Burst-Suppression EEG Reactivity to Photic Stimulation—A Translational Biomarker in Hypoxic–Ischemic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
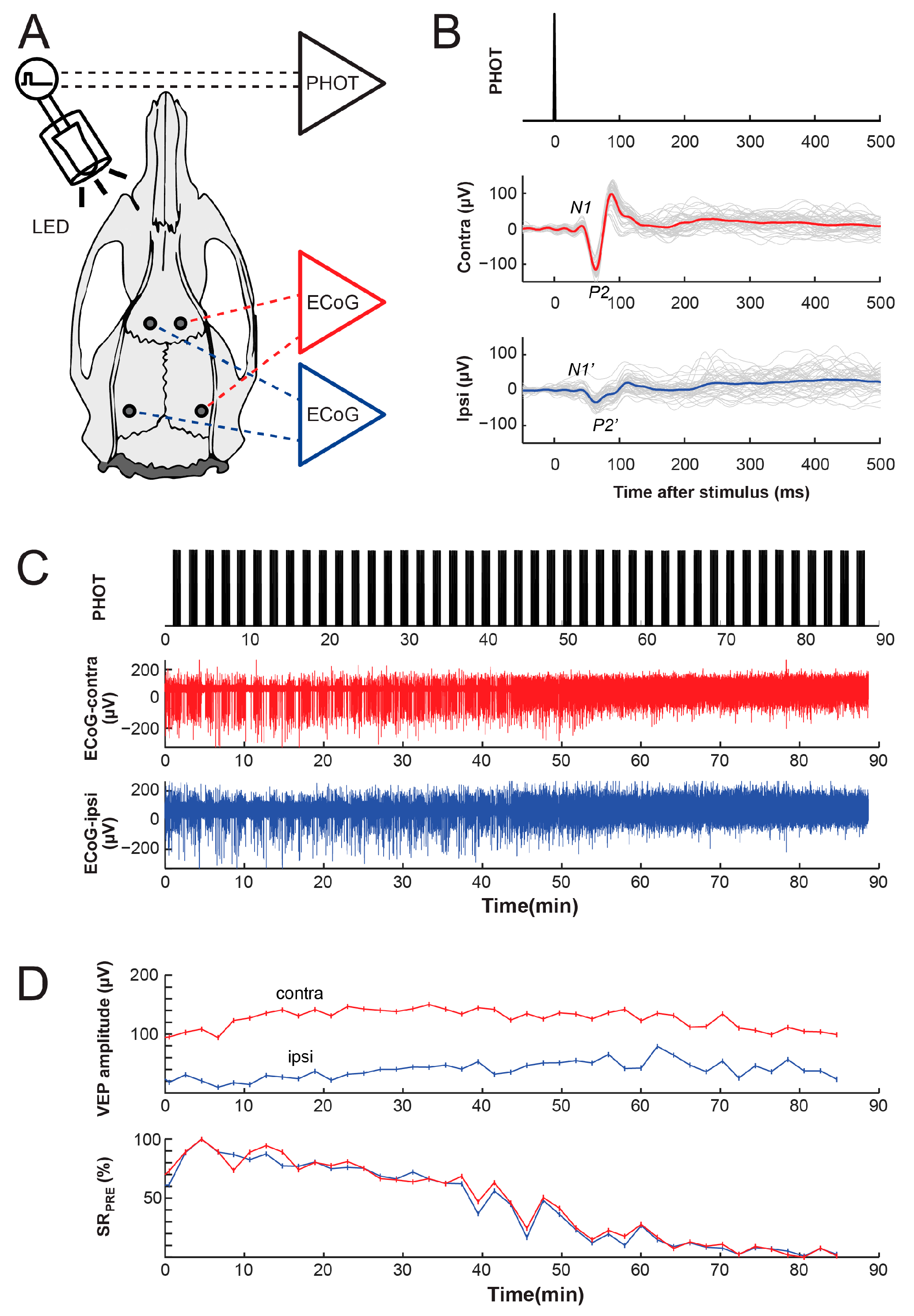
2.2. EEG Setup
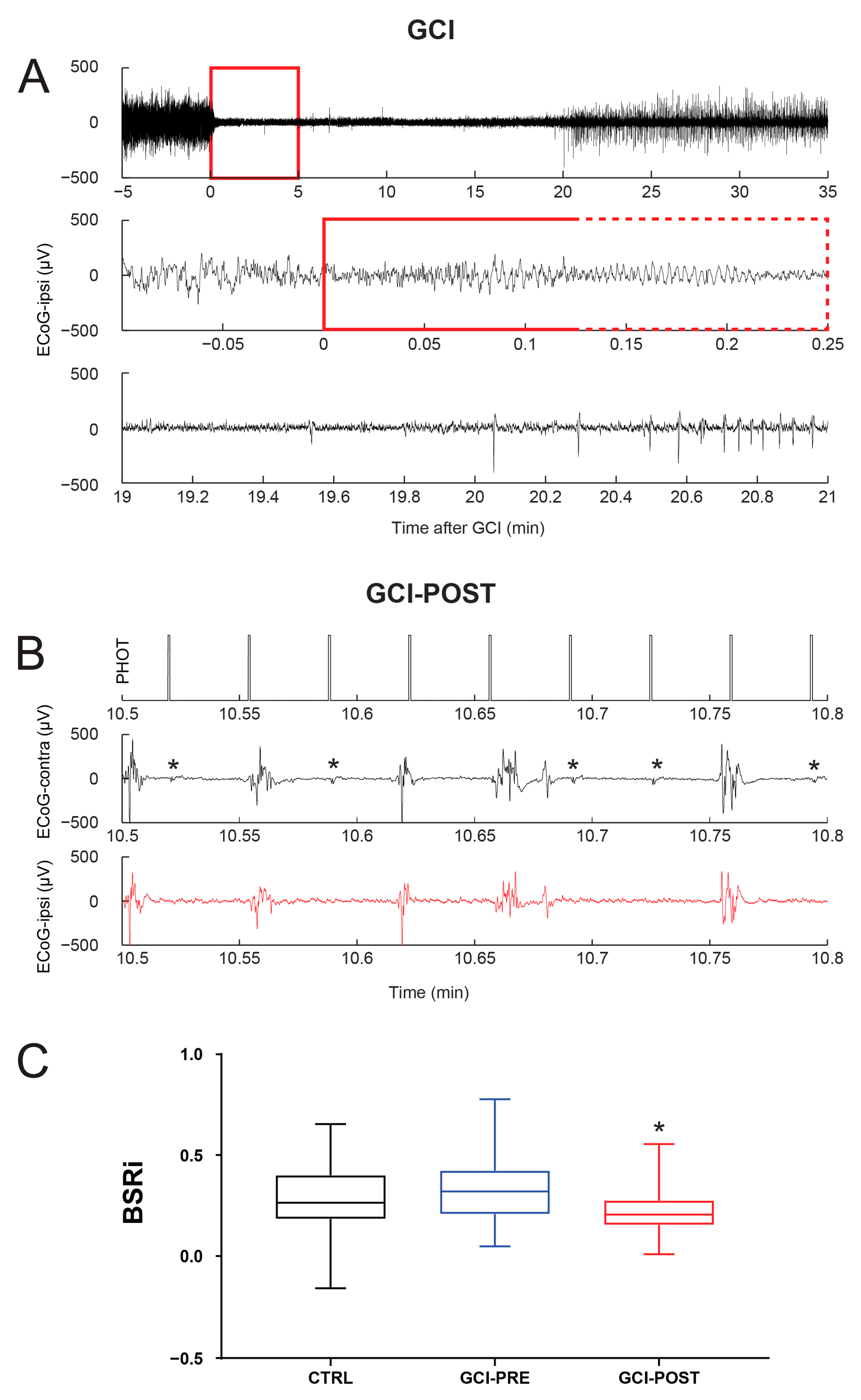
2.3. Transient Global Cerebral Ischemia (GCI) Model
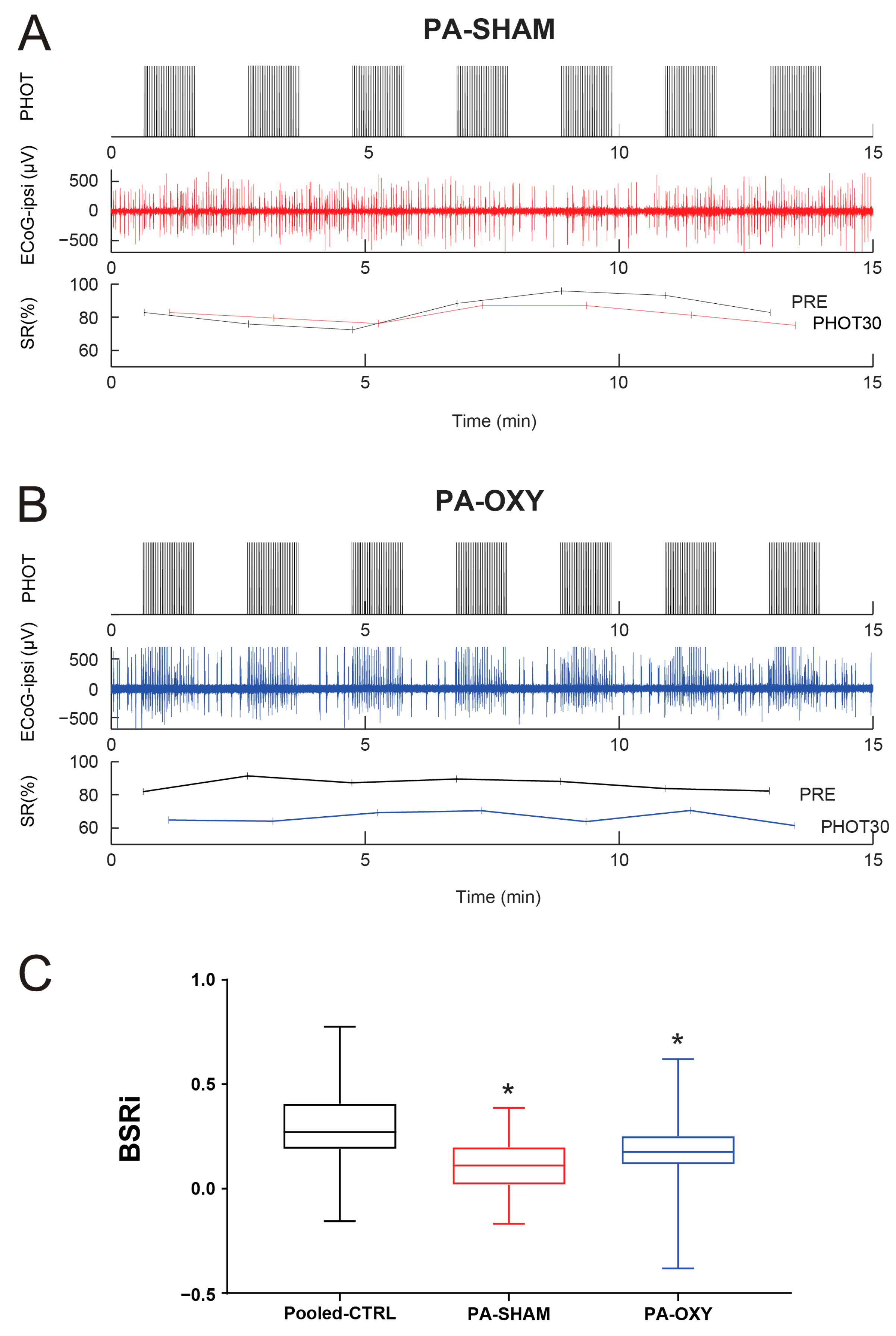
2.4. Experimental Perinatal Asphyxia (PA) Model
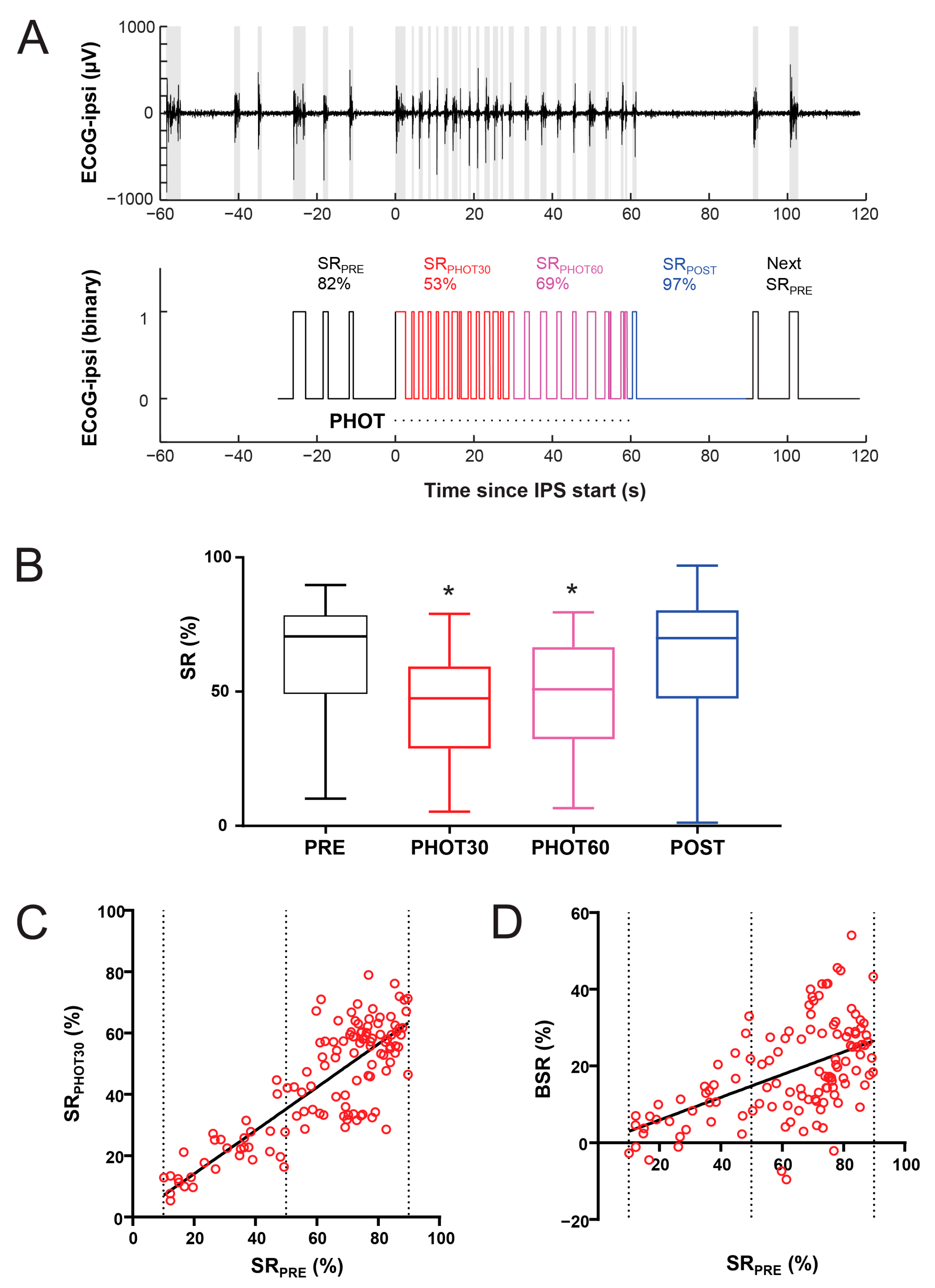
2.5. EEG Reactivity Recordings
2.6. Cortical EEG Reactivity Measurements
2.7. Data and Statistics
3. Results
3.1. Reactivity of Anesthetic Burst-Suppression Patterns to Photic Stimulation
3.2. Reactivity of Burst-Suppression Patterns Following Global Cerebral Ischemia (GCI)
3.3. Reactivity of Burst-Suppression Patterns Following Perinatal Asphyxia (PA)
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landucci, E.; Pellegrini-Giampietro, D.E.; Facchinetti, F. Experimental Models for Testing the Efficacy of Pharmacological Treatments for Neonatal Hypoxic-Ischemic Encephalopathy. Biomedicines 2022, 10, 937. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.C.; Dodson, S.C.; Rosen, C.L.; Huber, J.D. The Science of Cerebral Ischemia and the Quest for Neuroprotection: Navigating Past Failure to Future Success. J. Neurosurg. 2013, 118, 1072–1085. [Google Scholar] [CrossRef]
- Hoiland, R.L.; Rikhraj, K.J.K.; Thiara, S.; Fordyce, C.; Kramer, A.H.; Skrifvars, M.B.; Wellington, C.L.; Griesdale, D.E.; Fergusson, N.A.; Sekhon, M.S. Neurologic Prognostication After Cardiac Arrest Using Brain Biomarkers: A Systematic Review and Meta-Analysis. JAMA Neurol. 2022, 79, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Chalak, L.; Hellstrom-Westas, L.; Bonifacio, S.; Tsuchida, T.; Chock, V.; El-Dib, M.; Massaro, A.N.; Garcia-Alix, A. Newborn Brain Society Guidelines and Publications Bedside and Laboratory Neuromonitoring in Neonatal Encephalopathy. Semin. Fetal Neonatal Med. 2021, 26, 101273. [Google Scholar] [CrossRef] [PubMed]
- Fenter, H.; Rossetti, A.O.; Beuchat, I. Continuous versus Routine Electroencephalography in the Intensive Care Unit: A Review of Current Evidence. Eur. Neurol. 2024, 87, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Duez, C.H.V.; Ebbesen, M.Q.; Benedek, K.; Fabricius, M.; Atkins, M.D.; Beniczky, S.; Kjaer, T.W.; Kirkegaard, H.; Johnsen, B. Large Inter-Rater Variability on EEG-Reactivity Is Improved by a Novel Quantitative Method. Clin. Neurophysiol. 2018, 129, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Caroyer, S.; Depondt, C.; Rikir, E.; Mavroudakis, N.; Peluso, L.; Silvio Taccone, F.; Legros, B.; Gaspard, N. Assessment of a Standardized EEG Reactivity Protocol after Cardiac Arrest. Clin. Neurophysiol. 2021, 132, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Cho, S.-M.; Geocadin, R.; Ritzl, E.K. Methods of Evaluating EEG Reactivity in Adult Intensive Care Units: A Review. J. Clin. Neurophysiol. 2024. [Google Scholar] [CrossRef]
- Turella, S.; Dankiewicz, J.; Ben-Hamouda, N.; Bernhard Nilsen, K.; Düring, J.; Endisch, C.; Engstrøm, M.; Flügel, D.; Gaspard, N.; Grejs, A.M.; et al. EEG for Good Outcome Prediction after Cardiac Arrest: A Multicentre Cohort Study. Resuscitation 2024, 110319. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Tian, F.; Chen, W.; Cui, L.; Jiang, M.; Zhang, Y.; Gao, K.; Su, Y.; Wang, H. Quantitative EEG Reactivity Induced by Electrical Stimulation Predicts Good Outcome in Comatose Patients after Cardiac Arrest. Ann. Intensive Care 2024, 14, 99. [Google Scholar] [CrossRef]
- Timofeev, I.; Grenier, F.; Bazhenov, M.; Sejnowski, T.J.; Steriade, M. Origin of Slow Cortical Oscillations in Deafferented Cortical Slabs. Cereb. Cortex 2000, 10, 1185–1199. [Google Scholar] [CrossRef]
- Fischer-Williams, M.; Last, S.T.; Lyberi, G.; Northfield, D.W.C. Clinico-EEG Study of 128 Gliomas and 50 Intracranial Metastatic Tumors. Brain 1962, 85, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Scharfetter, C.; Schmoigl, S. The Isoelectric Electroencephalogram. Electroencephalogr. Clin. Neurophysiol. 1968, 24, 92. [Google Scholar]
- Kroeger, D.; Florea, B.; Amzica, F. Human Brain Activity Patterns beyond the Isoelectric Line of Extreme Deep Coma. PLoS ONE 2013, 8, e75257. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, K.; Rorarius, M.; Mäkelä, K.; Peräkylä, J.; Varila, E.; Jäntti, V. Visually Evoked Bursts during Isoflurane Anaesthesia. Br. J. Anaesth. 1995, 74, 681–685. [Google Scholar] [CrossRef]
- Akrawi, W.P.; Drummond, J.C.; Kalkman, C.J.; Patel, P.M. A Comparison of the Electrophysiologic Characteristics of EEG Burst-Suppression as Produced by Isoflurane, Thiopental, Etomidate, and Propofol. J. Neurosurg. Anesthesiol. 1996, 8, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Vijn, P.C.; Sneyd, J.R.I.v. Anaesthesia and EEG Burst Suppression in Rats: Bolus Injections and Closed-Loop Infusions. Br. J. Anaesth. 1998, 81, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Ching, S.; Liberman, M.Y.; Chemali, J.J.; Westover, M.B.; Kenny, J.D.; Solt, K.; Purdon, P.L.; Brown, E.N. Real-Time Closed-Loop Control in a Rodent Model of Medically Induced Coma Using Burst Suppression. Anesthesiology 2013, 119, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, K.M.; Rorarius, M.; Peräkylä, J.J.; Laippala, P.J.; Jäntti, V. Cortical Reactivity during Isoflurane Burst-Suppression Anesthesia. Anesth. Analg. 1995, 81, 1223–1228. [Google Scholar] [CrossRef]
- Liu, G.; Tian, F.; Zhu, Y.; Jiang, M.; Cui, L.; Zhang, Y.; Wang, Y.; Su, Y. The Predictive Value of EEG Reactivity by Electrical Stimulation and Quantitative Analysis in Critically Ill Patients with Large Hemispheric Infarction. J. Crit. Care 2023, 78, 154358. [Google Scholar] [CrossRef]
- Călin, A.; Kumaraswamy, V.M.; Braver, D.; Nair, D.G.; Moldovan, M.; Simon, M.V. Intraoperative Somatosensory Evoked Potential Monitoring Decreases EEG Burst Suppression Ratio during Deep General Anesthesia. J. Clin. Neurophysiol. 2014, 31, 133–137. [Google Scholar] [CrossRef]
- Nita, D.A.; Moldovan, M.; Sharma, R.; Avramescu, S.; Otsubo, H.; Hahn, C.D. Burst-Suppression Is Reactive to Photic Stimulation in Comatose Children with Acquired Brain Injury. Clin. Neurophysiol. 2016, 127, 2921–2930. [Google Scholar] [CrossRef]
- Pulsinelli, W.A.; Brierley, J.B. A New Model of Bilateral Hemispheric Ischemia in the Unanesthetized Rat. Stroke 1979, 10, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Ilie, A.; Ciocan, D.; Zagrean, A.-M.; Nita, D.A.; Zagrean, L.; Moldovan, M. Endogenous Activation of Adenosine A(1) Receptors Accelerates Ischemic Suppression of Spontaneous Electrocortical Activity. J. Neurophysiol. 2006, 96, 2809–2814. [Google Scholar] [CrossRef]
- Helmy, M.M.; Tolner, E.A.; Vanhatalo, S.; Voipio, J.; Kaila, K. Brain Alkalosis Causes Birth Asphyxia Seizures, Suggesting Therapeutic Strategy. Ann. Neurol. 2011, 69, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, A.M.; Isac, S.; Pavel, B.; Ilie, A.S.; Ceanga, M.; Totan, A.; Zagrean, L.; Peltecu, G.; Zagrean, A.M. Oxytocin Reduces Seizure Burden and Hippocampal Injury in a Rat Model of Perinatal Asphyxia. Acta Endocrinol. 2018, 14, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Ilie, A.; Spulber, S.; Avramescu, S.; Nita, D.A.; Zagrean, A.-M.; Zagrean, L.; Moldovan, M. Delayed Ischemic Electrocortical Suppression during Rapid Repeated Cerebral Ischemia and Kainate-Induced Seizures in Rat. Eur. J. Neurosci. 2006, 23, 2135–2144. [Google Scholar] [CrossRef]
- Lee, J.M.; Grabb, M.C.; Zipfel, G.J.; Choi, D.W. Brain Tissue Responses to Ischemia. J. Clin. Investig. 2000, 106, 723–731. [Google Scholar] [CrossRef]
- Baron, J.-C.; Yamauchi, H.; Fujioka, M.; Endres, M. Selective Neuronal Loss in Ischemic Stroke and Cerebrovascular Disease. J. Cereb. Blood Flow Metab. 2014, 34, 2–18. [Google Scholar] [CrossRef]
- Ilie, A.; Ciocan, D.; Constantinescu, A.O.; Zagrean, A.-M.; Nita, D.A.; Zagrean, L.; Moldovan, M. Endogenous Activation of Adenosine A1 Receptors Promotes Post-Ischemic Electrocortical Burst Suppression. Neuroscience 2009, 159, 1070–1078. [Google Scholar] [CrossRef]
- Ala-Kurikka, T.; Pospelov, A.; Summanen, M.; Alafuzoff, A.; Kurki, S.; Voipio, J.; Kaila, K. A Physiologically Validated Rat Model of Term Birth Asphyxia with Seizure Generation after, Not during, Brain Hypoxia. Epilepsia 2021, 62, 908–919. [Google Scholar] [CrossRef]
- Neumann, I.D.; Maloumby, R.; Beiderbeck, D.I.; Lukas, M.; Landgraf, R. Increased Brain and Plasma Oxytocin after Nasal and Peripheral Administration in Rats and Mice. Psychoneuroendocrinology 2013, 38, 1985–1993. [Google Scholar] [CrossRef]
- Rampil, I.J.; Laster, M.J. No Correlation between Quantitative Electroencephalographic Measurements and Movement Response to Noxious Stimuli during Isoflurane Anesthesia in Rats. Anesthesiology 1992, 77, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.L.; Guo, Y.; Kelman, S.E.; Flower, R.W.; Johnson, M.A. Functional and Cellular Responses in a Novel Rodent Model of Anterior Ischemic Optic Neuropathy. Invest. Ophthalmol. Vis. Sci. 2003, 44, 4153–4162. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, M.; Westerlund, U.; Langmoen, I.A.; Svensson, M. Methylprednisolone Treatment Does Not Influence Axonal Regeneration or Degeneration Following Optic Nerve Injury in the Adult Rat. J. Neuroophthalmol. 2004, 24, 11–18. [Google Scholar] [CrossRef]
- Goto, Y.; Furuta, A.; Tobimatsu, S. Magnesium Deficiency Differentially Affects the Retina and Visual Cortex of Intact Rats. J. Nutr. 2001, 131, 2378–2381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amzica, F. Basic Physiology of Burst-Suppression. Epilepsia 2009, 50 (Suppl. 12), 38–39. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.; Calin, A.; Kumaraswamy, V.M.; Braver, D.; Simon, M.V. Burst-Suppression Ratio on Electrocorticography Depends on Interelectrode Distance. J. Clin. Neurophysiol. 2016, 33, 127–132. [Google Scholar] [CrossRef]
- Constantinescu, A.O.; Ilie, A.; Ciocan, D.; Zagrean, A.-M.; Zagrean, L.; Moldovan, M. Endogenous Adenosine A1 Receptor Activation Underlies the Transient Post-Ischemic Rhythmic Delta EEG Activity. Clin. Neurophysiol. 2011, 122, 1117–1126. [Google Scholar] [CrossRef]
- Onofrj, M.; Harnois, C.; Bodis-Wollner, I. The Hemispheric Distribution of the Transient Rat VEP: A Comparison of Flash and Pattern Stimulation. Exp. Brain Res. 1985, 59, 427–433. [Google Scholar] [CrossRef]
- Lewis, L.D.; Ching, S.; Weiner, V.S.; Peterfreund, R.A.; Eskandar, E.N.; Cash, S.S.; Brown, E.N.; Purdon, P.L. Local Cortical Dynamics of Burst Suppression in the Anaesthetized Brain. Brain 2013, 136, 2727–2737. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, J.; Hussain, M.S.; Brindley, P.G.; Jacka, M.J.; Gross, D.W. The Role of the Standard 20 Minute EEG Recording in the Comatose Patient. J. Clin. Neurosci. 2010, 17, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Gavaret, M.; Marchi, A.; Lefaucheur, J.-P. Clinical Neurophysiology of Stroke. Handb. Clin. Neurol. 2019, 161, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Neumar, R.; Hsu, C.H.; Hirsch, K.G.; Aneman, A.; Becker, L.B.; Couper, K.; Callaway, C.W.; Hoedemaekers, C.W.E.; Lim, S.L.; et al. Improving Outcomes After Post-Cardiac Arrest Brain Injury: A Scientific Statement From the International Liaison Committee on Resuscitation. Circulation 2024. [Google Scholar] [CrossRef] [PubMed]
- Hartings, J.A.; Williams, A.J.; Tortella, F.C. Occurrence of Nonconvulsive Seizures, Periodic Epileptiform Discharges, and Intermittent Rhythmic Delta Activity in Rat Focal Ischemia. Exp. Neurol. 2003, 179, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Zayachkivsky, A.; Lehmkuhle, M.J.; Ekstrand, J.J.; Dudek, F.E. Background Suppression of Electrical Activity Is a Potential Biomarker of Subsequent Brain Injury in a Rat Model of Neonatal Hypoxia-Ischemia. J. Neurophysiol. 2022, 128, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, A.O.; Claassen, J.; Gaspard, N. Status Epilepticus in the ICU. Intensive Care Med. 2024, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Koenig, M.A.; Kaplan, P.W.; Thakor, N.V. Clinical Neurophysiologic Monitoring and Brain Injury from Cardiac Arrest. Neurol. Clin. 2006, 24, 89–106. [Google Scholar] [CrossRef]
- Barkhuizen, M.; Vles, J.S.H.; van Mechelen, R.; Vermeer, M.; Kramer, B.W.; Chedraui, P.; Bergs, P.; van Kranen-Mastenbroek, V.H.J.M.; Gavilanes, A.W.D. Preterm Perinatal Hypoxia-Ischemia Does Not Affect Somatosensory Evoked Potentials in Adult Rats. Diagnostics 2019, 9, 123. [Google Scholar] [CrossRef]
- Kroeger, D.; Amzica, F. Hypersensitivity of the Anesthesia-Induced Comatose Brain. J. Neurosci. 2007, 27, 10597–10607. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Alvarez, A.A.; Rodríguez, J.J.; Lafuente, H.; Canduela, M.J.; Hind, W.; Blanco-Bruned, J.L.; Alonso-Alconada, D.; Hilario, E. Effects of Cannabidiol, Hypothermia, and Their Combination in Newborn Rats with Hypoxic-Ischemic Encephalopathy. eNeuro 2023, 10, ENEURO.0417-22.2023. [Google Scholar] [CrossRef] [PubMed]
- Kamrani-Sharif, R.; Hayes, A.W.; Gholami, M.; Salehirad, M.; Allahverdikhani, M.; Motaghinejad, M.; Emanuele, E. Oxytocin as Neuro-Hormone and Neuro-Regulator Exert Neuroprotective Properties: A Mechanistic Graphical Review. Neuropeptides 2023, 101, 102352. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Benameur, T.; Porro, C. Hypothalamic Neuropeptide Brain Protection: Focus on Oxytocin. J. Clin. Med. Res. 2020, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, M.I.; Grigoras, I.-F.; Ionescu, R.-B.; Chitimus, D.M.; Haret, R.M.; Ianosi, B.; Ceanga, M.; Zagrean, A.-M. Oxytocin Exhibits Neuroprotective Effects on Hippocampal Cultures under Severe Oxygen-Glucose Deprivation Conditions. Curr. Issues Mol. Biol. 2024, 46, 6223–6236. [Google Scholar] [CrossRef] [PubMed]
- Ceanga, M.; Spataru, A.; Zagrean, A.-M. Oxytocin Is Neuroprotective against Oxygen-Glucose Deprivation and Reoxygenation in Immature Hippocampal Cultures. Neurosci. Lett. 2010, 477, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Deng, S.; Jiang, J.; Tian, M.; Xiao, L.; Gong, Y. Oxytocin Improves Intracerebral Hemorrhage Outcomes by Suppressing Neuronal Pyroptosis and Mitochondrial Fission. Stroke 2023, 54, 1888–1900. [Google Scholar] [CrossRef]
- Erbas, O.; Yılmaz, M.; Korkmaz, H.A.; Bora, S.; Evren, V.; Peker, G. Oxytocin Inhibits Pentylentetrazol-Induced Seizures in the Rat. Peptides 2013, 40, 141–144. [Google Scholar] [CrossRef]
- Ching, S.; Purdon, P.L.; Vijayan, S.; Kopell, N.J.; Brown, E.N. A Neurophysiological-Metabolic Model for Burst Suppression. Proc. Natl. Acad. Sci. USA 2012, 109, 3095–3100. [Google Scholar] [CrossRef]
- Saklani, P.; Khan, H.; Gupta, S.; Kaur, A.; Singh, T.G. Neuropeptides: Potential Neuroprotective Agents in Ischemic Injury. Life Sci. 2022, 288, 120186. [Google Scholar] [CrossRef]
- Cahen, R.L.; Wikler, A. Effects of Morphine on Cortical Electrical Activity of the Rat. Yale J. Biol. Med. 1944, 16, 239–244.2. [Google Scholar]
- Maheshwari, A. Rodent EEG: Expanding the Spectrum of Analysis. Epilepsy Curr. 2020, 20, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, P.C.; Brenner, R.P. Correlation of EEG, Computerized Tomography, and Clinical Findings. Study of 100 Patients with Focal Delta Activity. Arch. Neurol. 1981, 38, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, S.P.; Rose, S.E.; Chalk, J.B. Rapid EEG Changes Indicate Reperfusion after Tissue Plasminogen Activator Injection in Acute Ischaemic Stroke. Clin. Neurophysiol. 2006, 117, 2338–2339. [Google Scholar] [CrossRef]
- De Mendonça, A.; Sebastião, A.M.; Ribeiro, J.A. Adenosine: Does It Have a Neuroprotective Role after All? Brain Res. Brain Res. Rev. 2000, 33, 258–274. [Google Scholar] [CrossRef]
- Sun, H.; Gonzalez, F.; McQuillen, P.S. Caffeine Restores Background EEG Activity Independent of Infarct Reduction after Neonatal Hypoxic Ischemic Brain Injury. Dev. Neurosci. 2020, 42, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Bouchereau, E.; Marchi, A.; Hermann, B.; Pruvost-Robieux, E.; Guinard, E.; Legouy, C.; Schimpf, C.; Mazeraud, A.; Baron, J.-C.; Ramdani, C.; et al. Quantitative Analysis of Early-Stage EEG Reactivity Predicts Awakening and Recovery of Consciousness in Patients with Severe Brain Injury. Br. J. Anaesth. 2023, 130, e225–e232. [Google Scholar] [CrossRef]
- Johnsen, B.; Jeppesen, J.; Duez, C.H.V. Common Patterns of EEG Reactivity in Post-Anoxic Coma Identified by Quantitative Analyses. Clin. Neurophysiol. 2022, 142, 143–153. [Google Scholar] [CrossRef]
- Shoaib, M.; Choudhary, R.C.; Chillale, R.K.; Kim, N.; Miyara, S.J.; Haque, S.; Yin, T.; Frankfurt, M.; Molmenti, E.P.; Zanos, S.; et al. Metformin-Mediated Mitochondrial Protection Post-Cardiac Arrest Improves EEG Activity and Confers Neuroprotection and Survival Benefit. FASEB J. 2022, 36, e22307. [Google Scholar] [CrossRef]
- Sandroni, C.; D’Arrigo, S.; Cacciola, S.; Hoedemaekers, C.W.E.; Kamps, M.J.A.; Oddo, M.; Taccone, F.S.; Di Rocco, A.; Meijer, F.J.A.; Westhall, E.; et al. Prediction of Poor Neurological Outcome in Comatose Survivors of Cardiac Arrest: A Systematic Review. Intensive Care Med. 2020, 46, 1803–1851. [Google Scholar] [CrossRef]
- Hofmeijer, J.; Tjepkema-Cloostermans, M.C.; van Putten, M.J.A.M. Burst-Suppression with Identical Bursts: A Distinct EEG Pattern with Poor Outcome in Postanoxic Coma. Clin. Neurophysiol. 2014, 125, 947–954. [Google Scholar] [CrossRef]
- Abend, N.S.; Licht, D.J. Predicting Outcome in Children with Hypoxic Ischemic Encephalopathy. Pediatr. Crit. Care Med. 2008, 9, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Amzica, F. What Does Burst Suppression Really Mean? Epilepsy Behav. 2015, 49, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Șerban, C.-A.; Barborică, A.; Roceanu, A.-M.; Mîndruță, I.-R.; Ciurea, J.; Stancu, M.; Pâslaru, A.C.; Zăgrean, A.-M.; Zăgrean, L.; Moldovan, M. Towards an Electroencephalographic Measure of Awareness Based on the Reactivity of Oscillatory Macrostates to Hearing a Subject’s Own Name. Eur. J. Neurosci. 2024, 59, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Serban, C.-A.; Barborica, A.; Roceanu, A.-M.; Mindruta, I.; Ciurea, J.; Pâslaru, A.C.; Zăgrean, A.-M.; Zăgrean, L.; Moldovan, M. A Method to Assess the Default EEG Macrostate and Its Reactivity to Stimulation. Clin. Neurophysiol. 2022, 134, 50–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pâslaru, A.-C.; Călin, A.; Morozan, V.-P.; Stancu, M.; Tofan, L.; Panaitescu, A.M.; Zăgrean, A.-M.; Zăgrean, L.; Moldovan, M. Burst-Suppression EEG Reactivity to Photic Stimulation—A Translational Biomarker in Hypoxic–Ischemic Brain Injury. Biomolecules 2024, 14, 953. https://doi.org/10.3390/biom14080953
Pâslaru A-C, Călin A, Morozan V-P, Stancu M, Tofan L, Panaitescu AM, Zăgrean A-M, Zăgrean L, Moldovan M. Burst-Suppression EEG Reactivity to Photic Stimulation—A Translational Biomarker in Hypoxic–Ischemic Brain Injury. Biomolecules. 2024; 14(8):953. https://doi.org/10.3390/biom14080953
Chicago/Turabian StylePâslaru, Alexandru-Cătălin, Alexandru Călin, Vlad-Petru Morozan, Mihai Stancu, Laurențiu Tofan, Anca Maria Panaitescu, Ana-Maria Zăgrean, Leon Zăgrean, and Mihai Moldovan. 2024. "Burst-Suppression EEG Reactivity to Photic Stimulation—A Translational Biomarker in Hypoxic–Ischemic Brain Injury" Biomolecules 14, no. 8: 953. https://doi.org/10.3390/biom14080953
APA StylePâslaru, A.-C., Călin, A., Morozan, V.-P., Stancu, M., Tofan, L., Panaitescu, A. M., Zăgrean, A.-M., Zăgrean, L., & Moldovan, M. (2024). Burst-Suppression EEG Reactivity to Photic Stimulation—A Translational Biomarker in Hypoxic–Ischemic Brain Injury. Biomolecules, 14(8), 953. https://doi.org/10.3390/biom14080953













