Circular RNA in Cardiovascular Diseases: Biogenesis, Function and Application
Abstract
:1. Introduction
2. Characteristics of CircRNAs
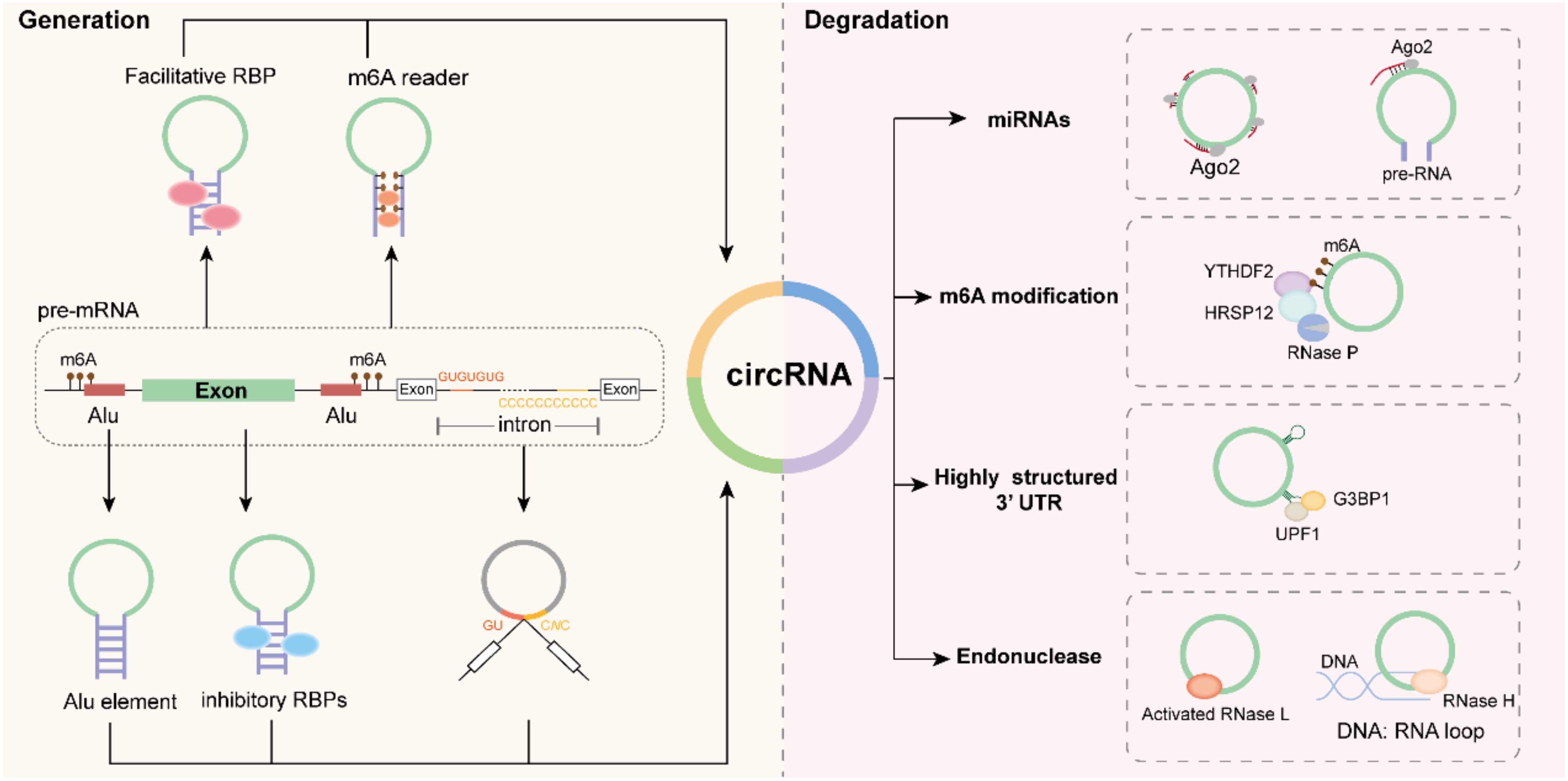
3. Biogenesis of CircRNAs
3.1. Flanking Inverted-Repeat Sequences
3.2. RBPs
3.3. Lariat Precursor Construction
3.4. m6A-Mediated CircRNA Biosynthesis
4. Degradation of CircRNAs
4.1. miRNA-Mediated Degradation
4.2. m6A Modification-Mediated Degradation
4.3. Exonuclease-Mediated Degradation
4.4. UPF1/G3BP1-Mediated Structure-Dependent Degradation
4.5. GW182-Mediated Degradation
5. Biological Functions of CircRNAs
5.1. miRNA Sponge
5.2. Binding Proteins
5.3. Translating Proteins
5.3.1. Internal Ribosome Entry Site (IRES) Driving circRNA Translation
5.3.2. N6-Methyladenosine (m6A) Driving circRNA Translation
5.4. Mediating mRNA Translation
5.5. Regulation of Gene Transcription
5.6. Competitive Cleaving with Parental Messenger RNA
6. CircRNAs and CVDs
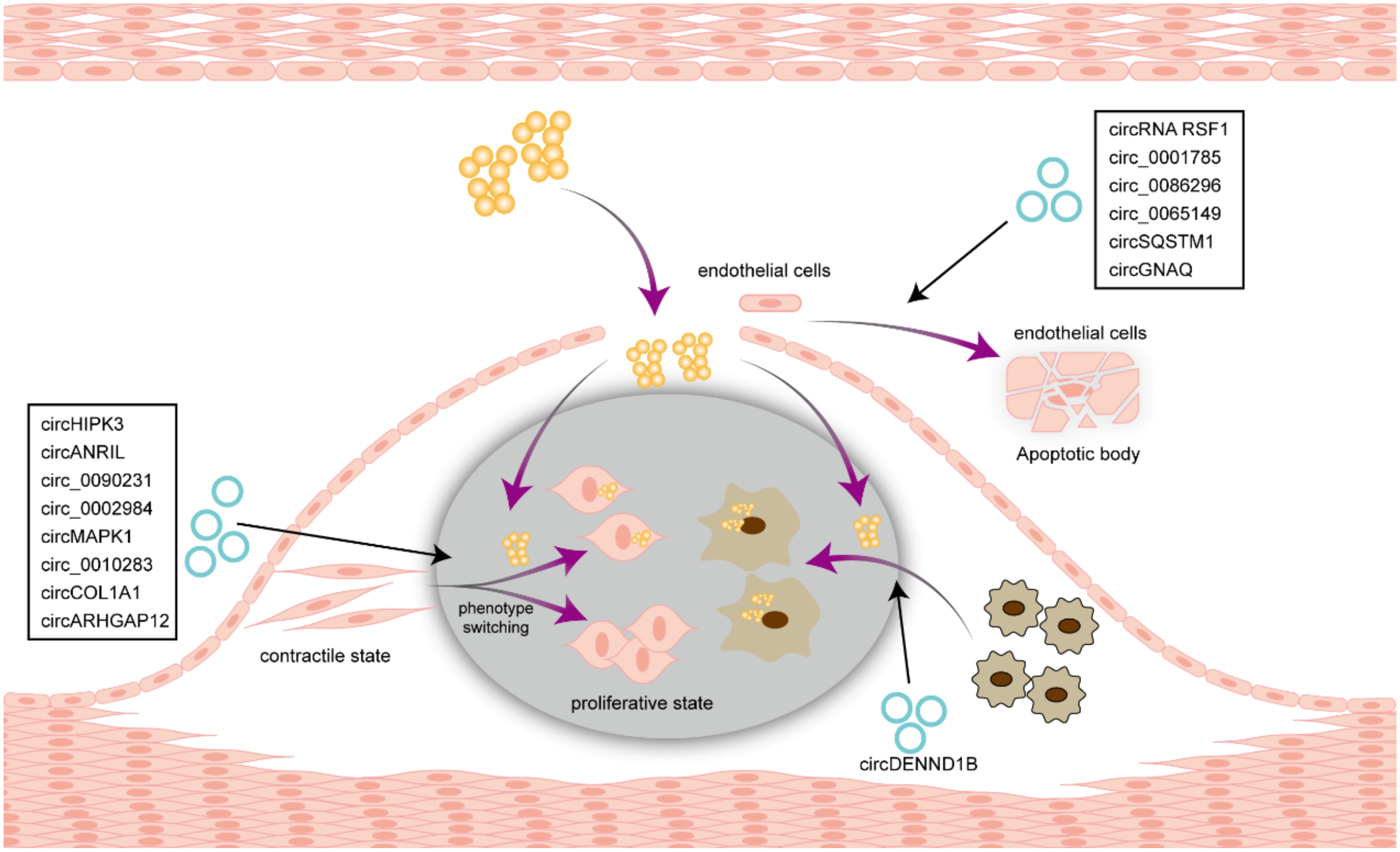
6.1. Atherosclerosis
6.2. Arterial Injury
6.3. Aortic Aneurysm or Dissection
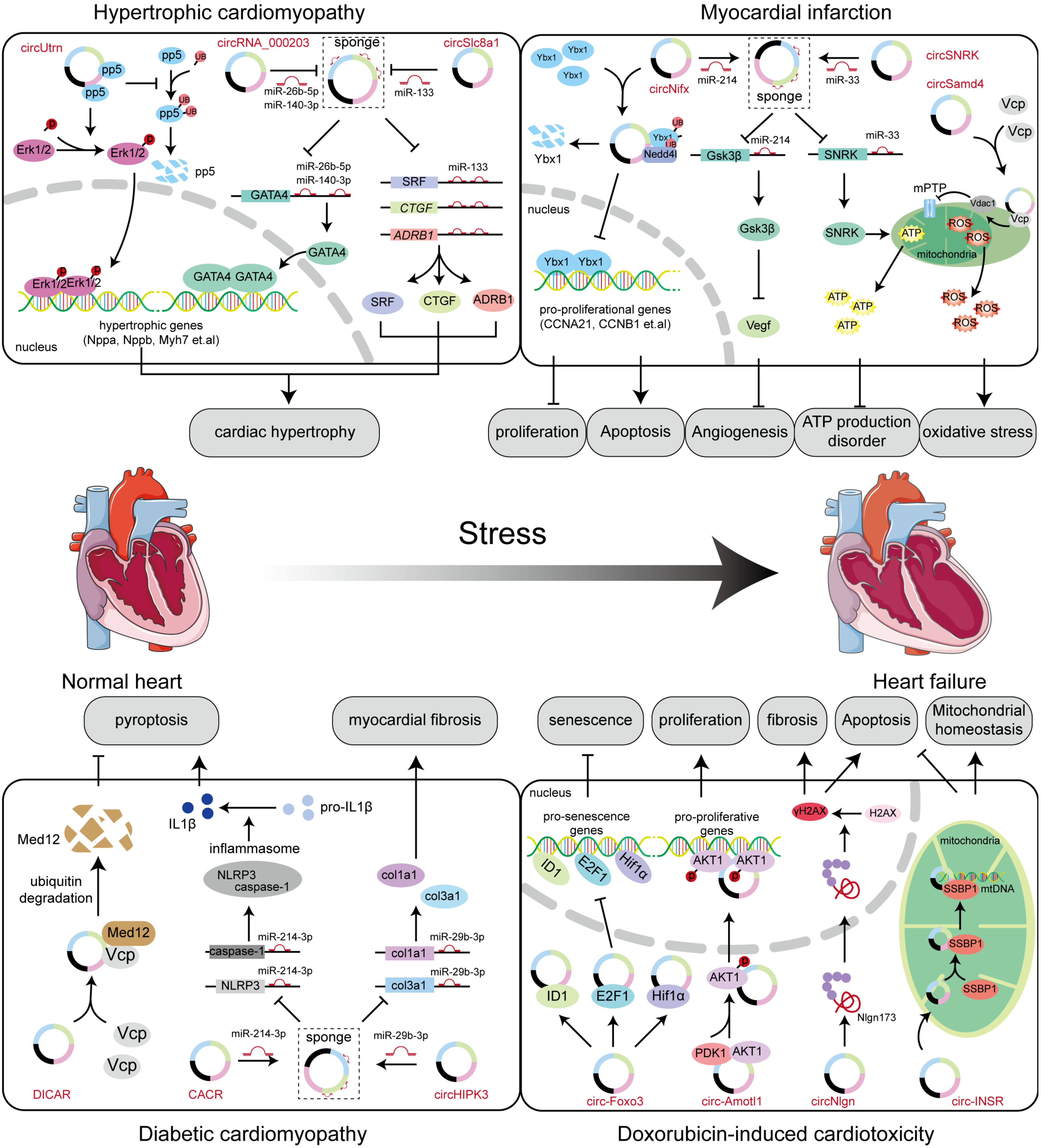
6.4. Myocardial Infarction (MI)
6.5. Hypertrophic Cardiomyopathy
6.6. Dilated Cardiomyopathy (DCM)
6.7. Diabetic Cardiomyopathy
6.8. Doxorubicin (DOX)-Induced Cardiotoxicity
7. Application of circRNAs in CVDs
7.1. CircRNA as a Biomarker
7.2. Therapeutic Role of CircRNAs
8. Limitations and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Fleg, J.L.; Aronow, W.S.; Frishman, W.H. Cardiovascular drug therapy in the elderly: Benefits and challenges. Nat. Rev. Cardiol. 2011, 8, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, Y.; Zeng, Y.; Gao, G.F.; Liang, X.; Zhou, M.; Wan, X.; Yu, S.; Jiang, Y.; Naghavi, M.; et al. Rapid health transition in China, 1990-2010: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 381, 1987–2015. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, C.; Zhou, M.; Wang, L.; Zhang, Y.; Luo, L. Burden of Ischaemic heart disease and attributable risk factors in China from 1990 to 2015: Findings from the global burden of disease 2015 study. BMC Cardiovasc. Disord. 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Diaz Canestro, C.; Libby, P.; Luscher, T.F.; Camici, G.G. The Aging Cardiovascular System: Understanding It at the Cellular and Clinical Levels. J. Am. Coll. Cardiol. 2017, 69, 1952–1967. [Google Scholar] [CrossRef]
- Lu, J.; Lu, Y.; Wang, X.; Li, X.; Linderman, G.C.; Wu, C.; Cheng, X.; Mu, L.; Zhang, H.; Liu, J.; et al. Prevalence, awareness, treatment, and control of hypertension in China: Data from 1.7 million adults in a population-based screening study (China PEACE Million Persons Project). Lancet 2017, 390, 2549–2558. [Google Scholar] [CrossRef] [PubMed]
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA 1976, 73, 3852–3856. [Google Scholar] [CrossRef]
- Cocquerelle, C.; Mascrez, B.; Hetuin, D.; Bailleul, B. Mis-splicing yields circular RNA molecules. FASEB J. 1993, 7, 155–160. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Q.; Shen, J.; Yang, B.B.; Ding, X. Circbank: A comprehensive database for circRNA with standard nomenclature. RNA Biol. 2019, 16, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Holdt, L.M.; Kohlmaier, A.; Teupser, D. Molecular roles and function of circular RNAs in eukaryotic cells. Cell. Mol. Life Sci. 2018, 75, 1071–1098. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.S.; Ai, Y.; Wilusz, J.E. Biogenesis and Functions of Circular RNAs Come into Focus. Trends Cell Biol. 2020, 30, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sharpless, N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014, 32, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Lu, L.; Wu, C.; Pan, X.; Liu, B.; Zhang, Y.; Wang, Y.; Wu, W.; Yan, B.; Zhang, Y.; et al. circHIPK3 prevents cardiac senescence by acting as a scaffold to recruit ubiquitin ligase to degrade HuR. Theranostics 2022, 12, 7550–7566. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhang, Y.; Liang, C.; Liu, B.; Ding, F.; Wang, Y.; Zhu, B.; Zhao, R.; Yu, X.Y.; Li, Y. Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/FOXO3a pathway. Theranostics 2020, 10, 6728–6742. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ma, X.X.; Luo, J.; Chung, H.K.; Kwon, M.S.; Yu, T.X.; Rao, J.N.; Kozar, R.; Gorospe, M.; Wang, J.Y. Circular RNA CircHIPK3 Promotes Homeostasis of the Intestinal Epithelium by Reducing MicroRNA 29b Function. Gastroenterology 2021, 161, 1303–1317.e3. [Google Scholar] [CrossRef]
- Shang, Z.; Luo, Z.; Wang, Y.; Liu, Q.; Xin, Y.; Zhang, M.; Li, X.; Zeng, S.; Yu, L.; Zhang, X.; et al. CircHIPK3 contributes to cisplatin resistance in gastric cancer by blocking autophagy-dependent ferroptosis. J. Cell. Physiol. 2023, 238, 2407–2424. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, R.; Su, W.; Yang, X.; Geng, Q.; Guo, C.; Wang, Z.; Wang, J.; Kresty, L.A.; Beer, D.G.; et al. Circular RNA circHIPK3 modulates autophagy via MIR124-3p-STAT3-PRKAA/AMPKalpha signaling in STK11 mutant lung cancer. Autophagy 2020, 16, 659–671. [Google Scholar] [CrossRef]
- Glazar, P.; Papavasileiou, P.; Rajewsky, N. circBase: A database for circular RNAs. RNA 2014, 20, 1666–1670. [Google Scholar] [CrossRef]
- Zhang, X.O.; Wang, H.B.; Zhang, Y.; Lu, X.; Chen, L.L.; Yang, L. Complementary sequence-mediated exon circularization. Cell 2014, 159, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Rybak-Wolf, A.; Stottmeister, C.; Glazar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R.; et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell 2015, 58, 870–885. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.; Baird, A.M.; Brady, L.; Lim, M.; Gray, S.G.; McDermott, R.; Finn, S.P. Circular RNAs: Biogenesis, Function and Role in Human Diseases. Front. Mol. Biosci. 2017, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.C.; De, S.; Grammatikakis, I.; Munk, R.; Yang, X.; Piao, Y.; Dudekula, D.B.; Abdelmohsen, K.; Gorospe, M. High-purity circular RNA isolation method (RPAD) reveals vast collection of intronic circRNAs. Nucleic Acids Res. 2017, 45, e116. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.O.; Chen, T.; Xiang, J.F.; Yin, Q.F.; Xing, Y.H.; Zhu, S.; Yang, L.; Chen, L.L. Circular intronic long noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef]
- Hagan, C.R.; Sheffield, R.F.; Rudin, C.M. Human Alu element retrotransposition induced by genotoxic stress. Nat. Genet. 2003, 35, 219–220. [Google Scholar] [CrossRef] [PubMed]
- De Koning, A.P.; Gu, W.; Castoe, T.A.; Batzer, M.A.; Pollock, D.D. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet. 2011, 7, e1002384. [Google Scholar] [CrossRef]
- Ivanov, A.; Memczak, S.; Wyler, E.; Torti, F.; Porath, H.T.; Orejuela, M.R.; Piechotta, M.; Levanon, E.Y.; Landthaler, M.; Dieterich, C.; et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015, 10, 170–177. [Google Scholar] [CrossRef]
- Zheng, Q.; Bao, C.; Guo, W.; Li, S.; Chen, J.; Chen, B.; Luo, Y.; Lyu, D.; Li, Y.; Shi, G.; et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016, 7, 11215. [Google Scholar] [CrossRef]
- Yu, J.; Xu, Q.G.; Wang, Z.G.; Yang, Y.; Zhang, L.; Ma, J.Z.; Sun, S.H.; Yang, F.; Zhou, W.P. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J. Hepatol. 2018, 68, 1214–1227. [Google Scholar] [CrossRef]
- Yoshimoto, R.; Rahimi, K.; Hansen, T.B.; Kjems, J.; Mayeda, A. Biosynthesis of Circular RNA ciRS-7/CDR1as Is Mediated by Mammalian-wide Interspersed Repeats. iScience 2020, 23, 101345. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Aktas, T.; Avsar Ilik, I.; Maticzka, D.; Bhardwaj, V.; Pessoa Rodrigues, C.; Mittler, G.; Manke, T.; Backofen, R.; Akhtar, A. DHX9 suppresses RNA processing defects originating from the Alu invasion of the human genome. Nature 2017, 544, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, T.; Li, C.; Li, W.; Zhou, X.; Li, Y.; Luo, D.; Zhang, N.; Chen, B.; Wang, L.; et al. CircRNA-CREIT inhibits stress granule assembly and overcomes doxorubicin resistance in TNBC by destabilizing PKR. J. Hematol. Oncol. 2022, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, N.; Chai, J.; Wang, T.; Ma, C.; Han, L.; Yang, M. CircPDIA4 Induces Gastric Cancer Progression by Promoting ERK1/2 Activation and Enhancing Biogenesis of Oncogenic circRNAs. Cancer Res. 2023, 83, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, Z.; Peng, Y.; Li, H.; Lin, T.; Zhao, Y.; Hu, Z.; Zhou, Z.; Zhou, W.; Liu, Y.; et al. Circular RNA circBNC2 inhibits epithelial cell G2-M arrest to prevent fibrotic maladaptive repair. Nat. Commun. 2022, 13, 6502. [Google Scholar] [CrossRef]
- Yan, D.; Dong, W.; He, Q.; Yang, M.; Huang, L.; Kong, J.; Qin, H.; Lin, T.; Huang, J. Corrigendum to “Circular RNA circPICALM sponges miR-1265 to inhibit bladder cancer metastasis and influence FAK phosphorylation”: [EBioMedicine 48 (2019) 316-331]. EBioMedicine 2021, 64, 103226. [Google Scholar] [CrossRef]
- Nichols, P.J.; Bevers, S.; Henen, M.; Kieft, J.S.; Vicens, Q.; Vogeli, B. Recognition of non-CpG repeats in Alu and ribosomal RNAs by the Z-RNA binding domain of ADAR1 induces A-Z junctions. Nat. Commun. 2021, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Zhang, J.; Liu, L.; Chen, Y.; Yang, X.; Liao, X.; He, M.; Jia, Z.; Fan, J.; et al. ADAR1 regulates vascular remodeling in hypoxic pulmonary hypertension through N1-methyladenosine modification of circCDK17. Acta Pharm. Sin. B 2023, 13, 4840–4855. [Google Scholar] [CrossRef]
- Zhou, B.; Xue, J.; Wu, R.; Meng, H.; Li, R.; Mo, Z.; Zhai, H.; Chen, X.; Liu, R.; Lai, G.; et al. CREBZF mRNA nanoparticles suppress breast cancer progression through a positive feedback loop boosted by circPAPD4. J. Exp. Clin. Cancer Res. 2023, 42, 138. [Google Scholar] [CrossRef]
- Errichelli, L.; Dini Modigliani, S.; Laneve, P.; Colantoni, A.; Legnini, I.; Capauto, D.; Rosa, A.; De Santis, R.; Scarfo, R.; Peruzzi, G.; et al. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat. Commun. 2017, 8, 14741. [Google Scholar] [CrossRef] [PubMed]
- Conn, S.J.; Pillman, K.A.; Toubia, J.; Conn, V.M.; Salmanidis, M.; Phillips, C.A.; Roslan, S.; Schreiber, A.W.; Gregory, P.A.; Goodall, G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell 2015, 160, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Garg, A.; Bar, C.; Chatterjee, S.; Foinquinos, A.; Milting, H.; Streckfuss-Bomeke, K.; Fiedler, J.; Thum, T. Quaking Inhibits Doxorubicin-Mediated Cardiotoxicity Through Regulation of Cardiac Circular RNA Expression. Circ. Res. 2018, 122, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Xu, C.; Liu, J.; Chen, J.; Li, G.; Huang, B.; Pan, Y.; Zhang, Y.; Wei, Q.; et al. Circular RNA circGlis3 protects against islet beta-cell dysfunction and apoptosis in obesity. Nat. Commun. 2023, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Lee, H.O.; Jawerth, L.; Maharana, S.; Jahnel, M.; Hein, M.Y.; Stoynov, S.; Mahamid, J.; Saha, S.; Franzmann, T.M.; et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 2015, 162, 1066–1077. [Google Scholar] [CrossRef]
- Ashwal-Fluss, R.; Meyer, M.; Pamudurti, N.R.; Ivanov, A.; Bartok, O.; Hanan, M.; Evantal, N.; Memczak, S.; Rajewsky, N.; Kadener, S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 2014, 56, 55–66. [Google Scholar] [CrossRef]
- Kong, Y.; Luo, Y.; Zheng, S.; Yang, J.; Zhang, D.; Zhao, Y.; Zheng, H.; An, M.; Lin, Y.; Ai, L.; et al. Mutant KRAS Mediates circARFGEF2 Biogenesis to Promote Lymphatic Metastasis of Pancreatic Ductal Adenocarcinoma. Cancer Res. 2023, 83, 3077–3094. [Google Scholar] [CrossRef]
- Li, Y.; Kong, Y.; An, M.; Luo, Y.; Zheng, H.; Lin, Y.; Chen, J.; Yang, J.; Liu, L.; Luo, B.; et al. ZEB1-mediated biogenesis of circNIPBL sustains the metastasis of bladder cancer via Wnt/beta-catenin pathway. J. Exp. Clin. Cancer Res. 2023, 42, 191. [Google Scholar] [CrossRef]
- Liu, D.; Dredge, B.K.; Bert, A.G.; Pillman, K.A.; Toubia, J.; Guo, W.; Dyakov, B.J.A.; Migault, M.M.; Conn, V.M.; Conn, S.J.; et al. ESRP1 controls biogenesis and function of a large abundant multiexon circRNA. Nucleic Acids Res. 2024, 52, 1387–1403. [Google Scholar] [CrossRef]
- Song, J.; Zhao, W.; Zhang, X.; Tian, W.; Zhao, X.; Ma, L.; Cao, Y.; Yin, Y.; Zhang, X.; Deng, X.; et al. Mutant RIG-I enhances cancer-related inflammation through activation of circRIG-I signaling. Nat. Commun. 2022, 13, 7096. [Google Scholar] [CrossRef]
- Wei, W.; Liu, K.; Huang, X.; Tian, S.; Wang, H.; Zhang, C.; Ye, J.; Dong, Y.; An, Z.; Ma, X.; et al. EIF4A3-mediated biogenesis of circSTX6 promotes bladder cancer metastasis and cisplatin resistance. J. Exp. Clin. Cancer Res. 2024, 43, 2. [Google Scholar] [CrossRef]
- Yang, M.; Hu, H.; Wu, S.; Ding, J.; Yin, B.; Huang, B.; Li, F.; Guo, X.; Han, L. EIF4A3-regulated circ_0087429 can reverse EMT and inhibit the progression of cervical cancer via miR-5003-3p-dependent upregulation of OGN expression. J. Exp. Clin. Cancer Res. 2022, 41, 165. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.P.; Wang, P.L.; Salzman, J. Circular RNA biogenesis can proceed through an exon-containing lariat precursor. Elife 2015, 4, e07540. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, T.Q.; Fang, K.; Zeng, Z.C.; Ye, H.; Chen, Y.Q. N6-methyladenosine methyltransferases: Functions, regulation, and clinical potential. J. Hematol. Oncol. 2021, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Wiener, D.; Schwartz, S. The epitranscriptome beyond m(6)A. Nat. Rev. Genet. 2021, 22, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Ranjan, P.; Suleiman, Z.G.; Goswami, S.K.; Li, J.; Prasad, R.; Verma, S.K. mRNA modifications in cardiovascular biology and disease: With a focus on m6A modification. Cardiovasc. Res. 2022, 118, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Molinie, B.; Daneshvar, K.; Pondick, J.V.; Wang, J.; Van Wittenberghe, N.; Xing, Y.; Giallourakis, C.C.; Mullen, A.C. Genome-Wide Maps of m6A circRNAs Identify Widespread and Cell-Type-Specific Methylation Patterns that Are Distinct from mRNAs. Cell Rep. 2017, 20, 2262–2276. [Google Scholar] [CrossRef] [PubMed]
- Di Timoteo, G.; Dattilo, D.; Centron-Broco, A.; Colantoni, A.; Guarnacci, M.; Rossi, F.; Incarnato, D.; Oliviero, S.; Fatica, A.; Morlando, M.; et al. Modulation of circRNA Metabolism by m(6)A Modification. Cell Rep. 2020, 31, 107641. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, D.; Di Timoteo, G.; Setti, A.; Giuliani, A.; Peruzzi, G.; Beltran Nebot, M.; Centron-Broco, A.; Mariani, D.; Mozzetta, C.; Bozzoni, I. The m(6)A reader YTHDC1 and the RNA helicase DDX5 control the production of rhabdomyosarcoma-enriched circRNAs. Nat. Commun. 2023, 14, 1898. [Google Scholar] [CrossRef]
- Kong, Z.; Lu, Y.; Yang, Y.; Chang, K.; Lin, Y.; Huang, Y.; Wang, C.; Zhang, L.; Xu, W.; Zhao, S.; et al. m6A-Mediated Biogenesis of circDDIT4 Inhibits Prostate Cancer Progression by Sequestrating ELAVL1/HuR. Mol. Cancer Res. 2023, 21, 1342–1355. [Google Scholar] [CrossRef]
- Chen, C.; Yuan, W.; Zhou, Q.; Shao, B.; Guo, Y.; Wang, W.; Yang, S.; Guo, Y.; Zhao, L.; Dang, Q.; et al. N6-methyladenosine-induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics 2021, 11, 4298–4315. [Google Scholar] [CrossRef] [PubMed]
- Piwecka, M.; Glazar, P.; Hernandez-Miranda, L.R.; Memczak, S.; Wolf, S.A.; Rybak-Wolf, A.; Filipchyk, A.; Klironomos, F.; Cerda Jara, C.A.; Fenske, P.; et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science 2017, 357, 1254. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Li, G.F.; Sun, M.L.; Xie, L.; Liu, D.; Zhang, Q.; Yang, X.X.; Xia, S.; Liu, X.; Zhou, H.; et al. MicroRNA-1224 Splicing CircularRNA-Filip1l in an Ago2-Dependent Manner Regulates Chronic Inflammatory Pain via Targeting Ubr5. J. Neurosci. 2019, 39, 2125–2143. [Google Scholar] [CrossRef] [PubMed]
- Park, O.H.; Ha, H.; Lee, Y.; Boo, S.H.; Kwon, D.H.; Song, H.K.; Kim, Y.K. Endoribonucleolytic Cleavage of m(6)A-Containing RNAs by RNase P/MRP Complex. Mol. Cell 2019, 74, 494–507.e8. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, Y.; Chen, C.; Fan, D.; Wu, X.; Zhao, L.; Shao, B.; Sun, Z.; Ji, Z. Circ3823 contributes to growth, metastasis and angiogenesis of colorectal cancer: Involvement of miR-30c-5p/TCF7 axis. Mol. Cancer 2021, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.N.; Chen, Z.Y.; Chen, X.Y.; Chen, M.; Yi, Y.C.; Zhu, J.S.; Zhang, J. METTL14-mediated m(6)A modification of circORC5 suppresses gastric cancer progression by regulating miR-30c-2-3p/AKT1S1 axis. Mol. Cancer 2022, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Li, X.; Nan, F.; Jiang, S.; Gao, X.; Guo, S.K.; Xue, W.; Cui, Y.; Dong, K.; Ding, H.; et al. Structure and Degradation of Circular RNAs Regulate PKR Activation in Innate Immunity. Cell 2019, 177, 865–880.e821. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.M. Circular RNAs and RNase L in PKR activation and virus infection. Cell Biosci. 2019, 9, 43. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.L.; Lei, Y.N.; Liu, X.Q.; Xue, W.; Zhang, Y.; Nan, F.; Gao, X.; Zhang, J.; Wei, J.; et al. Linking circular intronic RNA degradation and function in transcription by RNase H1. Sci. China Life Sci. 2021, 64, 1795–1809. [Google Scholar] [CrossRef]
- Fischer, J.W.; Busa, V.F.; Shao, Y.; Leung, A.K.L. Structure-Mediated RNA Decay by UPF1 and G3BP1. Mol. Cell 2020, 78, 70–84.e6. [Google Scholar] [CrossRef]
- Ding, L.; Han, M. GW182 family proteins are crucial for microRNA-mediated gene silencing. Trends Cell Biol. 2007, 17, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rivas, F.V.; Wohlschlegel, J.; Yates, J.R., 3rd; Parker, R.; Hannon, G.J. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 2005, 7, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Xiao, M.S.; Li, Z.; Shan, G.; Huang, C. Defining an evolutionarily conserved role of GW182 in circular RNA degradation. Cell Discov. 2019, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, F.; Xiao, X.; Xie, F.; Tao, D.; Huang, C.; Liu, D.; Wang, M.; Wang, L.; Zeng, F.; et al. CircHIPK3 sponges miR-558 to suppress heparanase expression in bladder cancer cells. EMBO Rep. 2022, 23, e56102. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Li, W.; Zhuge, Y.; Xu, S.; Wang, Y.; Chen, Y.; Shen, G.; Wang, F. Inhibition of circHIPK3 prevents angiotensin II-induced cardiac fibrosis by sponging miR-29b-3p. Int. J. Cardiol. 2019, 292, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zhao, Y.; Lim, G.; Lin, H.; Zhang, X.; Zhang, X. Circular RNA PVT1 acts as a competing endogenous RNA for miR-497 in promoting non-small cell lung cancer progression. Biomed. Pharmacother. 2019, 111, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Denzler, R.; Agarwal, V.; Stefano, J.; Bartel, D.P.; Stoffel, M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol. Cell 2014, 54, 766–776. [Google Scholar] [CrossRef]
- Aufiero, S.; Reckman, Y.J.; Pinto, Y.M.; Creemers, E.E. Circular RNAs open a new chapter in cardiovascular biology. Nat. Rev. Cardiol. 2019, 16, 503–514. [Google Scholar] [CrossRef]
- Barrett, S.P.; Salzman, J. Circular RNAs: Analysis, expression and potential functions. Development 2016, 143, 1838–1847. [Google Scholar] [CrossRef]
- Hansen, T.B.; Wiklund, E.D.; Bramsen, J.B.; Villadsen, S.B.; Statham, A.L.; Clark, S.J.; Kjems, J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011, 30, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Wu, S.; Zhou, Z.; Ding, X.; Shi, R.; Thorne, R.F.; Zhang, X.D.; Hu, W.; Wu, M. CircACC1 Regulates Assembly and Activation of AMPK Complex under Metabolic Stress. Cell Metab. 2019, 30, 157–173.e157. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Du, W.W.; Awan, F.M.; Dong, J.; Yang, B.B. The circular RNA circ-Ccnb1 dissociates Ccnb1/Cdk1 complex suppressing cell invasion and tumorigenesis. Cancer Lett. 2019, 459, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Cai, Q.; Ma, M.; Jin, L.Y.; Weng, M.; Zhou, D.; Tang, Z.; Wang, J.D.; Quan, Z. Circular RNA FOXP1 promotes tumor progression and Warburg effect in gallbladder cancer by regulating PKLR expression. Mol. Cancer 2019, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Unbehaun, A.; Spahn, C.M.T. Ribosomal Chamber Music: Toward an Understanding of IRES Mechanisms. Trends Biochem. Sci. 2017, 42, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Cheng, R.; Demeter, J.; Chen, J.; Weingarten-Gabbay, S.; Jiang, L.; Snyder, M.P.; Weissman, J.S.; Segal, E.; Jackson, P.K.; et al. Structured elements drive extensive circular RNA translation. Mol. Cell 2021, 81, 4300–4318.e13. [Google Scholar] [CrossRef] [PubMed]
- Legnini, I.; Di Timoteo, G.; Rossi, F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M.; et al. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol. Cell 2017, 66, 22–37.e29. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, X.; Zhang, M.; Yan, S.; Sun, C.; Xiao, F.; Huang, N.; Yang, X.; Zhao, K.; Zhou, H.; et al. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. J. Natl. Cancer Inst. 2018, 110, 304–315. [Google Scholar] [CrossRef]
- Xia, X.; Li, X.; Li, F.; Wu, X.; Zhang, M.; Zhou, H.; Huang, N.; Yang, X.; Xiao, F.; Liu, D.; et al. A novel tumor suppressor protein encoded by circular AKT3 RNA inhibits glioblastoma tumorigenicity by competing with active phosphoinositide-dependent Kinase-1. Mol. Cancer 2019, 18, 131. [Google Scholar] [CrossRef]
- Li, J.; Ma, M.; Yang, X.; Zhang, M.; Luo, J.; Zhou, H.; Huang, N.; Xiao, F.; Lai, B.; Lv, W.; et al. Circular HER2 RNA positive triple negative breast cancer is sensitive to Pertuzumab. Mol. Cancer 2020, 19, 142. [Google Scholar] [CrossRef]
- Wu, N.; Yuan, Z.; Du, K.Y.; Fang, L.; Lyu, J.; Zhang, C.; He, A.; Eshaghi, E.; Zeng, K.; Ma, J.; et al. Translation of yes-associated protein (YAP) was antagonized by its circular RNA via suppressing the assembly of the translation initiation machinery. Cell Death Differ. 2019, 26, 2758–2773. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Wang, P.; Long, F.; Wang, T. Mutual regulation between N6-methyladenosine (m6A) modification and circular RNAs in cancer: Impacts on therapeutic resistance. Mol. Cancer 2022, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xu, N.; Qiu, D.; Yang, X.; Zhao, S.; Zhao, H. Comprehensive analysis of m6A-modified circRNAs in peritoneal metastasis of high grade serious carcinoma of ovary. Front. Oncol. 2022, 12, 988578. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, X.; Mao, M.; Song, X.; Wu, P.; Zhang, Y.; Jin, Y.; Yang, Y.; Chen, L.L.; Wang, Y.; et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017, 27, 626–641. [Google Scholar] [CrossRef]
- Zeng, K.; Peng, J.; Xing, Y.; Zhang, L.; Zeng, P.; Li, W.; Zhang, W.; Pan, Z.; Zhou, C.; Lin, J. A positive feedback circuit driven by m(6)A-modified circular RNA facilitates colorectal cancer liver metastasis. Mol. Cancer 2023, 22, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Wu, X.; Gao, Y.; Chen, J.; Zhang, M.; Zhou, H.; Yang, J.; Xiao, F.; Yang, X.; Huang, N.; et al. Circular RNA encoded MET variant promotes glioblastoma tumorigenesis. Nat. Commun. 2023, 14, 4467. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, B.; Zhao, J.; Li, Q.; Chen, S.; Guo, T.; Li, Y.; Lai, H.; Chen, Z.; Meng, Z.; et al. HNRNPL Circularizes ARHGAP35 to Produce an Oncogenic Protein. Adv. Sci. 2021, 8, 2001701. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Wang, W.T.; Zeng, Z.C.; Chen, T.Q.; Han, C.; Pan, Q.; Huang, W.; Fang, K.; Sun, L.Y.; Zhou, Y.F.; et al. circMYBL2, a circRNA from MYBL2, regulates FLT3 translation by recruiting PTBP1 to promote FLT3-ITD AML progression. Blood 2019, 134, 1533–1546. [Google Scholar] [CrossRef]
- Rossi, F.; Beltran, M.; Damizia, M.; Grelloni, C.; Colantoni, A.; Setti, A.; Di Timoteo, G.; Dattilo, D.; Centron-Broco, A.; Nicoletti, C.; et al. Circular RNA ZNF609/CKAP5 mRNA interaction regulates microtubule dynamics and tumorigenicity. Mol. Cell 2022, 82, 75–89.e9. [Google Scholar] [CrossRef]
- Chen, L.; Kong, R.; Wu, C.; Wang, S.; Liu, Z.; Liu, S.; Li, S.; Chen, T.; Mao, C.; Liu, S. Circ-MALAT1 Functions as Both an mRNA Translation Brake and a microRNA Sponge to Promote Self-Renewal of Hepatocellular Cancer Stem Cells. Adv. Sci. 2020, 7, 1900949. [Google Scholar] [CrossRef]
- Ding, L.; Wang, R.; Zheng, Q.; Shen, D.; Wang, H.; Lu, Z.; Luo, W.; Xie, H.; Ren, L.; Jiang, M.; et al. circPDE5A regulates prostate cancer metastasis via controlling WTAP-dependent N6-methyladenisine methylation of EIF3C mRNA. J. Exp. Clin. Cancer Res. 2022, 41, 187. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, X.; Zhang, J.; Wu, W.; Zhang, B.; Zhao, F. circVAMP3 Drives CAPRIN1 Phase Separation and Inhibits Hepatocellular Carcinoma by Suppressing c-Myc Translation. Adv. Sci. 2022, 9, e2103817. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.; Singh, A.; Choudhary, A.; Dalavi, R.; Ralte, L.; Chawngthu, R.L.; Senthil Kumar, N.; Vijay, N.; Chande, A. HIV-1 Vpr induces ciTRAN to prevent transcriptional repression of the provirus. Sci. Adv. 2023, 9, eadh9170. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hu, A.; Li, D.; Wang, J.; Guo, Y.; Liu, Y.; Li, H.; Chen, Y.; Wang, X.; Huang, K.; et al. Circ-HuR suppresses HuR expression and gastric cancer progression by inhibiting CNBP transactivation. Mol. Cancer 2019, 18, 158. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, J.; Tian, Y.; Gao, Y.; Dong, X.; Chen, W.; Yuan, X.; Yin, W.; Xu, J.; Chen, K.; et al. CircRNA inhibits DNA damage repair by interacting with host gene. Mol. Cancer 2020, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Kobiyama, K.; Ley, K. Atherosclerosis. Circ. Res. 2018, 123, 1118–1120. [Google Scholar] [CrossRef]
- Samani, N.J.; Erdmann, J.; Hall, A.S.; Hengstenberg, C.; Mangino, M.; Mayer, B.; Dixon, R.J.; Meitinger, T.; Braund, P.; Wichmann, H.E.; et al. Genomewide association analysis of coronary artery disease. N. Engl. J. Med. 2007, 357, 443–453. [Google Scholar] [CrossRef]
- Holdt, L.M.; Stahringer, A.; Sass, K.; Pichler, G.; Kulak, N.A.; Wilfert, W.; Kohlmaier, A.; Herbst, A.; Northoff, B.H.; Nicolaou, A.; et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016, 7, 12429. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Y.; Wang, Z.; Gong, W.; Zhang, C. Hsa_circ_0030042 regulates abnormal autophagy and protects atherosclerotic plaque stability by targeting eIF4A3. Theranostics 2021, 11, 5404–5417. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Liu, S.; Xue, Y.; Li, Z.; Wang, T.; Jiao, L.; An, Q.; Liu, B.; Wang, J.; et al. Exosomes From IgE-Stimulated Mast Cells Aggravate Asthma-Mediated Atherosclerosis through circRNA CDR1as-Mediated Endothelial Cell Dysfunction in Mice. Arterioscler. Thromb. Vasc. Biol. 2024, 44, e99–e115. [Google Scholar] [CrossRef] [PubMed]
- Mineo, C. Lipoprotein receptor signalling in atherosclerosis. Cardiovasc. Res. 2020, 116, 1254–1274. [Google Scholar] [CrossRef] [PubMed]
- Kianmehr, A.; Qujeq, D.; Bagheri, A.; Mahrooz, A. Oxidized LDL-regulated microRNAs for evaluating vascular endothelial function: Molecular mechanisms and potential biomarker roles in atherosclerosis. Crit. Rev. Clin. Lab. Sci. 2022, 59, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, J.; Zhang, Q.; Luo, Q.; Liu, B. CircRNA RSF1 regulated ox-LDL induced vascular endothelial cells proliferation, apoptosis and inflammation through modulating miR-135b-5p/HDAC1 axis in atherosclerosis. Biol. Res. 2021, 54, 11. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, Y.; Zhu, J.; Xie, Y.; Wu, R.; Zhong, J.; Qiu, Z.; Jiang, L. circ_0086296 induced atherosclerotic lesions via the IFIT1/STAT1 feedback loop by sponging miR-576-3p. Cell Mol. Biol. Lett. 2022, 27, 80. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Zhang, Y.; Che, P.; Qin, W.; Wu, X.; Liu, Y.; Hu, B. Circ_0090231 knockdown protects vascular smooth muscle cells from ox-LDL-induced proliferation, migration and invasion via miR-942-5p/PPM1B axis during atherosclerosis. Mol. Cell Biochem. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jiang, Q.; Zheng, Y. Circ_0002984 induces proliferation, migration and inflammation response of VSMCs induced by ox-LDL through miR-326-3p/VAMP3 axis in atherosclerosis. J. Cell. Mol. Med. 2021, 25, 8028–8038. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y.; Tian, Y.; Lei, X. Circular RNA circ_0010283 regulates the viability and migration of oxidized low-density lipoprotein-induced vascular smooth muscle cells via an miR-370-3p/HMGB1 axis in atherosclerosis. Int. J. Mol. Med. 2020, 46, 1399–1408. [Google Scholar] [CrossRef]
- Wei, M.Y.; Lv, R.R.; Teng, Z. Circular RNA circHIPK3 as a novel circRNA regulator of autophagy and endothelial cell dysfunction in atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12849–12858. [Google Scholar] [CrossRef]
- Yuan, R.; Xin, Q.; Ma, X.; Yu, M.; Miao, Y.; Chen, K.; Cong, W. Identification of a Novel Angiogenesis Signalling circSCRG1/miR-1268b/NR4A1 Pathway in Atherosclerosis and the Regulatory Effects of TMP-PF In Vitro. Molecules 2023, 28, 1271. [Google Scholar] [CrossRef]
- Fu, Y.; Jia, Q.; Ren, M.; Bie, H.; Zhang, X.; Zhang, Q.; He, S.; Li, C.; Zhou, H.; Wang, Y.; et al. Circular RNA ZBTB46 depletion alleviates the progression of Atherosclerosis by regulating the ubiquitination and degradation of hnRNPA2B1 via the AKT/mTOR pathway. Immun. Ageing 2023, 20, 66. [Google Scholar] [CrossRef]
- Hou, X.; Dai, H.; Zheng, Y. Circular RNA hsa_circ_0008896 accelerates atherosclerosis by promoting the proliferation, migration and invasion of vascular smooth muscle cells via hsa-miR-633/CDC20B (cell division cycle 20B) axis. Bioengineered 2022, 13, 5987–5998. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Huang, H.; Chen, F.; Tang, Y. CircDLGAP4 induces autophagy and improves endothelial cell dysfunction in atherosclerosis by targeting PTPN4 with miR-134-5p. Environ. Toxicol. 2023, 38, 2952–2966. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chun, Y.; Lian, Z.Q.; Yong, Z.W.; Lan, Y.M.; Huan, L.; Xi, C.Y.; Juan, L.S.; Qing, Z.W.; Jia, C.; et al. circRNA-0006896-miR1264-DNMT1 axis plays an important role in carotid plaque destabilization by regulating the behavior of endothelial cells in atherosclerosis. Mol. Med. Rep. 2021, 23, 311. [Google Scholar] [CrossRef]
- Tong, X.; Dang, X.; Liu, D.; Wang, N.; Li, M.; Han, J.; Zhao, J.; Wang, Y.; Huang, M.; Yang, Y.; et al. Exosome-derived circ_0001785 delays atherogenesis through the ceRNA network mechanism of miR-513a-5p/TGFBR3. J. Nanobiotechnol. 2023, 21, 362. [Google Scholar] [CrossRef]
- Kang, L.; Jia, H.; Huang, B.; Lu, S.; Chen, Z.; Shen, J.; Zou, Y.; Wang, C.; Sun, Y. Identification of Differently Expressed mRNAs in Atherosclerosis Reveals CDK6 Is Regulated by circHIPK3/miR-637 Axis and Promotes Cell Growth in Human Vascular Smooth Muscle Cells. Front. Genet. 2021, 12, 596169. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jin, W.; Sun, L.; Wu, J.; Hu, H.; Ma, L. Circ_0065149 Alleviates Oxidized Low-Density Lipoprotein-Induced Apoptosis and Inflammation in Atherosclerosis by Targeting miR-330-5p. Front. Genet. 2021, 12, 590633. [Google Scholar] [CrossRef]
- Fu, X.; Niu, T.; Yang, T.; Li, X. CircMAPK1 promotes the proliferation and migration of vascular smooth muscle cells through miR-22-3p/methyl-CpG binding protein 2 axis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2189–2198. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, R.; Zhu, Y.; Huang, Z.; Yang, X.; Li, Q.; Zhong, M.; Zhang, W.; Chen, L.; Wu, W.; et al. A novel circular RNA, circSQSTM1, protects the endothelial function in atherosclerosis. Free Radic. Biol. Med. 2023, 209, 301–319. [Google Scholar] [CrossRef]
- Ye, M.; Ni, Q.; Wang, H.; Wang, Y.; Yao, Y.; Li, Y.; Wang, W.; Yang, S.; Chen, J.; Lv, L.; et al. CircRNA circCOL1A1 Acts as a Sponge of miR-30a-5p to Promote Vascular Smooth Cell Phenotype Switch through Regulation of Smad1 Expression. Thromb. Haemost. 2023, 123, 97–107. [Google Scholar] [CrossRef]
- Miao, R.; Qi, C.; Fu, Y.; Wang, Y.; Lang, Y.; Liu, W.; Zhang, Y.; Zhang, Z.; Liu, A.; Chai, H.; et al. Silencing of circARHGAP12 inhibits the progression of atherosclerosis via miR-630/EZH2/TIMP2 signal axis. J. Cell. Physiol. 2022, 237, 1057–1069. [Google Scholar] [CrossRef]
- Xu, F.; Shen, L.; Chen, H.; Wang, R.; Zang, T.; Qian, J.; Ge, J. circDENND1B Participates in the Antiatherosclerotic Effect of IL-1beta Monoclonal Antibody in Mouse by Promoting Cholesterol Efflux via miR-17-5p/Abca1 Axis. Front. Cell Dev. Biol. 2021, 9, 652032. [Google Scholar] [CrossRef]
- Wu, W.P.; Zhou, M.Y.; Liu, D.L.; Min, X.; Shao, T.; Xu, Z.Y.; Jing, X.; Cai, M.Y.; Xu, S.; Liang, X.; et al. circGNAQ, a circular RNA enriched in vascular endothelium, inhibits endothelial cell senescence and atherosclerosis progression. Mol. Ther. Nucleic Acids 2021, 26, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Wang, X.; Li, H.; Zhang, C.; Wang, A.; Zhang, S. Atherosclerosis-associated endothelial dysfunction is promoted by miR-199a-5p/SIRT1 axis regulated by circHIF1a. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Tian, M.; Cao, N.; Yang, P.; Xu, Z.; Zheng, S.; Liao, Q.; Chen, C.; Zeng, C.; Jose, P.A.; et al. Circular RNA circEsyt2 regulates vascular smooth muscle cell remodeling via splicing regulation. J. Clin. Investig. 2021, 131, e147031. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Xia, L.; Fan, S.; Zheng, J.; Qin, J.; Fan, X.; Liu, Y.; Tao, J.; Liu, Y.; Li, K.; et al. Circular RNA CircMAP3K5 Acts as a MicroRNA-22-3p Sponge to Promote Resolution of Intimal Hyperplasia Via TET2-Mediated Smooth Muscle Cell Differentiation. Circulation 2021, 143, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Chen, R.; Yang, L.Y.; Gong, M.; Du, M.Y.; Mu, S.Q.; Jiang, Z.A.; Li, H.H.; Yang, Y.; Wang, X.H.; et al. Hsa_circ_0001402 alleviates vascular neointimal hyperplasia through a miR-183-5p-dependent regulation of vascular smooth muscle cell proliferation, migration, and autophagy. J. Adv. Res. 2023, 60, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Cui, X.B.; Li, Y.; Chen, S.Y. CircSOD2: A Novel Regulator for Smooth Muscle Proliferation and Neointima Formation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.H.; Chang, N.B.; Yao, Q.P.; Li, T.; Zhu, X.L.; Cao, Y.; Jiang, M.J.; Cheng, Y.S.; Jiang, R.; Jiang, J. Suppression of circDcbld1 Alleviates Intimal Hyperplasia in Rat Carotid Artery by Targeting miR-145-3p/Neuropilin-1. Mol. Ther. Nucleic Acids 2019, 18, 999–1008. [Google Scholar] [CrossRef]
- Kong, P.; Yu, Y.; Wang, L.; Dou, Y.Q.; Zhang, X.H.; Cui, Y.; Wang, H.Y.; Yong, Y.T.; Liu, Y.B.; Hu, H.J.; et al. circ-Sirt1 controls NF-kappaB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res. 2019, 47, 3580–3593. [Google Scholar] [CrossRef]
- Song, H.; Yang, Y.; Sun, Y.; Wei, G.; Zheng, H.; Chen, Y.; Cai, D.; Li, C.; Ma, Y.; Lin, Z.; et al. Circular RNA Cdyl promotes abdominal aortic aneurysm formation by inducing M1 macrophage polarization and M1-type inflammation. Mol. Ther. 2022, 30, 915–931. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, X.; Han, Y.; Chen, G.; Xu, T.; Cai, D.; Sun, Y.; Wang, S.; Lai, Y.; Teng, Z.; et al. CircRNA Chordc1 protects mice from abdominal aortic aneurysm by contributing to the phenotype and growth of vascular smooth muscle cells. Mol. Ther. Nucleic Acids 2022, 27, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sui, R.; Ge, L.; Xia, D. CircRNA_0079586 and circRNA_RanGAP1 are involved in the pathogenesis of intracranial aneurysms rupture by regulating the expression of MPO. Sci. Rep. 2021, 11, 19800. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Zhu, T.; Yang, J.; Si, Y.; Xu, X.; Fang, Y.; Fu, W. CircCBFB-mediated miR-28-5p facilitates abdominal aortic aneurysm via LYPD3 and GRIA4. Life Sci. 2020, 253, 117533. [Google Scholar] [CrossRef]
- Wang, C.; Luo, Y.; Tang, H.; Yan, Y.; Chang, X.; Zhao, R.; Li, Q.; Yang, P.; Hong, B.; Xu, Y.; et al. Hsa_circ_0031608: A Potential Modulator of VSMC Phenotype in the Rupture of Intracranial Aneurysms. Front. Mol. Neurosci. 2022, 15, 842865. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, J.; Lu, Q.; Feng, X.; Liu, J.; Cui, C.; Song, C. Hsa_circ_0087352 promotes the inflammatory response of macrophages in abdominal aortic aneurysm by adsorbing hsa-miR-149-5p. Int. Immunopharmacol. 2022, 107, 108691. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, Z.; Meng, G.; Hua, L. Circular RNA CCDC66 facilitates abdominal aortic aneurysm through the overexpression of CCDC66. Cell Biochem. Funct. 2020, 38, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Fasolo, F.; Winski, G.; Li, Z.; Wu, Z.; Winter, H.; Ritzer, J.; Glukha, N.; Roy, J.; Hultgren, R.; Pauli, J.; et al. The circular RNA Ataxia Telangiectasia Mutated regulates oxidative stress in smooth muscle cells in expanding abdominal aortic aneurysms. Mol. Ther. Nucleic Acids 2023, 33, 848–865. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Liu, X. Circ_0020397 regulates the viability of vascular smooth muscle cells by up-regulating GREM1 expression via miR-502-5p in intracranial aneurysm. Life Sci. 2021, 265, 118800. [Google Scholar] [CrossRef]
- Huang, M.X.; Piao, H.L.; Wang, Y.; Zhu, Z.C.; Xu, R.H.; Wang, T.C.; Li, D.; Liu, K.X. Circ_0022920 Maintains the Contractile Phenotype of Human Aortic Vascular Smooth Muscle Cells Via Sponging microRNA-650 and Promoting Transforming Growth Factor Beta Receptor 1 Expression in Angiotensin II-Induced Models for Aortic Dissection. J. Am. Heart Assoc. 2023, 12, e027425. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011, 124, 2574–2609. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Mintz, G.S.; Park, D.W.; Lee, S.W.; Kim, Y.H.; Whan Lee, C.; Han, K.H.; Kim, J.J.; Park, S.W.; Park, S.J. Mechanisms of in-stent restenosis after drug-eluting stent implantation: Intravascular ultrasound analysis. Circ. Cardiovasc. Interv. 2011, 4, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Authors/Task Force, m.; Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.; Head, S.J.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2014, 35, 2541–2619. [Google Scholar] [CrossRef]
- Cassese, S.; Byrne, R.A.; Tada, T.; Pinieck, S.; Joner, M.; Ibrahim, T.; King, L.A.; Fusaro, M.; Laugwitz, K.L.; Kastrati, A. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart 2014, 100, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.F.; Climent, M.; Quintavalle, M.; Farina, F.M.; Schorn, T.; Zani, S.; Carullo, P.; Kunderfranco, P.; Civilini, E.; Condorelli, G.; et al. Circ_Lrp6, a Circular RNA Enriched in Vascular Smooth Muscle Cells, Acts as a Sponge Regulating miRNA-145 Function. Circ. Res. 2019, 124, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Lino Cardenas, C.L.; Lindsay, M.E. Hereditary Influence in Thoracic Aortic Aneurysm and Dissection. Circulation 2016, 133, 2516–2528. [Google Scholar] [CrossRef]
- Milewicz, D.M.; Prakash, S.K.; Ramirez, F. Therapeutics Targeting Drivers of Thoracic Aortic Aneurysms and Acute Aortic Dissections: Insights from Predisposing Genes and Mouse Models. Annu. Rev. Med. 2017, 68, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Nienaber, C.A.; Clough, R.E.; Sakalihasan, N.; Suzuki, T.; Gibbs, R.; Mussa, F.; Jenkins, M.P.; Thompson, M.M.; Evangelista, A.; Yeh, J.S.; et al. Aortic dissection. Nat. Rev. Dis. Primers 2016, 2, 16053. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Shi, Z.; Cai, L.; Li, X.; Ding, Y.; Xie, T.; Fu, W. Circular RNA expression profile and its potential regulative role in human abdominal aortic aneurysm. BMC Cardiovasc. Disord. 2020, 20, 70. [Google Scholar] [CrossRef]
- Liu, B.; Wang, B.; Zhang, X.; Lock, R.; Nash, T.; Vunjak-Novakovic, G. Cell type-specific microRNA therapies for myocardial infarction. Sci. Transl. Med. 2021, 13, eabd0914. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Wang, S.; Cheng, H.; Xu, L.; Pei, G.; Wang, Y.; Fu, C.; Jiang, Y.; He, C.; et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct. Target. Ther. 2022, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, Y.; Jaffe, A.S. Type 2 Myocardial Infarction: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 1846–1860. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, P.; Sun, L.; Lu, Y.; Zhu, W.; Zhang, J.; Xiang, C.; Mao, Y.; Chen, Q.; Zhang, F. Overexpression of circRNA SNRK targets miR-103-3p to reduce apoptosis and promote cardiac repair through GSK3beta/beta-catenin pathway in rats with myocardial infarction. Cell Death Discov. 2021, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Li, X.M.; Zhao, X.M.; Li, F.H.; Wang, S.C.; Wang, K.; Li, R.F.; Zhou, L.Y.; Liang, L.; Wang, Y.; et al. Circular RNA FEACR inhibits ferroptosis and alleviates myocardial ischemia/reperfusion injury by interacting with NAMPT. J. Biomed. Sci. 2023, 30, 45. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Hang, Y.; Lu, Y.; Li, D.; Shen, F.; Guan, P.; Dong, J.; Shi, L.; Hu, W. CircRNA circ-NNT mediates myocardial ischemia/reperfusion injury through activating pyroptosis by sponging miR-33a-5p and regulating USP46 expression. Cell Death Discov. 2021, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, X.; Zheng, H.; Si, X.; Li, B.; Wei, G.; Li, C.; Chen, Y.; Chen, Y.; Liao, W.; et al. Loss of Super-Enhancer-Regulated circRNA Nfix Induces Cardiac Regeneration After Myocardial Infarction in Adult Mice. Circulation 2019, 139, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ai, C.; Chen, Q.; Wang, Q.; Zhu, Y.; Li, M.; Chen, K.; He, M.; Shen, M.; Chen, L.; et al. CircMap4k2 reactivated by aneurysm plication alleviates residual cardiac remodeling after SVR by enhancing cardiomyocyte proliferation in post-MI mice. J. Adv. Res. 2023, in press. [CrossRef]
- Yellon, D.M.; Beikoghli Kalkhoran, S.; Davidson, S.M. The RISK pathway leading to mitochondria and cardioprotection: How everything started. Basic Res. Cardiol. 2023, 118, 22. [Google Scholar] [CrossRef]
- Gao, F.; Liang, T.; Lu, Y.W.; Pu, L.; Fu, X.; Dong, X.; Hong, T.; Zhang, F.; Liu, N.; Zhou, Y.; et al. Reduced Mitochondrial Protein Translation Promotes Cardiomyocyte Proliferation and Heart Regeneration. Circulation 2023, 148, 1887–1906. [Google Scholar] [CrossRef]
- Zheng, H.; Huang, S.; Wei, G.; Sun, Y.; Li, C.; Si, X.; Chen, Y.; Tang, Z.; Li, X.; Chen, Y.; et al. CircRNA Samd4 induces cardiac repair after myocardial infarction by blocking mitochondria-derived ROS output. Mol. Ther. 2022, 30, 3477–3498. [Google Scholar] [CrossRef]
- Funabashi, S.; Fujino, M.; Morita, Y.; Noguchi, T. Infarction at a Distance: Simultaneous Acute Myocardial Infarction Due to Non-Infarct-Related Chronic Artery Occlusion. JACC Cardiovasc. Interv. 2021, 14, 1734–1735. [Google Scholar] [CrossRef] [PubMed]
- Dauerman, H.L.; Ibanez, B. The Edge of Time in Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Garikipati, V.N.S.; Verma, S.K.; Cheng, Z.; Liang, D.; Truongcao, M.M.; Cimini, M.; Yue, Y.; Huang, G.; Wang, C.; Benedict, C.; et al. Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF-A axis. Nat. Commun. 2019, 10, 4317. [Google Scholar] [CrossRef]
- Long, X.; Qiu, Z.; Li, C.; Wang, Y.; Li, J.; Zhao, R.; Rong, J.; Gu, N.; Yuan, J.; Ge, J.; et al. CircERBB2IP promotes post-infarction revascularization via the miR-145a-5p/Smad5 axis. Mol. Ther. Nucleic Acids 2022, 28, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, J.D.; Fang, X.H.; Zhu, J.N.; Yang, J.; Pan, R.; Yuan, S.J.; Zeng, N.; Yang, Z.Z.; Yang, H.; et al. Circular RNA circRNA_000203 aggravates cardiac hypertrophy via suppressing miR-26b-5p and miR-140-3p binding to Gata4. Cardiovasc. Res. 2020, 116, 1323–1334. [Google Scholar] [CrossRef]
- Wang, L.; Feng, J.; Feng, X.; Meng, D.; Zhao, X.; Wang, J.; Yu, P.; Xu, G.E.; Hu, M.; Wang, T.; et al. Exercise-induced circular RNA circUtrn is required for cardiac physiological hypertrophy and prevents myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2023, 119, 2638–2652. [Google Scholar] [CrossRef]
- Guo, H.M.; Liu, Z.P. Up-regulation of circRNA_0068481 promotes right ventricular hypertrophy in PAH patients via regulating miR-646/miR-570/miR-885. J. Cell. Mol. Med. 2021, 25, 3735–3743. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Wang, X. Silencing of circHIPK3 Inhibits Pressure Overload-Induced Cardiac Hypertrophy and Dysfunction by Sponging miR-185-3p. Drug Des. Dev. Ther. 2020, 14, 5699–5710. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, L.; Zhang, W.; Lei, X.; Lu, Q.; Ma, A. Circular RNA circ_0001006 aggravates cardiac hypertrophy via miR-214-3p/PAK6 axis. Aging 2022, 14, 2210–2220. [Google Scholar] [CrossRef]
- Xu, Q.R.; Liu, J.L.; Zhu, R.R.; Huang, W.X.; Huang, H.; Liu, J.C.; Xu, X.P.; Zhou, X.L. NSD2 promotes pressure overload-induced cardiac hypertrophy via activating circCmiss1/TfR1/ferroptosis signaling. Life Sci. 2023, 328, 121873. [Google Scholar] [CrossRef]
- Wang, K.; Long, B.; Liu, F.; Wang, J.X.; Liu, C.Y.; Zhao, B.; Zhou, L.Y.; Sun, T.; Wang, M.; Yu, T.; et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Heart J. 2016, 37, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zheng, C.; Zhang, R.; Yan, J.; Li, M.; Ma, S.; Chen, K.; Chen, L.; Liu, J.; Xiu, J.; et al. Circ-Ddx60 contributes to the antihypertrophic memory of exercise hypertrophic preconditioning. J. Adv. Res. 2023, 46, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, W.; Wang, L.; Li, Y. Circ_0001052 promotes cardiac hypertrophy via elevating Hipk3. Aging 2023, 15, 1025–1038. [Google Scholar] [CrossRef]
- Ma, C.X.; Wei, Z.R.; Sun, T.; Yang, M.H.; Sun, Y.Q.; Kai, K.L.; Shi, J.C.; Zhou, M.J.; Wang, Z.W.; Chen, J.; et al. Circ-sh3rf3/GATA-4/miR-29a regulatory axis in fibroblast-myofibroblast differentiation and myocardial fibrosis. Cell. Mol. Life Sci. 2023, 80, 50. [Google Scholar] [CrossRef]
- Lim, T.B.; Aliwarga, E.; Luu, T.D.A.; Li, Y.P.; Ng, S.L.; Annadoray, L.; Sian, S.; Ackers-Johnson, M.A.; Foo, R.S. Targeting the highly abundant circular RNA circSlc8a1 in cardiomyocytes attenuates pressure overload induced hypertrophy. Cardiovasc. Res. 2019, 115, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Zhang, D.; Ding, F.; Ma, J.; Xiang, Y.K.; Zhao, M. Silencing of circCacna1c Inhibits ISO-Induced Cardiac Hypertrophy through miR-29b-2-5p/NFATc1 Axis. Cells 2023, 12, 1667. [Google Scholar] [CrossRef]
- Wu, N.; Li, F.; Yang, W.; Du, W.W.; Awan, F.M.; Zhang, C.; Lyu, J.; Misir, S.; Zeng, K.; Eshaghi, E.; et al. Silencing mouse circular RNA circSlc8a1 by circular antisense cA-circSlc8a1 induces cardiac hepatopathy. Mol. Ther. 2023, 31, 1688–1704. [Google Scholar] [CrossRef]
- Fang, X.; Ao, X.; Xiao, D.; Wang, Y.; Jia, Y.; Wang, P.; Li, M.; Wang, J. Circular RNA-circPan3 attenuates cardiac hypertrophy via miR-320-3p/HSP20 axis. Cell. Mol. Biol. Lett. 2024, 29, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.; Yang, M.; Wu, C.; Lan, R.; Wang, W.; Li, Y. Circ-SIRT1 inhibits cardiac hypertrophy via activating SIRT1 to promote autophagy. Cell Death Dis. 2021, 12, 1069. [Google Scholar] [CrossRef]
- Zuo, H.; Li, L.; Wang, X.; Chen, S.; Liao, Z.; Wei, S.; Ruan, H.; Li, T.; Chen, J. A novel circ_0018553 protects against angiotensin-induced cardiac hypertrophy in cardiomyocytes by modulating the miR-4731/SIRT2 signaling pathway. Hypertens. Res. 2023, 46, 421–436. [Google Scholar] [CrossRef]
- Lavenniah, A.; Luu, T.D.A.; Li, Y.P.; Lim, T.B.; Jiang, J.; Ackers-Johnson, M.; Foo, R.S. Engineered Circular RNA Sponges Act as miRNA Inhibitors to Attenuate Pressure Overload-Induced Cardiac Hypertrophy. Mol. Ther. 2020, 28, 1506–1517. [Google Scholar] [CrossRef]
- Wu, N.; Xu, J.; Du, W.W.; Li, X.; Awan, F.M.; Li, F.; Misir, S.; Eshaghi, E.; Lyu, J.; Zhou, L.; et al. YAP Circular RNA, circYap, Attenuates Cardiac Fibrosis via Binding with Tropomyosin-4 and Gamma-Actin Decreasing Actin Polymerization. Mol. Ther. 2021, 29, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Du, W.W.; Li, X.; Xu, J.; Wu, N.; Awan, F.M.; Yang, Y.; Alashti, F.A.; Wang, S.; Yang, B.B. A Novel Circular RNA circITGa9 Predominantly Generated in Human Heart Disease Induces Cardiac Remodeling and Fibrosis. Research 2024, 7, 0303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Zhao, R.; Qiu, Z.; Shen, C.; Wang, Z.; Liu, W.; Zhang, W.; Ge, J.; Shi, B. CircUbe3a from M2 macrophage-derived small extracellular vesicles mediates myocardial fibrosis after acute myocardial infarction. Theranostics 2021, 11, 6315–6333. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Zheng, H.; Wei, G.; Li, M.; Li, W.; Wang, H.; Guo, H.; Sun, J.; Li, C.; Zhong, S.; et al. circRNA Hipk3 Induces Cardiac Regeneration after Myocardial Infarction in Mice by Binding to Notch1 and miR-133a. Mol. Ther. Nucleic Acids 2020, 21, 636–655. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Li, C.; Jia, X.; Xie, J.; Tang, Z.; Jin, M.; Chen, Q.; Sun, Y.; He, S.; Li, X.; et al. Extracellular vesicle-derived CircWhsc1 promotes cardiomyocyte proliferation and heart repair by activating TRIM59/STAT3/Cyclin B2 pathway. J. Adv. Res. 2023, 53, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wang, M.Y.; Wu, Y.B.; Cui, H.M.; Wei, S.X.; Liu, B.; Wang, R. Circular RNA POSTN Promotes Myocardial Infarction-Induced Myocardial Injury and Cardiac Remodeling by Regulating miR-96-5p/BNIP3 Axis. Front. Cell Dev. Biol. 2020, 8, 618574. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.P.; Zhang, N.J.; Wang, H.J.; Hu, S.G.; Geng, X. Knockdown of circROBO2 attenuates acute myocardial infarction through regulating the miR-1184/TRADD axis. Mol. Med. 2021, 27, 21. [Google Scholar] [CrossRef]
- Pang, P.; Si, W.; Wu, H.; Wang, C.; Liu, K.; Jia, Y.; Zhang, Z.; Zhang, F.; Kong, X.; Yang, Y.; et al. The circular RNA circHelz enhances cardiac fibrosis by facilitating the nuclear translocation of YAP1. Transl. Res. 2023, 257, 30–42. [Google Scholar] [CrossRef]
- Wang, D.; Tian, L.; Wang, Y.; Gao, X.; Tang, H.; Ge, J. Circ_0001206 regulates miR-665/CRKL axis to alleviate hypoxia/reoxygenation-induced cardiomyocyte injury in myocardial infarction. ESC Heart Fail. 2022, 9, 998–1007. [Google Scholar] [CrossRef]
- Sun, G.; Shen, J.F.; Wei, X.F.; Qi, G.X. Circular RNA Foxo3 Relieves Myocardial Ischemia/Reperfusion Injury by Suppressing Autophagy via Inhibiting HMGB1 by Repressing KAT7 in Myocardial Infarction. J. Inflamm. Res. 2021, 14, 6397–6407. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, X.X.; Deng, Y.F. Negative feedback of SNRK to circ-SNRK regulates cardiac function post-myocardial infarction. Cell Death Differ. 2022, 29, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Qian, G.; Wu, H.; Pan, H.; Xie, S.; Sun, Z.; Shao, P.; Tang, G.; Hu, H.; Zhang, S. Knockdown of circ_0060745 alleviates acute myocardial infarction by suppressing NF-kappaB activation. J. Cell. Mol. Med. 2020, 24, 12401–12410. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, X.; Sun, H.; Xu, B.; Song, R.; Tian, Y.; Zhao, L.; Xu, Y.; Zhao, Y.; Yang, F.; et al. Oxidant stress-sensitive circRNA Mdc1 controls cardiomyocyte chromosome stability and cell cycle re-entry during heart regeneration. Pharmacol. Res. 2022, 184, 106422. [Google Scholar] [CrossRef]
- Gao, X.Q.; Liu, C.Y.; Zhang, Y.H.; Wang, Y.H.; Zhou, L.Y.; Li, X.M.; Wang, K.; Chen, X.Z.; Wang, T.; Ju, J.; et al. The circRNA CNEACR regulates necroptosis of cardiomyocytes through Foxa2 suppression. Cell Death Differ. 2022, 29, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Vausort, M.; Salgado-Somoza, A.; Zhang, L.; Leszek, P.; Scholz, M.; Teren, A.; Burkhardt, R.; Thiery, J.; Wagner, D.R.; Devaux, Y. Myocardial Infarction-Associated Circular RNA Predicting Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2016, 68, 1247–1248. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Qi, B.; Wu, X.; Peng, S.; Zhou, G.; Wei, Y.; Xu, J.; Chen, S.; Liu, S. Circular RNA Ttc3 regulates cardiac function after myocardial infarction by sponging miR-15b. J. Mol. Cell. Cardiol. 2019, 130, 10–22. [Google Scholar] [CrossRef]
- Hu, X.; Ma, R.; Cao, J.; Du, X.; Cai, X.; Fan, Y. CircSAMD4A aggravates H/R-induced cardiomyocyte apoptosis and inflammatory response by sponging miR-138-5p. J. Cell. Mol. Med. 2022, 26, 1776–1784. [Google Scholar] [CrossRef]
- Liu, B.; Guo, K. CircRbms1 knockdown alleviates hypoxia-induced cardiomyocyte injury via regulating the miR-742-3p/FOXO1 axis. Cell. Mol. Biol. Lett. 2022, 27, 31. [Google Scholar] [CrossRef]
- Bian, Y.; Pang, P.; Li, X.; Yu, S.; Wang, X.; Liu, K.; Ju, J.; Wu, H.; Gao, Y.; Liu, Q.; et al. CircHelz activates NLRP3 inflammasome to promote myocardial injury by sponging miR-133a-3p in mouse ischemic heart. J. Mol. Cell. Cardiol. 2021, 158, 128–139. [Google Scholar] [CrossRef]
- Luo, C.; Ling, G.X.; Lei, B.F.; Feng, X.; Xie, X.Y.; Fang, C.; Li, Y.G.; Cai, X.W.; Zheng, B.S. Circular RNA PVT1 silencing prevents ischemia-reperfusion injury in rat by targeting microRNA-125b and microRNA-200a. J. Mol. Cell. Cardiol. 2021, 159, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Long, T.Y.; Bi, S.S.; Sheikh, S.A.; Zhang, C.L. circPAN3 exerts a profibrotic role via sponging miR-221 through FoxO3/ATG7-activated autophagy in a rat model of myocardial infarction. Life Sci. 2020, 257, 118015. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gan, T.Y.; Li, N.; Liu, C.Y.; Zhou, L.Y.; Gao, J.N.; Chen, C.; Yan, K.W.; Ponnusamy, M.; Zhang, Y.H.; et al. Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ. 2017, 24, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Zhai, M.; Huang, Y.; Xu, S.; An, T.; Wang, Y.H.; Zhang, R.C.; Liu, C.Y.; Dong, Y.H.; Wang, M.; et al. The circular RNA ACR attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the Pink1/FAM65B pathway. Cell Death Differ. 2019, 26, 1299–1315. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liang, Y.; Zhang, M.; Yang, P.C.; Hinek, A.; Mao, S. Extracellular vesicle-derived circCEBPZOS attenuates postmyocardial infarction remodeling by promoting angiogenesis via the miR-1178-3p/PDPK1 axis. Commun. Biol. 2023, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Xue, J.; Dai, C.; Jiang, E.; Zhu, B.; Pang, H. CircSLC8A1 and circNFIX can be used as auxiliary diagnostic markers for sudden cardiac death caused by acute ischemic heart disease. Sci. Rep. 2021, 11, 4695. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dou, B.; Tang, W.; Yang, H.; Chen, K.; Wang, Y.; Qin, J.; Yang, F. Cardioprotective effects of circ_0002612 in myocardial ischemia/reperfusion injury correlate with disruption of miR-30a-5p-dependent Ppargc1a inhibition. Int. Immunopharmacol. 2023, 117, 110006. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wang, J.; Xu, G.E.; Zhao, X.; Cui, X.; Feng, J.; Sun, J.; Wang, T.; Spanos, M.; Lehmann, H.I.; et al. RNA m(6)A-Regulated circ-ZNF609 Suppression Ameliorates Doxorubicin-Induced Cardiotoxicity by Upregulating FTO. JACC Basic Transl. Sci. 2023, 8, 677–698. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Bu, X.; Wang, J.; Li, L.; Yang, Z. Circ-LTBP1 is involved in doxorubicin-induced intracellular toxicity in cardiomyocytes via miR-107/ADCY1 signal. Mol. Cell. Biochem. 2022, 477, 1127–1138. [Google Scholar] [CrossRef]
- Ji, X.; Ding, W.; Xu, T.; Zheng, X.; Zhang, J.; Liu, M.; Liu, G.; Wang, J. MicroRNA-31-5p attenuates doxorubicin-induced cardiotoxicity via quaking and circular RNA Pan3. J. Mol. Cell. Cardiol. 2020, 140, 56–67. [Google Scholar] [CrossRef]
- Zeng, Y.; Du, W.W.; Wu, Y.; Yang, Z.; Awan, F.M.; Li, X.; Yang, W.; Zhang, C.; Yang, Q.; Yee, A.; et al. A Circular RNA Binds To and Activates AKT Phosphorylation and Nuclear Localization Reducing Apoptosis and Enhancing Cardiac Repair. Theranostics 2017, 7, 3842–3855. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Chatterjee, S.; Xiao, K.; Riedel, I.; Huang, C.K.; Costa, A.; Cushman, S.; Neufeldt, D.; Rode, L.; Schmidt, A.; et al. A circular RNA derived from the insulin receptor locus protects against doxorubicin-induced cardiotoxicity. Eur. Heart J. 2022, 43, 4496–4511. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, Y.; Wang, Y.; Dai, X.; Zhou, T.; Chen, J.; Tao, B.; Zhang, J.; Cao, F. The Tumor-Suppressive Human Circular RNA CircITCH Sponges miR-330-5p to Ameliorate Doxorubicin-Induced Cardiotoxicity Through Upregulating SIRT6, Survivin, and SERCA2a. Circ. Res. 2020, 127, e108–e125. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, W.W.; Wu, N.; Li, F.; Li, X.; Xie, Y.; Wang, S.; Yang, B.B. The circular RNA circNlgnmediates doxorubicin-inducedcardiac remodeling and fibrosis. Mol. Ther. Nucleic Acids 2022, 28, 175–189. [Google Scholar] [CrossRef]
- Du, W.W.; Yang, W.; Chen, Y.; Wu, Z.K.; Foster, F.S.; Yang, Z.; Li, X.; Yang, B.B. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur. Heart J. 2017, 38, 1402–1412. [Google Scholar] [CrossRef]
- Yang, F.; Li, A.; Qin, Y.; Che, H.; Wang, Y.; Lv, J.; Li, Y.; Li, H.; Yue, E.; Ding, X.; et al. A Novel Circular RNA Mediates Pyroptosis of Diabetic Cardiomyopathy by Functioning as a Competing Endogenous RNA. Mol. Ther. Nucleic Acids 2019, 17, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, M.; Yu, Q.; Gong, M.; Wang, Y.; Yang, X.; Liu, L.; Liu, D.; Tan, Z.; Zhang, Y.; et al. CircRNA CDR1as promotes cardiomyocyte apoptosis through activating hippo signaling pathway in diabetic cardiomyopathy. Eur. J. Pharmacol. 2022, 922, 174915. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, S.; Xu, L.; Feng, Y.; Wu, X.; Zhang, M.; Yu, Z.; Zhou, X. Involvement of circHIPK3 in the pathogenesis of diabetic cardiomyopathy in mice. Diabetologia 2021, 64, 681–692. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, J.; Lin, Z.; Li, Y.; Qin, G. CircularRNA circ_0071269 knockdown protects against from diabetic cardiomyopathy injury by microRNA-145/gasdermin A axis. Bioengineered 2022, 13, 2398–2411. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, H.; Yang, C. Circ-AMOTL1 enhances cardiac fibrosis through binding with EIF4A3 and stabilizing MARCKS expression in diabetic cardiomyopathy. Cell. Signal. 2023, 111, 110853. [Google Scholar] [CrossRef]
- Yuan, Q.; Sun, Y.; Yang, F.; Yan, D.; Shen, M.; Jin, Z.; Zhan, L.; Liu, G.; Yang, L.; Zhou, Q.; et al. CircRNA DICAR as a novel endogenous regulator for diabetic cardiomyopathy and diabetic pyroptosis of cardiomyocytes. Signal Transduct. Target. Ther. 2023, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wu, Y.; Li, L.; Zhang, L.; Liu, G.; Wang, R. CircMAP3K5 promotes cardiomyocyte apoptosis in diabetic cardiomyopathy by regulating miR-22-3p/DAPK2 Axis. J. Diabetes 2024, 16, e13471. [Google Scholar] [CrossRef]
- Maron, B.J. Clinical Course and Management of Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2018, 379, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, K.; Wilczek, A.L.; de Gonzalo-Calvo, D.; Pfanne, A.; Derda, A.A.; Zwadlo, C.; Bavendiek, U.; Bauersachs, J.; Fiedler, J.; Thum, T. Serum circular RNAs act as blood-based biomarkers for hypertrophic obstructive cardiomyopathy. Sci. Rep. 2019, 9, 20350. [Google Scholar] [CrossRef] [PubMed]
- Neufeldt, D.; Schmidt, A.; Mohr, E.; Lu, D.; Chatterjee, S.; Fuchs, M.; Xiao, K.; Pan, W.; Cushman, S.; Jahn, C.; et al. Circular RNA circZFPM2 regulates cardiomyocyte hypertrophy and survival. Basic Res. Cardiol. 2024, in press. [Google Scholar] [CrossRef]
- Heymans, S.; Lakdawala, N.K.; Tschope, C.; Klingel, K. Dilated cardiomyopathy: Causes, mechanisms, and current and future treatment approaches. Lancet 2023, 402, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Reckman, Y.J.; Aufiero, S.; van den Hoogenhof, M.M.; van der Made, I.; Beqqali, A.; Koolbergen, D.R.; Rasmussen, T.B.; van der Velden, J.; Creemers, E.E.; et al. RBM20 Regulates Circular RNA Production From the Titin Gene. Circ. Res. 2016, 119, 996–1003. [Google Scholar] [CrossRef]
- Lin, Z.; Zhao, Y.; Dai, F.; Su, E.; Li, F.; Yan, Y. Analysis of changes in circular RNA expression and construction of ceRNA networks in human dilated cardiomyopathy. J. Cell. Mol. Med. 2021, 25, 2572–2583. [Google Scholar] [CrossRef]
- Phang, R.J.; Ritchie, R.H.; Hausenloy, D.J.; Lees, J.G.; Lim, S.Y. Cellular interplay between cardiomyocytes and non-myocytes in diabetic cardiomyopathy. Cardiovasc. Res. 2023, 119, 668–690. [Google Scholar] [CrossRef]
- Dannenberg, L.; Weske, S.; Kelm, M.; Levkau, B.; Polzin, A. Cellular mechanisms and recommended drug-based therapeutic options in diabetic cardiomyopathy. Pharmacol. Ther. 2021, 228, 107920. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, Y.; Liang, H.; Du, W.; Wang, L. Preliminary evidence for the presence of multiple forms of cell death in diabetes cardiomyopathy. Acta Pharm. Sin. B 2022, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, L.; Du, Y.; Zhang, Y.; Ren, J. Mitochondrial quality control mechanisms as therapeutic targets in doxorubicin-induced cardiotoxicity. Trends Pharmacol. Sci. 2023, 44, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B.; Sardao, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, W.; Deng, F.; Chen, S.; Zhu, P.; Wang, M.; Chen, X.; Wang, Y.; Hu, X.; Zhao, B.; et al. Identification of Circular RNA hsa_circ_0001599 as a Novel Biomarker for Large-Artery Atherosclerotic Stroke. DNA Cell Biol. 2021, 40, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.B.; Wu, Y.T.; Guo, X.X.; Xiang, C.; Chen, P.S.; Qin, W.; Shi, Z.S. Circular RNA hsa_circ_0007990 as a blood biomarker for unruptured intracranial aneurysm with aneurysm wall enhancement. Front. Immunol. 2022, 13, 1061592. [Google Scholar] [CrossRef]
- Chen, X.; Yang, S.; Yang, J.; Liu, Q.; Li, M.; Wu, J.; Wang, H.; Wang, S. Circular RNA circDUS2 Is a Potential Biomarker for Intracranial Aneurysm. Front. Aging Neurosci. 2021, 13, 632448. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Duan, J.; Zhou, H. Perspectives of circular RNAs in diabetic complications from biological markers to potential therapeutic targets (Review). Mol. Med. Rep. 2023, 28, 194. [Google Scholar] [CrossRef]
- Wesselhoeft, R.A.; Kowalski, P.S.; Parker-Hale, F.C.; Huang, Y.; Bisaria, N.; Anderson, D.G. RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In Vivo. Mol. Cell 2019, 74, 508–520.e4. [Google Scholar] [CrossRef]
- Seephetdee, C.; Bhukhai, K.; Buasri, N.; Leelukkanaveera, P.; Lerdwattanasombat, P.; Manopwisedjaroen, S.; Phueakphud, N.; Kuhaudomlarp, S.; Olmedillas, E.; Saphire, E.O.; et al. A circular mRNA vaccine prototype producing VFLIP-X spike confers a broad neutralization of SARS-CoV-2 variants by mouse sera. Antivir. Res. 2022, 204, 105370. [Google Scholar] [CrossRef]
- Qu, L.; Yi, Z.; Shen, Y.; Lin, L.; Chen, F.; Xu, Y.; Wu, Z.; Tang, H.; Zhang, X.; Tian, F.; et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 2022, 185, 1728–1744.e16. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhu, X.; Ye, S.; Zhang, J.; Liao, J.; Zhang, N.; Zeng, X.; Wang, J.; Yang, B.; Zhang, Y.; et al. Tumour circular RNAs elicit anti-tumour immunity by encoding cryptic peptides. Nature 2024, 625, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, K.; Liu, X.; An, R.; Komiyama, M.; Liang, X. Preferential production of RNA rings by T4 RNA ligase 2 without any splint through rational design of precursor strand. Nucleic Acids Res. 2020, 48, e54. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Guo, S.K.; Nan, F.; Xu, Y.F.; Yang, L.; Chen, L.L. RNA circles with minimized immunogenicity as potent PKR inhibitors. Mol. Cell 2022, 82, 420–434.e6. [Google Scholar] [CrossRef] [PubMed]
- He, A.T.; Liu, J.; Li, F.; Yang, B.B. Targeting circular RNAs as a therapeutic approach: Current strategies and challenges. Signal Transduct. Target. Ther. 2021, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef]
- Chen, Y.G.; Kim, M.V.; Chen, X.; Batista, P.J.; Aoyama, S.; Wilusz, J.E.; Iwasaki, A.; Chang, H.Y. Sensing Self and Foreign Circular RNAs by Intron Identity. Mol. Cell 2017, 67, 228–238.e5. [Google Scholar] [CrossRef]


| CircRNA | Expression | Targeting Cells | Functions and Mechanisms |
|---|---|---|---|
| Atherosclerosis | |||
| circ_0030042 [110] | ↓ | HUVECs | Inhibiting HUVECs autophagy; sponging eIF4A3 |
| circHIPK3 [119] | ↑ | HUVECs | Activating autophagy; targeting miR-190b/ATG7 axis |
| circRSF1 [114] | ↓ | HUVECs | Inducing cell proliferation, inhibited apoptosis and inflammation; modulating miR-135b-5p/HDAC1 axis |
| circSCRG1 [120] | ↑ | HUVECs | Promoting angiogenesis; targeting miR-1268b/NR4A1 axis |
| circZBTB46 [121] | ↑ | HUVECs; HCASMC | Increasing the atherosclerotic plaque area; interacting with hnRNPA2B1 and regulating the PTEN/AKT/mTOR pathway |
| circ_0008896 [122] | ↑ | VSMC | Enhancing proliferation, migration; sponging hsa-miR-633/CDC20B axis |
| circDLGAP4 [123] | ↑ | HUVECs | Promoting cell proliferation; targeting miR-134-5p/PTPN4 |
| circRNA-0006896 [124] | ↑ | HUVECs | Enhancing proliferation, migration; targeting miR-1264/DNMT1 |
| circ_0001785 [125] | ↑ | HUVECs | Reducing endothelial cell injury; targeting miR-513a-5p/TGFBR3 |
| CDR1as [111] | ↑ | HUVECs | Promoting the endothelial adhesion function; targeting FUS-phos-p65 |
| circHIPK3 [126] | ↑ | VSMC | Promoting cell growth; targeting miR-637/CDK6 |
| circ_0086296 [115] | ↑ | HUVECs | Promoting ECs injury; sponging miR-576-3p/IFIT1-STAT1 axis |
| circ_0065149 [127] | ↓ | HUVECs | Promoting the migration and invasion of HUVECs; targeting miR-330-5p |
| circANRIL [109] | ↑ | VSMC | Resulting in the induction of apoptosis; binding to PES1 |
| circ_0090231 [116] | ↑ | VSMC | Promoting proliferation and migration; sequestering miR-942-5p/PPM1B |
| circ_0002984 [117] | ↓ | VSMC | Promoting VMSC viability, migration and inflammation; modulating miR-326-3p and VAMP3 |
| circMAPK1 [128] | ↑ | VSMC | Promoting proliferation and migration of VSMCs; targeting miR-22-3p/MECP2 axis |
| circSQSTM1 [129] | ↑ | HUVECs | Reducing inflammation, and promoting autophagy; sponging miR-23b-3p/Sirt1; interacting with eIF4A3 |
| circ_0010283 [118] | ↑ | VSMC | Promoting cell viability and migration; targeting miR-370-3p/HMGB1 |
| circCOL1A1 [130] | ↑ | VSMC | Promoting VSMC phenotype switch; targeting miR-30a-5p/SMAD1 |
| circARHGAP12 [131] | ↑ | MASMC | Promoting VSMC phenotype switch; binding with miR-630 |
| circDENND1B [132] | ↑ | RAW264.7 | Alleviating foam-cell formation; targeting miR-17-5p/Abca1 Axis |
| circGNAQ [133] | ↓ | HUVECs | Reducing endothelial cell senescence, enhancing cell proliferation and angiogenesis; targeting miR-146a-5p-PLK2 |
| circHIF1α [134] | ↓ | HUVECs | Reducing ox-LDL-induced disabilities of endothelial proliferation; targeting miR-199a-5p/SIRT1 axis |
| Vascular injury | |||
| circEsyt2 [135] | ↑ | VSMC | Enhancing cell proliferation and migration and inhibiting apoptosis and differentiation; interacting with PCBP1 |
| circMAP3K5 [136] | ↓ | VSMC | Inhibiting VSMCs proliferation; targeting miR-22-3p/TET2 axis |
| Hsa_circ_0001402 [137] | ↓ | VSMC | Inhibiting VSMC proliferation and migration; activating VSMC autophagy; acting as a miR-183-5p sponge |
| circSOD2 [138] | ↑ | VSMC | Blocking SMC proliferation; sponging miR-206/NOTCH3 |
| circDcbld1 [139] | ↑ | VSMC | Promoting VSMC phenotype switch; targeting miR-145-3p/Nrp1 |
| circ-Sirt1 [140] | ↓ | VSMC | Repressing VSMC inflammatory response; interacting with NF-κB p65; binding to miR-132/212 |
| Arterial aneurysm | |||
| circCdyl [141] | ↑ | macrophages | Promoting M1 polarization and M1-type inflammation; inhibitingIRF4 entry into the nucleus; acting as a let-7c sponge |
| circChordc1 [142] | ↓ | VSMC | Facilitating the VSMC phenotype; binding to vimentin and ANXA2 |
| circRanGAP1 [143] | ↑ | HUVECs | Targeting miR-183-5p/miR-877-3p/MPO axis |
| circCBFB [144] | ↓ | VSMC | Repressing VSMC apoptosis; serving as a sponge of miR-28-5p |
| hsa_circ_0031608 [145] | ↑ | VSMC | Promoting the migration and proliferation capacity of VSMCs |
| hsa_circ_0087352 [146] | ↑ | VSMC | Inducing VSMC apoptosis; adsorbing hsa-miR-149-5p |
| circCCDC66 [147] | ↓ | VSMC | Inducing proliferation facilitation; sponging miR-342-3p |
| cATM [148] | ↑ | VSMC | Enhancing oxidative stress |
| circ_0020397 [149] | ↓ | VSMC | Promoting VSMC viability; targeting miR-502-5p/GREM1 axis |
| circ_0022920 [150] | ↓ | VSMC | Inhibiting HASMC proliferation and migration; sponging microRNA-650 |
| circRNAs | Expression | Functions and Mechanisms |
|---|---|---|
| Hypertrophic cardiomyopathy | ||
| circrna_000203 [175] | ↑ | Aggravating cardiac hypertrophy; suppressing mir-26b-5p-mir-140-3p/Gata4 |
| circUtrn [176] | ↑ | Preventing acute myocardial injury and pathological cardiac remodeling; binding to PP5 |
| circrna_0068481 [177] | ↑ | Promoting right ventricular hypertrophy; sponging mir-646, mir-570 and mir-885 |
| circHIPK3 [178] | ↑ | Inhibiting cardiac hypertrophy and dysfunction; sponging mir-185-3p |
| circ_0001006 [179] | ↑ | Aggravating cardiac hypertrophy; sponging mir-214-3p/PAK6 |
| circCMISS1 [180] | ↑ | Activating the ferroptosis and promoting cardiac hypertrophy; interacting with EIF4A3 |
| HRCR [181] | ↓ | Inhibiting cardiac hypertrophy and heart failure; sponging mir-223/ARC axis |
| circ-Ddx60 [182] | ↑ | Aggravating cardiac hypertrophy; binding and activating eef2 |
| circ_0001052 [183] | ↑ | Promotes cardiac hypertrophy; sponging mir-148a-3p and mir-124-3p |
| circ-SH3RF3 [184] | ↓ | Inhibiting myocardial fibrosis; interacting with GATA4 |
| circSlc8a1 [185] | ↑ | Promoting cardiac hypertrophy; sponging mir-133a |
| circCACNA1c [186] | ↑ | Promoting pathological hypertrophy; binding to mir-29b-2-5p |
| ca-circSlc8a1 [187] | ↓ | Inhibiting congestive heart failure and maintaining heart function; translocating into mitochondria to drive ATP synthesis |
| circPAN3 [188] | ↓ | Attenuating cardiomyocyte hypertrophy; targeting mir-320-3p |
| circ-SIRT1 [189] | ↓ | Attenuating autophagy and cardiac hypertrophy; sponging mir-3681-3p/mir-5195-3p and stabilizing SIRT1 protein by recruiting USP22 |
| circ_0018553 [190] | ↓ | Attenuating Ang II-induced cardiac hypertrophy; sponging mir-4731/SIRT2 signaling pathway |
| Engineered circular RNA [191] | ↓ | Attenuating cardiomyocyte hypertrophy; sponging mir-132 and -212 |
| circYAP [192] | ↓ | Attenuating cardiac fibrosis; binding with TMP4 and ACTG |
| circITGA9 [193] | ↑ | Promoting cardiac fibrosis; binding with tropomyosin 3 |
| Myocardial infarction | ||
| circSamd4 [170] | ↓ | Inducing CM proliferation and preventing CM apoptosis; inducing the mitochondrial translocation of the Vcp protein |
| circNfix [166] | ↑ | Inhibiting cardiomyocyte proliferation and angiogenesis and promoting cardiomyocyte apoptosis; interacting with Ybx1; acting as a sponge for mir-214 |
| circUBE3a [194] | ↑ | Promoting CF proliferation, migration, and phenotypic transformation; exacerbating myocardial fibrosis; sponging mir-138-5p |
| circFEACR [164] | ↓ | Inhibiting hypoxia and reoxygenation-induced ferroptosis; mediating NAMPT-Sirt1-FOXO1-FTH1 signaling axis |
| circHIPK3 [195] | ↓ | Attenuating cardiac dysfunction and decreasing fibrotic area; binding to Notch1 and mir-133a |
| circSNRK [163] | ↓ | Reducing apoptosis and promoting cardiac repair; targeting mir-103-3p/SNRK axis |
| circWHSC1 [196] | ↑ | Inducing CM proliferation, alleviating cardiac fibrosis and restoring cardiac function; reinforcing the binding of TRIM59 to STAT3 by enhancing TRIM59 phosphorylation |
| circPOSTN [197] | ↑ | Promoting myocardial injury and cardiac remodeling; serving as a mir-96-5p/BNIP3 axis |
| circ-NNT [165] | ↑ | Activating pyroptosis; sponging mir-33a-5p/USP46 axis |
| circFndc3b [173] | ↓ | Reducing cardiomyocyte apoptosis, enhancing neovascularization; interacting with FUS to regulate VEGF expression |
| circROBO2 [198] | ↑ | Promoting myocardial apoptosis; targeting mir-1184/TRADD |
| circHELZ [199] | ↑ | Exacerbating myocardial fibroblast proliferation and differentiation; facilitating YAP localization in the nucleus |
| circ_0001206 [200] | ↓ | Promoting cell viability and inhibiting cardiomyocyte apoptosis; sponging mir-665 and regulating CRKL expression |
| circ Foxo3 [201] | ↓ | Ameliorating cardiac autophagy, apoptosis, inflammation; inhibiting HMGB1 by repressing KAT7 |
| circ-SNRK [202] | ↓ | Improving the cardiac function by improving the ATP synthesis; sponging mir-33/SNRK axis |
| circ_0060745 [203] | ↑ | Promoting macrophage migration and cardiomyocyte apoptosis; activating NF-κB |
| circMDC1 [204] | ↑ | Blunting the regenerative capacity of neonatal hearts; binding to PABP |
| CNEACR [205] | ↓ | Attenuating myocardial necrosis; binding to HDAC7 and increasing FOXA2 expression |
| MICRA [206] | ↓ | Predicting LV dysfunction |
| circTtc3 [207] | ↑ | Counteracting hypoxia-induced ATP depletion and apoptotic death; sponging mir-15b-5p/Arl2 axis |
| circERBB2IP [174] | ↓ | Promoting CMEC proliferation, migration, and tube formation; targeting mir-145a-5p/Smad5 axis |
| circSAMD4a [208] | ↑ | Aggravating H/R-induced cardiomyocyte apoptosis and inflammatory response; sponging mir-138-5p |
| circrBMS1 [209] | ↑ | Aggravating hypoxia-induced cardiomyocyte injury; sponging mir-742-3p/FOXO1 axis |
| circHELZ [210] | ↑ | Activating NLRP3 inflammasome; sponging mir-133a-3p |
| circMAP4K2 [167] | ↑ | Promoting cardiomyocyte proliferation; binding to mir-106a-3p |
| circPVT1 [211] | ↑ | Inhibiting cardiomyocyte proliferation and viability, and promoting apoptosis; sponging mir-125b and mir-200a |
| circPan3 [212] | ↑ | Increasing cell proliferation and migration; sponging mir-221 through foxo3/ATG7-activated autophagy |
| MFACR [213] | ↑ | Promoting mitochondrial fission and cardiomyocyte apoptosis; sponging mir-652-3p and increasing MTP18 expression |
| ACR [214] | ↓ | Attenuating myocardial ischemia/reperfusion injury by suppressing autophagy activating Pink1 expression; binding to Dnmt3B and |
| circCEBPZOS [215] | ↓ | Attenuating myocardia remodeling by promoting angiogenesis; targeting mir-1178-3p/PDPK1 |
| circSlc8a1/circNfix [216] | ↑/↓ | Auxiliary diagnostic markers for SCD caused by acute IHD |
| circ_0002612 [217] | ↓ | Promoting cardiomyocyte viability; targeting mir-30a-5p/Ppargc1a axis |
| Myocardial injury | ||
| circ-ZNF609 [218] | ↑ | Aggravating cardiomyocyte apoptosis, promoting ROS production and increasing iron overload; repressing FTO expression |
| circ-Ltbp1 [219] | ↑ | Aggravating DOX-induced proliferation inhibition, inflammation, apoptosis, and oxidative stress; increasing ADCY1 expression by sponging mir-107 |
| circPan3 [220] | ↓ | Inhibiting DOX-induced myocardial apoptosis |
| circ-Amotl1 [221] | ↓ | Protecting against Dox-induced cardiomyopathy; increasing the nuclear fraction of pAKT |
| circ-INSR [222] | ↓ | Reducing cardiomyocyte cell death and mitochondrial damage; interacting with SSBP1 |
| circITCH [223] | ↓ | Alleviating oxidative stress and DNA damage induced by doxorubicin; sponging mir-330-5p |
| circNlgn [224] | ↑ | Decreasing cardiac function and inducing cardiac fibrosis; binding and activating H2AX |
| Quaking Inhibits Doxorubicin-Mediated Cardiotoxicity Through Regulation of Cardiac Circular RNA Expression [43] | ||
| Circ-Foxo3 [225] | ↑ | Inducing cellular senescence of mefs; interacting with ID-1 and E2F1 |
| Diabetic cardiomyopathy | ||
| CACR [226] | ↑ | Aggravating DCM pyroptosis; sponging mir-214-3p |
| CDR1as [227] | ↑ | Promoting cardiomyocyte apoptosis; activating the Hippo signaling pathway |
| circHIPK3 [228] | ↑ | Increasing myocardial fibrosis; sponging mir-29b-3p |
| circ_0071269 [229] | ↑ | Increasing myocardial damage; sponging mir-145 |
| circ-Amotl1 [230] | ↑ | Enhancing cardiac fibrosis; binding with EIF4A3 and stabilizing MARCKS expression |
| DICAR [231] | ↓ | Alleviating diabetic cardiomyocyte pyroptosis; binding to VCP |
| circMAP3K5 [232] | ↑ | Promoting cardiomyocyte cell apoptosis; sponging mir-22-3p |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, S.; Ma, X.; Zhou, L.; Wuyun, Q.; Cai, Z.; Yan, J.; Ding, H. Circular RNA in Cardiovascular Diseases: Biogenesis, Function and Application. Biomolecules 2024, 14, 952. https://doi.org/10.3390/biom14080952
Mei S, Ma X, Zhou L, Wuyun Q, Cai Z, Yan J, Ding H. Circular RNA in Cardiovascular Diseases: Biogenesis, Function and Application. Biomolecules. 2024; 14(8):952. https://doi.org/10.3390/biom14080952
Chicago/Turabian StyleMei, Shuai, Xiaozhu Ma, Li Zhou, Qidamugai Wuyun, Ziyang Cai, Jiangtao Yan, and Hu Ding. 2024. "Circular RNA in Cardiovascular Diseases: Biogenesis, Function and Application" Biomolecules 14, no. 8: 952. https://doi.org/10.3390/biom14080952





