Inositol Hexaphosphate in Bone Health and Disease
Abstract
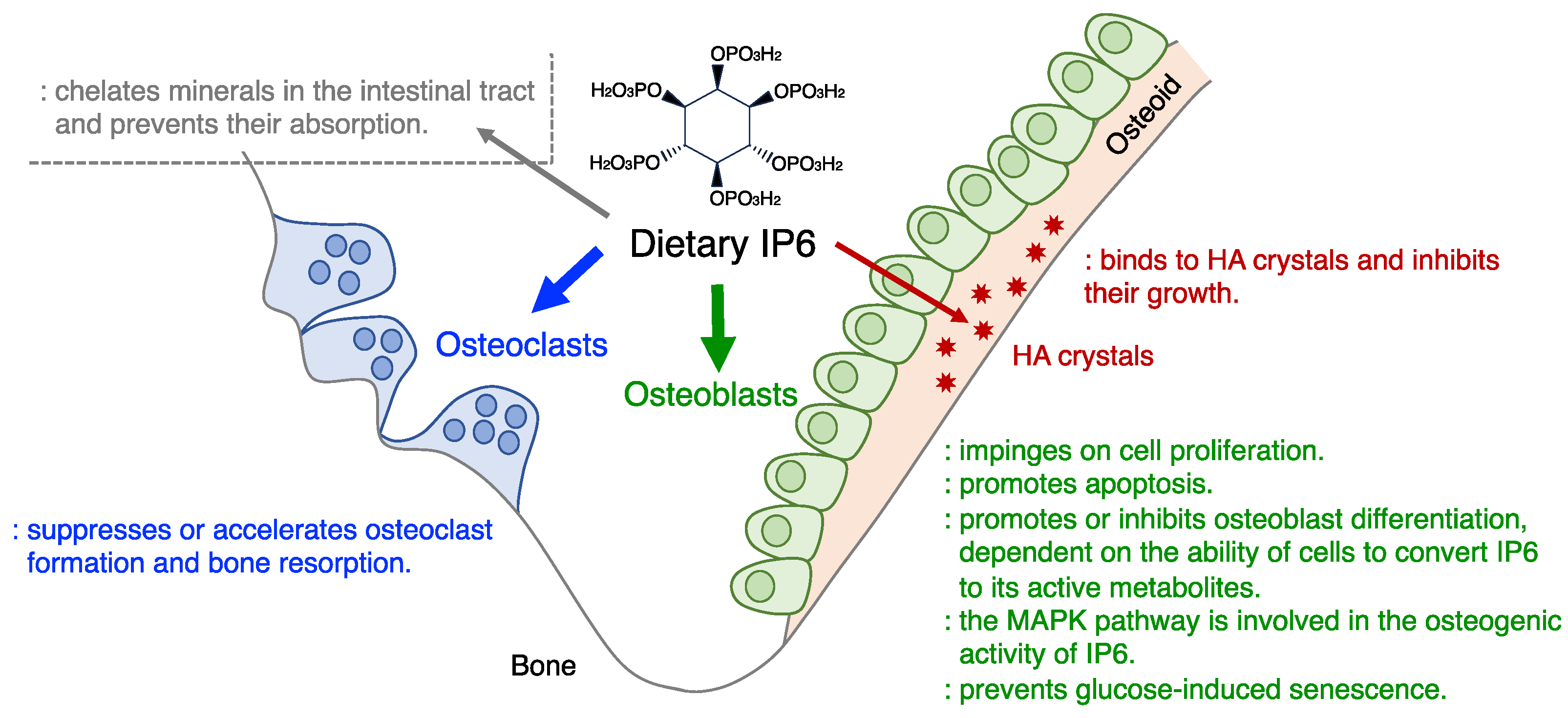
1. Introduction
2. The Effects of IP6 on Bone Cells (Osteoblasts and Osteoclasts) In Vitro
| Cell Type | IP6 Type | Effect | Model | Additional Information | Ref. | |
|---|---|---|---|---|---|---|
| Mode | ||||||
| Osteoblasts | Sodium IP6 | Nagative | Apoptosis | MC3T3 cells under serum starvation | IP6 at concentrations 10 times or higher than normal (50 µM) was used. | [41] |
| Sodium IP6 | Mineralization | MC3T3-E1 cells with osteogenic medium | Neither osteoblast differentiation, ALP activity, nor marker gene expression (except Spp1) was affected. | [42] | ||
| Sodium IP6 | Marker gene expression including Alpl | MC3T3-E1 cells with osteogenic medium | ALP activity was not affected. | [43] | ||
| IP6 | Positive | DNA synthesis | Newborn rat calvaria cells under mechanical stress | Intracellular IPs including IP6 were involved in the transduction of mechanical strain into cellular proliferation. | [37] | |
| Sodium IP6 | ALPL gene expression | Human MSCs with osteogenic medium | − | [43] | ||
| Sodium IP6 | ALP activity, ECM mineralization | Human MSCs with osteogenic medium | − | [44] | ||
| Ca IP6 | Cell proliferation, marker gene expression, ALP activity, ECM mineralization | Human T2DM BMSCs with osteogenic medium/high glucose/palmitic acid | IP6 improved impaired osteoblastogenesis under culture conditions mimicking T2DM. | [45] | ||
| Ca IP6 | ALP activity, marker gene expression, ECM mineralization, cell senescence | Human MSCs with osteogenic medium/high glucose | [46] | |||
| IP6 | ECM mineralization (negative for cell viability, ALP activity) | Human PDL fibroblasts with osteogenic medium/high glucose | [48] | |||
| IP6 | ALP activity, marker gene expression | MC3T3-E1 cells on IP6-coated titanium | IP6 was introduced into titanium for implants and scaffolds for bone regeneration. It has been suggested that these effects may be mediated by the release of IP6 from carriers. | [49] | ||
| IP6 | Cell adhesion, cell proliferation, ALP activity, marker gene expression | Human BMSCs on IP6/Mg-coated titanium with or without osteogenic medium | [50] | |||
| Ca IP6 | ALP activity, ECM mineralization | Human BMSCs on micro/nanostructured Ca IP6-coated titanium with osteogenic medium | [51] | |||
| Ca IP6 | Cell proliferation, ALP activity, ECM mineralization, marker gene expression | Human BMSCs on Ca IP6-coated titanium with or without osteogenic medium | [52] | |||
| IP6 | Cell proliferation, marker gene expression, collagen secretion, ECM mineralization | Mouse BMSCs on IP6/copper-coated titanium | [53] | |||
| IP6 | Cell adhesion, cell proliferation, ALP activity, marker gene expression | Mouse BMSCs on IP6/curcumin-hydrogel composite membrane | [54] | |||
| IP6 | Cell adhesion, cell proliferation, marker gene expression, ALP activity, ECM mineralization | Rat BMSCs on IP6- and IP6/magnesium-coated PKFs | [55] | |||
| IP6 | Cell adhesion, ALP activity, ECM mineralization | BMSCs in IP6-soaked poly-L-lactide scaffolds | [56] | |||
| SNF472 | No effect | ALP activity, ECM mineralization | Rat bone marrow stromal cells with osteogenic medium | Bone marrow stromal cells include non-osteogenic cells. Some marker genes were affected. | [32] | |
| IP6 | Cell viability, ALP activity | MC3T3-E1 cells | ALP activity decreased with days in culture, suggesting that this model does not mimic osteoblastogenesis. | [57] | ||
| Osteoclasts | IP6 | Negative | Calcium release (bone resorption) | Organ cultures of rat long bones with PTH | − | [59] |
| IP6 | Osteoclast formation | RAW264.7 cells and human PBMCs with TNFSF11 and TNFSF11/CSF1/DEX, respectively | − | [60] | ||
| BRE | Osteoclast formation | Mouse BMMs with TNFSF11/CSF1 | − | [58] | ||
| Sodium IP6 | Positive | Osteoclast formation | BMMs, from rats fed high sodium IP6 and low calcium diet, with TNFSF11/CSF1 | BMMs were isolated from rats with renal dysfunction, bone loss, etc. IP6 was not added to the culture. | [7] | |
3. The Effects of IP6 on Bone in Laboratory Animals
| IP6 | Effect | Model | Additional Information | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | Ad Route | Mode | Animal | Diet | Period | |||
| Phytin | 0.13% | Oral | Negative | Mineral content, skeleton size, rib-cage deformities | Wistar rats (3-wk-old) | Pure flour | 60 d | The pure flour diet has large gaps in its nutrient profile, including low levels of Ca and vitamin D, compared to control diets tested. | [62] |
| IP6 | The ratio of IP6 to Zn, 18:1 | Oral | Tr Thk | Adult Wistar rats | AIN-93G + vitamin/mineral mix, ±Zn | 3 wk | Ca and Mg contents were unaffected. Tr ThK was measured by scanning electron microscopy. | [67] | |
| Sodium IP6 | 1, 3 or 5% | Oral | BMD, BV/TV, BS/TV, Tr No, osteoclast numbers, TNFSF11, TNFRSF11B, etc. | Weaning SD rats | AIN-93G | 12 wk | IP6 (3% and 5%) may represent more than 4 times the average human intake in the US and UK. Ca supplementation improved skeletal anomalies in 3% IP6. No effect was seen in 1% IP6. | [7] | |
| Ca/Mg IP6 | 12.99 g/kg | Oral | Positive | Ca and P contents, BMD, Urinary DPD (decreased) | OVX Wistar rats | AIN-76A | 12 wk | These have been evaluated in models of diseases and bone defects. | [68] |
| Ca IP6 | Dental repair films with 10 μL of 34 μM Ca IP6 | Scaffold | BV/TV | Parietal bone defects in T2DM-SD rats | HFD + STZ | 8 wk | [45] | ||
| IP6 | Human BMSCs with 4 μM IP6 and collagens | Local | BV/TV, BS/TV, Tr No, Tr Thk, MAR, osteoblast markers. | Cranial defects in T2DM-male SD rats | HFD + STZ | 12 wk | [47] | ||
| IP6 | Mouse BMSCs with 4 μM IP6 | Local | BV/TV | Tooth extraction sites in T2DM-male C57/BL6 mice | HFD + STZ | 9 d | [46] | ||
| IP6 | IP6- or IP6/Mg-coated PKFs with rat BMSCs | Scaffold | Accumulation of M2 macrophages involved in bone formation, BMD and BV/TV | Air pouches in SD rats Ligament-bone defects in New Zealand rabbits | − | 14 d 12 wk | [55] | ||
| IP6 | IP6/Calcium-decorated titanium | Scaffold | BV, Tr No, Tr Sep, bone implant contact | Bone marrow cavities in SD rats | HFD + STZ | 8 wk | [52] | ||
| IP6 | IP6-soaked poly-L-lactide | Scaffold | Osteoid and mineralized bone areas | Muscle pockets in male SD rats | − | 6 wk | [56] | ||
| Sodium IP6 | 3, or 5% | Oral | No effect | Bone ash, Ca, Mg, and P contents | Male CH3 mice (5-wk-old) | L-488F + 1% Cr2O3 | 2 wk | − | [63] |
| Sodium IP6 | 0.15, 0.3, or 0.6% | Oral | BMC, Ca and P contents, bone histomorphometric parameters | Weaning male SD rats | AIN-76 + 0.2, 0.4, or 0.8% Ca | 8 wk | Negative effects were seen only in 0.8% Ca and 0.6% sodium IP6. The authors did not clarify whether this was due to IP6 or sodium. | [64] | |
| Sodium IP6 | 1% | Oral | Ca, Mg, Fe, and Mn contents | Wistar rats | AIN-76A | 16 wk | Zn content was affected. Zn levels in this model were 10-fold higher than those in rats fed the common non-purified diet. | [65] | |
| SNF472 | 25 mg/kg, 3 times weekly | IV infusion | BV, Tr No, Tr Thk, cortical Thk, osteoclasts, etc. | Beagle dogs | − | 9 mth | − | [66] | |
4. The Effects of IP6 on Bone in Humans
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chatree, S.; Thongmaen, N.; Tantivejkul, K.; Sitticharoon, C.; Vucenik, I. Role of Inositols and Inositol Phosphates in Energy Metabolism. Molecules 2020, 25, 5079. [Google Scholar] [CrossRef] [PubMed]
- Maffucci, T.; Falasca, M. Signalling Properties of Inositol Polyphosphates. Molecules 2020, 25, 5281. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Sun, L.Y.; Yang, A.L.; Liao, J.; Yang, G.Y. Overview of Inositol and Inositol Phosphates on Chemoprevention of Colitis-Induced Carcinogenesis. Molecules 2020, 26, 31. [Google Scholar] [CrossRef]
- Qi, J.; Shi, L.; Zhu, L.; Chen, Y.; Zhu, H.; Cheng, W.; Chen, A.F.; Fu, C. Functions, Mechanisms, and Therapeutic Applications of the Inositol Pyrophosphates 5PP-InsP5 and InsP8 in Mammalian Cells. J. Cardiovasc. Transl. Res. 2024, 17, 197–215. [Google Scholar] [CrossRef]
- Bruce, H.M.; Callow, R.K. Cereals and Rickets. The Rôle of Inositolhexaphosphoric Acid. Biochem. J. 1934, 28, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.R.P. Cereals, Phytic Acid and Calcification. Lancet 1951, 2, 244–248. [Google Scholar] [CrossRef]
- Kim, O.-H.; Booth, C.J.; Choi, H.S.; Lee, J.; Kang, J.; Hur, J.; Jung, W.J.; Jung, Y.-S.; Choi, H.J.; Kim, H.; et al. High-Phytate/Low-Calcium Diet Is a Risk Factor for Crystal Nephropathies, Renal Phosphate Wasting, and Bone Loss. eLife 2020, 9, e52709. [Google Scholar] [CrossRef]
- Grases, F.; Simonet, B.M.; Prieto, R.M.; March, J.G. Dietary Phytate and Mineral Bioavailability. J. Trace Elem. Med. Biol. 2001, 15, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Humer, E.; Schwarz, C.; Schedle, K. Phytate in Pig and Poultry Nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 605–625. [Google Scholar] [CrossRef]
- Sahu, A.; Verma, R.; Gupta, U.; Kashyap, S.; Sanyal, I. An Overview of Targeted Genome Editing Strategies for Reducing the Biosynthesis of Phytic Acid: An Anti-Nutrient in Crop Plants. Mol. Biotechnol. 2023, 66, 11–25. [Google Scholar] [CrossRef]
- Pettifor, J.M. Calcium and Vitamin D Metabolism in Children in Developing Countries. Ann. Nutr. Metab. 2014, 64 (Suppl. S2), 15–22. [Google Scholar] [CrossRef]
- Pujol, A.; Sanchis, P.; Grases, F.; Masmiquel, L. Phytate Intake, Health and Disease: “Let Thy Food Be Thy Medicine and Medicine Be Thy Food”. Antioxidants 2023, 12, 146. [Google Scholar] [CrossRef]
- Dilworth, L.; Stennett, D.; Omoruyi, F. Cellular and Molecular Activities of IP6 in Disease Prevention and Therapy. Biomolecules 2023, 13, 972. [Google Scholar] [CrossRef]
- Mukherjee, S.; Haubner, J.; Chakraborty, A. Targeting the Inositol Pyrophosphate Biosynthetic Enzymes in Metabolic Diseases. Molecules 2020, 25, 1403. [Google Scholar] [CrossRef]
- Vucenik, I.; Druzijanic, A.; Druzijanic, N. Inositol Hexaphosphate (IP6) and Colon Cancer: From Concepts and First Experiments to Clinical Application. Molecules 2020, 25, 5931. [Google Scholar] [CrossRef]
- Rizzoli, R. Vitamin D Supplementation: Upper Limit for Safety Revisited? Aging Clin. Exp. Res. 2021, 33, 19–24. [Google Scholar] [CrossRef]
- Roberts, A.H.; Yudkin, J. Effect of Phytate and Other Dietary Factors on Intestinal Phytase and Bone Calcification in the Rat. Br. J. Nutr. 1961, 15, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Irvine, R.F.; Schell, M.J. Back in the Water: The Return of the Inositol Phosphates. Nat. Rev. Mol. Cell Biol. 2001, 2, 327–338. [Google Scholar] [CrossRef]
- Grases, F.; Simonet, B.M.; Prieto, R.M.; March, J.G. Variation of InsP(4), InsP(5) and InsP(6) Levels in Tissues and Biological Fluids Depending on Dietary Phytate. J. Nutr. Biochem. 2001, 12, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Simonet, B.M.; Prieto, R.M.; March, J.G. Phytate Levels in Diverse Rat Tissues: Influence of Dietary Phytate. Br. J. Nutr. 2001, 86, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzalez, A.A.; Grases, F.; Perello, J.; Tur, F.; Costa-Bauza, A.; Monroy, N.; Mari, B.; Vicente-Herrero, T. Phytate Levels and Bone Parameters: A Retrospective Pilot Clinical Trial. Front. Biosci. 2010, 2, 1093–1098. [Google Scholar] [CrossRef][Green Version]
- López-González, A.A.; Grases, F.; Monroy, N.; Marí, B.; Vicente-Herrero, M.T.; Tur, F.; Perelló, J. Protective Effect of Myo-Inositol Hexaphosphate (Phytate) on Bone Mass Loss in Postmenopausal Women. Eur. J. Nutr. 2013, 52, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.A.L.; Grases, F.; Mari, B.; Tomas-Salva, M.; Rodriguez, A. Urinary Phytate Concentration and Risk of Fracture Determined by the FRAX Index in a Group of Postmenopausal Women. Turk. J. Med. Sci. 2019, 49, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T. Ultrastructure and Biological Function of Matrix Vesicles in Bone Mineralization. Histochem. Cell Biol. 2018, 149, 289–304. [Google Scholar] [CrossRef]
- Arnold, A.; Dennison, E.; Kovacs, C.S.; Mannstadt, M.; Rizzoli, R.; Brandi, M.L.; Clarke, B.; Thakker, R.V. Hormonal Regulation of Biomineralization. Nat. Rev. Endocrinol. 2021, 17, 261–275. [Google Scholar] [CrossRef]
- Turner, M.E.; Beck, L.; Hill Gallant, K.M.; Chen, Y.; Moe, O.W.; Kuro-o, M.; Moe, S.M.; Aikawa, E. Phosphate in Cardiovascular Disease: From New Insights into Molecular Mechanisms to Clinical Implications. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 584–602. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauza, A. Key Aspects of Myo-Inositol Hexaphosphate (Phytate) and Pathological Calcifications. Molecules 2019, 24, 4434. [Google Scholar] [CrossRef]
- Raggi, P.; Bellasi, A.; Sinha, S.; Bover, J.; Rodriguez, M.; Ketteler, M.; Bushinsky, D.A.; Garg, R.; Perelló, J.; Gold, A.; et al. Effects of SNF472, a Novel Inhibitor of Hydroxyapatite Crystallization in Patients Receiving Hemodialysis—Subgroup Analyses of the CALIPSO Trial. Kidney Int. Rep. 2020, 5, 2178–2182. [Google Scholar] [CrossRef]
- Sinha, S.; Raggi, P.; Chertow, G.M. SNF472: Mechanism of Action and Results from Clinical Trials. Curr. Opin. Nephrol. Hypertens. 2021, 30, 424–429. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Raggi, P.; Bover, J.; Ketteler, M.; Bellasi, A.; Rodriguez, M.; Sinha, S.; Garg, R.; Perelló, J.; Gold, A.; et al. Effects of Myo-Inositol Hexaphosphate (SNF472) on Bone Mineral Density in Patients Receiving Hemodialysis: An Analysis of the Randomized, Placebo-Controlled CaLIPSO Study. Clin. J. Am. Soc. Nephrol. 2021, 16, 736–745. [Google Scholar] [CrossRef]
- Sinha, S.; Gould, L.J.; Nigwekar, S.U.; Serena, T.E.; Brandenburg, V.; Moe, S.M.; Aronoff, G.; Chatoth, D.K.; Hymes, J.L.; Miller, S.; et al. The CALCIPHYX Study: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Clinical Trial of SNF472 for the Treatment of Calciphylaxis. Clin. Kidney J. 2022, 15, 136–144. [Google Scholar] [CrossRef]
- Perelló, J.; Ferrer, M.D.; Del Mar Pérez, M.; Kaesler, N.; Brandenburg, V.M.; Behets, G.J.; D’Haese, P.C.; Garg, R.; Isern, B.; Gold, A.; et al. Mechanism of Action of SNF472, a Novel Calcification Inhibitor to Treat Vascular Calcification and Calciphylaxis. Br. J. Pharmacol. 2020, 177, 4400–4415. [Google Scholar] [CrossRef]
- Thomas, W.C., Jr.; Tilden, M.T. Inhibition of Mineralization by Hydrolysates of Phytic Acid. Johns. Hopkins Med. J. 1972, 131, 133–142. [Google Scholar] [PubMed]
- Magrill, D.S. Phytate Inhibition of Enamel Hardening by Mineralizing Solutions. J. Dent. Res. 1973, 52, 1342. [Google Scholar] [CrossRef]
- Grenby, T.H.; Bull, J.M. Chemical Studies of the Protective Action of Phosphate Compounds against the Demineralization of Human Dental Enamel in Vitro. Caries Res. 1980, 14, 210–220. [Google Scholar] [CrossRef]
- Parkinson, C.R.; Burnett, G.R.; Creeth, J.E.; Lynch, R.J.M.; Budhawant, C.; Lippert, F.; Hara, A.T.; Zero, D.T. Effect of Phytate and Zinc Ions on Fluoride Toothpaste Efficacy Using an in Situ Caries Model. J. Dent. 2018, 73, 24–31. [Google Scholar] [CrossRef]
- Brighton, C.T.; Sennett, B.J.; Farmer, J.C.; Iannotti, J.P.; Hansen, C.A.; Williams, J.L.; Williamson, J. The Inositol Phosphate Pathway as a Mediator in the Proliferative Response of Rat Calvarial Bone Cells to Cyclical Biaxial Mechanical Strain. J. Orthop. Res. 1992, 10, 385–393. [Google Scholar] [CrossRef]
- Hidaka, K.; Kanematsu, T.; Caffrey, J.J.; Takeuchi, H.; Shears, S.B.; Hirata, M. The Importance to Chondrocyte Differentiation of Changes in Expression of the Multiple Inositol Polyphosphate Phosphatase. Exp. Cell Res. 2003, 290, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Yang, X.; Kingsley, P.D.; O’Keefe, R.J.; Puzas, J.E.; Rosier, R.N.; Shears, S.B.; Reynolds, P.R. Targeted Deletion of Minpp1 Provides New Insight into the Activity of Multiple Inositol Polyphosphate Phosphatase In Vivo. Mol. Cell Biol. 2000, 20, 6496–6507. [Google Scholar] [CrossRef] [PubMed]
- Ucuncu, E.; Rajamani, K.; Wilson, M.S.C.; Medina-Cano, D.; Altin, N.; David, P.; Barcia, G.; Lefort, N.; Banal, C.; Vasilache-Dangles, M.-T.; et al. MINPP1 Prevents Intracellular Accumulation of the Chelator Inositol Hexakisphosphate and Is Mutated in Pontocerebellar Hypoplasia. Nat. Commun. 2020, 11, 6087. [Google Scholar] [CrossRef]
- Agarwal, R.; Mumtaz, H.; Ali, N. Role of Inositol Polyphosphates in Programmed Cell Death. Mol. Cell Biochem. 2009, 328, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Addison, W.N.; McKee, M.D. Inositol Hexakisphosphate Inhibits Mineralization of MC3T3-E1 Osteoblast Cultures. Bone 2010, 46, 1100–1107. [Google Scholar] [CrossRef]
- Arriero, M.D.M.; Ramis, J.M.; Perelló, J.; Monjo, M. Differential Response of MC3T3-E1 and Human Mesenchymal Stem Cells to Inositol Hexakisphosphate. Cell Physiol. Biochem. 2012, 30, 974–986. [Google Scholar] [CrossRef]
- Asensio, G.; Martín-Del-Campo, M.; Ramírez, R.A.; Rojo, L.; Vázquez-Lasa, B. New Insights into the In Vitro Antioxidant Routes and Osteogenic Properties of Sr/Zn Phytate Compounds. Pharmaceutics 2023, 15, 339. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wang, Q.; Liu, D.; Chu, C.H.; Zhou, H.; Li, G.; Wu, J.; Cai, K.; Tang, C. Calcium Phytate Reverses High Glucose-Inhibited Osteogenesis of BMSCs via the MAPK/JNK Pathway. Oral. Dis. 2024, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Y.; Wu, J.; Zhou, H.-Y.; Lv, J.-X.; Cai, K.-Z.; Tang, C.-B. Phytic Acid Improves Osteogenesis and Inhibits the Senescence of Human Bone Marrow Mesenchymal Stem Cells under High-Glucose Conditions via the ERK Pathway. Chem. Biol. Interact. 2023, 387, 110818. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Nie, H.; Shen, Y.; Guo, Z.; Huihan Chu, C.; Cai, K.; Tang, C. Phytic Acid Promotes High Glucose-Mediated Bone Marrow Mesenchymal Stem Cells Osteogenesis via Modulating CircEIF4B That Sponges MiR-186-5p and Complexes with IGF2BP3. Biochem. Pharmacol. 2024, 222, 116118. [Google Scholar] [CrossRef] [PubMed]
- Aryal AC, S.; Nassar, M.; Rani KG, A.; Al-Rawi, A.M.; Nassar, R.; Islam, M.S. Phytic Acid Effect on Periodontal Ligament Fibroblast: An in-Vitro Study. PLoS ONE 2023, 18, e0295612. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, A.; Hierro-Oliva, M.; Pacha-Olivenza, M.Á.; Fernández-Calderón, M.C.; Perelló, J.; Isern, B.; González-Martín, M.L.; Monjo, M.; Ramis, J.M. Direct Covalent Grafting of Phytate to Titanium Surfaces through Ti–O–P Bonding Shows Bone Stimulating Surface Properties and Decreased Bacterial Adhesion. ACS Appl. Mater. Interfaces 2016, 8, 11326–11335. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Zhang, H.; Wu, Y.; Tang, C. Covalent Immobilization of the Phytic Acid-Magnesium Layer on Titanium Improves the Osteogenic and Antibacterial Properties. Colloids Surf. B Biointerfaces 2021, 203, 111768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, K.; Lu, M.; Liu, L.; Yan, Y.; Chu, Z.; Ge, Y.; Wang, T.; Qiu, J.; Bu, S.; et al. Micro/Nanostructured Calcium Phytate Coating on Titanium Fabricated by Chemical Conversion Deposition for Biomedical Application. Mater. Sci. Eng. C 2021, 118, 111402. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhao, T.; Wu, W.; Zhang, Z.; Wu, J.; Cai, K.; Li, G.; Lv, J.; Zhou, H.; Tang, C. Sandblasted/Acid-Etched Titanium Surface Modified with Calcium Phytate Enhances Bone Regeneration in a High-Glucose Microenvironment by Regulating Reactive Oxygen Species and Cell Senescence. ACS Biomater. Sci. Eng. 2023, 9, 4720–4734. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Qin, W.; Zhang, C.; Jiao, T. One-Step in Situ Deposition of Phytic Acid-Metal Coordination Complexes for Combined Porphyromonas Gingivalis Infection Prevention and Osteogenic Induction. J. Mater. Chem. B 2022, 10, 4293–4305. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Zhu, Z.; Sun, T.; Liu, M.; Lu, L.; Zhou, C.; Luo, B. Drug-Loaded and Anisotropic Wood-Derived Hydrogel Periosteum with Super Antibacterial, Anti-Inflammatory, and Osteogenic Activities. ACS Appl. Mater. Interfaces 2022, 14, 50485–50498. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, P.; Xie, E.; Ji, Y.; Niu, Y.; Li, F.; Wei, J. Phytic Acid/Magnesium Ion Complex Coating on PEEK Fiber Woven Fabric as an Artificial Ligament with Anti-Fibrogenesis and Osteogenesis for Ligament-Bone Healing. Biomater. Adv. 2022, 140, 213079. [Google Scholar] [CrossRef]
- Li, Y.; Tang, S.; Luo, Z.; Liu, K.; Luo, Y.; Wen, W.; Ding, S.; Li, L.; Liu, M.; Zhou, C.; et al. Chitin Whisker/Chitosan Liquid Crystal Hydrogel Assisted Scaffolds with Bone-like ECM Microenvironment for Bone Regeneration. Carbohydr. Polym. 2024, 332, 121927. [Google Scholar] [CrossRef]
- Nassar, M.; Hiraishi, N.; Tamura, Y.; Otsuki, M.; Aoki, K.; Tagami, J. Phytic Acid: An Alternative Root Canal Chelating Agent. J. Endod. 2015, 41, 242–247. [Google Scholar] [CrossRef]
- Kang, M.; Song, J.-H.; Park, S.-H.; Lee, J.-H.; Park, H.W.; Kim, T.-W. Effects of Brown Rice Extract Treated with Lactobacillus Sakei Wikim001 on Osteoblast Differentiation and Osteoclast Formation. Prev. Nutr. Food Sci. 2014, 19, 353–357. [Google Scholar] [CrossRef]
- Gomes, B.C.; Kaufman, H.W.; Bloom, J.R.; Navon, J.; Wilkens, T.J.; Rifkin, R.A. Inhibitory Effect of Inositol Phosphates on Parathyroid Hormone-Induced Bone Resorption in Organ Cultures. J. Dent. Res. 1984, 63, 890–893. [Google Scholar] [CrossRef]
- Arriero, M. del M.; Ramis, J.M.; Perelló, J.; Monjo, M. Inositol Hexakisphosphate Inhibits Osteoclastogenesis on RAW 264.7 Cells and Human Primary Osteoclasts. PLoS ONE 2012, 7, e43187. [Google Scholar] [CrossRef]
- Sanchis, P.; López-González, Á.-A.; Costa-Bauzá, A.; Busquets-Cortés, C.; Riutord, P.; Calvo, P.; Grases, F. Understanding the Protective Effect of Phytate in Bone Decalcification Related-Diseases. Nutrients 2021, 13, 2859. [Google Scholar] [CrossRef] [PubMed]
- Sasson, A.; Etzion, Z.; Shany, S.; Berlyne, G.M.; Yagil, R. Growth and Bone Mineralisation as Affected by Dietary Calcium, Phytic Acid and Vitamin D. Comp. Biochem. Physiol. A Comp. Physiol. 1982, 72, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Shinoda, S.; Matsumoto, T.; Watari, S. Feed Digestibility and Mineral Balance of the Diet of Young Mice Kept in Mouse Cages inside or Outside an Isolator, Using Varied Concentrations of Sodium Phytate. J. Nutr. Sci. Vitaminol. 1982, 28, 401–410. [Google Scholar] [CrossRef]
- Kunkel, M.E.; Powers, D.L.; Hord, N.G. Comparison of Chemical, Histomorphometric, and Absorptiometric Analyses of Bones of Growing Rats Subjected to Dietary Calcium Stress. J. Am. Coll. Nutr. 1990, 9, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Simonet, B.M.; Perelló, J.; Costa-Bauzá, A.; Prieto, R.M. Effect of Phytate on Element Bioavailability in the Second Generation of Rats. J. Trace Elem. Med. Biol. 2004, 17, 229–234. [Google Scholar] [CrossRef]
- McClung, J.P.; Stahl, C.H.; Marchitelli, L.J.; Morales-Martinez, N.; Mackin, K.M.; Young, A.J.; Scrimgeour, A.G. Effects of Dietary Phytase on Body Weight Gain, Body Composition and Bone Strength in Growing Rats Fed a Low-Zinc Diet. J. Nutr. Biochem. 2006, 17, 190–196. [Google Scholar] [CrossRef]
- Dilworth, L.; Omoruyi, F.O.; Reid, W.; Asemota, H.N. Bone and Faecal Minerals and Scanning Electron Microscopic Assessments of Femur in Rats Fed Phytic Acid Extract from Sweet Potato (Ipomoea Batatas). BioMetals 2008, 21, 133–141. [Google Scholar] [CrossRef]
- Grases, F.; Sanchis, P.; Prieto, R.M.; Perelló, J.; López-González, Á.A. Effect of Tetracalcium Dimagnesium Phytate on Bone Characteristics in Ovariectomized Rats. J. Med. Food 2010, 13, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- López-González, A.A.; Grases, F.; Roca, P.; Mari, B.; Vicente-Herrero, M.T.; Costa-Bauzá, A. Phytate (Myo-Inositol Hexaphosphate) and Risk Factors for Osteoporosis. J. Med. Food 2008, 11, 747–752. [Google Scholar] [CrossRef]
- López-González, Á.A.; Grases, F.; Marí, B.; Vicente-Herrero, M.T.; Costa-Bauzá, A.; Monroy, N. Influencia Del Consumo de Fitato Sobre La Masa Ósea En Mujeres Posmenopáusicas de Mallorca. Reumatol. Clin. 2011, 7, 220–223. [Google Scholar] [CrossRef]
- Sanchis, P.; Prieto, R.M.; Konieczna, J.; Grases, F.; Abete, I.; Salas-Salvadó, J.; Martín, V.; Ruiz-Canela, M.; Babio, N.; García-Gavilán, J.F.; et al. Estimated Phytate Intake Is Associated with Bone Mineral Density in Mediterranean Postmenopausal Women. Nutrients 2023, 15, 1791. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Castrellon, P.G.; Rivas, R.; Gutiérrez, C.J.; Garcia, L.D.; Jimenez, J.E.; Anzo, A.; Hegar, B.; Alarcon, P. Safety of Soya-Based Infant Formulas in Children. Br. J. Nutr. 2014, 111, 1340–1360. [Google Scholar] [CrossRef]
- Reiss, A.B.; Miyawaki, N.; Moon, J.; Kasselman, L.J.; Voloshyna, I.; D’Avino, R.; De Leon, J. CKD, Arterial Calcification, Atherosclerosis and Bone Health: Inter-Relationships and Controversies. Atherosclerosis 2018, 278, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Guimerà, J.; Martínez, A.; Bauza, J.L.; Sanchís, P.; Pieras, E.; Grases, F. Effect of Phytate on Hypercalciuria Secondary to Bone Resorption in Patients with Urinary Stones: Pilot Study. Urolithiasis 2022, 50, 685–690. [Google Scholar] [CrossRef]
- Palacios, C. The Role of Nutrients in Bone Health, from A to Z. Crit. Rev. Food Sci. Nutr. 2006, 46, 621–628. [Google Scholar] [CrossRef]
- Alabadi, B.; Civera, M.; Moreno-Errasquin, B.; Cruz-Jentoft, A. Nutrition-Based Support for Osteoporosis in Postmenopausal Women: A Review of Recent Evidence. Int. J. Womens Health 2024, 16, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Pramitha, J.L.; Rana, S.; Aggarwal, P.R.; Ravikesavan, R.; Joel, A.J.; Muthamilarasan, M. Diverse Role of Phytic Acid in Plants and Approaches to Develop Low-Phytate Grains to Enhance Bioavailability of Micronutrients. Adv. Genet. 2021, 107, 89–120. [Google Scholar] [CrossRef]
- Wu, L.; Luthringer, B.J.C.; Feyerabend, F.; Zhang, Z.; Machens, H.G.; Maeda, M.; Taipaleenmäki, H.; Hesse, E.; Willumeit-Römer, R.; Schilling, A.F. Increased Levels of Sodium Chloride Directly Increase Osteoclastic Differentiation and Resorption in Mice and Men. Osteoporos. Int. 2017, 28, 3215–3228. [Google Scholar] [CrossRef]
- Suzuki, T.; Nishioka, T.; Ishizuka, S.; Hara, H. A Novel Mechanism Underlying Phytate-Mediated Biological Action-Phytate Hydrolysates Induce Intracellular Calcium Signaling by a Gαq Protein-Coupled Receptor and Phospholipase C-Dependent Mechanism in Colorectal Cancer Cells. Mol. Nutr. Food Res. 2010, 54, 947–955. [Google Scholar] [CrossRef]
- Schantl, A.E.; Verhulst, A.; Neven, E.; Behets, G.J.; D’Haese, P.C.; Maillard, M.; Mordasini, D.; Phan, O.; Burnier, M.; Spaggiari, D.; et al. Inhibition of Vascular Calcification by Inositol Phosphates Derivatized with Ethylene Glycol Oligomers. Nat. Commun. 2020, 11, 721. [Google Scholar] [CrossRef]
- Mora-Boza, A.; López-Donaire, M.L.; Saldaña, L.; Vilaboa, N.; Vázquez-Lasa, B.; San Román, J. Glycerylphytate Compounds with Tunable Ion Affinity and Osteogenic Properties. Sci. Rep. 2019, 9, 11491. [Google Scholar] [CrossRef] [PubMed]
- Mora-Boza, A.; García-Fernández, L.; Barbosa, F.A.; Oliveira, A.L.; Vázquez-Lasa, B.; San Román, J. Glycerylphytate Crosslinker as a Potential Osteoinductor of Chitosan-Based Systems for Guided Bone Regeneration. Carbohydr. Polym. 2020, 241, 116269. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshiko, Y.; Vucenik, I. Inositol Hexaphosphate in Bone Health and Disease. Biomolecules 2024, 14, 1072. https://doi.org/10.3390/biom14091072
Yoshiko Y, Vucenik I. Inositol Hexaphosphate in Bone Health and Disease. Biomolecules. 2024; 14(9):1072. https://doi.org/10.3390/biom14091072
Chicago/Turabian StyleYoshiko, Yuji, and Ivana Vucenik. 2024. "Inositol Hexaphosphate in Bone Health and Disease" Biomolecules 14, no. 9: 1072. https://doi.org/10.3390/biom14091072
APA StyleYoshiko, Y., & Vucenik, I. (2024). Inositol Hexaphosphate in Bone Health and Disease. Biomolecules, 14(9), 1072. https://doi.org/10.3390/biom14091072





