The Role of Epigenetic Mechanisms in the Pathogenesis of Hepatitis C Infection
Abstract
:1. Introduction
2. Epigenetic Mechanisms Regulating Hepatitis C Infection and Progression to Hepatocellular Carcinoma
2.1. DNA Methylation
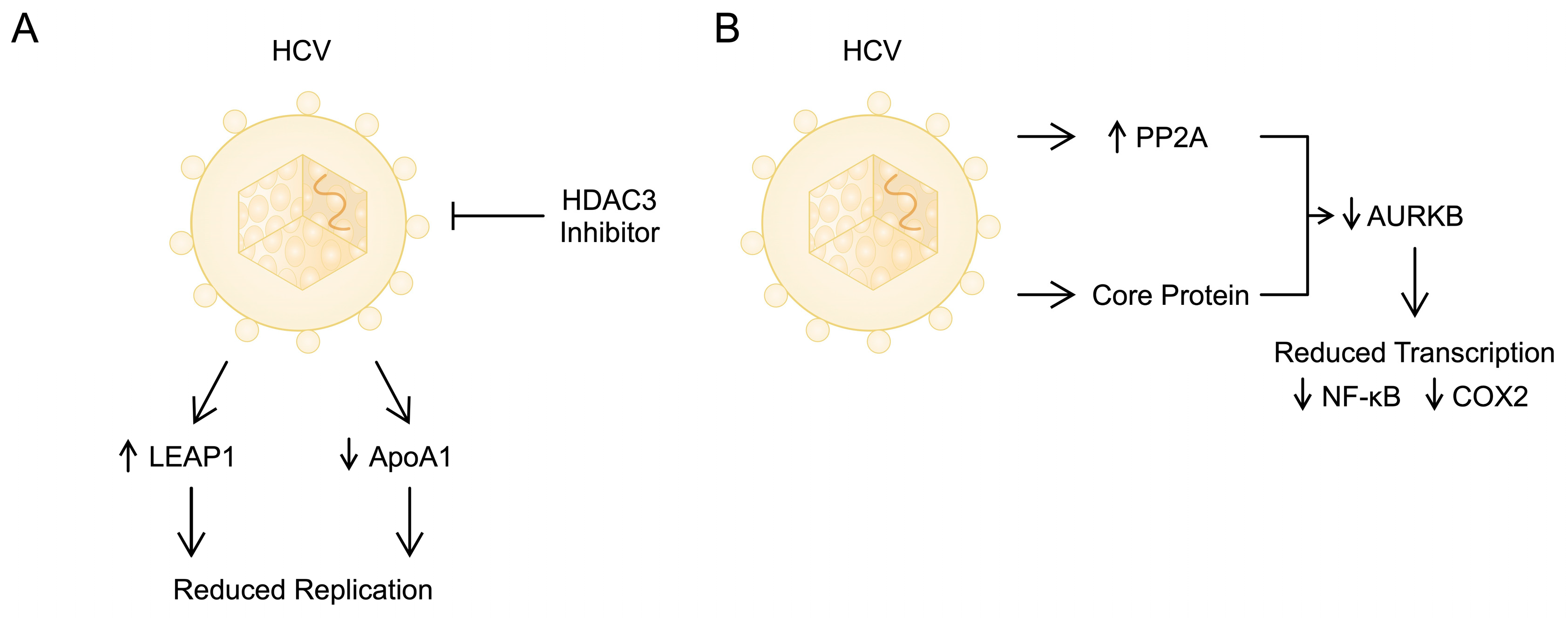
2.2. Histone Modifications
2.3. Non-Coding RNAs
2.3.1. MicroRNA
2.3.2. Long Non-Coding RNA
2.3.3. Circular RNA
3. HCV Infection and Immune Cells
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.H.; Kao, J.H. Acute hepatitis C virus infection: Clinical update and remaining challenges. Clin. Mol. Hepatol. 2023, 29, 623–642. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, H.I.; Lu, S.N.; Jen, C.L.; You, S.L.; Wang, L.Y.; Wang, C.H.; Chen, W.J.; Chen, C.J.; R.E.V.E.A.L.-HCV Study Group. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: A community-based long-term prospective study. J. Infect. Dis. 2012, 206, 469–477. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Kim, D.W.; Talati, C.; Kim, R. Hepatocellular carcinoma (HCC): Beyond sorafenib-chemotherapy. J. Gastrointest. Oncol. 2017, 8, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Bu, P. Non-coding RNA in cancer. Essays Biochem. 2021, 65, 625–639. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Isaac, C.; Patel, T.R.; Zovoilis, A. Non-coding RNAs in virology: An RNA genomics approach. Biotechnol. Genet. Eng. Rev. 2018, 34, 90–106. [Google Scholar] [CrossRef]
- Kiselev, I.S.; Kulakova, O.G.; Boyko, A.N.; Favorova, O.O. DNA Methylation As an Epigenetic Mechanism in the Development of Multiple Sclerosis. Acta Naturae 2021, 13, 45–57. [Google Scholar] [CrossRef]
- Harris, C.J.; Scheibe, M.; Wongpalee, S.P.; Liu, W.; Cornett, E.M.; Vaughan, R.M.; Li, X.; Chen, W.; Xue, Y.; Zhong, Z.; et al. A DNA methylation reader complex that enhances gene transcription. Science 2018, 362, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Lotfi-Shahreza, M.; Nikpour, P. Integrative analysis of DNA methylation and gene expression through machine learning identifies stomach cancer diagnostic and prognostic biomarkers. J. Cell Mol. Med. 2023, 27, 714–726. [Google Scholar] [CrossRef] [PubMed]
- NZekri, A.R.; Raafat, A.M.; Elmasry, S.; Bahnassy, A.A.; Saad, Y.; Dabaon, H.A.; El-Kassas, M.; Shousha, H.I.; Nassar, A.A.; El-Dosouky, M.A.; et al. Promotor methylation: Does it affect response to therapy in chronic hepatitis C (G4) or fibrosis? Ann. Hepatol. 2014, 13, 518–524. [Google Scholar]
- Xu, G.; Zhou, X.; Xing, J.; Xiao, Y.; Jin, B.; Sun, L.; Yang, H.; Du, S.; Xu, H.; Mao, Y. Identification of RASSF1A promoter hypermethylation as a biomarker for hepatocellular carcinoma. Cancer Cell Int. 2020, 20, 547. [Google Scholar] [CrossRef] [PubMed]
- Dammann, R.; Li, C.; Yoon, J.H.; Chin, P.L.; Bates, S.; Pfeifer, G.P. Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat. Genet. 2000, 25, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tommasi, S.; Lee, D.H.; Dammann, R.; Pfeifer, G.P. Control of microtubule stability by the RASSF1A tumor suppressor. Oncogene 2003, 22, 8125–8136. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.D.; Ellis, C.A.; Bell, A.; Birrer, M.J.; Clark, G.J. Ras uses the novel tumor suppressor RASSF1 as an effector to mediate apoptosis. J. Biol. Chem. 2000, 275, 35669–35672. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.Y.; Kim, J.S.; Kim, G.J.; Park, Y.N. RASSF1A-mediated regulation of AREG via the Hippo pathway in hepatocellular carcinoma. Mol. Cancer Res. 2013, 11, 748–758. [Google Scholar] [CrossRef]
- Kwak, J.; Shim, J.H.; Tiwari, I.; Jang, K.L. Hepatitis C virus core protein inhibits E6AP expression via DNA methylation to escape from ubiquitin-dependent proteasomal degradation. Cancer Lett. 2016, 380, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.G.; Ludwig, S.; Ehrhardt, C.; Albrecht, U.; Erhardt, A.; Schaper, F.; Heinrich, P.C.; Häussinger, D. IFN-α antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J. 2003, 17, 488–490. [Google Scholar] [CrossRef]
- Zhao, L.J.; He, S.F.; Liu, Y.; Zhao, P.; Bian, Z.Q.; Qi, Z.T. Inhibition of STAT Pathway Impairs Anti-Hepatitis C Virus Effect of Interferon Alpha. Cell Physiol. Biochem. 2016, 40, 77–90. [Google Scholar] [CrossRef]
- Shen, L.; Kang, L.; Wang, D.; Xun, J.; Chen, C.; Du, L.; Zhang, M.; Gong, J.; Mi, X.; Yue, S.; et al. Legumain-deficient macrophages promote senescence of tumor cells by sustaining JAK1/STAT1 activation. Cancer Lett. 2020, 472, 40–49. [Google Scholar] [CrossRef]
- Zakir, U.; Siddiqui, N.N.; Naqvi, F.U.; Khan, R. Aberrant STAT1 methylation as a non-invasive biomarker in blood of HCV induced hepatocellular carcinoma. Cancer Biomark. 2022, 34, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Denard, B.; Seemann, J.; Chen, Q.; Gay, A.; Huang, H.; Chen, Y.; Ye, J. The membrane-bound transcription factor CREB3L1 is activated in response to virus infection to inhibit proliferation of virus-infected cells. Cell Host Microbe 2011, 10, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Denard, B.; Huang, H.; Ye, J. Epigenetic silencing of antiviral genes renders clones of Huh-7 cells permissive for hepatitis C virus replication. J. Virol. 2013, 87, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Imgenberg-Kreuz, J.; Sandling, J.K.; Almlof, J.C.; Nordlund, J.; Signer, L.; Norheim, K.B.; Omdal, R.; Ronnblom, L.; Eloranta, M.L.; Syvanen, A.C.; et al. Genome-wide DNA methylation analysis in multiple tissues in primary Sjogren’s syndrome reveals regulatory effects at interferon-induced genes. Ann. Rheum. Dis. 2016, 75, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wu, L.F.; Mo, X.B.; Lu, X.; Tang, H.; Zhu, X.W.; Xia, W.; Guo, Y.F.; Wang, M.J.; Zeng, K.Q.; et al. Rheumatoid arthritis-associated DNA methylation sites in peripheral blood mononuclear cells. Ann. Rheum. Dis. 2019, 78, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Shirakura, M.; Murakami, K.; Ichimura, T.; Suzuki, R.; Shimoji, T.; Fukuda, K.; Abe, K.; Sato, S.; Fukasawa, M.; Yamakawa, Y.; et al. E6AP ubiquitin ligase mediates ubiquitylation and degradation of hepatitis C virus core protein. J. Virol. 2007, 81, 1174–1185. [Google Scholar] [CrossRef]
- Lai, W.K.M.; Pugh, B.F. Understanding nucleosome dynamics and their links to gene expression and DNA replication. Nat. Rev. Mol. Cell Biol. 2017, 18, 548–562. [Google Scholar] [CrossRef]
- Kimura, H. Histone modifications for human epigenome analysis. J. Hum. Genet. 2013, 58, 439–445. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Z.; Jia, J.; Du, T.; Zhang, N.; Tang, Y.; Fang, Y.; Fang, D. Overview of Histone Modification. In Histone Mutations and Cancer; Advances in Experimental Medicine and Biology Series; Springer: Singapore, 2021; Volume 1283, pp. 1–16. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Knipe, D.M.; Lieberman, P.M.; Jung, J.U.; McBride, A.A.; Morris, K.V.; Ott, M.; Margolis, D.; Nieto, A.; Nevels, M.; Parks, R.J.; et al. Snapshots: Chromatin control of viral infection. Virology 2013, 435, 141–156. [Google Scholar] [CrossRef]
- Lilley, C.E.; Chaurushiya, M.S.; Weitzman, M.D. Chromatin at the intersection of viral infection and DNA damage. Biochim. Biophys. Acta 2010, 1799, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Domovitz, T.; Gal-Tanamy, M. Tracking Down the Epigenetic Footprint of HCV-Induced Hepatocarcinogenesis. J. Clin. Med. 2021, 10, 551. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, F.; Wan, T.; Xu, H.; Zhao, Q. Overexpression of long noncoding RNA. Onco Targets Ther. 2018, 11, 5209–5217. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bolatkan, A.; Kaneko, S.; Ikawa, N.; Asada, K.; Komatsu, M.; Hayami, S.; Ojima, H.; Abe, N.; Yamaue, H.; et al. Deregulation of the Histone Lysine-Specific Demethylase 1 Is Involved in Human Hepatocellular Carcinoma. Biomolecules 2019, 9, 810. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zhou, Y.; Zhuang, X.; Zhu, Y.; Wu, Z.; Lu, Y.; Li, S.; Zeng, Y.; Lu, Q.R.; Huo, Y.; et al. HDAC3 Deficiency Promotes Liver Cancer through a Defect in H3K9ac/H3K9me3 Transition. Cancer Res. 2019, 79, 3676–3688. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xia, J.; Zhou, Y.J.; Wan, J.; Li, L.; Bao, J.; Shi, Y.J.; Bu, H. Proportions of acetyl-histone-positive hepatocytes indicate the functional status and prognosis of cirrhotic patients. World J. Gastroenterol. 2015, 21, 6665–6674. [Google Scholar] [CrossRef]
- Zhao, P.; Malik, S.; Xing, S. Epigenetic Mechanisms Involved in HCV-Induced Hepatocellular Carcinoma (HCC). Front. Oncol. 2021, 11, 677926. [Google Scholar] [CrossRef] [PubMed]
- El-Maraghy, S.A.; Adel, O.; Zayed, N.; Yosry, A.; El-Nahaas, S.M.; Gibriel, A.A. Circulatory miRNA-484, 524, 615 and 628 expression profiling in HCV mediated HCC among Egyptian patients; implications for diagnosis and staging of hepatic cirrhosis and fibrosis. J. Adv. Res. 2020, 22, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, X.; Shen, Y.; Wang, Y.; Xia, X.; Zhang, A.M. STAT3 signaling pathway plays importantly genetic and functional roles in HCV infection. Mol. Genet. Genom. Med. 2019, 7, e821. [Google Scholar] [CrossRef]
- Xue, X.; Gao, W.; Sun, B.; Xu, Y.; Han, B.; Wang, F.; Zhang, Y.; Sun, J.; Wei, J.; Lu, Z.; et al. Vasohibin 2 is transcriptionally activated and promotes angiogenesis in hepatocellular carcinoma. Oncogene 2013, 32, 1724–1734. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Yang, Q.; Tang, J.; Xu, C.; Gai, D.; Chen, X.; Chen, J. Histone Deacetylase 3 Inhibitor Suppresses Hepatitis C Virus Replication by Regulating Apo-A1 and LEAP-1 Expression. Virol. Sin. 2018, 33, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Wrensch, F.; Crouchet, E.; Ligat, G.; Zeisel, M.B.; Keck, Z.Y.; Foung, S.K.H.; Schuster, C.; Baumert, T.F. Hepatitis C Virus (HCV)-Apolipoprotein Interactions and Immune Evasion and Their Impact on HCV Vaccine Design. Front. Immunol. 2018, 9, 1436. [Google Scholar] [CrossRef] [PubMed]
- Mancone, C.; Steindler, C.; Santangelo, L.; Simonte, G.; Vlassi, C.; Longo, M.A.; D’Offizi, G.; Di Giacomo, C.; Pucillo, L.P.; Amicone, L.; et al. Hepatitis C virus production requires apolipoprotein A-I and affects its association with nascent low-density lipoproteins. Gut 2011, 60, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chiu, D.K.; Tsang, F.H.; Law, C.T.; Cheng, C.L.; Au, S.L.; Lee, J.M.; Wong, C.C.; Ng, I.O.; Wong, C.M. Histone methyltransferase G9a promotes liver cancer development by epigenetic silencing of tumor suppressor gene RARRES3. J. Hepatol. 2017, 67, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Duong, F.H.; Christen, V.; Berke, J.M.; Penna, S.H.; Moradpour, D.; Heim, M.H. Upregulation of protein phosphatase 2Ac by hepatitis C virus modulates NS3 helicase activity through inhibition of protein arginine methyltransferase 1. J. Virol. 2005, 79, 15342–15350. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, F.M.; Déry, U.; Masson, J.Y.; Richard, S. Arginine methylation of MRE11 by PRMT1 is required for DNA damage checkpoint control. Genes Dev. 2005, 19, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Sif, S. Interplay between chromatin remodelers and protein arginine methyltransferases. J. Cell Physiol. 2007, 213, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Bernsmeier, C.; Duong, F.H.; Christen, V.; Pugnale, P.; Negro, F.; Terracciano, L.; Heim, M.H. Virus-induced over-expression of protein phosphatase 2A inhibits insulin signalling in chronic hepatitis C. J. Hepatol. 2008, 49, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Duong, F.H.; Christen, V.; Lin, S.; Heim, M.H. Hepatitis C virus-induced up-regulation of protein phosphatase 2A inhibits histone modification and DNA damage repair. Hepatology 2010, 51, 741–751. [Google Scholar] [CrossRef]
- Madejón, A.; Sheldon, J.; Francisco-Recuero, I.; Perales, C.; Domínguez-Beato, M.; Lasa, M.; Sánchez-Perez, I.; Muntané, J.; Domingo, E.; García-Samaniego, J.; et al. Hepatitis C virus-mediated Aurora B kinase inhibition modulates inflammatory pathway and viral infectivity. J. Hepatol. 2015, 63, 312–319. [Google Scholar] [CrossRef] [PubMed]
- García-Crespo, C.; Francisco-Recuero, I.; Gallego, I.; Camblor-Murube, M.; Soria, M.E.; López-López, A.; de Ávila, A.I.; Madejón, A.; García-Samaniego, J.; Domingo, E.; et al. Hepatitis C virus fitness can influence the extent of infection-mediated epigenetic modifications in the host cells. Front. Cell Infect. Microbiol. 2023, 13, 1057082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, J.; Li, Y.; An, Y.; Du, W.; Yang, Q.; Huang, M.; Cai, X. Overexpression of Aurora Kinase B Is Correlated with Diagnosis and Poor Prognosis in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2024, 25, 2199. [Google Scholar] [CrossRef] [PubMed]
- Poreba, E.; Broniarczyk, J.K.; Gozdzicka-Jozefiak, A. Epigenetic mechanisms in virus-induced tumorigenesis. Clin. Epigenetics 2011, 2, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Schueller, F.; Roy, S.; Vucur, M.; Trautwein, C.; Luedde, T.; Roderburg, C. The Role of miRNAs in the Pathophysiology of Liver Diseases and Toxicity. Int. J. Mol. Sci. 2018, 19, 261. [Google Scholar] [CrossRef] [PubMed]
- Li, H.C.; Yang, C.H.; Lo, S.Y. Roles of microRNAs in Hepatitis C Virus Replication and Pathogenesis. Viruses 2022, 14, 1776. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Jiang, D.; Rao, H.Y.; Zhao, J.M.; Wang, Y.; Wei, L. Absolute quantification of serum microRNA-122 and its correlation with liver inflammation grade and serum alanine aminotransferase in chronic hepatitis C patients. Int. J. Infect. Dis. 2015, 30, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.L.; Yi, M.; Lancaster, A.M.; Lemon, S.M.; Sarnow, P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 2005, 309, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Machlin, E.S.; Sarnow, P.; Sagan, S.M. Masking the 5’ terminal nucleotides of the hepatitis C virus genome by an unconventional microRNA-target RNA complex. Proc. Natl. Acad. Sci. USA 2011, 108, 3193–3198. [Google Scholar] [CrossRef] [PubMed]
- Schult, P.; Roth, H.; Adams, R.L.; Mas, C.; Imbert, L.; Orlik, C.; Ruggieri, A.; Pyle, A.M.; Lohmann, V. microRNA-122 amplifies hepatitis C virus translation by shaping the structure of the internal ribosomal entry site. Nat. Commun. 2018, 9, 2613. [Google Scholar] [CrossRef]
- Wang, X.; Feng, L.; Lu, Y.; Zhang, H. miR-122/PPARβ axis is involved in hypoxic exercise and modulates fatty acid metabolism in skeletal muscle of obese rats. Heliyon 2024, 10, e26572. [Google Scholar] [CrossRef]
- Trebicka, J.; Anadol, E.; Elfimova, N.; Strack, I.; Roggendorf, M.; Viazov, S.; Wedemeyer, I.; Drebber, U.; Rockstroh, J.; Sauerbruch, T.; et al. Hepatic and serum levels of miR-122 after chronic HCV-induced fibrosis. J. Hepatol. 2013, 58, 234–239. [Google Scholar] [CrossRef]
- Elemeery, M.N.; Mohamed, M.A.; Madkour, M.A.; Shamseya, M.M.; Issa, N.M.; Badr, A.N.; Ghareeb, D.A.; Pan, C.H. MicroRNA signature in patients with hepatocellular carcinoma associated with type 2 diabetes. World J. Gastroenterol. 2019, 25, 6322–6341. [Google Scholar] [CrossRef]
- Deng, P.; Li, M.; Wu, Y. The Predictive Efficacy of Serum Exosomal microRNA-122 and microRNA-148a for Hepatocellular Carcinoma Based on Smart Healthcare. J. Healthc. Eng. 2022, 2022, 5914541. [Google Scholar] [CrossRef]
- Bandiera, S.; Pfeffer, S.; Baumert, T.F.; Zeisel, M.B. miR-122--a key factor and therapeutic target in liver disease. J. Hepatol. 2015, 62, 448–457. [Google Scholar] [CrossRef]
- Li, Y.P.; Gottwein, J.M.; Scheel, T.K.; Jensen, T.B.; Bukh, J. MicroRNA-122 antagonism against hepatitis C virus genotypes 1-6 and reduced efficacy by host RNA insertion or mutations in the HCV 5’ UTR. Proc. Natl. Acad. Sci. USA 2011, 108, 4991–4996. [Google Scholar] [CrossRef]
- Janssen, H.L.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; van der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013, 368, 1685–1694. [Google Scholar] [CrossRef]
- Ottosen, S.; Parsley, T.B.; Yang, L.; Zeh, K.; van Doorn, L.J.; van der Veer, E.; Raney, A.K.; Hodges, M.R.; Patick, A.K. In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrob. Agents Chemother. 2015, 59, 599–608. [Google Scholar] [CrossRef]
- Zhu, H.; Geng, Y.; He, Q.; Li, M. miRNAs regulate immune response and signaling during hepatitis C virus infection. Eur. J. Med. Res. 2018, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.R.; Tang, L.J.; He, Y.; Garcia, R.C. An update on the role of miRNA-155 in pathogenic microbial infections. Microbes Infect. 2015, 17, 613–621. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, W.; Cheng, N.; Wang, K.; Li, B.; Jiang, X.; Sun, S. Hepatitis C virus-induced up-regulation of microRNA-155 promotes hepatocarcinogenesis by activating Wnt signaling. Hepatology 2012, 56, 1631–1640. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Ren, J.P.; Zhao, J.; Wang, J.M.; Zhou, Y.; Li, G.Y.; Moorman, J.P.; Yao, Z.Q. MicroRNA-155 regulates interferon-γ production in natural killer cells via Tim-3 signalling in chronic hepatitis C virus infection. Immunology 2015, 145, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Tilahun, Y.; Taha, O.; Alao, H.; Kodys, K.; Catalano, D.; Szabo, G. Increased microRNA-155 expression in the serum and peripheral monocytes in chronic HCV infection. J. Transl. Med. 2012, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Yosry, A.; Zayed, N.; Dawood, R.M.; Ibrahim, M.K.; Elsharkawy, M.; Ekladious, S.M.; Khairy, A.; Elsharkawy, A.; Khairy, M.; Abdel Alem, S.; et al. Highly Sensitive Serum miRNA Panel for the Diagnosis of Hepatocellular Carcinoma in Egyptian Patients with HCV-Related HCC. Lab. Med. 2022, 53, 523–529. [Google Scholar] [CrossRef]
- Hyrina, A.; Olmstead, A.D.; Steven, P.; Krajden, M.; Tam, E.; Jean, F. Treatment-Induced Viral Cure of Hepatitis C Virus-Infected Patients Involves a Dynamic Interplay among three Important Molecular Players in Lipid Homeostasis: Circulating microRNA (miR)-24, miR-223, and Proprotein Convertase Subtilisin/Kexin Type 9. EBioMedicine 2017, 23, 68–78. [Google Scholar] [CrossRef]
- El-Guendy, N.M.; Helwa, R.; El-Halawany, M.S.; Abdel Rahman Ali, S.; Tantawy Aly, M.; Hasan Alieldin, N.; Fouad, S.A.; Saeid, H.; Abdel-Wahab, A.H. The Liver MicroRNA Expression Profiles Associated With Chronic Hepatitis C Virus (HCV) Genotype-4 Infection: A Preliminary Study. Hepat. Mon. 2016, 16, e33881. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Zhang, T.; Lou, G.; Liu, Y. Role of miR-223 in the pathophysiology of liver diseases. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Yuan, X.; Liu, M.; Xue, B. miRNA-223-3p regulates NLRP3 to promote apoptosis and inhibit proliferation of hep3B cells. Exp. Ther. Med. 2018, 15, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, C.; Che, X.; Wang, L.; Yu, D.; Zhang, T.; Huang, L.; Li, H.; Tan, W.; Wang, C.; et al. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol. Carcinog. 2011, 50, 136–142. [Google Scholar] [CrossRef]
- Öksüz, Z.; Gragnani, L.; Lorini, S.; Temel, G.; Serin, M.S.; Zignego, A.L. Evaluation of Plasma miR-17-5p, miR-24-3p and miRNA-223-3p Profile of Hepatitis C Virus-Infected Patients after Treatment with Direct-Acting Antivirals. J. Pers. Med. 2023, 13, 1188. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Sun, L.; Zhou, L.; Ma, T.C.; Song, L.; Wu, J.G.; Li, J.L.; Ho, W.Z. Toll-like receptor 3-activated macrophages confer anti-HCV activity to hepatocytes through exosomes. FASEB J. 2016, 30, 4132–4140. [Google Scholar] [CrossRef]
- Ullah, A.; Rehman, I.U.; Ommer, K.; Ahmed, N.; Odenthal, M.; Yu, X.; Ahmad, J.; Nadeem, T.; Ali, Q.; Ahmad, B. Circulating miRNA-192 and miR-29a as Disease Progression Biomarkers in Hepatitis C Patients with a Prevalence of HCV Genotype 3. Genes 2023, 14, 1056. [Google Scholar] [CrossRef]
- Yu, X.; Elfimova, N.; Müller, M.; Bachurski, D.; Koitzsch, U.; Drebber, U.; Mahabir, E.; Hansen, H.P.; Friedman, S.L.; Klein, S.; et al. Autophagy-Related Activation of Hepatic Stellate Cells Reduces Cellular miR-29a by Promoting Its Vesicular Secretion. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 1701–1716. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Friedman, R.C.; Marquez, R.T.; Keck, K.; Kong, B.; Icardi, M.S.; Brown, K.E.; Burge, C.B.; Schmidt, W.N.; Wang, Y.; et al. Hepatitis C virus infection and hepatic stellate cell activation downregulate miR-29: miR-29 overexpression reduces hepatitis C viral abundance in culture. J. Infect. Dis. 2011, 203, 1753–1762. [Google Scholar] [CrossRef]
- Hoffmann, T.W.; Duverlie, G.; Bengrine, A. MicroRNAs and hepatitis C virus: Toward the end of miR-122 supremacy. Virol. J. 2012, 9, 109. [Google Scholar] [CrossRef]
- Pedersen, I.M.; Cheng, G.; Wieland, S.; Volinia, S.; Croce, C.M.; Chisari, F.V.; David, M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature 2007, 449, 919–922. [Google Scholar] [CrossRef]
- Kwon, J.J.; Factora, T.D.; Dey, S.; Kota, J. A Systematic Review of miR-29 in Cancer. Mol. Ther. Oncolytics 2019, 12, 173–194. [Google Scholar] [CrossRef]
- Xiong, Y.; Fang, J.H.; Yun, J.P.; Yang, J.; Zhang, Y.; Jia, W.H.; Zhuang, S.M. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 2010, 51, 836–845. [Google Scholar] [CrossRef]
- Badami, E.; Carcione, C.; Chinnici, C.M.; Tinnirello, R.; Conaldi, P.G.; Iannolo, G. HCV Interplay With Mir34a: Implications in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 803278. [Google Scholar] [CrossRef]
- Cabral, B.C.A.; Hoffmann, L.; Bottaro, T.; Costa, P.F.; Ramos, A.L.A.; Coelho, H.S.M.; Villela-Nogueira, C.A.; Ürményi, T.P.; Faffe, D.S.; Silva, R. Circulating microRNAs associated with liver fibrosis in chronic hepatitis C patients. Biochem. Biophys. Rep. 2020, 24, 100814. [Google Scholar] [CrossRef]
- Smith, J.L.; Jeng, S.; McWeeney, S.K.; Hirsch, A.J. A MicroRNA Screen Identifies the Wnt Signaling Pathway as a Regulator of the Interferon Response during Flavivirus Infection. J. Virol. 2017, 91, e02388-16. [Google Scholar] [CrossRef]
- Badami, E.; Busà, R.; Douradinha, B.; Russelli, G.; Miceli, V.; Gallo, A.; Zito, G.; Conaldi, P.G.; Iannolo, G. Hepatocellular carcinoma, hepatitis C virus infection and miRNA involvement: Perspectives for new therapeutic approaches. World J. Gastroenterol. 2022, 28, 2417–2428. [Google Scholar] [CrossRef] [PubMed]
- Cermelli, S.; Ruggieri, A.; Marrero, J.A.; Ioannou, G.N.; Beretta, L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS ONE 2011, 6, e23937. [Google Scholar] [CrossRef] [PubMed]
- Carnero, E.; Fortes, P. HCV infection, IFN response and the coding and non-coding host cell genome. Virus Res. 2016, 212, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Unfried, J.P.; Fortes, P. LncRNAs in HCV Infection and HCV-Related Liver Disease. Int. J. Mol. Sci. 2020, 21, 2255. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Li, L.; Gao, Y.; Wu, G.; Hou, Z.; Liu, S. Long noncoding RNA UCA1 regulates HCV replication and antiviral response via miR-145-5p/SOCS7/IFN pathway. Int. J. Biol. Sci. 2021, 17, 2826–2840. [Google Scholar] [CrossRef] [PubMed]
- Carnero, E.; Barriocanal, M.; Prior, C.; Pablo Unfried, J.; Segura, V.; Guruceaga, E.; Enguita, M.; Smerdou, C.; Gastaminza, P.; Fortes, P. Long noncoding RNA EGOT negatively affects the antiviral response and favors HCV replication. EMBO Rep. 2016, 17, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Valadkhan, S.; Gunawardane, L.S. lncRNA-mediated regulation of the interferon response. Virus Res. 2016, 212, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Perry, J.W.; Lauring, A.S.; Neddermann, P.; De Francesco, R.; Tai, A.W. Oxysterol-binding protein is a phosphatidylinositol 4-kinase effector required for HCV replication membrane integrity and cholesterol trafficking. Gastroenterology 2014, 146, 1373–1385.e11. [Google Scholar] [CrossRef]
- Liu, X.; Duan, X.; Holmes, J.A.; Li, W.; Lee, S.H.; Tu, Z.; Zhu, C.; Salloum, S.; Lidofsky, A.; Schaefer, E.A.; et al. A Long Noncoding RNA Regulates Hepatitis C Virus Infection Through Interferon Alpha-Inducible Protein 6. Hepatology 2019, 69, 1004–1019. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, Y.; Zhao, H.; Chen, H.; Yu, Q.Q.; Cui, H. The roles of lncRNA functions and regulatory mechanisms in the diagnosis and treatment of hepatocellular carcinoma. Front. Cell Dev. Biol. 2022, 10, 1051306. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Pal, A.; Ghosh, S.; Goel, A.; Aggarwal, R.; Banerjee, S.; Das, S. LncRNA NEAT1 regulates HCV-induced Hepatocellular carcinoma by modulating the miR-9-BGH3 axis. J. Gen. Virol. 2022, 103, 001809. [Google Scholar] [CrossRef]
- Moon, S.L.; Blackinton, J.G.; Anderson, J.R.; Dozier, M.K.; Dodd, B.J.; Keene, J.D.; Wilusz, C.J.; Bradrick, S.S.; Wilusz, J. XRN1 stalling in the 5’ UTR of Hepatitis C virus and Bovine Viral Diarrhea virus is associated with dysregulated host mRNA stability. PLoS Pathog. 2015, 11, e1004708. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, L.; Du, Y.; Zhu, P.; Huang, G.; Luo, J.; Yan, X.; Ye, B.; Li, C.; Xia, P.; et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell 2015, 16, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, X.; Liu, Y.; Zhao, J.; Ma, S.; Yin, H.; Huang, S.; Zhao, Y.; He, X. Gain of LINC00624 Enhances Liver Cancer Progression by Disrupting the Histone Deacetylase 6/Tripartite Motif Containing 28/Zinc Finger Protein 354C Corepressor Complex. Hepatology 2021, 73, 1764–1782. [Google Scholar] [CrossRef]
- Gaber, D.A.; Shaker, O.; Younis, A.T.; El-Kassas, M. LncRNA HULC and miR-122 Expression Pattern in HCC-Related HCV Egyptian Patients. Genes 2022, 13, 1669. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, X.; Li, X.; Song, S.; Meng, Q.; Wang, L.; Lu, Y.; Xin, X.; Pu, H.; Gui, X.; et al. Long noncoding RNA HULC accelerates the growth of human liver cancer stem cells by upregulating CyclinD1 through miR675-PKM2 pathway via autophagy. Stem Cell Res. Ther. 2020, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Tripathi, S.K.; Das, S. lncRNA HULC facilitates efficient loading of HCV-core protein onto lipid droplets and subsequent virus-particle release. Cell Microbiol. 2019, 21, e13086. [Google Scholar] [CrossRef]
- Yao, Y.; Shu, F.; Wang, F.; Wang, X.; Guo, Z.; Wang, H.; Li, L.; Lv, H. Long noncoding RNA LINC01189 is associated with HCV-hepatocellular carcinoma and regulates cancer cell proliferation and chemoresistance through hsa-miR-155-5p. Ann. Hepatol. 2021, 22, 100269. [Google Scholar] [CrossRef] [PubMed]
- Mofed, D.; Sabet, S.; Baiomy, A.A.; Salem, T.Z. The Transgene Expression of the Immature Form of the HCV Core Protein (C191) and the LncRNA MEG3 Increases Apoptosis in HepG2 Cells. Curr. Issues Mol. Biol. 2022, 44, 3632–3647. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Lv, Y.; Zhang, C.; Guo, S. LncRNA meg3 suppresses hepatocellular carcinoma in vitro and vivo studies. Am. J. Transl. Res. 2019, 11, 4089–4099. [Google Scholar]
- Gan, Y.; Han, N.; He, X.; Yu, J.; Zhang, M.; Zhou, Y.; Liang, H.; Deng, J.; Zheng, Y.; Ge, W.; et al. Long non-coding RNA CASC2 regulates cell biological behaviour through the MAPK signalling pathway in hepatocellular carcinoma. Tumour Biol. 2017, 39, 1010428317706229. [Google Scholar] [CrossRef] [PubMed]
- Refai, N.S.; Louka, M.L.; Halim, H.Y.; Montasser, I. Long non-coding RNAs (CASC2 and TUG1) in hepatocellular carcinoma: Clinical significance. J. Gene Med. 2019, 21, e3112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, X.; Qi, Q.; Gao, Y.; Wei, Q.; Han, S. lncRNA-HEIH in serum and exosomes as a potential biomarker in the HCV-related hepatocellular carcinoma. Cancer Biomark. 2018, 21, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, X.; Li, X.; Xu, L.; Yu, W. LncRNA-HEIH is a Novel Diagnostic and Predictive Biomarker in Gastric Cancer. Genet. Test. Mol. Biomarkers 2021, 25, 284–292. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, L.; Huo, X.S.; Yuan, J.H.; Xu, D.; Yuan, S.X.; Zhu, N.; Zhou, W.P.; Yang, G.S.; Wang, Y.Z.; et al. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology 2011, 54, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, M.; Nakashima, Y.; Nakashima, O.; Kojiro, M. Immunohistologic study on the expressions of α-fetoprotein and protein induced by vitamin K absence or antagonist II in surgically resected small hepatocellular carcinoma. Hepatology 2001, 34, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Toraih, E.A.; Ellawindy, A.; Fala, S.Y.; Al Ageeli, E.; Gouda, N.S.; Fawzy, M.S.; Hosny, S. Oncogenic long noncoding RNA MALAT1 and HCV-related hepatocellular carcinoma. Biomed. Pharmacother. 2018, 102, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, Y.Q.; You, S.; Zhang, C.M.; Wu, L.; Zhao, W.; Wang, X.M. Long Non-Coding RNA MALAT1 as a Detection and Diagnostic Molecular Marker in Various Human Cancers: A Pooled Analysis Based on 3255 Subjects. Onco Targets Ther 2020, 13, 5807–5817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, C.; Zhao, Y.; Li, M.; Wu, L.; Yang, X.; Wan, X.; Wang, A.; Zhang, M.Q.; Sang, X.; et al. Long non-coding RNA expression profiles of hepatitis C virus-related dysplasia and hepatocellular carcinoma. Oncotarget 2015, 6, 43770–43778. [Google Scholar] [CrossRef]
- Yan, L.; Chen, Y.G. Circular RNAs in Immune Response and Viral Infection. Trends Biochem. Sci. 2020, 45, 1022–1034. [Google Scholar] [CrossRef]
- Tan, K.E.; Lim, Y.Y. Viruses join the circular RNA world. FEBS J. 2021, 288, 4488–4502. [Google Scholar] [CrossRef]
- Zebardast, A.; Latifi, T.; Shirzad, M.; Goodarzi, G.; Ebrahimi Fana, S.; Samavarchi Tehrani, S.; Yahyapour, Y. Critical involvement of circular RNAs in virus-associated cancers. Genes Dis. 2023, 10, 2296–2305. [Google Scholar] [CrossRef] [PubMed]
- Jost, I.; Shalamova, L.A.; Gerresheim, G.K.; Niepmann, M.; Bindereif, A.; Rossbach, O. Functional sequestration of microRNA-122 from Hepatitis C Virus by circular RNA sponges. RNA Biol. 2018, 15, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Breuer, J.; Rossbach, O. Production and Purification of Artificial Circular RNA Sponges for Application in Molecular Biology and Medicine. Methods Protoc. 2020, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Xu, H.; Ji, M.; Shang, D.; Lu, Z.; Wu, Y.; Tu, Z.; Liu, H. Circular RNAs in hepatocellular carcinoma: Biomarkers, functions and mechanisms. Life Sci. 2019, 231, 116660. [Google Scholar] [CrossRef]
- Aborehab, N.M.; Kandeil, M.A.; Sabry, D.; Rabie, R.; Ibrahim, I.T. Circular SERPINA3 and its target microRNA-944 as potential biomarkers in hepatitis C virus-induced hepatocellular carcinoma in Egyptian population. Noncoding RNA Res. 2023, 8, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Bedair, H.M.; El-Banna, E.A.; Ahmed, E.A.; Elhelbawy, M.G.; Abdelfattah, A.; Khalaf, F.A.; Abdel-Samiee, M.; El Sharnoby, A. Evaluation of Circular RNA SMARCA5 as a Novel Biomarker for Hepatocellular Carcinoma. Asian Pac. J. Cancer Prev. 2024, 25, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, B.; Wang, T.; Li, W.; Wang, Z.; Zhang, H.; Song, Y.; Li, N. Serum microRNA expression profiling identifies serum biomarkers for HCV-related hepatocellular carcinoma. Cancer Biomark. 2019, 26, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Quan, Y.; Fan, S.; Wang, H.; Liang, J.; Huang, L.; Chen, L.; Liu, Q.; He, P.; Ye, Y. Exosome-transmitted circular RNA hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett. 2020, 475, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bhowmik, S.; Majumdar, S.; Goswami, A.; Chakraborty, J.; Gupta, S.; Aggarwal, S.; Ray, S.; Chatterjee, R.; Bhattacharyya, S.; et al. The exosome encapsulated microRNAs as circulating diagnostic marker for hepatocellular carcinoma with low alpha-fetoprotein. Int. J. Cancer 2020, 147, 2934–2947. [Google Scholar] [CrossRef]
- Su, X.; Su, J.; He, H.; Zhan, Y.; Liu, H. Hsa_circ_0070269 inhibits hepatocellular carcinoma progression through modulating miR-182/NPTX1 axis. Biomed. Pharmacother. 2019, 120, 109497. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Deng, Y.; Liang, J.; Hu, Z.; Li, X.; Liu, H.; Wang, G.; Fu, B.; Zhang, T.; Zhang, Q.; et al. Circular RNA circ-102,166 acts as a sponge of miR-182 and miR-184 to suppress hepatocellular carcinoma proliferation and invasion. Cell Oncol. 2021, 44, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Chigbu, D.I.; Loonawat, R.; Sehgal, M.; Patel, D.; Jain, P. Hepatitis C Virus Infection: Host⁻Virus Interaction and Mechanisms of Viral Persistence. Cells 2019, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Jinushi, M.; Takehara, T.; Tatsumi, T.; Kanto, T.; Miyagi, T.; Suzuki, T.; Kanazawa, Y.; Hiramatsu, N.; Hayashi, N. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J. Immunol. 2004, 173, 6072–6081. [Google Scholar] [CrossRef] [PubMed]
- Nattermann, J.; Feldmann, G.; Ahlenstiel, G.; Langhans, B.; Sauerbruch, T.; Spengler, U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut 2006, 55, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Tacke, R.S.; Lee, H.C.; Goh, C.; Courtney, J.; Polyak, S.J.; Rosen, H.R.; Hahn, Y.S. Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology 2012, 55, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Sreenarasimhaiah, J.; Jaramillo, A.; Crippin, J.; Lisker-Melman, M.; Chapman, W.C.; Mohanakumar, T. Lack of optimal T-cell reactivity against the hepatitis C virus is associated with the development of fibrosis/cirrhosis during chronic hepatitis. Hum. Immunol. 2003, 64, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Kotsari, M.; Dimopoulou, V.; Koskinas, J.; Armakolas, A. Immune System and Hepatocellular Carcinoma (HCC): New Insights into HCC Progression. Int. J. Mol. Sci. 2023, 24, 11471. [Google Scholar] [CrossRef] [PubMed]
- Barili, V.; Fisicaro, P.; Montanini, B.; Acerbi, G.; Filippi, A.; Forleo, G.; Romualdi, C.; Ferracin, M.; Guerrieri, F.; Pedrazzi, G.; et al. Targeting p53 and histone methyltransferases restores exhausted CD8+ T cells in HCV infection. Nat. Commun. 2020, 11, 604. [Google Scholar] [CrossRef]
- Chen, J.; Wang, N.; Dong, M.; Guo, M.; Zhao, Y.; Zhuo, Z.; Zhang, C.; Chi, X.; Pan, Y.; Jiang, J.; et al. The Metabolic Regulator Histone Deacetylase 9 Contributes to Glucose Homeostasis Abnormality Induced by Hepatitis C Virus Infection. Diabetes 2015, 64, 4088–4098. [Google Scholar] [CrossRef]
- Han, T.; Wan, Y.; Wang, J.; Zhao, P.; Yuan, Y.; Wang, L.; She, Y.; Broering, R.; Lu, M.; Ye, L.; et al. Set7 facilitates hepatitis C virus replication via enzymatic activity-dependent attenuation of the IFN-related pathway. J. Immunol. 2015, 194, 2757–2768. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Tai, J.J.; Wong, W.C.; Han, H.; Sem, X.; Yeap, W.H.; Kourilsky, P.; Wong, S.C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011, 118, e16–e31. [Google Scholar] [CrossRef] [PubMed]
- Hammad, R.; Eldosoky, M.A.; Elmadbouly, A.A.; Aglan, R.B.; AbdelHamid, S.G.; Zaky, S.; Ali, E.; Abd El Hakam, F.E.; Mosaad, A.M.; Abdelmageed, N.A.; et al. Monocytes subsets altered distribution and dysregulated plasma hsa-miR-21-5p and hsa-miR-155-5p in HCV-linked liver cirrhosis progression to hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 15349–15364. [Google Scholar] [CrossRef]
- Ren, J.P.; Ying, R.S.; Cheng, Y.Q.; Wang, L.; El Gazzar, M.; Li, G.Y.; Ning, S.B.; Moorman, J.P.; Yao, Z.Q. HCV-induced miR146a controls SOCS1/STAT3 and cytokine expression in monocytes to promote regulatory T-cell development. J. Viral Hepat. 2016, 23, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Marcos, M.; Kodys, K.; Csak, T.; Catalano, D.; Mandrekar, P.; Szabo, G. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor α (TNFα) production via increased mRNA half-life in alcoholic liver disease. J. Biol. Chem. 2011, 286, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Kogure, T.; Ninomiya, M.; Fukuda, R.; Monma, N.; Ikeo, K.; Tanaka, Y. The reduction of miR146b-5p in monocytes and T cells could contribute to the immunopathogenesis of hepatitis C virus infection. Sci. Rep. 2019, 9, 13393. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- Ren, J.P.; Wang, L.; Zhao, J.; Ning, S.B.; El Gazzar, M.; Moorman, J.P.; Yao, Z.Q. Decline of miR-124 in myeloid cells promotes regulatory T-cell development in hepatitis C virus infection. Immunology 2017, 150, 213–220. [Google Scholar] [CrossRef]
- Wang, L.; Cao, D.; Zhao, J.; Nguyen, L.N.; Dang, X.; Ji, Y.; Wu, X.Y.; Morrison, Z.D.; Xie, Q.; El Gazzar, M.; et al. HCV-associated exosomes promote myeloid-derived suppressor cell expansion via inhibiting miR-124 to regulate T follicular cell differentiation and function. Cell Discov. 2018, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Wang, H.; Shi, J.; Wu, K.; Liu, S.; Liu, Y.; Wu, J. HCV-induced miR-21 contributes to evasion of host immune system by targeting MyD88 and IRAK1. PLoS Pathog. 2013, 9, e1003248. [Google Scholar] [CrossRef]
- Kambara, H.; Fukuhara, T.; Shiokawa, M.; Ono, C.; Ohara, Y.; Kamitani, W.; Matsuura, Y. Establishment of a novel permissive cell line for the propagation of hepatitis C virus by expression of microRNA miR122. J. Virol. 2012, 86, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, H.; Zhang, W.J.; Jie, S.H.; Tong, Q.X.; Lu, M.J.; Yang, D.L. Inhibitory effect of miR-125b on hepatitis C virus core protein-induced TLR2/MyD88 signaling in THP-1 cells. World J. Gastroenterol. 2016, 22, 4354–4361. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, M.; Thibault, P.A.; Wilson, J.A. MicroRNA 122 Affects both the Initiation and the Maintenance of Hepatitis C Virus Infections. J. Virol. 2022, 96, e0190321. [Google Scholar] [CrossRef]
- Li, S.; Duan, X.; Li, Y.; Liu, B.; McGilvray, I.; Chen, L. MicroRNA-130a inhibits HCV replication by restoring the innate immune response. J. Viral Hepat. 2014, 21, 121–128. [Google Scholar] [CrossRef]
- Li, G.Y.; Zhou, Y.; Ying, R.S.; Shi, L.; Cheng, Y.Q.; Ren, J.P.; Griffin, J.W.; Jia, Z.S.; Li, C.F.; Moorman, J.P.; et al. Hepatitis C virus-induced reduction in miR-181a impairs CD4(+) T-cell responses through overexpression of DUSP6. Hepatology 2015, 61, 1163–1173. [Google Scholar] [CrossRef]
- Chen, S.; Huang, X.; Xie, Q.; Liu, Q.; Zhu, H. The Role of Long Noncoding RNA BST2-2 in the Innate Immune Response to Viral Infection. J. Virol. 2022, 96, e0020722. [Google Scholar] [CrossRef]
- Khatun, M.; Zhang, J.; Ray, R.; Ray, R.B. Hepatitis C Virus Evades Interferon Signaling by Suppressing Long Noncoding RNA Linc-Pint Involving C/EBP-β. J. Virol. 2021, 95, e0095221. [Google Scholar] [CrossRef]
- Thakuri, B.K.C.; Zhang, J.; Zhao, J.; Nguyen, L.N.; Nguyen, L.N.T.; Khanal, S.; Cao, D.; Dang, X.; Schank, M.; Wu, X.Y.; et al. LncRNA HOTAIRM1 promotes MDSC expansion and suppressive functions through the HOXA1-miR124 axis during HCV infection. Sci. Rep. 2020, 10, 22033. [Google Scholar] [CrossRef]
- Lefkowitz, R.B.; Miller, C.M.; Martinez-Caballero, J.D.; Ramos, I. Epigenetic Control of Innate Immunity: Consequences of Acute Respiratory Virus Infection. Viruses 2024, 16, 197. [Google Scholar] [CrossRef] [PubMed]
- Torne, A.S.; Robertson, E.S. Epigenetic Mechanisms in Latent Epstein-Barr Virus Infection and Associated Cancers. Cancers 2024, 16, 991. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Koh, J.Y.; Lee, D.H.; Kim, H.D.; Choi, S.J.; Ko, Y.Y.; Lee, H.S.; Lee, J.S.; Choi, I.A.; Lee, E.Y.; et al. Epigenetic scars in regulatory T cells are retained after successful treatment of chronic hepatitis C with direct-acting antivirals. J. Hepatol. 2024; in press. [Google Scholar] [CrossRef]
- Goncharova, I.A.; Zarubin, A.A.; Babushkina, N.P.; Koroleva, I.A.; Nazarenko, M.S. Changes in DNA methylation profile in liver tissue during progression of HCV-induced fibrosis to hepatocellular carcinoma. Vavilovskii Zhurnal Genet. Sel. 2023, 27, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Callegari, E.; Domenicali, M.; Shankaraiah, R.C.; D’Abundo, L.; Guerriero, P.; Giannone, F.; Baldassarre, M.; Bassi, C.; Elamin, B.K.; Zagatti, B.; et al. MicroRNA-Based Prophylaxis in a Mouse Model of Cirrhosis and Liver Cancer. Mol. Ther. Nucleic Acids 2019, 14, 239–250. [Google Scholar] [CrossRef] [PubMed]
| microRNA | Expression | Mechanism Linking to HCV Infection | Association with HCV-Related Hepatocellular Carcinoma | References |
|---|---|---|---|---|
| miRNA-122 | elevated | Leads to increased translation and stabilisation of HCV RNA | Decreased miR-122 levels may be associated with poor prognosis | [59,64] |
| miRNA-122 | decreased | Decreases significantly with the progression of fibrosis severity | Decreased miR-122 levels may be associated with liver cancer metastasis | [62,65] |
| miRNA-155 | increased in both serum and PMBC | Affects replication and life cycle of virus | Mediates proliferation and carcinogenesis due to increased Wnt signalling | [70,71,72] |
| miRNA-223 | elevated in plasma, decreased in liver cells | Long-term immune response | Affects fibrosis through NOD receptors | [75,76,78] |
| miRNA-29 | elevated in serum, decreased in liver cells | Suppression of HCV replication | Suppressive role | [82,83,87] |
| miRNA-34 | elevated in serum | Cell cycle regulation | Suppressive role | [89,91,92] |
| LncRNA | Functions | Mechanism | References |
|---|---|---|---|
| GAS5 | Inhibiting HCV replication | Blocks NS3 | [95] |
| UCA1 | Antiviral response | Affects miR-145-5p/SOCS7/INF pathway | [96] |
| EGOT | Antiviral response | Reduces IFN | [97] |
| HOTAIR | Viral release | Affects lipid metabolism | [95] |
| IGF2-AS | Viral release | Increases PI4P | [95] |
| 7SK | Viral release | Increases PI4P | [95] |
| IFI6 | Increasing HCV replication | Modifies histones | [101] |
| Linc-Pint | Inhibiting HCV replication | Binds to serine-arginine protein kinase 2 (SRPK2) | [101] |
| NEAT1 | HCC oncogene | Increases BGH3 concentration | [102] |
| lncTCF7 | Tumour propagation | Activates Wnt signalling | [104] |
| LINC00624 | Removing inhibitory effect on transcription in HCC | Disrupts formation of HDAC6-TRIM28-ZNF354C transcriptional corepressor complex | [105] |
| HULC | Promoting HCC cells growth and autophagy | Stimulates cyclin D1 and inhibits P21 WAF1/CIP1 in human liver cancer stem cells Manipulates pool of lipids HULC transcription may be regulated by HCV core protein via RXRA | [107,108] |
| MEG3 | Tumour suppressor | Upregulation of tumour suppressors miRNA152 and p53 and downregulation of oncogenes TGF-b, BCL-2, and DNMT1 Induces apoptosis in HCC cell line Regulates expression of miRNA-10a-5p and PTEN | [110,111] |
| CASC2 | Tumour suppressor | Inhibits MAPK pathway | [112] |
| LINC01189 | Reducing 5-FU chemoresistance and inhibiting HCC proliferation | Inhibits hsa-miR-155-5p | [109] |
| HEIH | Biomarker | HCV-related HCC, gastric cancer, cirrhosis | [114,115,116] |
| TUG1 | Biomarker | HCV-related HCC, gastric cancer, osteosarcoma, urothelial carcinoma of the bladder, esophageal squamous cell carcinoma | [113] |
| MALAT1 | Biomarker | HCV-related HCC, breast cancer, lung cancer | [118] |
| LINC01419 | Biomarker | HCV- and HBV-related HCC | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żychowska, J.; Ćmil, M.; Skórka, P.; Olejnik-Wojciechowska, J.; Plewa, P.; Bakinowska, E.; Kiełbowski, K.; Pawlik, A. The Role of Epigenetic Mechanisms in the Pathogenesis of Hepatitis C Infection. Biomolecules 2024, 14, 986. https://doi.org/10.3390/biom14080986
Żychowska J, Ćmil M, Skórka P, Olejnik-Wojciechowska J, Plewa P, Bakinowska E, Kiełbowski K, Pawlik A. The Role of Epigenetic Mechanisms in the Pathogenesis of Hepatitis C Infection. Biomolecules. 2024; 14(8):986. https://doi.org/10.3390/biom14080986
Chicago/Turabian StyleŻychowska, Justyna, Maciej Ćmil, Patryk Skórka, Joanna Olejnik-Wojciechowska, Paulina Plewa, Estera Bakinowska, Kajetan Kiełbowski, and Andrzej Pawlik. 2024. "The Role of Epigenetic Mechanisms in the Pathogenesis of Hepatitis C Infection" Biomolecules 14, no. 8: 986. https://doi.org/10.3390/biom14080986





