BMP2 Diminishes Angiotensin II-Induced Atrial Fibrillation by Inhibiting NLRP3 Inflammasome Signaling in Atrial Fibroblasts
Abstract
:1. Introduction
2. Methods
2.1. Patient Study
2.2. Animal Study
2.3. Cell Culture
2.4. mRNA Sequence
2.5. qRT-PCR
2.6. Western Blot
2.7. Histology
2.8. Elisa Assay
2.9. Statistical Analysis
3. Results
3.1. BMP2 Expression Upregulated in Different AF Model
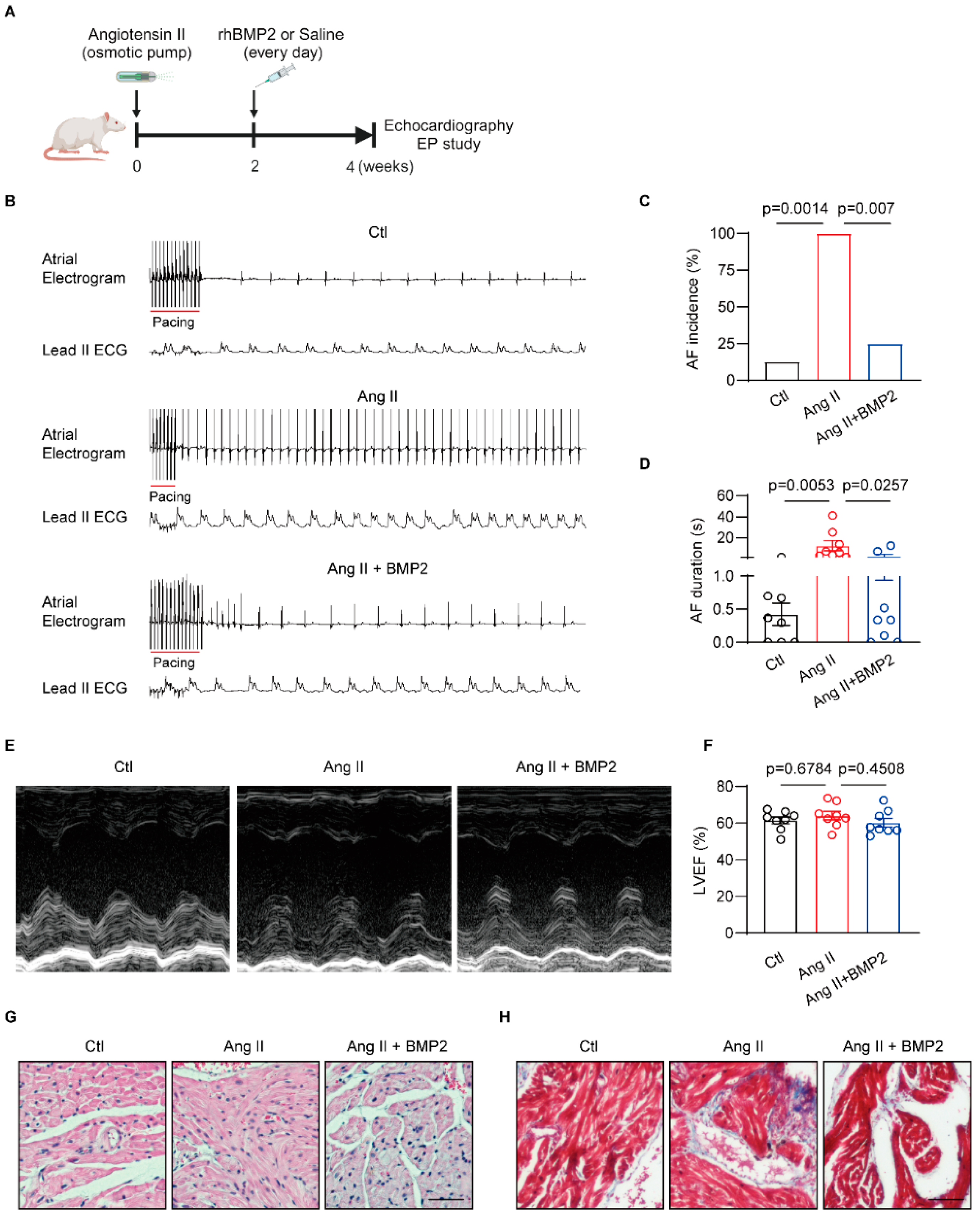
3.2. BMP2 Attenuated Angiotensin II Induced-AF Inducibility via Preventing Atrial Fibrosis In Vivo
3.3. RNA-Sequence Identify the Negative Role of BMP2 in Rat Atrial Inflammation
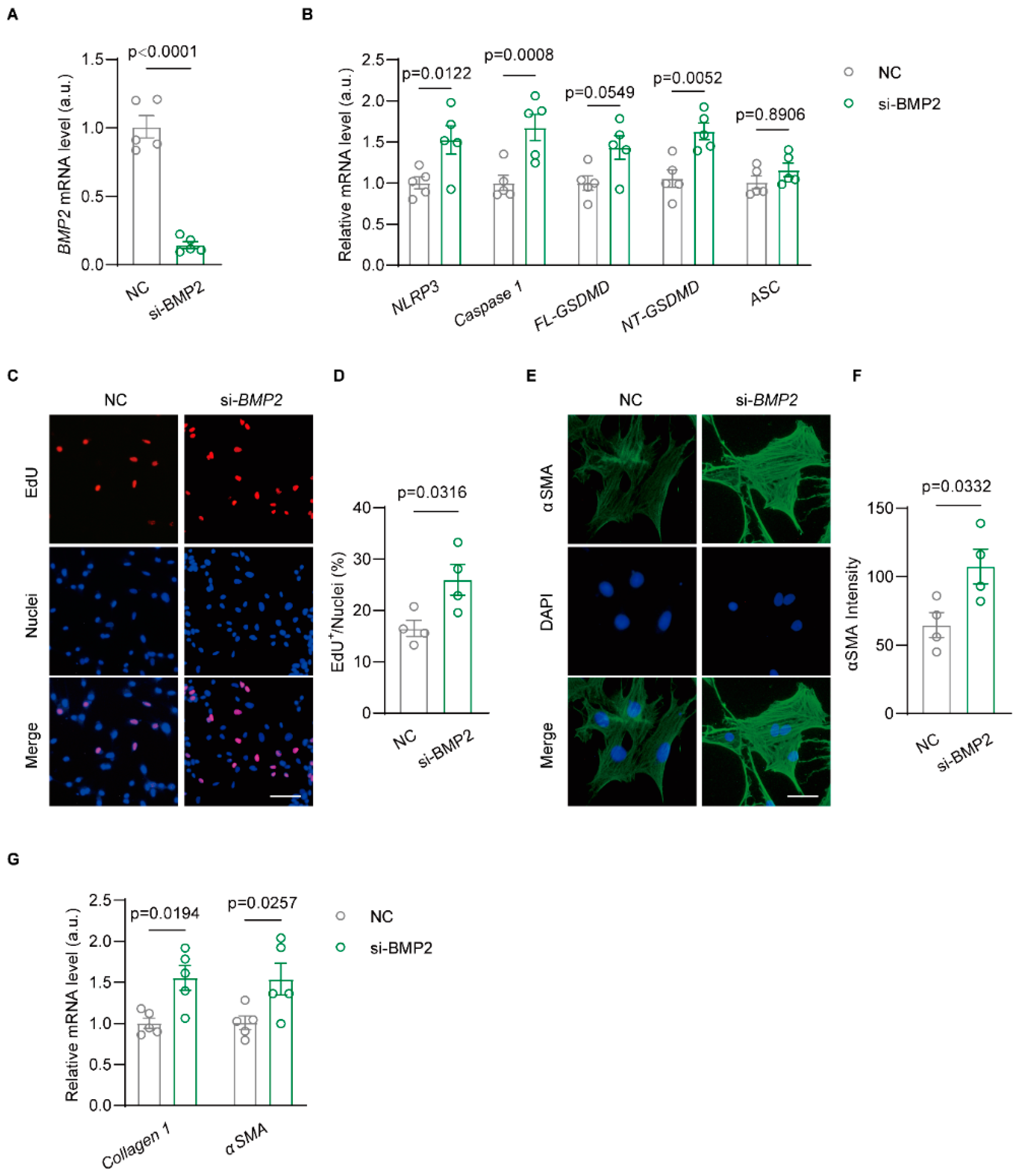
3.4. Knockdown BMP2 Enhances NLRP3-Dependent Inflammasome Activation in Rat Atrial Fibroblast
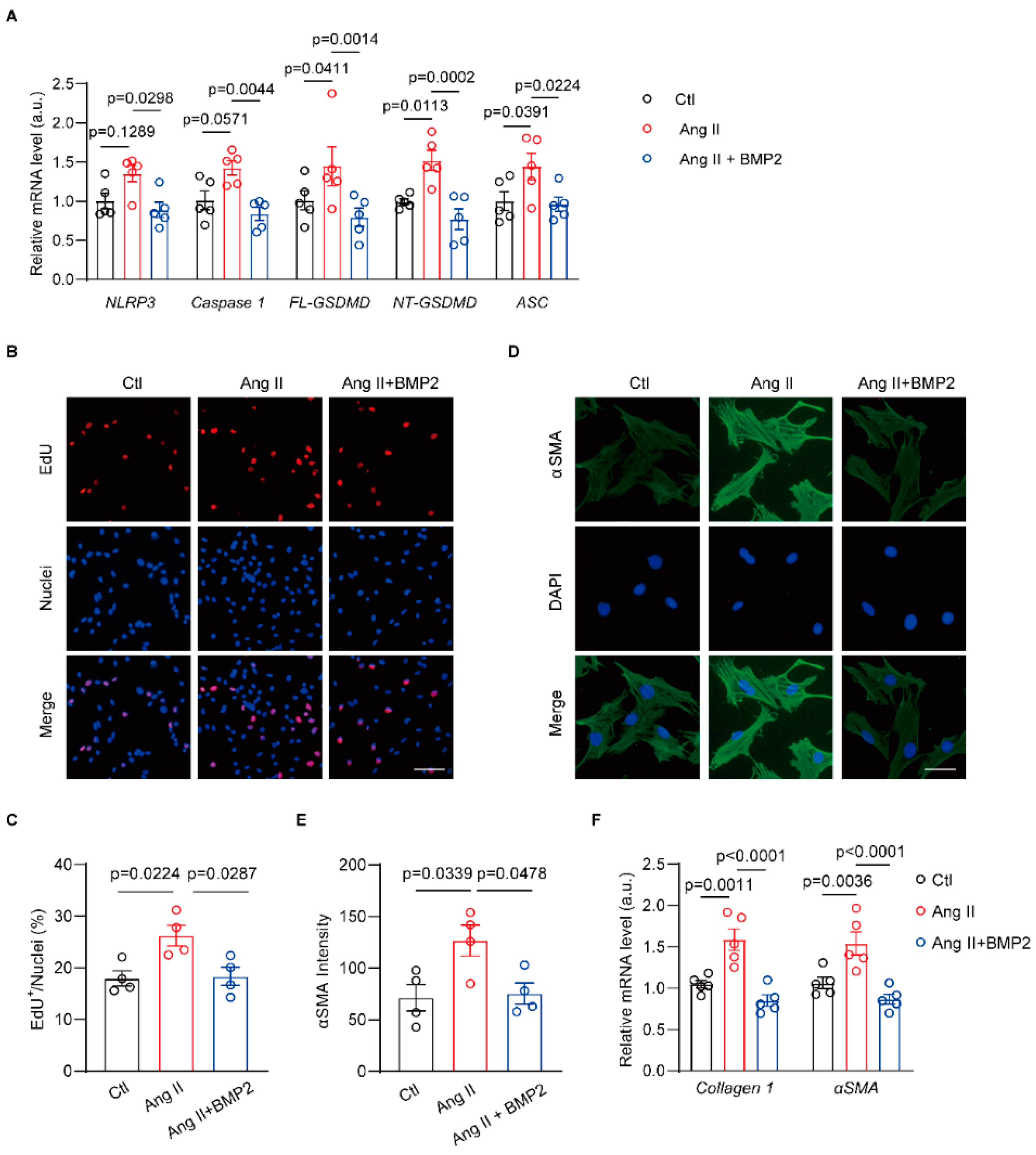
3.5. BMP2 Prevents Ang II-Induced NLRP3 Inflammasome Signaling in Neonatal Rat Atrial Fibroblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kornej, J.; Benjamin, E.J.; Magnani, J.W. Atrial fibrillation: Global burdens and global opportunities. Heart 2021, 107, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhang, Q.; Much, A.A.; Maor, E.; Segev, A.; Beinart, R.; Adawi, S.; Lu, Y.; Bragazzi, N.L.; Wu, J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: Results from the Global Burden of Disease Study 2017. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 574–582. [Google Scholar] [CrossRef]
- Dieleman, J.L.; Cao, J.; Chapin, A.; Chen, C.; Li, Z.; Liu, A.; Horst, C.; Kaldjian, A.; Matyasz, T.; Scott, K.W.; et al. US Health Care Spending by Payer and Health Condition, 1996–2016. JAMA 2020, 323, 863–884. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar]
- Gao, P.; Gao, X.; Xie, B.; Tse, G.; Liu, T. Aging and atrial fibrillation: A vicious circle. Int. J. Cardiol. 2024, 395, 131445. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [PubMed]
- Zaman, N.; Naccarelli, G.; Foy, A. A Comparison of Rate Control Agents for the Treatment of Atrial Fibrillation: Follow-Up Investigation of the AFFIRM Study. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 328–334. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, e199–e267. [Google Scholar] [CrossRef] [PubMed]
- Sohns, C.; Marrouche, N.F. Atrial fibrillation and cardiac fibrosis. Eur. Heart J. 2020, 41, 1123–1131. [Google Scholar] [CrossRef]
- Harada, M.; Nattel, S. Implications of Inflammation and Fibrosis in Atrial Fibrillation Pathophysiology. Card. Electrophysiol. Clin. 2021, 13, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin. Electrophysiol. 2017, 3, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Hu, Y.F.; Chou, C.Y.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Tuan, T.C.; Li, C.H.; Chao, T.F.; Chung, F.P.; et al. Transforming growth factor-β1 level and outcome after catheter ablation for nonparoxysmal atrial fibrillation. Heart Rhythm 2013, 10, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Leask, A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ. Res. 2010, 106, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Chouvarine, P.; Legchenko, E.; Hoffmann, N.; Geldner, J.; Borchert, P.; Jonigk, D.; Mozes, M.M.; Hansmann, G. PPARγ Links BMP2 and TGFβ1 Pathways in Vascular Smooth Muscle Cells, Regulating Cell Proliferation and Glucose Metabolism. Cell Metab. 2017, 25, 1118–1134.e7. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Iimuro, Y.; Otogawa, K.; Saika, S.; Inagaki, Y.; Nakajima, Y.; Kawada, N.; Fujimoto, J.; Friedman, S.L.; Ikeda, K. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut 2007, 56, 706–714. [Google Scholar] [CrossRef]
- Yang, Y.L.; Liu, Y.S.; Chuang, L.Y.; Guh, J.Y.; Lee, T.C.; Liao, T.N.; Hung, M.Y.; Chiang, T.A. Bone morphogenetic protein-2 antagonizes renal interstitial fibrosis by promoting catabolism of type I transforming growth factor-beta receptors. Endocrinology 2009, 150, 727–740. [Google Scholar] [CrossRef]
- Shlyonsky, V.; Soussia, I.B.; Naeije, R.; Mies, F. Opposing effects of bone morphogenetic protein-2 and endothelin-1 on lung fibroblast chloride currents. Am. J. Respir. Cell Mol. Biol. 2011, 45, 1154–1160. [Google Scholar] [CrossRef]
- Luna-Zurita, L.; Prados, B.; Grego-Bessa, J.; Luxán, G.; del Monte, G.; Benguría, A.; Adams, R.H.; Pérez-Pomares, J.M.; de la Pompa, J.L. Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J. Clin. Investig. 2010, 120, 3493–3507. [Google Scholar] [CrossRef]
- Chen, N.Y.; DCollum, S.; Luo, F.; Weng, T.; Le, T.T.; MHernandez, A.; Philip, K.; Molina, J.G.; Garcia-Morales, L.J.; Cao, Y.; et al. Macrophage bone morphogenic protein receptor 2 depletion in idiopathic pulmonary fibrosis and Group III pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L238–L254. [Google Scholar] [CrossRef] [PubMed]
- Morrell, N.W.; Bloch, D.B.; ten Dijke, P.; Goumans, M.J.; Hata, A.; Smith, J.; Yu, P.B.; Bloch, K.D. Targeting BMP signalling in cardiovascular disease and anaemia. Nat. Rev. Cardiol. 2016, 13, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.N.; Schoenhard, J.A.; Saleh, M.A.; Mukherjee, A.; Ryzhov, S.; McMaster, W.G., Jr.; Nolan, K.; Gumina, R.J.; Thompson, T.B.; Magnuson, M.A.; et al. BMP Antagonist Gremlin 2 Limits Inflammation After Myocardial Infarction. Circ. Res. 2016, 119, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Morine, K.J.; Qiao, X.; York, S.; Natov, P.S.; Paruchuri, V.; Zhang, Y.; Aronovitz, M.J.; Karas, R.H.; Kapur, N.K. Bone Morphogenetic Protein 9 Reduces Cardiac Fibrosis and Improves Cardiac Function in Heart Failure. Circulation 2018, 138, 513–526. [Google Scholar] [CrossRef]
- Lu, G.; Ge, Z.; Chen, X.; Ma, Y.; Yuan, A.; Xie, Y.; Pu, J. BMP6 knockdown enhances cardiac fibrosis in a mouse myocardial infarction model by upregulating AP-1/CEMIP expression. Clin. Transl. Med. 2023, 13, e1296. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Orriols, M.; Walther, F.J.; Laghmani, E.H.; Hoogeboom, A.M.; Hogen-Esch, A.C.B.; Hiemstra, P.S.; Folkerts, G.; Goumans, M.T.H.; Ten Dijke, P.; et al. Bone Morphogenetic Protein 9 Protects against Neonatal Hyperoxia-Induced Impairment of Alveolarization and Pulmonary Inflammation. Front. Physiol. 2017, 8, 486. [Google Scholar] [CrossRef]
- Qu, Q.; Sun, J.Y.; Zhang, Z.Y.; Su, Y.; Li, S.S.; Li, F.; Wang, R.X. Hub microRNAs and genes in the development of atrial fibrillation identified by weighted gene co-expression network analysis. BMC Med. Genom. 2021, 14, 271. [Google Scholar] [CrossRef]
- Lv, W.; Zhang, L.; Cheng, X.; Wang, H.; Qin, W.; Zhou, X.; Tang, B. Apelin Inhibits Angiotensin II-Induced Atrial Fibrosis and Atrial Fibrillation via TGF-β1/Smad2/α-SMA Pathway. Front. Physiol. 2020, 11, 583570. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, Y.; Han, X.; Yuan, Y.; Zhou, Y.; Gao, Y.; Yu, H.; Zhang, J.; Shi, Y.; Duan, Y.; et al. Akkermansia muciniphila prevents cold-related atrial fibrillation in rats by modulation of TMAO induced cardiac pyroptosis. EBioMedicine 2022, 82, 104087. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, J.; Gong, Y.; Wang, D.; Wang, X.; Yun, F.; Liu, Z.; Zhang, S.; Li, W.; Zhao, X.; et al. Autophagy exacerbates electrical remodeling in atrial fibrillation by ubiquitin-dependent degradation of L-type calcium channel. Cell Death Dis. 2018, 9, 873. [Google Scholar] [CrossRef]
- Zhang, J.M.; Yu, R.Q.; Wu, F.Z.; Qiao, L.; Wu, X.R.; Fu, Y.J.; Liang, Y.F.; Pang, Y.; Xie, C.Y. BMP-2 alleviates heart failure with type 2 diabetes mellitus and doxorubicin-induced AC16 cell injury by inhibiting NLRP3 inflammasome-mediated pyroptosis. Exp. Ther. Med. 2021, 22, 897. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, X.; Li, B.; Tang, Y. Fraxinellone alleviates inflammation and promotes osteogenic differentiation in lipopolysaccharide-stimulated periodontal ligament stem cells by regulating the bone morphogenetic protein 2/Smad pathway. Arch. Oral Biol. 2021, 121, 104927. [Google Scholar] [CrossRef]
- Ma, L.; Lu, M.F.; Schwartz, R.J.; Martin, J.F. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 2005, 132, 5601–5611. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Feliciano, J.; Tabin, C.J. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev. Biol. 2006, 295, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.D.; Nickel, J. Promiscuity and specificity in BMP receptor activation. FEBS Lett. 2012, 586, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.A.; Lee, E.J.; Kang, H.J.; Zhang, S.Y.; Kim, J.H.; Li, L.; Youn, S.W.; Lee, C.S.; Kim, K.H.; Won, J.Y.; et al. Impact of myocardial infarct proteins and oscillating pressure on the differentiation of mesenchymal stem cells: Effect of acute myocardial infarction on stem cell differentiation. Stem Cells 2008, 26, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Sagave, J.; Czibik, G.; Baysa, A.; Zihlavnikova Enayati, K.; Hillestad, V.; Dahl, C.P.; Fiane, A.; Gullestad, L.; Gravning, J.; et al. Connective tissue growth factor and bone morphogenetic protein 2 are induced following myocardial ischemia in mice and humans. Scand. J. Clin. Lab. Investig. 2017, 77, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Zhang, Q.; Deng, S.; Yu, Y.; Zhu, F.; Zhang, S.; Pan, Y.; Long, D.; Wang, J.; Liu, C. A BMP-2-triggered in vivo osteo-organoid for cell therapy. Sci. Adv. 2023, 9, eadd1541. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cao, Y.; Yang, W.; Duan, C.; Aronson, J.F.; Rastellini, C.; Chao, C.; Hellmich, M.R.; Ko, T.C. BMP2 inhibits TGF-β-induced pancreatic stellate cell activation and extracellular matrix formation. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G804–G813. [Google Scholar] [CrossRef]
- Xuan, L.; Fu, D.; Zhen, D.; Wei, C.; Bai, D.; Yu, L.; Gong, G. Extracellular vesicles derived from human bone marrow mesenchymal stem cells protect rats against acute myocardial infarction-induced heart failure. Cell Tissue Res. 2022, 389, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Espitia-Corredor, J.A.; Boza, P.; Espinoza-Pérez, C.; Lillo, J.M.; Rimassa-Taré, C.; Machuca, V.; Osorio-Sandoval, J.M.; Vivar, R.; Bolivar, S.; Pardo-Jiménez, V.; et al. Angiotensin II Triggers NLRP3 Inflammasome Activation by a Ca2+ Signaling-Dependent Pathway in Rat Cardiac Fibroblast Ang-II by a Ca2+-Dependent Mechanism Triggers NLRP3 Inflammasome in CF. Inflammation 2022, 45, 2498–2512. [Google Scholar] [CrossRef]
- Li, L.; Coarfa, C.; Yuan, Y.; Abu-Taha, I.; Wang, X.; Song, J.; Koirala, A.; Grimm, S.L.; Kamler, M.; Mullany, L.K.; et al. Fibroblast-specific inflammasome activation predisposes to atrial fibrillation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Hruska, K.A.; Mathew, S.; Saab, G. Bone morphogenetic proteins in vascular calcification. Circ. Res. 2005, 97, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, B.; Huo, R.; Wang, Y.C.; Yang, D.; Xing, Y.; Xiao, X.L.; Xie, X.; Dong, D.L. Bone morphogenetic protein-2 antagonizes bone morphogenetic protein-4 induced cardiomyocyte hypertrophy and apoptosis. J. Cell. Physiol. 2014, 229, 1503–1510. [Google Scholar] [CrossRef]
- Sun, X.; Gan, L.; Li, N.; Sun, S.; Li, N. Tabersonine ameliorates osteoblast apoptosis in rats with dexamethasone-induced osteoporosis by regulating the Nrf2/ROS/Bax signalling pathway. AMB Express 2020, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Qiao, W.; Cao, Y.; Fu, X.; Song, J. A sono-responsive antibacterial nanosystem co-loaded with metformin and bone morphogenetic protein-2 for mitigation of inflammation and bone loss in experimental peri-implantitis. Front. Bioeng. Biotechnol. 2024, 12, 1410230. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Zhang, H.; Xia, E.; Zhao, X.; Gao, Q.; Mu, H.; Liu, X.; Tian, Y.; Liu, L.; Shen, Q.; et al. BMP2 Diminishes Angiotensin II-Induced Atrial Fibrillation by Inhibiting NLRP3 Inflammasome Signaling in Atrial Fibroblasts. Biomolecules 2024, 14, 1053. https://doi.org/10.3390/biom14091053
Yuan Y, Zhang H, Xia E, Zhao X, Gao Q, Mu H, Liu X, Tian Y, Liu L, Shen Q, et al. BMP2 Diminishes Angiotensin II-Induced Atrial Fibrillation by Inhibiting NLRP3 Inflammasome Signaling in Atrial Fibroblasts. Biomolecules. 2024; 14(9):1053. https://doi.org/10.3390/biom14091053
Chicago/Turabian StyleYuan, Yue, Hang Zhang, Erwen Xia, Xinbo Zhao, Qiang Gao, Hongyuan Mu, Xingzuo Liu, Yuanye Tian, Lei Liu, Qiuling Shen, and et al. 2024. "BMP2 Diminishes Angiotensin II-Induced Atrial Fibrillation by Inhibiting NLRP3 Inflammasome Signaling in Atrial Fibroblasts" Biomolecules 14, no. 9: 1053. https://doi.org/10.3390/biom14091053





