Immunohistochemical and Ultrastructural Characterization of Telocytes in Normal and Diabetic Human Kidneys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Light Microscopy
2.2. Transmission Electron Microscopy
2.3. Statistical Analysis
3. Results
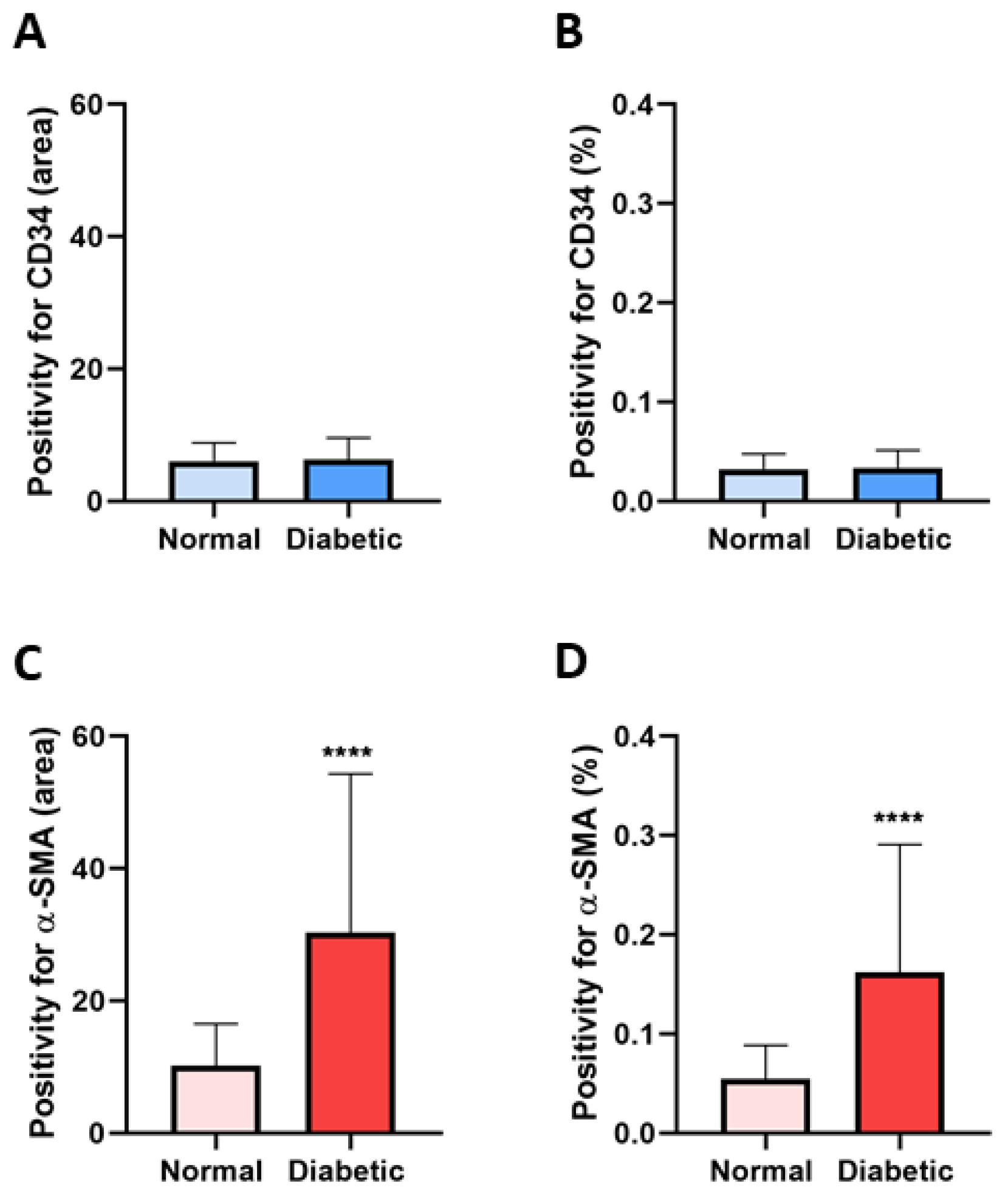
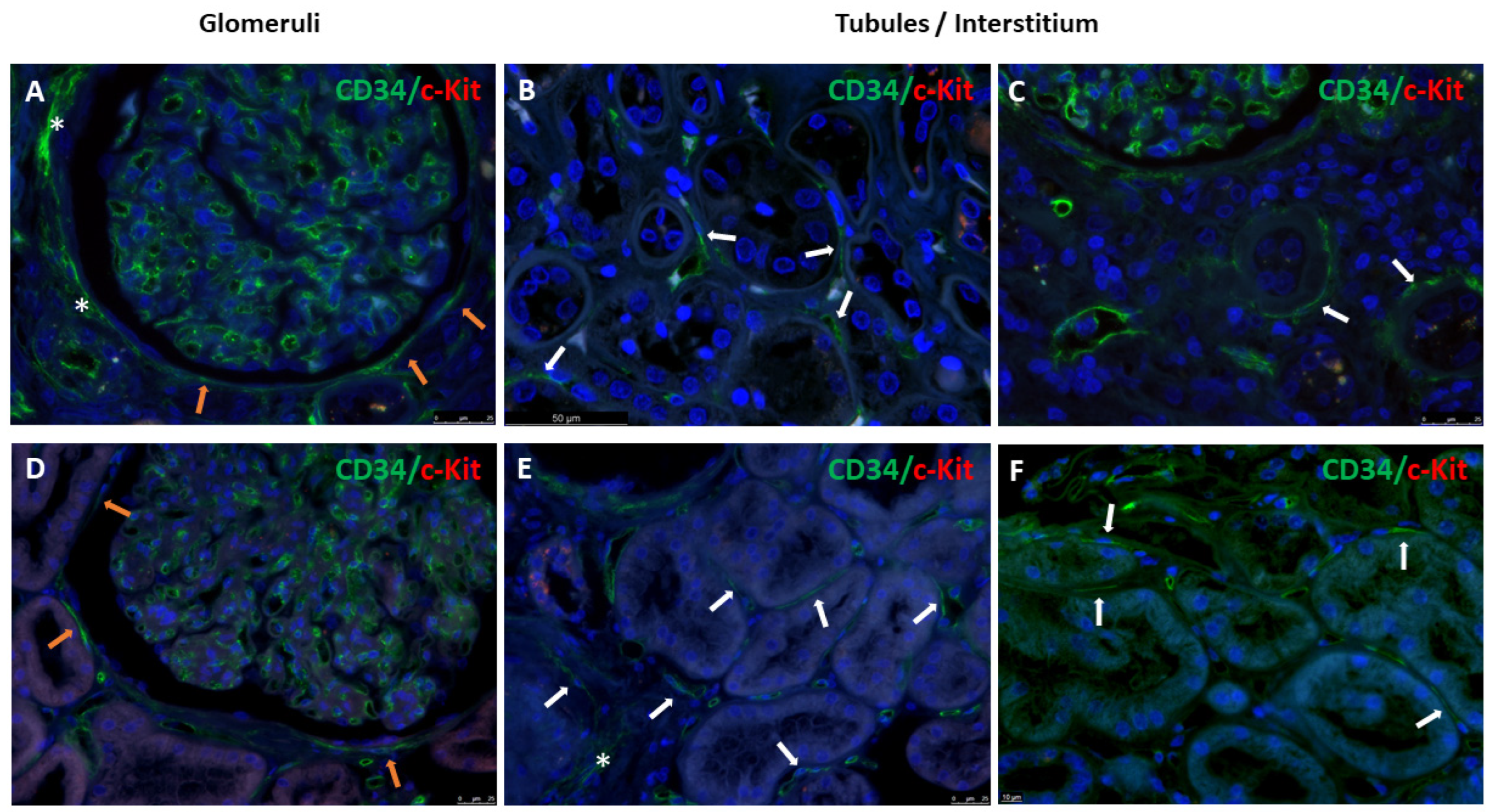
3.1. Immunophenotype and Localization of Telocytes in Normal and Diabetic Human Kidney Tissues
3.2. Ultrastructure of Telocytes in Normal and Diabetic Human Kidney Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaissling, B.; Le Hir, M. The renal cortical interstitium: Morphological and functional aspects. Histochem. Cell Biol. 2008, 130, 247–262. [Google Scholar] [CrossRef]
- Popescu, L.M.; Faussone-Pellegrini, M.S. TELOCYTES—A case of serendipity: The winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J. Cell Mol. Med. 2010, 14, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Cantarero, I.; Luesma, M.J.; Alvarez-Dotu, J.M.; Muñoz, E.; Junquera, C. Transmission electron microscopy as key technique for the characterization of telocytes. Curr. Stem Cell Res. Ther. 2016, 11, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Faussone-Pellegrini, M.S.; Popescu, L.M. Telocytes. Mol. Concepts 2011, 2, 481–489. [Google Scholar] [CrossRef]
- Faussone-Pellegrini, M.S.; Gherghiceanu, M. Telocyte’s contacts. Semin. Cell Dev. Biol. 2016, 55, 3–8. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, W.; Wu, S.M.; Xiao, J.; Kong, X. Telocytes in human heart valves. J. Cell Mol. Med. 2014, 18, 759–765. [Google Scholar] [CrossRef]
- Popescu, L.M.; Manole, C.G.; Gherghiceanu, M.; Ardelean, A.; Nicolescu, M.I.; Hinescu, M.E.; Kostin, S. Telocytes in human epicardium. J. Cell Mol. Med. 2010, 14, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zheng, Y.; Manole, C.G.; Wang, X.; Wang, Q. Telocytes in human oesophagus. J. Cell Mol. Med. 2013, 17, 1506–1512. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Traini, C.; Manetti, M.; Ibba-Manneschi, L.; Faussone-Pellegrini, M.S. Telocytes express PDGFRalpha in the human gastrointestinal tract. J. Cell Mol. Med. 2013, 17, 1099–1108. [Google Scholar] [CrossRef]
- Aschacher, T.; Schmidt, K.; Aschacher, O.; Eichmair, E.; Baranyi, U.; Winkler, B.; Grabenwoeger, M.; Spittler, A.; Enzmann, F.; Messner, B.; et al. Telocytes in the human ascending aorta: Char acterization and exosome-related KLF-4/VEGF-A expression. J. Cell Mol. Med. 2021, 25, 9697–9709. [Google Scholar] [CrossRef]
- Borges, L.F.; Falcão, R.S.P.; Taboga, S.R.; Gutierrez, P.S.; Michel, J.B. Are telocytes related to maintenance of vascular homeostasis in normal and pathological aorta? Cardiovasc. Pathol. 2024, 70, 107617. [Google Scholar] [CrossRef] [PubMed]
- Creţoiu, D.; Popescu, L.M. Human myometrium—The ultrastructural 3D network of telocytes. J. Cell Mol. Med. 2012, 16, 2844–2849. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.; Nardini, P.; Fioretto, B.S.; Guasti, D.; Romano, E.; Sgambati, E.; Marini, M.; Manetti, M. Immunohistochemical and ultrastructural identification of telocytes in the lamina propria of human vaginal mucosa. Acta Histochem. 2023, 125, 152094. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Rosa, I.; Guasti, D.; Gacci, M.; Sgambati, E.; Ibba-Manneschi, L.; Manetti, M. Reappraising the microscopic anatomy of human testis: Identification of telocyte networks in the peritubular and intertubular stromal space. Sci. Rep. 2018, 8, 14780. [Google Scholar] [CrossRef] [PubMed]
- Suciu, L.; Popescu, L.M.; Gherghiceanu, M.; Regalia, T.; Nicolescu, M.I.; Hinescu, M.E.; Faussone-Pellegrini, M.S. Telocytes in human term placenta: Morphology and phenotype. Cells Tissues Organs 2010, 192, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Ceafalan, L.; Gherghiceanu, M.; Popescu, L.M.; Simionescu, O. Telocytes in human skin—Are they involved in skin regeneration? J. Cell Mol. Med. 2012, 16, 1405–1420. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, L.; Zhang, R.; Jin, H.; Shi, H. Ultrastructural and immunohistochemical characteristics of telocytes in human scalp tissue. Sci. Rep. 2020, 10, 1693. [Google Scholar] [CrossRef]
- Rosa, I.; Ibba-Manneschi, L.; Guasti, D.; Perigli, G.; Faussone-Pellegrini, M.S.; Manetti, M. Morphologic evidence of telocytes in human thyroid stromal tissue. J. Cell Mol. Med. 2022, 26, 2477–2481. [Google Scholar] [CrossRef]
- Nicolescu, M.I.; Bucur, A.; Dinca, O.; Rusu, M.C.; Popescu, L.M. Telocytes in Parotid Glands. Anat. Rec. 2012, 295, 378–385. [Google Scholar] [CrossRef]
- Petre, N.; Rusu, M.C.; Pop, F.; Jianu, A.M. Telocytes of the mammary gland stroma. Folia Morphol. 2016, 75, 224–231. [Google Scholar] [CrossRef]
- Qi, G.; Lin, M.; Xu, M.; Manole, C.G.; Wang, X.; Zhu, T. Telocytes in the human kidney cortex. J. Cell Mol. Med. 2012, 16, 3116–3122. [Google Scholar] [CrossRef]
- Rusu, M.C.; Mogoantă, L.; Pop, F.; Dobra, M.A. Molecular phenotypes of the human kidney: Myoid stromal cells/telocytes and myoepithelial cells. Ann. Anat. 2018, 218, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kondo, A.; Kaestner, K.H. Emerging diverse roles of telocytes. Development 2019, 146, dev175018. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wei, L.; Zhong, C.; Fu, S.; Bei, Y.; Huică, R.I.; Wang, F.; Xiao, J. Cardiac telocytes are double positive for CD34/PDGFR-α. J. Cell Mol. Med. 2015, 19, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Suciu, L.C.; Popescu, B.O.; Kostin, S.; Popescu, L.M. Platelet-derived growth factor receptor-β-positive telocytes in skeletal muscle interstitium. J. Cell Mol. Med. 2012, 16, 701–707. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; González-Gómez, M.; García, M.P.; Díaz-Flores, L., Jr.; Carrasco, J.L.; Martín-Vasallo, P. CD34+ Stromal Cells/Telocytes as a Source of Cancer-Associated Fibroblasts (CAFs) in Invasive Lobular Carcinoma of the Breast. Int. J. Mol. Sci. 2021, 22, 3686. [Google Scholar] [CrossRef] [PubMed]
- Gherghiceanu, M.; Popescu, L.M. Heterocellular communication in the heart: Electron tomography of telocyte-myocyte junctions. J. Cell Mol. Med. 2011, 15, 1005–1011. [Google Scholar] [CrossRef]
- Cretoiu, D.; Xu, J.; Xiao, J.; Cretoiu, S.M. Telocytes and their extracellular vesicles: Evidence and hypotheses. Int. J. Mol. Sci. 2016, 17, 1322. [Google Scholar] [CrossRef]
- Marini, M.; Ibba-Manneschi, L.; Manetti, M. Cardiac telocyte-derived exosomes and their possible implications in cardiovascular pathophysiology. Adv. Exp. Med. Biol. 2017, 998, 237–254. [Google Scholar]
- Kang, Y.; Zhu, Z.; Zheng, Y.; Wan, W.; Manole, C.G.; Zhang, Q. Skin telocytes versus fibroblasts: Two distinct dermal cell populations. J. Cell Mol. Med. 2015, 19, 2530–2539. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, H. Telocytes and inflammation: A review. Medicine 2023, 102, e35983. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, X.; Qian, M.; Zhang, M.; Zhang, D.; Bai, C.; Wang, Q.; Wang, X. Human lung telocytes could promote the proliferation and angiogenesis of human pulmonary microvascular endothelial cells in vitro. Mol. Cell Ther. 2014, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Manole, C.G.; Gherghiceanu, M.; Ceafalan, L.C.; Hinescu, M.E. Dermal Telocytes: A Different Viewpoint of Skin Repairing and Regeneration. Cells 2022, 11, 3903. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G.; Bani, D.; Faussone-Pellegrini, M.S. Telocytes Contribute as Cell Progenitors and Differentiation Inductors in Tissue Regeneration. Curr. Stem Cell Res. Ther. 2016, 11, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Wang, F.; Yang, C.; Xiao, J. Telocytes in regenerative medicine. J. Cell Mol. Med. 2015, 19, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.; Marini, M.; Manetti, M. Telocytes: An Emerging Component of Stem Cell Niche Microenvironment. J. Histochem. Cytochem. 2021, 69, 795–818. [Google Scholar] [CrossRef] [PubMed]
- Ardeleanu, C.; Bussolati, G. Telocytes are the common cell of origin of both PEComas and GISTs: An evidence-supported hypothesis. J. Cell Mol. Med. 2011, 15, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Aschacher, T.; Aschacher, O.; Schmidt, K.; Enzmann, F.K.; Eichmair, E.; Winkler, B.; Arnold, Z.; Nagel, F.; Podesser, B.K.; Mitterbauer, A.; et al. The Role of Telocytes and Telocyte-Derived Exosomes in the Development of Thoracic Aortic Aneurysm. Int. J. Mol. Sci. 2022, 23, 4730. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wang, F.; Cao, Y.; Huang, Q.; Xiao, J.; Yang, C.; Popescu, L.M. Telocytes in human liver fibrosis. J. Cell Mol. Med. 2015, 19, 676–683. [Google Scholar] [CrossRef]
- Milia, A.F.; Ruffo, M.; Manetti, M.; Rosa, I.; Conte, D.; Fazi, M.; Messerini, L.; Ibba-Manneschi, L. Telocytes in Crohn’s disease. J. Cell Mol. Med. 2013, 17, 1525–1536. [Google Scholar] [CrossRef]
- Ibba-Manneschi, L.; Rosa, I.; Manetti, M. Telocyte implications in human pathology: An overview. Semin. Cell Dev. Biol. 2016, 55, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González-Gómez, M.; Rodríguez-Rodriguez, R.; Hernández-León, N.; Díaz-Flores, L., Jr.; Carrasco, J.L. Cd34+ Stromal Cells/Telocytes in Normal and Pathological Skin. Int. J. Mol. Sci. 2021, 22, 7342. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.; Brodsky, S.V.; Satoskar, A.A.; Nadasdy, G.; Nadasdy, T. The relevance of periglomerular fibrosis in the evaluation of routine needle core renal biopsies. Arch. Pathol. Lab. Med. 2011, 135, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Makni, K.; Jarraya, F.; Khabir, A.; Hentati, B.; Hmida, M.B.; Makni, H.; Boudawara, T.; Jlidi, R.; Hachicha, J.; Ayadi, H. Renal alpha-smooth muscle actin: A new prognostic factor for lupus nephritis. Nephrology 2009, 14, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Alpers, C.E.; Hudkins, K.L.; Gown, A.M.; Johnson, R.J. Enhanced expression of “muscle-specific” actin inglomerulonephritis. Kidney Int. 1992, 41, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Suzuki, J.; Sakai, N.; Tanji, M.; Suzuki, H. Predicting the prognosis of renal dysfunction by renal expression of alpha-smooth muscle actin in children with MPGN type 1. Am. J. Kidney Dis. 2003, 42, 1131–1138. [Google Scholar] [CrossRef]
- Saratlija Novakovic, Z.; Glavina Durdov, M.; Puljak, L.; Saraga, M.; Ljutic, D.; Filipovic, T.; Pastar, Z.; Bendic, A.; Vukojevic, K. The interstitial expression of alpha-smooth muscle actin in glomerulonephritis is associated with renal function. Med. Sci. Monit. 2012, 18, CR235–CR240. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, Y.; Kawamura, T.; Abe, A.; Imai, H.; Hirano, K.; Maruyama, N.; Hosoya, T.; Sakai, O. Significance of mesangial expression of alpha-smooth muscle actin in the progression of IgA nephropathy. Am. J. Kidney Dis. 1999, 34, 902–910. [Google Scholar] [CrossRef]
- Essawy, M.; Soylemezoglu, O.; Muchaneta-Kubara, E.C.; Shortland, J.; Brown, C.B.; El Nahas, A.M. Myofibroblasts and the progression of diabetic nephropathy. Nephrol. Dial. Transplant. 1997, 12, 43–50. [Google Scholar] [CrossRef]
- Sasi, A.; Romaine, A.; Erusappan, P.M.; Melleby, A.O.; Hasic, A.; Dahl, C.P.; Broch, K.; Almaas, V.M.; Puertas, R.D.; Roderick, H.L.; et al. Temporal expression and spatial distribution of the proteoglycan versican during cardiac fibrosis development. Matrix Biol. Plus 2023, 19–20, 100135. [Google Scholar] [CrossRef]
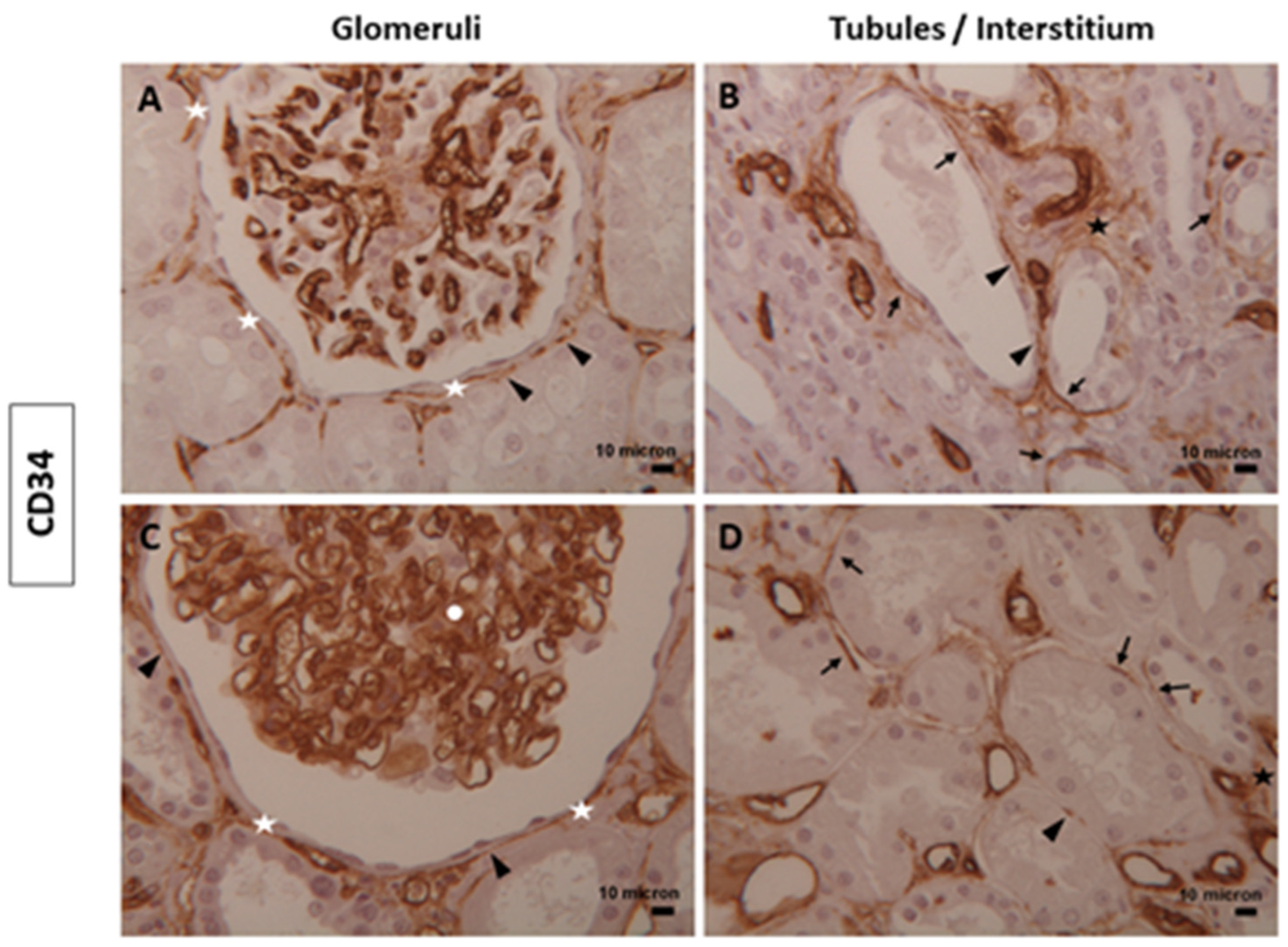

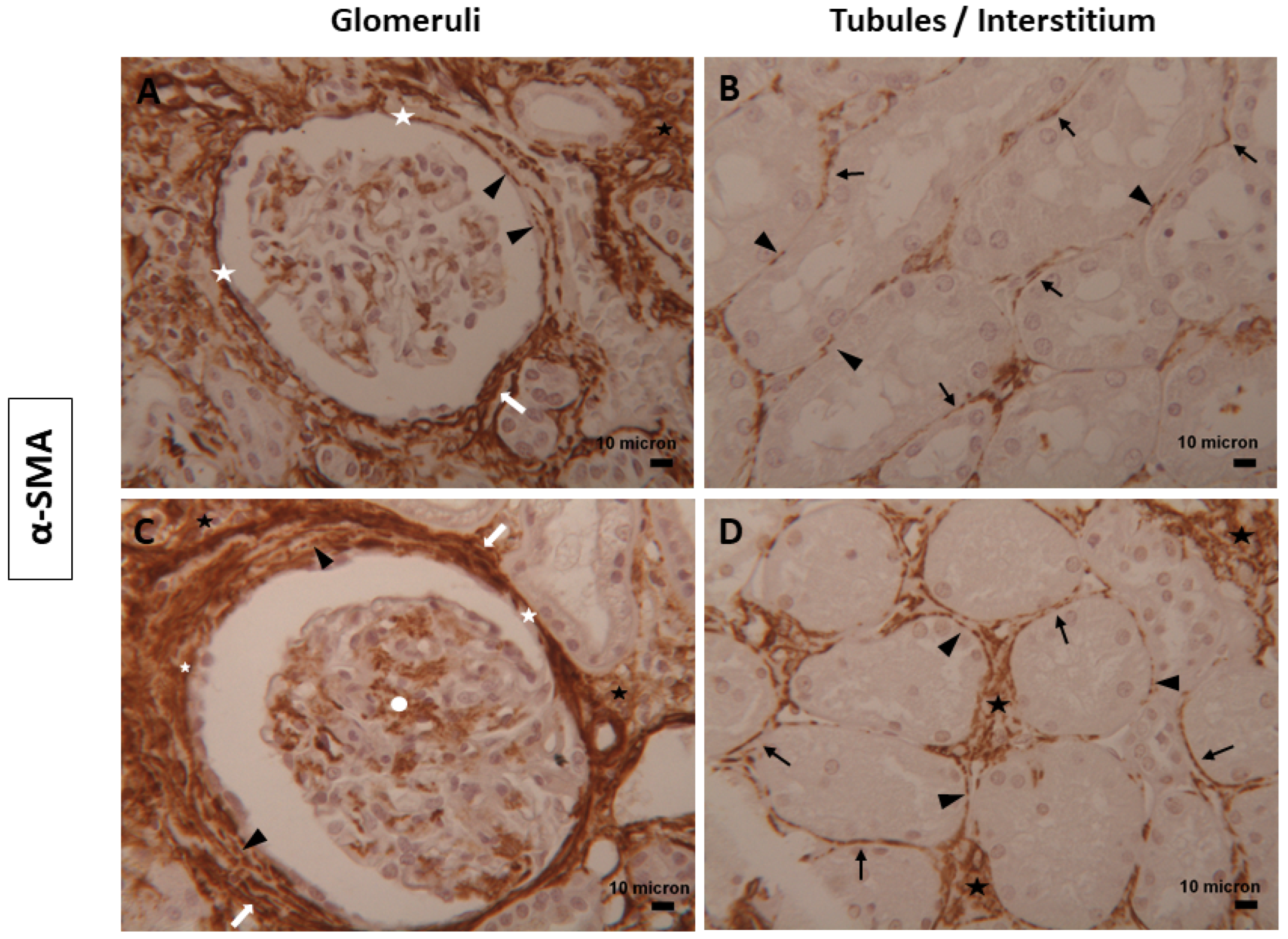

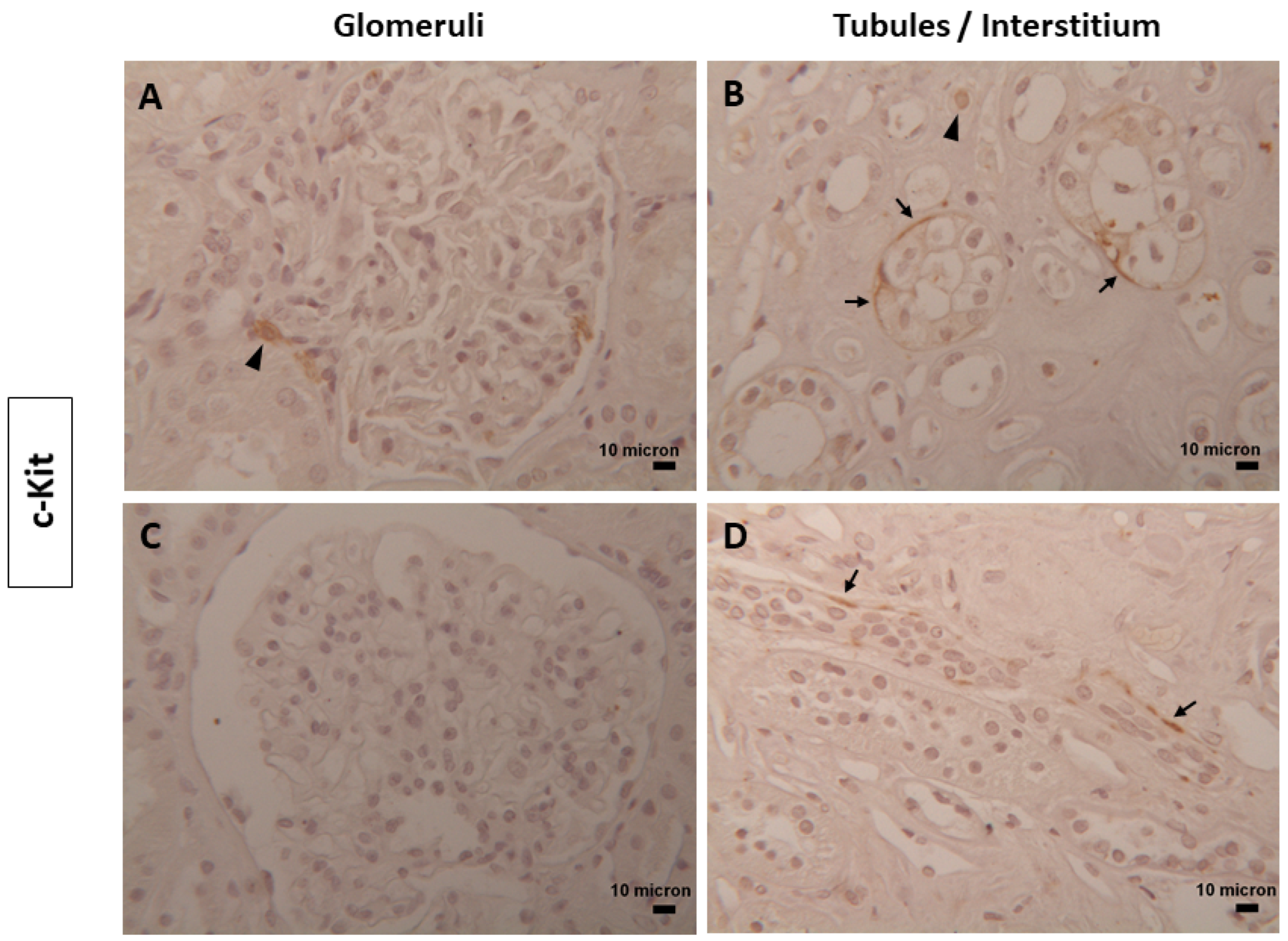

| Glomerulus | Tubule-Interstitium | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bowman’s Capsule | Mesangial Cells | Glomerular Endothelial Cells | Peritubular Capillaries | Tubules | Interstitium | |||||||
| Antibodies | N | D | N | D | N | D | N | D | N | D | N | D |
| CD34 | + | + | - | + | ++ | ++ | ++ | ++ | + | + | + | + |
| Nestin | + | + | ++ | + | ++ | + | +/- | + | + | + | +/- | +/- |
| α-SMA | ++ | ++ | + | + | +/- | +/- | + | ++ | + | ++ | ++ | ++ |
| Vimentin | ++ | ++ | + | + | ++ | ++ | + | ++ | + | ++ | + | ++ |
| c-Kit | +/- | - | - | - | - | - | - | - | +/- | +/- | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valente, S.; Villacampa Lahoz, M.; Vasuri, F.; Pasquinelli, G. Immunohistochemical and Ultrastructural Characterization of Telocytes in Normal and Diabetic Human Kidneys. Biomolecules 2024, 14, 968. https://doi.org/10.3390/biom14080968
Valente S, Villacampa Lahoz M, Vasuri F, Pasquinelli G. Immunohistochemical and Ultrastructural Characterization of Telocytes in Normal and Diabetic Human Kidneys. Biomolecules. 2024; 14(8):968. https://doi.org/10.3390/biom14080968
Chicago/Turabian StyleValente, Sabrina, Marta Villacampa Lahoz, Francesco Vasuri, and Gianandrea Pasquinelli. 2024. "Immunohistochemical and Ultrastructural Characterization of Telocytes in Normal and Diabetic Human Kidneys" Biomolecules 14, no. 8: 968. https://doi.org/10.3390/biom14080968





