V.-V.D.; Geurts, J.M.W.; van Weeghel, M.; Schrauwen, P.; Houtkooper, R.H.; Hoeks, J. Healthy aging and muscle function are positively associated with NAD+ abundance in humans. Nat. Aging 2022, 2, 254–263. [Google Scholar] [CrossRef]
- Elhassan, Y.S.; Kluckova, K.; Fletcher, R.S.; Schmidt, M.S.; Garten, A.; Doig, C.L.; Cartwright, D.M.; Oakey, L.; Burley, C.V.; Jenkinson, N.; et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Rep. 2019, 28, 1717–1728.e6. [Google Scholar] [CrossRef] [PubMed]
- Lautrup, S.; Hou, Y.; Fang, E.F.; Bohr, V.A. Roles of NAD+ in Health and Aging. Cold Spring Harb. Perspect. Med. 2024, 14, a041193. [Google Scholar] [CrossRef] [PubMed]
- Migliavacca, E.; Tay, S.K.H.; Patel, H.P.; Sonntag, T.; Civiletto, G.; McFarlane, C.; Forrester, T.; Barton, S.J.; Leow, M.K.; Antoun, E.; et al. Mitochondrial oxidative capacity and NAD+ biosynthesis are reduced in human sarcopenia across ethnicities. Nat. Commun. 2019, 10, 5808. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef] [PubMed]

| Centenarians | |
|---|---|
| Population number, n | 185 |
| Blood samples, n (%) | 135 (73%) |
| Age, mean (SD) | 100.1 (0.04) |
| Sex frequency, n (%) | |
| Women | 105 (77.8%) |
| Men | 30 (22.2%) |
| ADL disability score | |
| Completion frequency, n (%) * | 135 (100%) |
| Score range | 1 (Nondisabled), 2 (Moderately disabled), 3 (Severely disabled) |
| Nondisabled | |
| n (%) | 37 (27.4%) |
| Moderately disabled | |
| n (%) | 51 (37.8%) |
| Severely disabled | |
| n (%) | 47 (34.8%) |
| Grip strength | |
| Completion frequency, n (%) * | 126 (93.3%) |
| Score range | 2–30 |
| Mean (SD) | 12.38 (5.07) |
| Median (IQR) | 12 (6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Roman, I.; Ferrando, B.; Myrup Holst, C.; Mengel-From, J.; Hoei Rasmussen, S.; Thinggaard, M.; Bohr, V.A.; Christensen, K.; Stevnsner, T. Markers of Mitochondrial Function and DNA Repair Associated with Physical Function in Centenarians. Biomolecules 2024, 14, 909. https://doi.org/10.3390/biom14080909
Sanchez-Roman I, Ferrando B, Myrup Holst C, Mengel-From J, Hoei Rasmussen S, Thinggaard M, Bohr VA, Christensen K, Stevnsner T. Markers of Mitochondrial Function and DNA Repair Associated with Physical Function in Centenarians. Biomolecules. 2024; 14(8):909. https://doi.org/10.3390/biom14080909
Chicago/Turabian StyleSanchez-Roman, Ines, Beatriz Ferrando, Camilla Myrup Holst, Jonas Mengel-From, Signe Hoei Rasmussen, Mikael Thinggaard, Vilhelm A. Bohr, Kaare Christensen, and Tinna Stevnsner. 2024. "Markers of Mitochondrial Function and DNA Repair Associated with Physical Function in Centenarians" Biomolecules 14, no. 8: 909. https://doi.org/10.3390/biom14080909





