Effects of Synthetic Toll-Like Receptor 9 Ligand Molecules on Pulpal Immunomodulatory Response and Repair after Injuries
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vivo Experimental Procedures
2.1.1. Animals
2.1.2. Tooth Replantation
2.2. Tissue Preparation
2.3. Immunohistochemical Procedures
2.4. Cell Counting and Statistical Analysis
3. Results
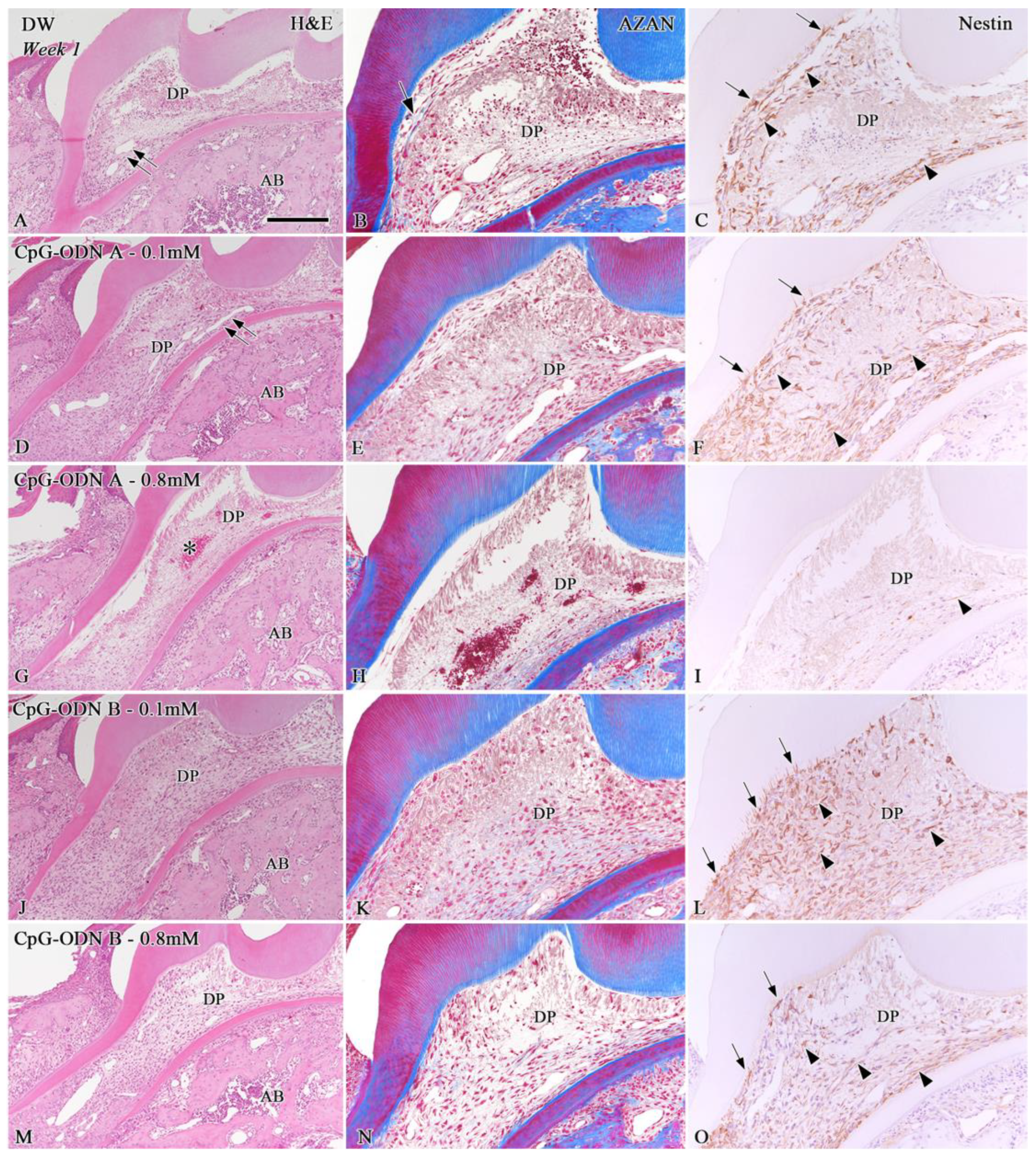
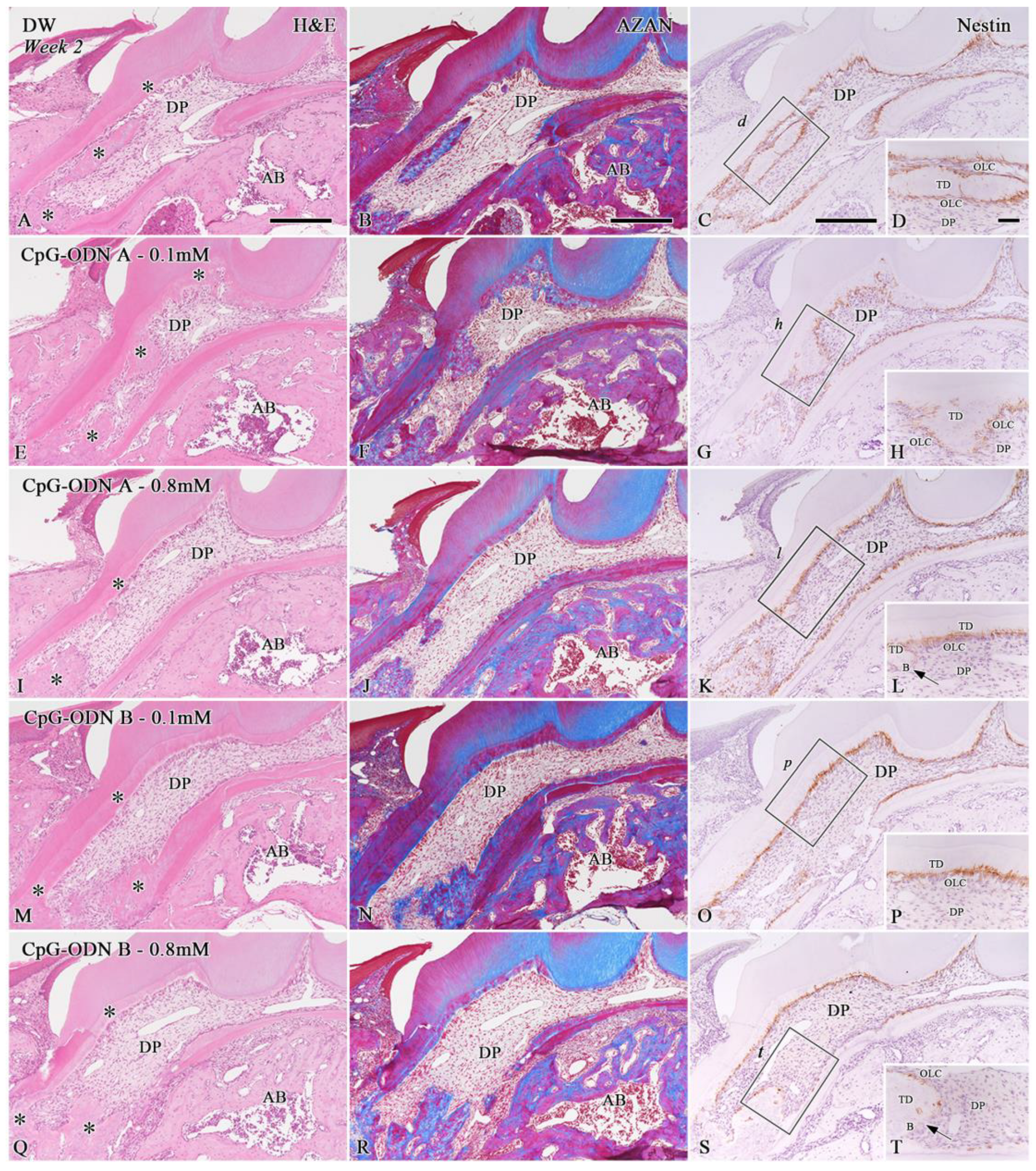
3.1. Histological Evaluation of the Dental Pulp Healing Process by H&E and AZAN Staining and Nestin Immunohistochemistry
3.2. Analysis of Cell Proliferation in the Pulpal Tissue by Ki-67 Immunohistochemistry
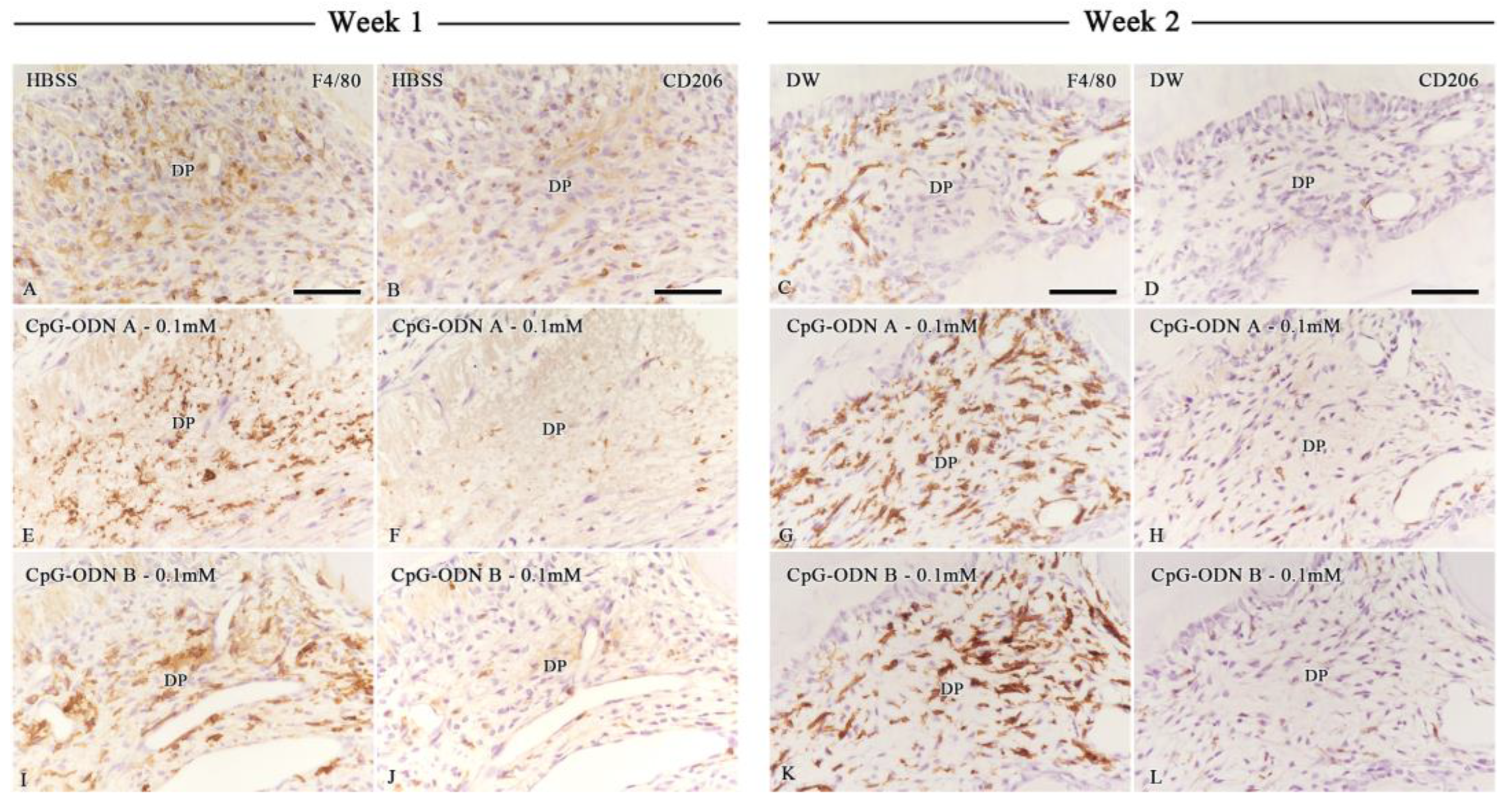
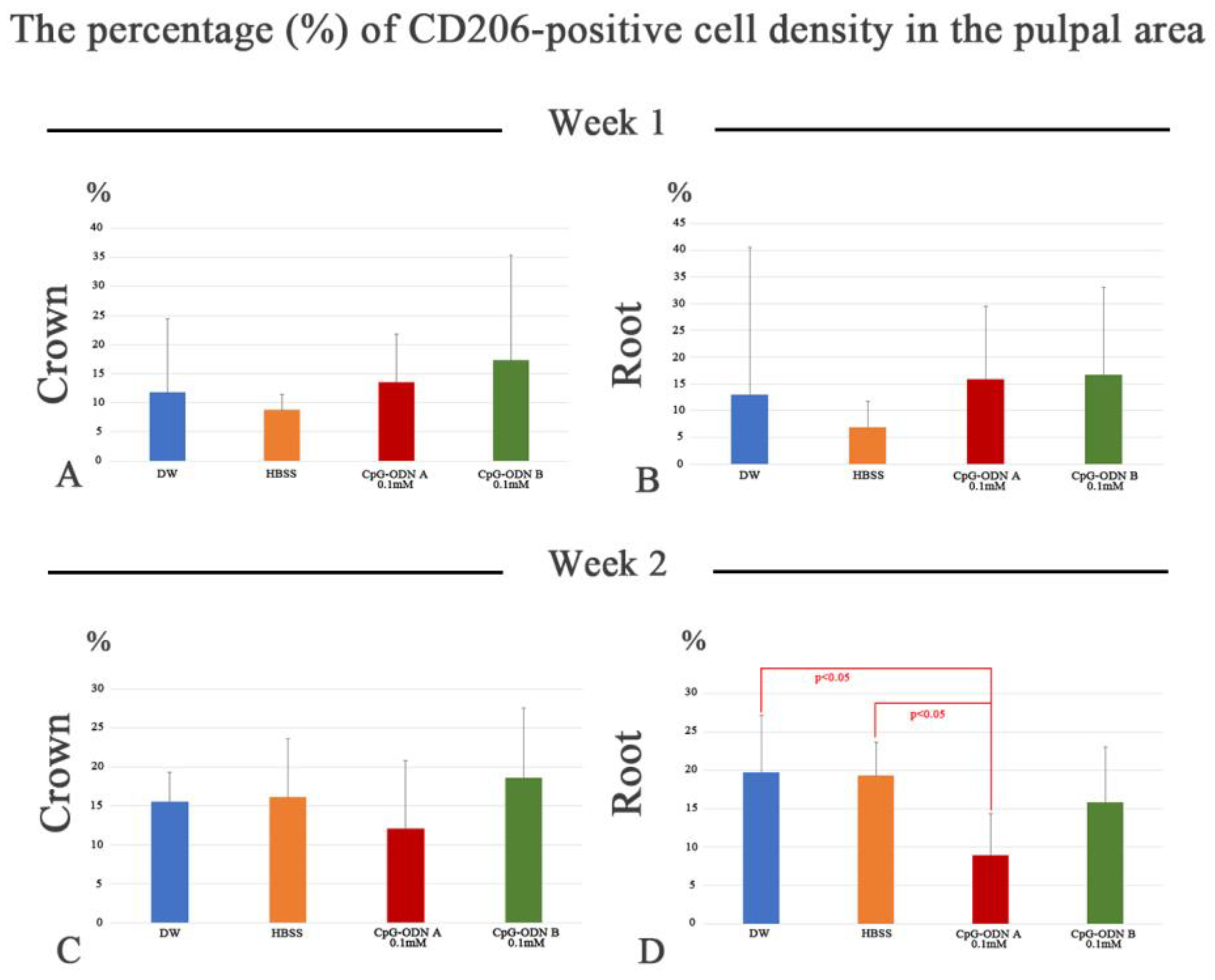
3.3. Assessment of Macrophage Activity in the Dental Pulp by F4/80 and CD206 Immunohistochemistry
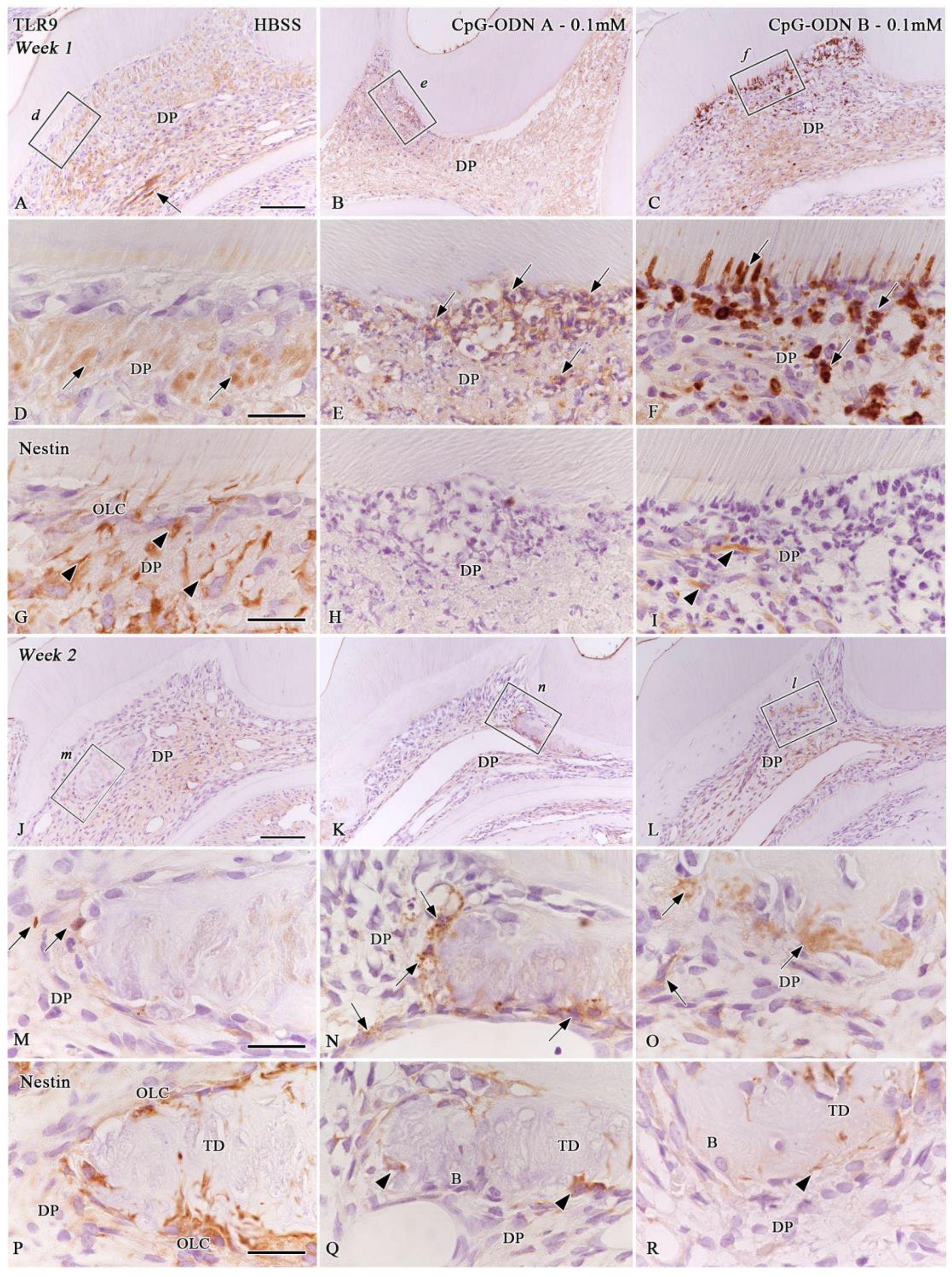
3.4. TLR9 Immunoexpression in the Afflicted Dental Pulp
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M.; Yi, A.K.; Matson, S.; Waldschmidt, T.J.; Bishop, G.A.; Teasdale, R.; Koretzky, G.A.; Klinman, D.M. Cpg motifs in bacterial-DNA trigger direct B-cell activation. Nature 1995, 374, 546–549. [Google Scholar] [CrossRef]
- Bauer, S.; Kirschning, C.J.; Häcker, H.; Redecke, V.; Hausmann, S.; Akira, S.; Wagner, H.; Lipford, G.B. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 2001, 98, 9237–9242. [Google Scholar] [CrossRef]
- Bauer, S.; Wagner, H. Bacterial CpG-DNA licenses TLR9. Toll-Like Recept. Fam. Memb. Their Ligands 2002, 270, 145–154. [Google Scholar]
- Klinman, D.M.; Currie, D.; Gursel, I.; Verthelyi, D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol. Rev. 2004, 199, 201–216. [Google Scholar] [CrossRef]
- Li, M.Q.; Yao, H.; Yi, K.; Lao, Y.H.; Shao, D.; Tao, Y. Emerging nanoparticle platforms for CpG oligonucleotide delivery. Biomater. Sci. 2024, 12, 2203–2228. [Google Scholar] [CrossRef] [PubMed]
- Weiner, G.J. The immunobiology and clinical potential of immunostimulatory CpG oligodeoxynucleotides. J. Leukoc. Biol. 2000, 68, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, F.M.; Kahler, B. Pulpal response after acute dental injury in the permanent dentition: Clinical implications—A review. J. Endod. 2015, 41, 299–308. [Google Scholar] [CrossRef]
- Andreasen, J.O.; Andreasen, F.M.; Andersson, L. Textbook and Color Atlas of Traumatic Injuries to the Teeth, 5th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019. [Google Scholar]
- Hargreaves, K.M.; Goodis, H.E.; Tay, F.R. Seltzer and Bender’s Dental Pulp, 2nd ed.; Quintessence Publishing: Batavia, IL, USA, 2012; 501p. [Google Scholar]
- Pääkkönen, V.; Rusanen, P.; Hagström, J.; Tjäderhane, L. Mature human odontoblasts express virus-recognizing toll-like receptors. Int. Endod. J. 2014, 47, 934–941. [Google Scholar] [CrossRef]
- Yumoto, H.; Hirao, K.; Hosokawa, Y.; Kuramoto, H.; Takegawa, D.; Nakanishi, T.; Matsuo, T. The roles of odontoblasts in dental pulp innate immunity. Jpn. Dent. Sci. Rev. 2018, 54, 105–117. [Google Scholar] [CrossRef]
- Cooper, P.R.; Takahashi, Y.; Graham, L.W.; Simon, S.; Imazato, S.; Smith, A.J. Inflammation-regeneration interplay in the dentine-pulp complex. J. Dent. 2010, 38, 687–697. [Google Scholar] [CrossRef]
- Jang, J.H.; Shin, H.W.; Lee, J.M.; Lee, H.W.; Kim, E.C.; Park, S.H. An overview of pathogen recognition receptors for innate immunity in dental pulp. Mediat. Inflamm. 2015, 2015, 794143. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Q.L.; Huang, P.; Yu, Q.; Wang, Z.H.; Cooper, P.R.; Smith, A.J.; He, W. CpG ODN-induced matrix metalloproteinase-13 expression is mediated via activation of the ERK and NF-kappaB signalling pathways in odontoblast cells. Int. Endod. J. 2013, 46, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Durand, S.H.; Flacher, V.; Roméas, A.; Carrouel, F.; Colomb, E.; Vincent, C.; Magloire, H.; Couble, M.L.; Bleicher, F.; Staquet, M.J.; et al. Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. J. Immunol. 2006, 176, 2880–2887. [Google Scholar] [CrossRef]
- Staquet, M.J.; Durand, S.H.; Colomb, E.; Roméas, A.; Vincent, C.; Bleicher, F.; Lebecque, S.; Farges, J.C. Different roles of odontoblasts and fibroblasts in immunity. J. Dent. Res. 2008, 87, 256–261. [Google Scholar] [CrossRef]
- He, W.X.; Yu, Q.; Zhou, Z.; Wang, P. CpG oligonucleotides induce an immune response of odontoblasts through the TLR9, MyD88 and NF-κB pathways. Biochem. Biophys. Res. Commun. 2010, 399, 274–278. [Google Scholar] [CrossRef] [PubMed]
- He, W.X.; Zhang, Y.; Zhang, J.; Yu, Q.; Wang, P.; Wang, Z.; Smith, A.J. Cytidine-phosphate-guanosine oligonucleotides induce interleukin-8 production through activation of TLR9, MyD88, NF-κB, and ERK pathways in odontoblast cells. J. Endod. 2012, 38, 780–785. [Google Scholar] [CrossRef]
- Quispe-Salcedo, A.; Ida-Yonemochi, H.; Ohshima, H. Use of a triple antibiotic solution affects the healing process of intentionally delayed replanted teeth in mice. J. Oral Biosci. 2013, 55, 91–100. [Google Scholar] [CrossRef]
- Quispe-Salcedo, A.; Ida-Yonemochi, H.; Ohshima, H. Effects of a triple antibiotic solution on pulpal dynamics after intentionally delayed tooth replantation in mice. J. Endod. 2014, 40, 1566–1572. [Google Scholar] [CrossRef]
- Sano, H.; Nakakura-Ohshima, K.; Quispe-Salcedo, A.; Okada, Y.; Sato, T.; Ohshima, H. Early revascularization activates quiescent dental pulp stem cells following tooth replantation in mice. Regen. Ther. 2023, 24, 582–591. [Google Scholar] [CrossRef]
- Suzuki-Barrera, K.; Makishi, S.; Nakatomi, M.; Saito, K.; Ida-Yonemochi, H.; Ohshima, H. Role of osteopontin in the process of pulpal healing following tooth replantation in mice. Regen. Ther. 2022, 21, 460–468. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthi, I.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 2012, 41, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, K.; Yamazaki, T.; Sugiyama, Y.; Tsukakoshi, K.; Tsugawa, W.; Sode, K.; Ikebukuro, K. G-quadruplex structure improves the immunostimulatory effects of CpG oligonucleotides. Nucleic Acid Ther. 2019, 29, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.A.; Sterin, E.H.; Day, E.S. Membrane-wrapped nanoparticles for nucleic acid delivery. Biomater. Sci. 2022, 10, 4378–4391. [Google Scholar] [CrossRef] [PubMed]
- Niger, S.; Shimosato, T. Cooperation of oligodeoxynucleotides and synthetic molecules as enhanced immune modulators. Front. Nutr. 2019, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [Google Scholar] [CrossRef]
- Suwarti, S.; Yamazaki, T.; Svetlana, C.; Hanagata, N. Recognition of CpG oligodeoxynucleotides by human Toll-like receptor 9 and subsequent cytokine induction. Biochem. Biophys. Res. Commun. 2013, 430, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.J.; Yamazaki, T.; Nishida, Y.; Hanagata, N. Nuclease-resistant immunostimulatory phosphodiester CpG oligodeoxynucleotides as human Toll-like receptor 9 agonists. BMC Biotechnol. 2011, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.Z.; Hu, Y.; Huang, S.; Ruiz, S.; Kawai, T.; Bai, Y.; Han, X. CpG ODN/mangiferin dual delivery through calcium alginate hydrogels inhibits immune-mediated osteoclastogenesis and promotes alveolar bone regeneration in mice. Biology 2023, 12, 976. [Google Scholar] [CrossRef]
- Chang, E.; Kobayashi, R.; Hagiwara-Hamano, M.; Kurita-Ochiai, T.; Komiya, M. Sublingual immunization with recombinant GroEL plus CpG-ODN inhibits Porphyromonas gingivalis-induced inflammation and alveolar bone loss. Mol. Oral Microbiol. 2022, 37, 31–41. [Google Scholar] [CrossRef]
- Bai, G.H.; Yu, H.; Guan, X.; Zeng, F.; Liu, X.; Chen, B.; Liu, J.; Tian, Y. CpG immunostimulatory oligodeoxynucleotide 1826 as a novel nasal ODN adjuvant enhanced the protective efficacy of the periodontitis gene vaccine in a periodontitis model in SD rats. BMC Oral Health 2021, 21, 403. [Google Scholar] [CrossRef]
- Klinman, D.M. Adjuvant activity of CpG oligodeoxynucleotides. Int. Rev. Immunol. 2006, 25, 135–154. [Google Scholar] [CrossRef]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef]
- Kadowaki, N.; Ho, S.; Antonenko, S.; Malefyt, R.W.; Kastelein, R.A.; Bazan, F.; Liu, Y.J. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001, 194, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Oxenius, A.; Martinic, M.M.; Hengartner, H.; Klenerman, P. CpG-containing oligonucleotides are efficient adjuvants for induction of protective antiviral immune responses with T-cell peptide vaccines. J. Virol. 1999, 73, 4120–4126. [Google Scholar] [CrossRef]
- Li, J.; Ren, H.; Zhang, Z.; Zhang, J.; Wei, F. Macrophage M2 polarization promotes pulpal inflammation resolution during orthodontic tooth movement. J. Cell. Mol. Med. 2024, 28, e18350. [Google Scholar] [CrossRef]
- Baldeon-Gutierrez, R.; Ohkura, N.; Yoshiba, K.; Yoshiba, N.; Tohma, A.; Takeuchi, R.; Belal, R.S.; Edanami, N.; Takahara, S.; Gomez-Kasimoto, S.; et al. Wound-healing processes after pulpotomy in the pulp tissue of type 1 diabetes mellitus model rats. J. Endod. 2024, 50, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Okamoto, M.; Watanabe, M.; Matsumoto, S.; Moriyama, K.; Komichi, S.; Ali, M.; Matayoshi, S.; Nomura, R.; Nakano, K.; et al. Development of rat caries-induced pulpitis model for vital pulp therapy. J. Dent. Res. 2023, 102, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, M.; Yoshida, S.; Itoyama, T.; Tomokiyo, A.; Hamano, S.; Hasegawa, D.; Sugii, H.; Kaneko, H.; Sugiura, R.; Maeda, H. Involvement of M1/M2 macrophage polarization in reparative dentin formation. Life 2022, 12, 1812. [Google Scholar] [CrossRef]
- Zhou, J.; Ou, M.H.; Wei, X.L.; Lan, B.Y.; Chen, W.J.; Song, S.J.; Chen, W.X. The role of different macrophages-derived conditioned media in dental pulp tissue regeneration. Tissue Cell 2022, 79, 101944. [Google Scholar] [CrossRef]
- Chen, Y.N.; Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 339–340. [Google Scholar] [CrossRef]
- Wu, J.H.; Su, W.; Powner, M.B.; Liu, J.; Copland, D.A.; Fruttiger, M.; Madeddu, P.; Dick, A.D.; Liu, L. Pleiotropic action of CpG-ODN on endothelium and macrophages attenuates angiogenesis through distinct pathways. Sci. Rep. 2016, 6, 31873. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.G.; Yang, S.; Zhao, Z.; Feng, L.; Sheng, J.; Deng, R.; Wang, B.; He, Y.; Luo, D.; Chen, M.; et al. Smart tumor cell-derived DNA nano-tree assembly for on-demand macrophages reprogramming. Adv. Sci. 2024, 11, 2307188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Wang, Y.; Jiang, K.; Gao, C.; Zhang, P.; Xie, Y.; Wang, B.; Zhao, Y.; Xiao, H.; et al. Regulating the surface topography of CpG nanoadjuvants coordination-driven self-assembly for enhanced tumor immunotherapy. Nanoscale Adv. 2023, 5, 4758–4769. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Dou, W.; Zhang, Y.; Qi, M. Targeted ferritin nanoparticle encapsulating CpG oligodeoxynucleotides induces tumor-associated macrophage M2 phenotype polarization into M1 phenotype and inhibits tumor growth. Nanoscale 2020, 12, 22268–22280. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Salcedo, A.; Ohshima, H. The role of dendritic cells during physiological and pathological dentinogenesis. J. Clin. Med. 2021, 10, 3348. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Ohshima, H. Biological characteristics of dental pulp stem cells and their potential use in regenerative medicine. J. Oral Biosci. 2022, 64, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.L.; Falkler, W.A., Jr.; Siegel, M.A. A study of T and B cells in pulpal pathosis. J. Endod. 1989, 15, 20–26. [Google Scholar] [CrossRef]
- Rungvechvuttivittaya, S.; Okiji, T.; Suda, H. Responses of macrophage-associated antigen-expressing cells in the dental pulp of rat molars to experimental tooth replantation. Arch Oral Biol. 1998, 43, 701–710. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quispe-Salcedo, A.; Yamazaki, T.; Ohshima, H. Effects of Synthetic Toll-Like Receptor 9 Ligand Molecules on Pulpal Immunomodulatory Response and Repair after Injuries. Biomolecules 2024, 14, 931. https://doi.org/10.3390/biom14080931
Quispe-Salcedo A, Yamazaki T, Ohshima H. Effects of Synthetic Toll-Like Receptor 9 Ligand Molecules on Pulpal Immunomodulatory Response and Repair after Injuries. Biomolecules. 2024; 14(8):931. https://doi.org/10.3390/biom14080931
Chicago/Turabian StyleQuispe-Salcedo, Angela, Tomohiko Yamazaki, and Hayato Ohshima. 2024. "Effects of Synthetic Toll-Like Receptor 9 Ligand Molecules on Pulpal Immunomodulatory Response and Repair after Injuries" Biomolecules 14, no. 8: 931. https://doi.org/10.3390/biom14080931
APA StyleQuispe-Salcedo, A., Yamazaki, T., & Ohshima, H. (2024). Effects of Synthetic Toll-Like Receptor 9 Ligand Molecules on Pulpal Immunomodulatory Response and Repair after Injuries. Biomolecules, 14(8), 931. https://doi.org/10.3390/biom14080931







