Biological Properties of Sandalwood Oil and Microbial Synthesis of Its Major Sesquiterpenoids
Abstract
1. Introduction
2. The Source of Sandalwood Essential Oil, Its Main Components, and Its Applications
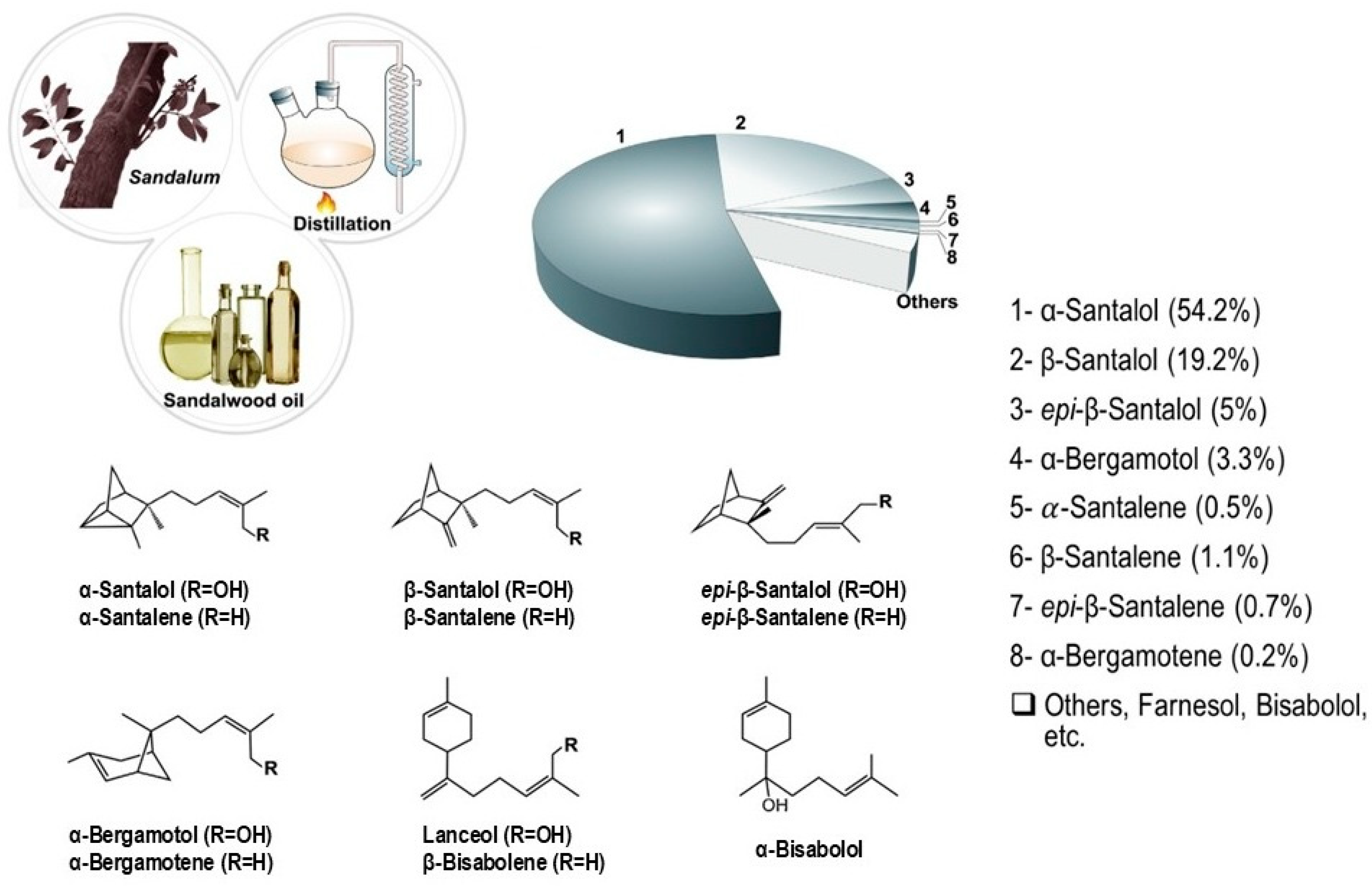
2.1. Antioxidant Properties of Sandalwood Oil
2.2. Cytotoxicity of Santalol
2.3. Sedative and Anxiolytic Effects of Sandalwood Oil
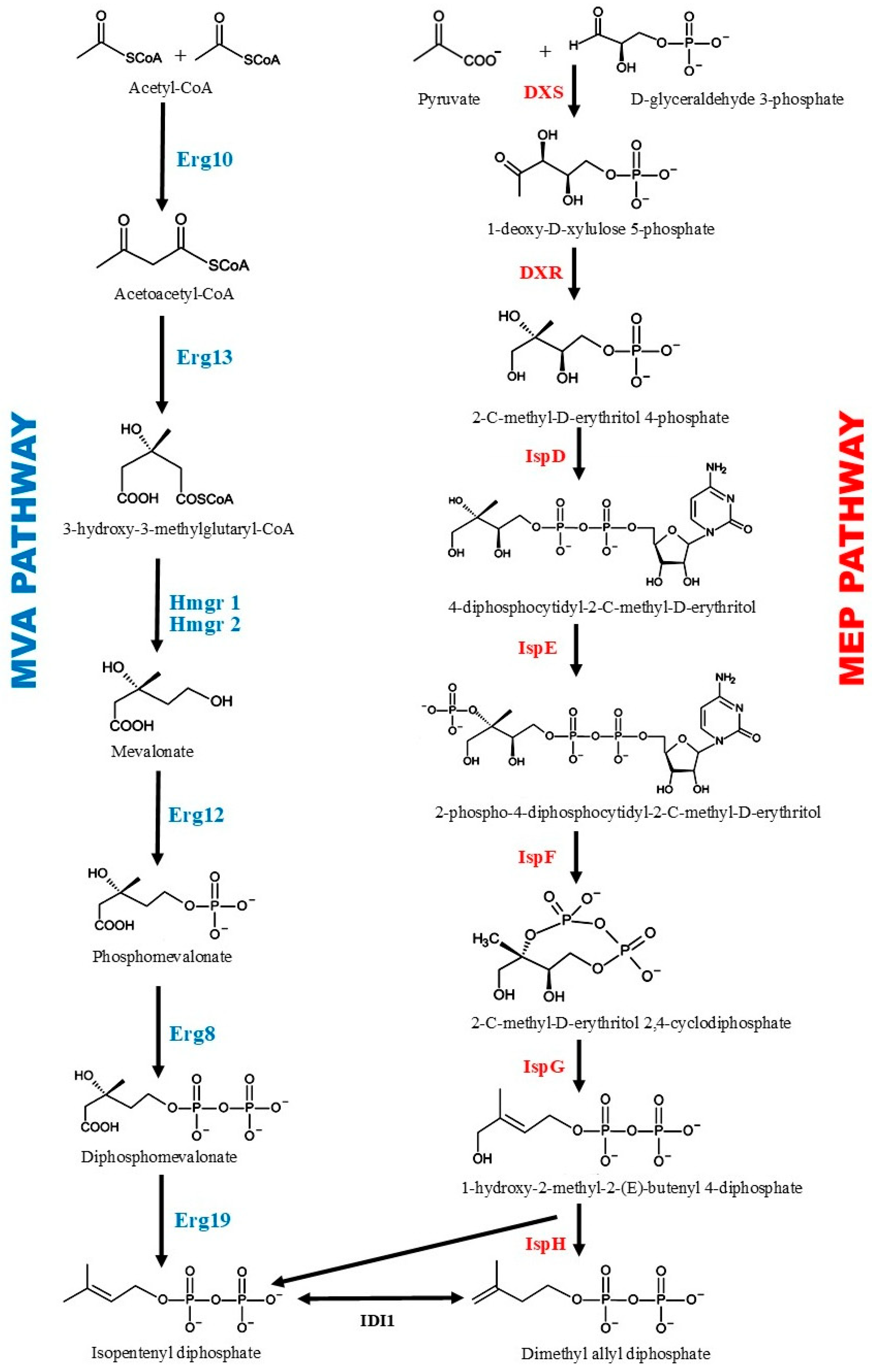
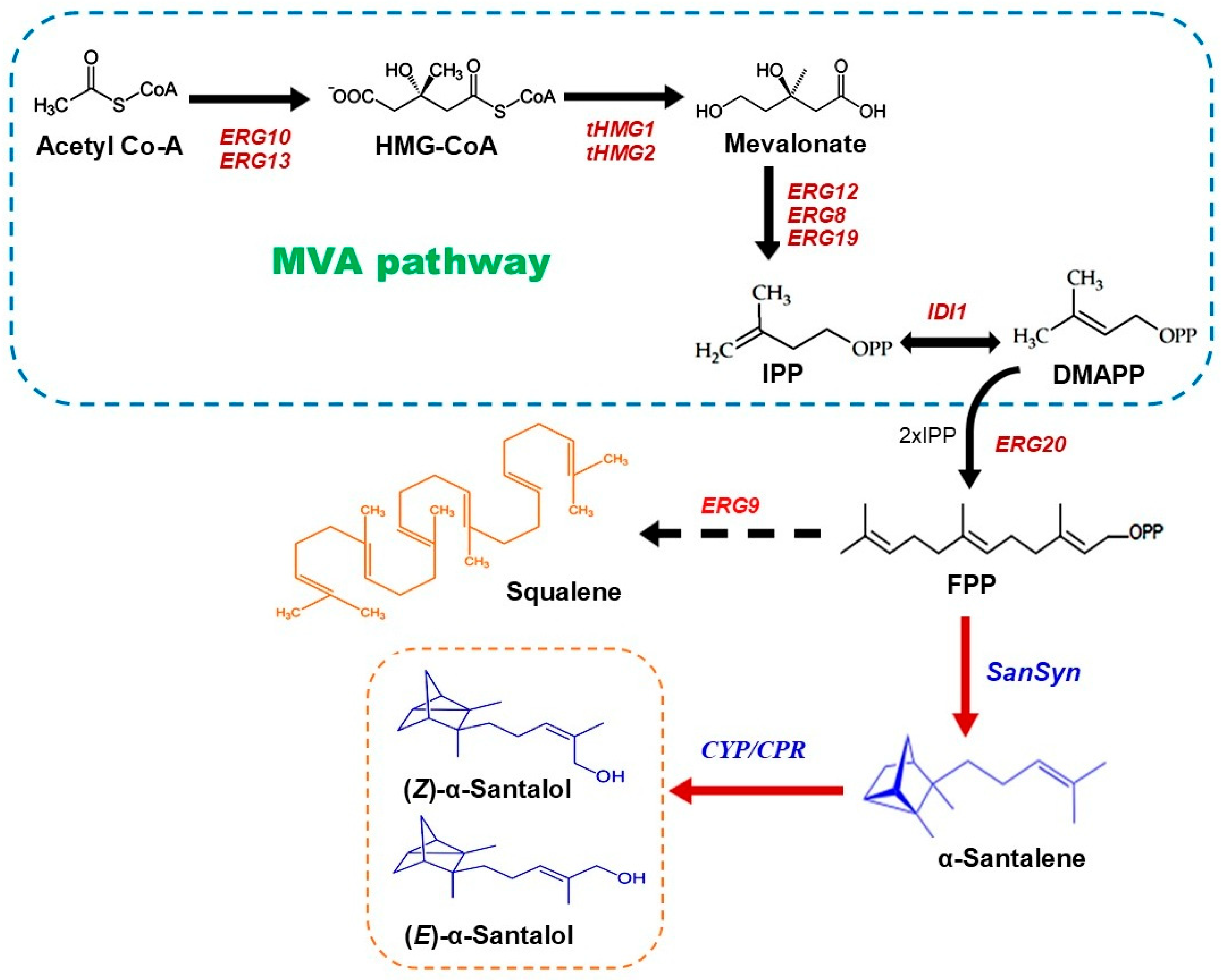
3. Biosynthesis of Santalene, Santalol, and Other Terpenoids Found in Sandalwood Oil
4. Enzyme Engineering to Improve Catalytic Properties
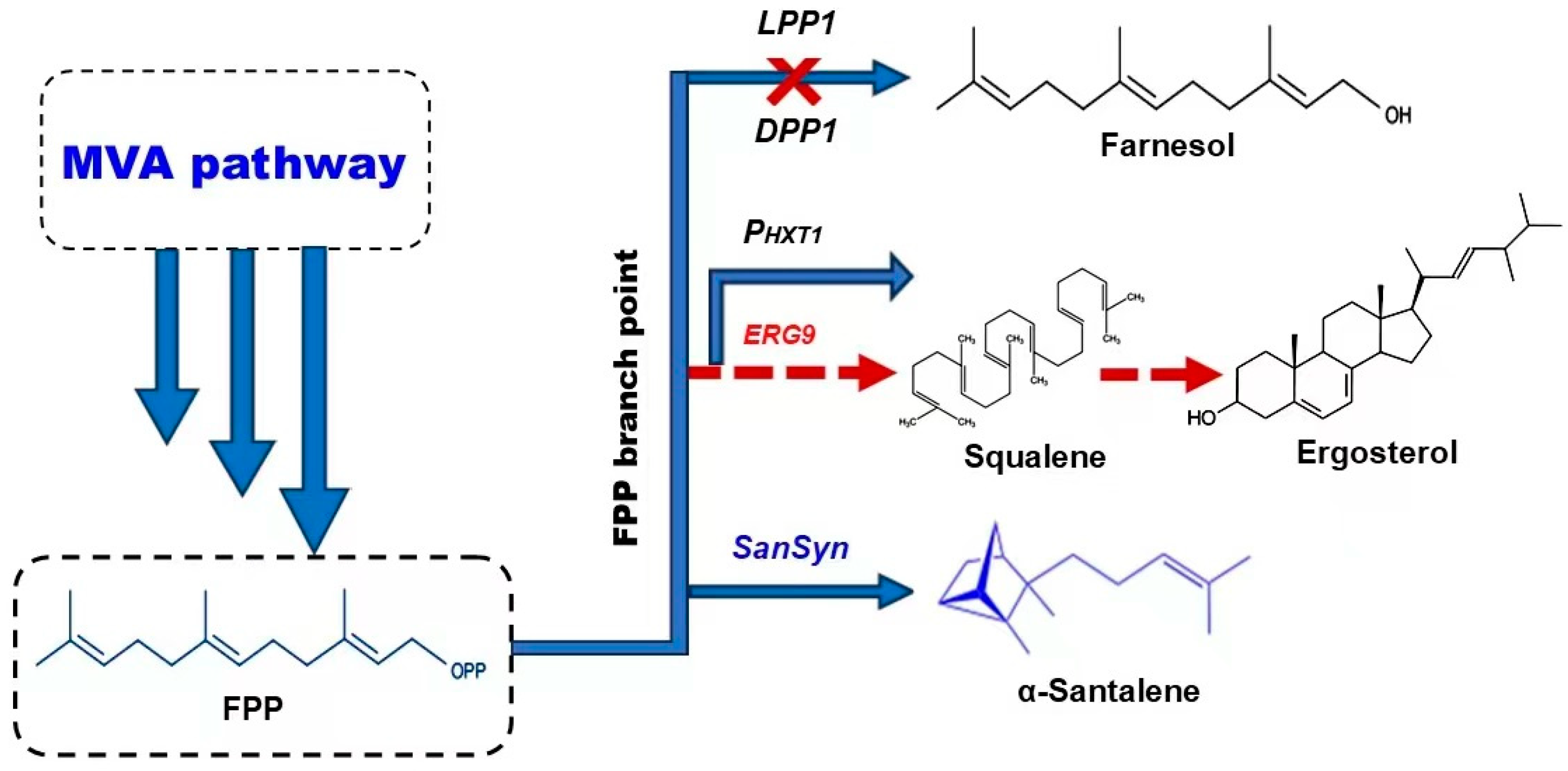
5. Metabolic Engineering Strategies to Increase the Yield of Santalene and Santalol in S. cerevisiae
5.1. Engineering of the Key Enzymes in the MVA Pathway
5.2. Engineering of FPP Branch Point
5.3. Manipulation of NADH and NADPH Cofactors
5.4. Engineering of the Acetyl-CoA Supply
5.5. Reconstructing Santalol Biosynthesis Pathway under the GAL Regulatory System
5.6. Fermentation Optimization
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, K.; Mishra, V.; Ranjan, K.R.; Yadav, N.; Sharma, M.A. Methodological approach of plant essential oils and their isolated bioactive components for antiviral activities. In Essential Oils; Wiley: Hoboken, NJ, USA, 2023; pp. 1–29. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Jindal, S.; Anand, R.; Sharma, N.; Ranjan, K.R.; Mukherjee, M.D.; Mishra, V. Plant essential oils and their constituents for therapeutic benefits. In Essential Oils; Wiley: Hoboken, NJ, USA, 2023; pp. 977–1008. [Google Scholar]
- Neagu, R.; Popovici, V.; Ionescu, L.E.; Ordeanu, V.; Popescu, D.M.; Ozon, E.A.; Gîrd, C.E. Antibacterial and antibiofilm effects of different samples of five commercially available essential oils. Antibiotics 2023, 12, 1191. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.J.P.; Souza, P.F.N.; Malveira, E.A.; Neto, N.A.S.; Silva, R.R.S.; Melo, G.L.C.; Silva, A.F.B.; Lima, L.B.; de Albuquerque, C.C.; Bastos, R.W.; et al. Antimicrobial activity of the essential oil from Croton Pluriglandulosus Carn. leaves against microorganisms of clinical interest. J. Fungi 2023, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.W.; Kim, B. Antimicrobial activity of essential oils and single fragrance ingredients for cosmetic applications. Asian J. Beauty Cosmetol. 2022, 20, 417–426. [Google Scholar] [CrossRef]
- Khan, S.; Ikram, M.; Faisal, M. Commercial, cosmetic, and medicinal importance of sandal (Santalum album): A valuable forest resource. In Non-Timber Forest Products; Springer International Publishing: Cham, Switzerland, 2021; pp. 129–144. [Google Scholar]
- Nautiyal, O.H. Sandalwood (Santalum album) oil. In Fruit Oils: Chemistry and Functionality; Springer International Publishing: Cham, Switzerland, 2019; pp. 711–740. [Google Scholar]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 2013, 496, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Fordjour, E.; Liu, C.; Hao, Y.; Sackey, I.; Yang, Y.; Liu, X.; Li, Y.; Tan, T.; Bai, Z. Engineering Escherichia coli BL21 (DE3) for high-yield production of germacrene A, a precursor of β-elemene via combinatorial metabolic engineering strategies. Biotechnol. Bioeng. 2023, 120, 3039–3056. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; An, T.; Li, T.; Zhu, J.; Gao, K.; Sun, Z.; Xu, W.; Lin, P.; Zi, J. Reconstruction of the biosynthetic pathway of santalols under control of the gal regulatory system in yeast. ACS Synth. Biol. 2020, 9, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Bureau, J.A.; Oliva, M.E.; Dong, Y.; Ignea, C. Engineering yeast for the production of plant terpenoids using synthetic biology approaches. Nat. Prod. Rep. 2023, 40, 1822–1848. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Wang, S.; Chen, R.; Ge, M.; Yan, X.; Qiao, J. Catalytic mechanism and heterologous biosynthesis application of sesquiterpene synthases. J. Agric. Food Chem. 2024, 72, 6871–6888. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, A.; Shanmugam, G.; Kalaiselvi, D.; Levenson, C.; Nivitha, S.; Thiruppathi, G.; Sundararaj, P. East Indian sandalwood (Santalum album L.) Oil confers neuroprotection and geroprotection in caenorhabditis elegans via activating SKN-1/Nrf2 signaling pathway. RSC Adv. 2018, 8, 33753–33774. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.K.; Sudharshan, S.J.; Nagegowda, D.A. Sandalwood fragrance pathway and its engineering for sustainable production of high-value santalols. In The Sandalwood Genome; Springer International Publishing: Cham, Switzerland, 2022; pp. 65–82. [Google Scholar]
- Marongiu, B.; Piras, A.; Porcedda, S.; Tuveri, E. Extraction of Santalum album and Boswellia carterii Birdw. Volatile oil by supercritical carbon dioxide: Influence of some process parameters. Flavour Fragr. J. 2006, 21, 718–724. [Google Scholar] [CrossRef]
- Kucharska, M.; Frydrych, B.; Wesolowski, W.; Szymanska, J.A.; Kilanowicz, A. A comparison of the composition of selected commercial sandalwood oils with the international standard. Molecules 2021, 26, 2249. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.S.; Ravindra, M.; Gayathri, D.N. Variability in yield and composition of oil from Indian sandalwood (Santalum album L.) trees grown in homogeneous conditions. Trop. Plant Res. 2019, 6, 31–36. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Mahfud, M. Chemical composition of essential oil of Indonesia sandalwood extracted by microwave-assisted hydrodistillation. AIP Conf. Proc. 2016, 1755, 050001. [Google Scholar] [CrossRef]
- Moniodis, J.; Jones, C.G.; Renton, M.; Plummer, J.A.; Barbour, E.L.; Ghisalberti, E.L.; Bohlmann, J. Sesquiterpene variation in West Australian sandalwood (Santalum spicatum). Molecules 2017, 22, 940. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.K.; Mukhopadhyay, S.; Tah, J. Santalol oil of sandalwood (white) grown in different edaphic factors in West Bengal, India. World, J. Adv. Res. Rev. 2023, 19, 1404–1413. [Google Scholar] [CrossRef]
- Srikantaprasad, D.; Gowda, A.P.M.; Pushpa, T.N.; Thimmegowda, M.N.; Umesha, K.; Ravikumar, R.L.; Prasanna, K.T. Identification of suitable host for sandalwood cultivation in the northern dry zone of Karnataka. Ind. Crops Prod. 2022, 182, 114874. [Google Scholar] [CrossRef]
- Brand, J.E.; Pronk, G.M. Influence of age on sandalwood (Santalum spicatum) oil content within different wood grades from five plantations in Western Australia. Aust. For. 2011, 74, 141–148. [Google Scholar] [CrossRef]
- Hasegawa, T.; Izumi, H.; Tajima, Y.; Yamada, H. Structure-odor relationships of α-santalol derivatives with modified side chains. Molecules 2012, 17, 2259–2270. [Google Scholar] [CrossRef]
- Blankenhorn, K.; Keating, A.; Oschal, J.; Maldonado, D.; Bommareddy, A. Cancer-preventive and antitumor effects of sandalwood oil and alpha-santalol. In Indian Sandalwood: A Compendium; Springer: Berlin/Heidelberg, Germany, 2022; pp. 407–421. [Google Scholar]
- Zhang, X.; Chen, W.; Guillermo, R.; Chandrasekher, G.; Kaushik, R.S.; Young, A.; Fahmy, H.; Dwivedi, C. Alpha-santalol, a chemopreventive agent against skin cancer, causes G 2/M cell cycle arrest in both P53-mutated human epidermoid carcinoma A431 cells and P53 wild-type human melanoma UACC-62 Cells. BMC Res. Notes 2010, 3, 220. [Google Scholar] [CrossRef] [PubMed]
- Bommareddy, A.; Knapp, K.; Nemeth, A.; Steigerwalt, J.; Landis, T.; Vanwert, A.L.; Gorijavolu, H.P.; Dwivedi, C. Alpha-santalol, a component of sandalwood oil inhibits migration of breast cancer cells by targeting the β-catenin pathway. Anticancer Res. 2018, 38, 4475–4480. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B.; Dey, S. Comparative phytochemical analysis and antibacterial efficacy of in vitro and in vivo extracts from east indian sandalwood tree (Santalum album L.). Lett. Appl. Microbiol. 2012, 55, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Zehra, Z.; Shamsi, A.; Beg, M.A.; Parray, Z.A.; Israil; Imam, M.A.; Gaur, N.A.; Hassan, M.I.; Chaudhary, A.A.; et al. Elucidating the role of santalol as a potent inhibitor of tyrosinase: In vitro and in silico approaches. Molecules 2022, 27, 8915. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.H.; Haque, A.U. Use of α- and β-Santalols Major Constituents of Sandalwood Oil, in the Treatment of Warts, Skin Blemishes and Other Viral-Induced Tumors. U.S. Patent US6406706B1, 11 August 2000. [Google Scholar]
- Koch, C.; Reichling, J.; Schneele, J.; Schnitzler, P. Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomedicine 2008, 15, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, H.; Ueda, R.; Matsumoto, K.; Kawanishi, K.; Kato, A. Effect of α-santalol and β-santalol from sandalwood on the central nervous system in mice. Phytomedicine 1995, 2, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Turgumbayeva, A.; Mertdinç, Z.; Tütüncü, S.; Aydar, E.F.; Özçelik, B.; Anna, S.W.; Mariola, S.; Koziróg, A.; et al. Santalum genus: Phytochemical constituents, biological activities and health promoting-effects. Z. Naturforsch.-Sect. C J. Biosci. 2023, 78, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kim, S.W. Shaking up ancient scents: Insights into santalol synthesis in engineered Escherichia coli. Process Biochem. 2015, 50, 1177–1183. [Google Scholar] [CrossRef]
- Kolanthan, V.L.; Brown, A.; Soobramaney, V.; Philibert, E.G.; Newton, V.F.; Hosenally, M.; Sokeechand, B.N.; Petkar, G.; Moga, A.; Andres, P.; et al. Clinical evaluation of Indian sandalwood oil and its protective effect on the skin against the detrimental effect of the exposome. Cosmetics 2022, 9, 35. [Google Scholar] [CrossRef]
- Francois-Newton, V.; Brown, A.; Andres, P.; Mandary, M.B.; Weyers, C.; Latouche-Veerapen, M.; Hettiarachchi, D. Antioxidant and anti-aging potential of Indian sandalwood oil against environmental stressors in vitro and ex vivo. Cosmetics 2021, 8, 53. [Google Scholar] [CrossRef]
- Misra, B.B.; Dey, S. Evaluation of in Vivo Anti-Hyperglycemic and Antioxidant Potentials of α-Santalol and Sandalwood Oil. Phytomedicine 2013, 20, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Umdale, S.; Ahire, M.; Aiwale, V.; Jadhav, A.; Mundada, P. Phytochemical investigation and antioxidant efficacy of wild, underutilized berries of economically important Indian sandalwood (Santalum album L.). Biocatal. Agric. Biotechnol. 2020, 27, 101705. [Google Scholar] [CrossRef]
- Santha, S.; Bommareddy, A.; Rule, B.; Guillermo, R.; Kaushik, R.S.; Young, A.; Dwivedi, C. Antineoplastic effects of α-santalol on estrogen receptor-positive and estrogen receptor-negative breast cancer cells through cell cycle arrest at G2/M phase and induction of apoptosis. PLoS ONE 2013, 8, e56982. [Google Scholar] [CrossRef]
- Santha, S.; Dwivedi, C. Properties of sandalwood oil. Anticancer Res. 2015, 35, 3137–3145. [Google Scholar] [PubMed]
- Dwivedi, C. Skin cancer chemoprevention by a-santalol. Front. Biosci. 2011, S3, 186. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Singh, A.; Liu, Y.; Sunderland, B.; Li, D. Comparative effects of sandalwood seed oil on fatty acid profiles and inflammatory factors in rats. Lipids 2013, 48, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Ogawa, Y.; Koike, K. Relationship between emotional behavior in mice and the concentration of (+)-α-santalol in the brain. Phyther. Res. 2015, 29, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Bommareddy, A.; Brozena, S.; Steigerwalt, J.; Landis, T.; Hughes, S.; Mabry, E.; Knopp, A.; VanWert, A.L.; Dwivedi, C. Medicinal properties of alpha-santalol, a naturally occurring constituent of sandalwood oil: Review. Nat. Prod. Res. 2019, 33, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, A.; Shinomiya, K.; Utsu, Y.; Tokunaga, S.; Hasegawa, Y.; Kamei, C. Effect of santalol on the sleep-wake cycle in sleep-disturbed rats. Jpn. J. Psychopharmacol. 2007, 27, 167–171. [Google Scholar]
- Roh, H.S.; Park, K.C.; Park, C.G. Repellent effect of santalol from sandalwood oil against Tetranychus urticae (Acari: Tetranychidae). J. Econ. Entomol. 2012, 105, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Basallo, O.; Perez, L.; Lucido, A.; Sorribas, A.; Marin-Saguino, A.; Vilaprinyo, E.; Perez-Fons, L.; Albacete, A.; Martínez-Andújar, C.; Fraser, P.D.; et al. Changing biosynthesis of terpenoid precursors in rice through synthetic biology. Front. Plant Sci. 2023, 14, 1133299. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.M.; McBee, D.P.; Trybala, T.N.; Hulsey, Z.N.; Gonzalez Curbelo, C.; Mazur, W.; Baccile, J.A. Membrane permeant analogs for the independent cellular introduction of the terpene precursors isopentenyl- and dimethylallyl-pyrophosphate. ChemBioChem 2023, 24, e202200512. [Google Scholar] [CrossRef] [PubMed]
- Drummond, L.; Haque, P.J.; Gu, B.; Jung, J.S.; Schewe, H.; Dickschat, J.S.; Buchhaupt, M. High versatility of IPP and DMAPP methyltransferases enables the synthesis of C6, C7 and C8 terpenoid building blocks. ChemBioChem 2022, 23, e202200091. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhang, H.; Cao, X.; Kong, S.; Zhu, B.; Lin, X.; Zhou, Y.J. Construction and optimization of nonclassical isoprenoid biosynthetic pathways in yeast peroxisomes for (+)-valencene production. J. Agric. Food Chem. 2023, 71, 11124–11130. [Google Scholar] [CrossRef] [PubMed]
- Ro, D.K.; Paradise, E.M.; Quellet, M.; Fisher, K.J.; Newman, K.L.; Ndungu, J.M.; Ho, K.A.; Eachus, R.A.; Ham, T.S.; Kirby, J.; et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006, 440, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, Q.; Liu, H.; Xian, M.; Liu, H. A novel MVA-mediated pathway for isoprene production in engineered E. coli. BMC Biotechnol. 2016, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Song, A.A.L.; Abdullah, J.O.; Abdullah, M.P.; Shafee, N.; Othman, R.; Tan, E.F.; Noor, N.M.; Raha, A.R. Overexpressing 3-hydroxy-3-methylglutaryl coenzyme a reductase (HMGR) in the lactococcal mevalonate pathway for heterologous plant sesquiterpene production. PLoS ONE 2012, 7, e52444. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhou, C.; Guo, X.; Du, Z.; Cheng, Y.; Wang, Z.; He, X. Enhancing fluxes through the mevalonate pathway in Saccharomyces cerevisiae by engineering the HMGR and β-alanine metabolism. Microb. Biotechnol. 2022, 15, 2292–2306. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.Z.; Rouvière, P.E.; LaRossa, R.A.; Suh, W. Chromosomal promoter replacement of the isoprenoid pathway for enhancing carotenoid production in E. coli. Metab. Eng. 2006, 8, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Ajikumar, P.K.; Xiao, W.H.; Tyo, K.E.J.; Wang, Y.; Simeon, F.; Leonard, E.; Mucha, O.; Phon, T.H.; Pfeifer, B.; Stephanopoulos, G. Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli. Science 2010, 330, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Liu, Y.; Wu, Y.; Zhao, L.; Pei, J. Functional characterization and screening of promiscuous kinases and isopentenyl phosphate kinases for the synthesis of DMAPP via a one-pot enzymatic cascade. Int. J. Mol. Sci. 2022, 23, 12904. [Google Scholar] [CrossRef] [PubMed]
- Szkopińska, A.; Płochocka, D. Farnesyl diphosphate synthase; regulation of product specificity. Acta Biochim. Pol. 2005, 52, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Moniodis, J.; Zulak, K.G.; Scaffidi, A.; Plummer, J.A.; Ghisalberti, E.L.; Barbour, E.L.; Bohlmann, J. Sandalwood fragrance biosynthesis involves sesquiterpene synthases of both the terpene synthase (TPS)-a and TPS-b Subfamilies, including santalene synthases. J. Biol. Chem. 2011, 286, 17445–17454. [Google Scholar] [CrossRef]
- Srivastava, P.L.; Daramwar, P.P.; Krithika, R.; Pandreka, A.; Shankar, S.S.; Thulasiram, H.V. Functional characterization of novel sesquiterpene synthases from Indian sandalwood, Santalum album. Sci. Rep. 2015, 5, 10095. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sahu, S.K.; Yang, T.; Mu, W.; Wei, J.; Cheng, L.; Yang, J.; Liu, J.; Zhao, Y.; Lisby, M.; et al. The Clausena lansium (Wampee) genome reveal new insights into the carbazole alkaloids biosynthesis pathway. Genomics 2021, 113, 3696–3704. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, A.; Durairaj, J.; van Houwelingen, A.; Verstappen, F.; Bosch, D.; Cankar, K.; Bouwmeester, H.; de Ridder, D.; van Dijk, A.D.J.; Beekwilder, J. The santalene synthase from Cinnamomum camphora: Reconstruction of a sesquiterpene synthase from a monoterpene synthase. Arch. Biochem. Biophys. 2020, 695, 108647. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Ravikumar, P.; Reddy, M.D.; Kush, A. Molecular regulation of santalol biosynthesis in Santalum album L. Gene 2013, 527, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Celedon, J.M.; Chiang, A.; Yuen, M.M.S.; Diaz-Chavez, M.L.; Madilao, L.L.; Finnegan, P.M.; Barbour, E.L.; Bohlmann, J. Heartwood-specific transcriptome and metabolite signatures of tropical sandalwood (Santalum album) reveal the final step of (z)-santalol fragrance biosynthesis. Plant J. 2016, 86, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Chavez, M.L.; Moniodis, J.; Madilao, L.L.; Jancsik, S.; Keeling, C.I.; Barbour, E.L.; Ghisalberti, E.L.; Plummer, J.A.; Jones, C.G.; Bohlmann, J. Biosynthesis of sandalwood oil: Santalum album CYP76F cytochromes p450 produce santalols and bergamotol. PLoS ONE 2013, 8, e75053. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, X.; Li, F.; Zuo, S.; Li, M.; Zhao, J.; Han, X.; Wen, M. Optimized biosynthesis of santalenes and santalols in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2021, 105, 8795–8804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Zhang, X.; Zhang, Y.; Wang, F.; Li, X. Sesquiterpene synthase engineering and targeted engineering of α-santalene overproduction in Escherichia coli. J. Agric. Food Chem. 2022, 70, 5377–5385. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Cao, X.; Yu, W.; Chen, Y.; Lin, X.; Zhu, B.; Zhou, Y.J. Global metabolic rewiring of yeast enables overproduction of sesquiterpene (+)-valencene. J. Agric. Food Chem. 2022, 70, 7180–7187. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liu, M.; Li, Z.H.; Tao, X.; Wei, D.Z.; Wang, F.Q. Significantly enhanced production of patchoulol in metabolically engineered Saccharomyces cerevisiae. J. Agric. Food Chem. 2019, 67, 8590–8598. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, X.; Cui, Z.; Wang, Z.; Qi, Q.; Hou, J. Engineering the oleaginous yeast Yarrowia lipolytica for production of α-farnesene. Biotechnol. Biofuels 2019, 12, 296. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; Zhang, F.; Shao, J.; Ma, X.; Zhu, J.; Sun, P.; Wu, R.; Zi, J. Rationally engineering santalene synthase to readjust the component ratio of sandalwood oil. Nat. Commun. 2022, 13, 2508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, Q.Y.; Ge, Y.; Huang, Z.Y.; Hong, R.; Li, A.; Xu, J.H.; Yu, H.L. Hydroxylases involved in terpenoid biosynthesis: A review. Bioresour. Bioprocess. 2023, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Renault, H.; Bassard, J.E.; Hamberger, B.; Werck-Reichhart, D. Cytochrome P450-mediated metabolic engineering: Current progress and future challenges. Curr. Opin. Plant Biol. 2014, 19, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.W.; Girvan, H.M.; Mason, A.E.; Dunford, A.J.; McLean, K.J. What makes a P450 tick? Trends Biochem. Sci. 2013, 38, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Schückel, J.; Rylott, E.L.; Grogan, G.; Bruce, N.C. A gene-fusion approach to enabling plant cytochromes P450 for biocatalysis. ChemBioChem 2012, 13, 2758–2763. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhao, X.; Zhou, J.; Lu, W.; Li, J.; Chen, J.; Du, G. Biosynthesis of high-active hemoproteins by the efficient heme-supply Pichia pastoris chassis. Adv. Sci. 2023, 10, 2302826. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, T.; Liu, B.; Zheng, C.; Huo, H.; Zhang, J. Comparative and phylogenetic analysis of the complete chloroplast genome sequences of Allium mongolicum. Sci. Rep. 2022, 12, 21676. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha Gowda, V.S.; Patil, K.B.; Ashwath, D.S. Manufacturing of sandalwood oil, market potential demand and use. J. Essent. Oil-Bearing Plants 2004, 7, 293–297. [Google Scholar] [CrossRef]
- Rahmat, E.; Kang, Y. Yeast metabolic engineering for the production of pharmaceutically important secondary metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 4659–4674. [Google Scholar] [CrossRef] [PubMed]
- Paramasivan, K.; Mutturi, S. Progress in terpene synthesis strategies through the engineering of Saccharomyces cerevisiae. Crit. Rev. Biotechnol. 2017, 37, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Du, M.M.; Zhang, G.G.; Zhu, Z.T.; Zhao, Y.Q.; Gao, B.; Tao, X.Y.; Wang, F.Q.; Wei, D.Z. Boosting the epoxidation of squalene to produce triterpenoids in Saccharomyces cerevisiae. Biotechnol. Biofuels Bioprod. 2023, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Durr, I.F.; Rudney, H. The reduction of beta-hydroxy-beta-methyl-glutaryl coenzyme a to mevalonic acid. J. Biol. Chem. 1960, 235, 2572–2578. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.J.; Durr, I.F.; Rudney, H. The biosynthesis of mevalonic acid. Proc. Natl. Acad. Sci. USA 1959, 45, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Friesen, J.A.; Rodwell, V.W. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases gene organization and evolutionary history. Genome Biol. 2004, 5, 248. [Google Scholar] [CrossRef] [PubMed]
- Basson, M.E.; Thorsness, M.; Rine, J. Saccharomyces cerevisiae contains two functional genes encoding 3-hydroxy-3-methylglutaryl-coenzyme-A reductase. Proc. Natl. Acad. Sci. USA 1986, 83, 5563–5567. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. The biosynthesis of C5-C25 terpenoid compounds. Nat. Prod. Rep. 1999, 16, 97–130. [Google Scholar] [CrossRef]
- Scalcinati, G.; Partow, S.; Siewers, V.; Schalk, M.; Daviet, L.; Nielsen, J. Combined metabolic engineering of precursor and co-factor supply to increase α-santalene production by Saccharomyces cerevisiae. Microb. Cell Fact. 2012, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Scalcinati, G.; Knuf, C.; Partow, S.; Chen, Y.; Maury, J.; Schalk, M.; Daviet, L.; Nielsen, J.; Siewers, V. Dynamic control of gene expression in Saccharomyces cerevisiae engineered for the production of plant sesquiterpene α-santalene in a fed-batch mode. Metab. Eng. 2012, 14, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, M.A.; Maury, J.; Schalk, M.; Clark, A.; Nielsen, J. Enhancement of farnesyl diphosphate pool as a direct precursor of sesquiterpenes through metabolic engineering of the mevalonate pathway in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2010, 106, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Xu, S.; Sun, J.; Zhang, C.; Li, D.; Lu, W. Yarrowia lipolytica construction for the heterologous synthesis of α-santalene and fermentation optimization. Appl. Microbiol. Biotechnol. 2019, 103, 3511–3520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Liu, Q.; Jeong, S.-H.; Zhu, L.; Yu, X.; Zheng, X.; Wei, G.; Kim, S.-W.; Wang, C. Metabolic engineering of Escherichia coli for production of α-santalene, a precursor of sandalwood oil. J. Agric. Food Chem. 2021, 69, 13135–13142. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Xiao, F.; Gao, J.; Ye, C.; Jiang, L.; Dong, C.; Lian, J. Establishing Komagataella phaffii as a cell factory for efficient production of sesquiterpenoid α-santalene. J. Agric. Food Chem. 2022, 70, 8024–8031. [Google Scholar] [CrossRef] [PubMed]
- Chambon, C.; Ladeveze, V.; Oulmouden, A.; Servouse, M.; Karst, F. Isolation and properties of yeast mutants affected in farnesyl diphosphate synthetase. Curr. Genet. 1990, 18, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Grabińska, K.; Palamarczyk, G. Dolichol biosynthesis in the yeast Saccharomyces cerevisiae: An insight into the regulatory role of farnesyl diphosphate synthase. FEMS Yeast Res. 2002, 2, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Daum, G.; Lees, N.D.; Bard, M.; Dickson, R. Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast 1998, 14, 1471–1510. [Google Scholar] [CrossRef]
- Szkopińska, A.; Świezewska, E.; Karst, F. The regulation of activity of main mevalonic acid pathway enzymes: Farnesyl diphosphate synthase, 3-hydroxy-3-methylglutaryl-CoA reductase, and squalene synthase in yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2000, 267, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.A.; Barbuch, R.; Bard, M. Transcriptional regulation of the squalene synthase gene (erg9) in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta-Gene Struct. Expr. 1999, 1445, 110–122. [Google Scholar] [CrossRef]
- Keesler, G.A.; Laster, S.M.; Parks, L.W. A defect in the sterol: Steryl ester interconversion in a mutant of the yeast, Saccharomyces cerevisiae. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1992, 1123, 127–132. [Google Scholar] [CrossRef]
- Asadollahi, M.A.; Maury, J.; Møller, K.; Nielsen, K.F.; Schalk, M.; Clark, A.; Nielsen, J. Production of plant sesquiterpenes in Saccharomyces cerevisiae: Effect of erg9 repression on sesquiterpene biosynthesis. Biotechnol. Bioeng. 2008, 99, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Paradise, E.M.; Kirby, J.; Chan, R.; Keasling, J.D. Redirection of flux through the FPP branch-point in Saccharomyces cerevisiae by down-regulating squalene synthase. Biotechnol. Bioeng. 2008, 100, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Toke, D.A.; Bennett, W.L.; Dillon, D.A.; Wu, W.I.; Chen, X.; Ostrander, D.B.; Oshiro, J.; Cremesti, A.; Voelker, D.R.; Fischl, A.S.; et al. Isolation and characterization of the Saccharomyces cerevisiae DPP1 gene encoding diacylglycerol pyrophosphate phosphatase. J. Biol. Chem. 1998, 273, 3278–3284. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yan, X.; Zhang, Y.; Liang, D.; Caiyin, Q.; Qiao, J. Characterization of trans-nerolidol synthase from Celastrus angulatus maxim and production of trans-nerolidol in engineered Saccharomyces cerevisiae. J. Agric. Food Chem. 2021, 69, 2236–2244. [Google Scholar] [CrossRef] [PubMed]
- Nowrouzi, B.; Torres-Montero, P.; Kerkhoven, E.J.; Martínez, J.L.; Rios-Solis, L. Rewiring Saccharomyces cerevisiae metabolism for optimized taxol® precursors production. Metab. Eng. Commun. 2024, 18, e00229. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Scalcinati, G.; Oldiges, M.; Vemuri, G.N. Metabolic impact of increased NADH availability in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2010, 76, 851–859. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nissen, T.L.; Kielland-Brandt, M.C.; Nielsen, J.; Villadsen, J. Optimization of ethanol production in Saccharomyces cerevisiae by metabolic engineering of the ammonium assimilation. Metab. Eng. 2000, 2, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Moreira Dos Santos, M.; Thygesen, G.; Kötter, P.; Olsson, L.; Nielsen, J. Aerobic Physiology of redox-engineered Saccharomyces cerevisiae strains modified in the ammonium assimilation for increased NADPH availability. FEMS Yeast Res. 2003, 4, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y. Modulating betulinic acid production in Saccharomyces cerevisiae by managing the intracellular supplies of the co-factor NADPH and oxygen. J. Biosci. Bioeng. 2015, 119, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Celton, M.; Goelzer, A.; Camarasa, C.; Fromion, V.; Dequin, S. A constraint-based model analysis of the metabolic consequences of increased NADPH oxidation in Saccharomyces cerevisiae. Metab. Eng. 2012, 14, 366–379. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; Van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 3920. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhou, J.; Shi, Z.; Liu, L.; Du, G.; Chen, J. Effect of acetyl-CoA synthase gene overexpression on physiological function of Saccharomyces cerevisiae. Acta Microbiol. Sin. 2010, 50, 1172–1179. [Google Scholar]
- Ding, J.; Holzwarth, G.; Penner, M.H.; Patton-Vogt, J.; Bakalinsky, A.T. Overexpression of acetyl-CoA synthetase in Saccharomyces cerevisiae increases acetic acid tolerance. FEMS Microbiol. Lett. 2015, 362, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Siewers, V.; Nielsen, J. Profiling of cytosolic and peroxisomal acetyl-CoA metabolism in Saccharomyces cerevisiae. PLoS ONE 2012, 7, e42475. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Daviet, L.; Schalk, M.; Siewers, V.; Nielsen, J. Establishing a platform cell factory through the engineering of yeast acetyl-CoA metabolism. Metab. Eng. 2013, 15, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Tippmann, S.; Scalcinati, G.; Siewers, V.; Nielsen, J. Production of farnesene and santalene by Saccharomyces cerevisiae using fed-batch cultivations with RQ-controlled feed. Biotechnol. Bioeng. 2016, 113, 72–81. [Google Scholar] [CrossRef] [PubMed]
| Native/Trade Name | Scientific Name | α-Santalol (%) | β-Santalol (%) |
|---|---|---|---|
| Indian sandalwood | Santalum album | 41–55 | 16–24 |
| New Caledonian sandalwood | Santalum austrocaledonicum | 48–49 | 20–22 |
| Fiji sandalwood | Santalum yasi | 37–39 | 26–28 |
| Australian sandalwood | Santalum spicatum | 15–25 | 5–20 |
| Pathway | Starting Carbon Source | Enzyme Catalyzed Steps | Precursor Formed |
|---|---|---|---|
| MVA pathway | Acetyl-CoA | 6 | IPP |
| MEP pathway | Pyruvate and G3P | 7 | IPP and DMAPP |
| Host Organism | Metabolic Engineering Strategy | Santalene Synthase | Redox System | Fermentation Scale | Yield | Ref. |
|---|---|---|---|---|---|---|
| S. cerevisiae | Altering ERG9 expression by replacing promoter with PHXT1; overexpression of santalol biosynthesis genes under GAL promotors. | SaSS and ClSS | CYP736A167-SaCPR2 | Fed-batch, 5 L bioreactor | 1.3 g/L santalol 1.2 g/L Z-α-santalol | [12] |
| S. cerevisiae | Integration of optimized P450-CPR redox system for santalols production; downregulating ERG9 gene. | SaSS | CYP736A167opt-46tATR1opt | 100 mL shake flasks | 164.7 mg/L santalene 68.8 mg/L santalol | [67] |
| S. cerevisiae | Optimization of precursor and cofactor supply; optimization of the FPP branch point; modulation of the MVA pathway; modification of the ammonium assimilation pathway; enhancing the activity of a transcriptional activator; continuous fermentation process optimization. | ClSS | - | 0.3 L fermenter | 0.036 Cmmol (g biomass)−1 h−1 of α-santalene | [88] |
| Y. lipolytica | Overexpression of MVA pathway genes; optimization of glucose concentration; fermentation optimization through genetic and feeding strategies. | ClSS | - | 5-L fermenter | 27.92 mg/L α-santalene | [91] |
| E. coli | Amplified flux toward FPP precursor; engineered santalene synthase through mutagenesis and fusion tag. | ClSS | - | 100 mL shake flasks Fed-batch 1.3 L bioreactor | 1272 mg/L α-santalene 2916 mg/L α-santalene | [68] |
| E. coli | Manipulated ribosome binding sites (RBSs) to optimize synthetic operon; deleted tnaA gene to increase α-santalene production | ClSS | - | 100 mL shake flasks | 599 mg/L α-santalene | [92] |
| Komagataella phaffii (Pichia pastoris) | Promoter optimization; gene overexpression; multi-copy integration for biosynthesis rewiring; medium optimization; bioprocess engineering. | SaSS | - | Shake flask Batch fermenter Fed-batch fermenter | 829.8 mg/L α-santalene 4.4 g/L α-santalene 21.5 g/L α-santalene | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; David, S.D.; Du, G.; Li, W.; Liang, D.; Nie, S.; Ge, M.; Wang, C.; Qiao, J.; Li, Y.; et al. Biological Properties of Sandalwood Oil and Microbial Synthesis of Its Major Sesquiterpenoids. Biomolecules 2024, 14, 971. https://doi.org/10.3390/biom14080971
Yan X, David SD, Du G, Li W, Liang D, Nie S, Ge M, Wang C, Qiao J, Li Y, et al. Biological Properties of Sandalwood Oil and Microbial Synthesis of Its Major Sesquiterpenoids. Biomolecules. 2024; 14(8):971. https://doi.org/10.3390/biom14080971
Chicago/Turabian StyleYan, Xiaoguang, Sichone Daniel David, Guangzhao Du, Weiguo Li, Dongmei Liang, Shengxin Nie, Mingyue Ge, Chen Wang, Jianjun Qiao, Yanni Li, and et al. 2024. "Biological Properties of Sandalwood Oil and Microbial Synthesis of Its Major Sesquiterpenoids" Biomolecules 14, no. 8: 971. https://doi.org/10.3390/biom14080971
APA StyleYan, X., David, S. D., Du, G., Li, W., Liang, D., Nie, S., Ge, M., Wang, C., Qiao, J., Li, Y., & Caiyin, Q. (2024). Biological Properties of Sandalwood Oil and Microbial Synthesis of Its Major Sesquiterpenoids. Biomolecules, 14(8), 971. https://doi.org/10.3390/biom14080971






