Genetics of Female Pelvic Organ Prolapse: Up to Date
Abstract
:1. Introduction
2. Materials and Methods
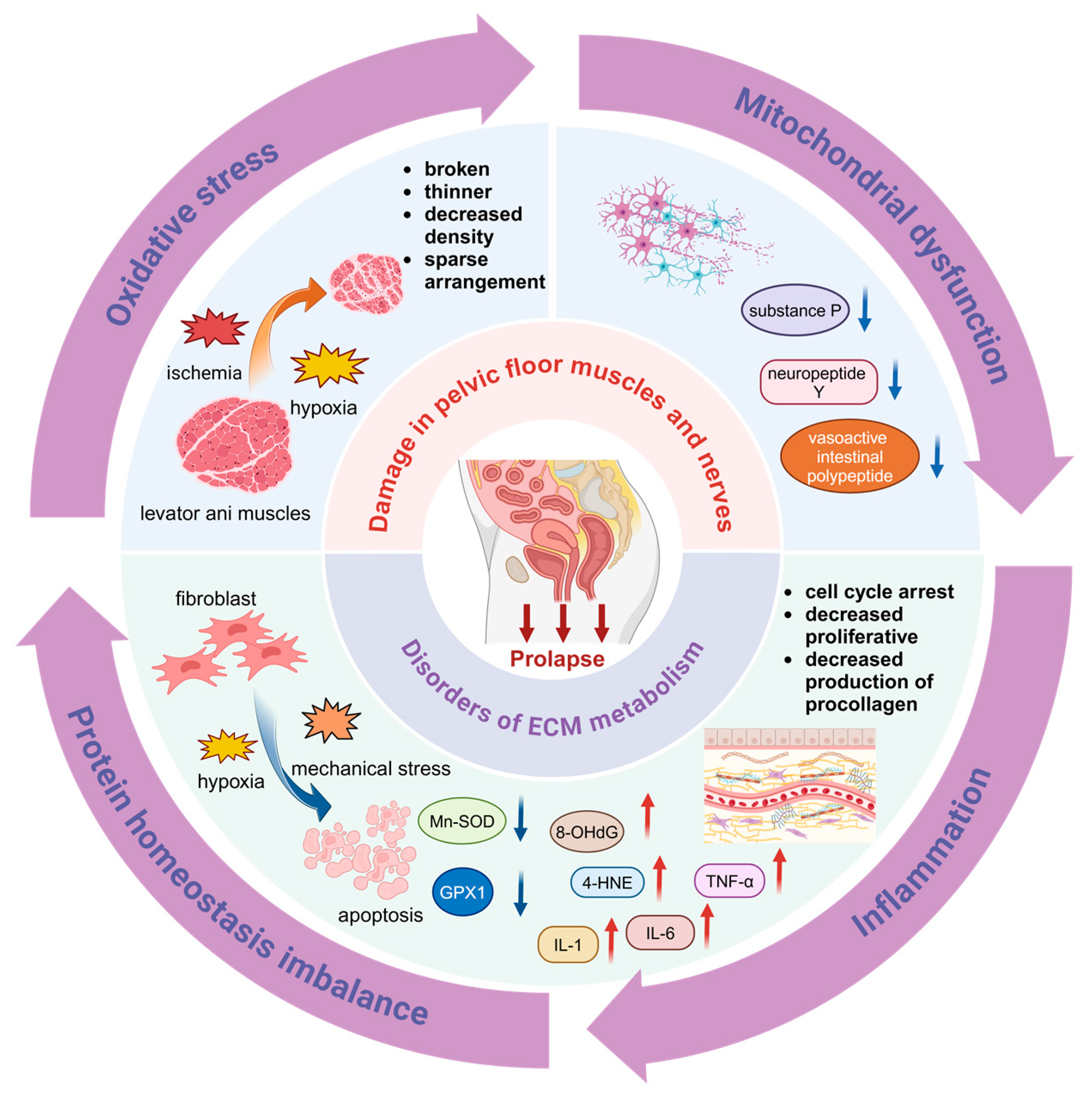
3. Pathogenesis of POP
3.1. Damage in Pelvic Floor Muscles and Nerves
3.2. Disorders of ECM Metabolism in the Pelvic Floor
3.2.1. Oxidative Stress (OS)
3.2.2. Mitochondrial Dysfunction
3.2.3. Hormonal Changes
3.2.4. Inflammation
4. Genetic Studies of POP
4.1. Linkage Analyses
4.2. Candidate Gene Association Studies
4.2.1. COL1A1
4.2.2. COL3A1
4.2.3. LAMC1
4.2.4. LOXL
4.2.5. FBLN5
4.2.6. MMPs and TIMPs
4.2.7. ER
4.3. Whole Exome Sequencing (WES)
4.4. Genome-Wide Association Studies (GWAS)
5. Epigenetic Studies of POP
5.1. DNA Methylation
5.2. MicroRNA
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jelovsek, J.E.; Maher, C.; Barber, M.D. Pelvic organ prolapse. Lancet 2007, 369, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.D. Pelvic organ prolapse. BMJ 2016, 354, i3853. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Vaughan, C.P.; Goode, P.S.; Redden, D.T.; Burgio, K.L.; Richter, H.E.; Markland, A.D. Prevalence and Trends of Symptomatic Pelvic Floor Disorders in U.S. Women. Obstet. Gynecol. 2014, 123, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Zhu, L.; Xu, T.; Liu, Q.; Li, Z.A.; Gong, J.; Wang, Y.L.; Wang, J.T.; Lai, T.; Wu, L.; et al. An epidemiologic study of pelvic organ prolapse in urban Chinese women: A population-based sample in China. Zhonghua Yi Xue Za Zhi 2019, 99, 857–861. [Google Scholar] [CrossRef]
- Drewes, P.G.; Yanagisawa, H.; Starcher, B.; Hornstra, I.; Csiszar, K.; Marinis, S.I.; Keller, P.; Word, R.A. Pelvic Organ Prolapse in Fibulin-5 Knockout Mice. Am. J. Pathol. 2007, 170, 578–589. [Google Scholar] [CrossRef]
- Tinelli, A.; Malvasi, A.; Rahimi, S.; Negro, R.; Vergara, D.; Martignago, R.; Pellegrino, M.; Cavallotti, C. Age-related pelvic floor modifications and prolapse risk factors in postmenopausal women. Menopause 2010, 17, 204–212. [Google Scholar] [CrossRef]
- Kieserman-Shmokler, C.; Swenson, C.W.; Chen, L.; Desmond, L.M.; Ashton-Miller, J.A.; DeLancey, J.O. From molecular to macro: The key role of the apical ligaments in uterovaginal support. Am. J. Obstet. Gynecol. 2020, 222, 427–436. [Google Scholar] [CrossRef]
- Chi, N.; Lozo, S.; Rathnayake, R.A.C.; Botros-Brey, S.; Ma, Y.; Damaser, M.; Wang, R.R. Distinctive structure, composition and biomechanics of collagen fibrils in vaginal wall connective tissues associated with pelvic organ prolapse. Acta Biomater. 2022, 152, 335–344. [Google Scholar] [CrossRef]
- Orlicky, D.J.; Guess, M.K.; Bales, E.S.; Rascoff, L.G.; Arruda, J.S.; Hutchinson-Colas, J.A.; Johnson, J.; Connell, K.A. Using the novel pelvic organ prolapse histologic quantification system to identify phenotypes in uterosacral ligaments in women with pelvic organ prolapse. Am. J. Obstet. Gynecol. 2021, 224, 67.e1–67.e18. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Q.; Fang, G.; Li, B.-S.; Wu, D.-B.; Guo, W.-J.; Hong, S.-S.; Hong, L. Collagen metabolic disorder induced by oxidative stress in human uterosacral ligament-derived fibroblasts: A possible pathophysiological mechanism in pelvic organ prolapse. Mol. Med. Rep. 2016, 13, 2999–3008. [Google Scholar] [CrossRef]
- Li, B.-S.; Guo, W.-J.; Hong, L.; Liu, Y.-D.; Liu, C.; Hong, S.-S.; Wu, D.-B.; Min, J. Role of mechanical strain-activated PI3K/Akt signaling pathway in pelvic organ prolapse. Mol. Med. Rep. 2016, 14, 243–253. [Google Scholar] [CrossRef]
- Jundt, K.; Kiening, M.; Fischer, P.; Bergauer, F.; Rauch, E.; Janni, W.; Peschers, U.; Dimpfl, T. Is the histomorphological concept of the female pelvic floor and its changes due to age and vaginal delivery correct? Neurourol. Urodyn. 2005, 24, 44–50. [Google Scholar] [CrossRef]
- Zhu, L.; Lang, J.H.; Chen, J.; Chen, J. Morphologic study on levator ani muscle in patients with pelvic organ prolapse and stress urinary incontinence. Int. Urogynecol. J. 2005, 16, 401–404. [Google Scholar] [CrossRef]
- DeLancey, J.O.L.; Morgan, D.M.; Fenner, D.E.; Kearney, R.; Guire, K.; Miller, J.M.; Hussain, H.; Umek, W.; Hsu, Y.; Ashton-Miller, J.A. Comparison of Levator Ani Muscle Defects and Function in Women with and without Pelvic Organ Prolapse. Obstet. Gynecol. 2007, 109, 295–302. [Google Scholar] [CrossRef]
- Huang, G.; He, Y.; Hong, L.; Zhou, M.; Zuo, X.; Zhao, Z. Restoration of NAD+ homeostasis protects C2C12 myoblasts and mouse levator ani muscle from mechanical stress-induced damage. Anim. Cells Syst. 2022, 26, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Wang, L.; Li, S.; Li, B.; Liu, C.; Hong, L. Effects of mechanical trauma on the differentiation and ArfGAP3 expression of C2C12 myoblast and mouse levator ani muscle. Int. Urogynecol. J. 2020, 31, 1913–1924. [Google Scholar] [CrossRef]
- Busacchi, P.; Perri, T.; Paradisi, R.; Oliverio, C.; Santini, D.; Guerrini, S.; Barbara, G.; Stanghellini, V.; Corinaldesi, R.; De Giorgio, R. Abnormalities of somatic peptide-containing nerves supplying the pelvic floor of women with genitourinary prolapse and stress urinary incontinence. Urology 2004, 63, 591–595. [Google Scholar] [CrossRef] [PubMed]
- McArdle, A.; Pollock, N.; Staunton, C.A.; Jackson, M.J. Aberrant redox signalling and stress response in age-related muscle decline: Role in inter- and intra-cellular signalling. Free Radic. Biol. Med. 2019, 132, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Lozanoska-Ochser, B.; Calvani, R.; Coelho-Júnior, H.J.; Leewenburgh, C.; Marzetti, E. Inflammatory, mitochondrial, and senescence-related markers: Underlying biological pathways of muscle aging and new therapeutic targets. Exp. Gerontol. 2023, 178, 112204. [Google Scholar] [CrossRef]
- Yiou, R.; Authier, F.-J.; Gherardi, R.; Abbou, C. Evidence of Mitochondrial Damage in the Levator Ani Muscle of Women with Pelvic Organ Prolapse. Eur. Urol. 2009, 55, 1241–1243. [Google Scholar] [CrossRef]
- Cao, L.L.; Yu, J.; Yang, Z.L.; Qiao, X.; Ye, H.; Xi, C.L.; Zhou, Q.C.; Hu, C.C.; Zhao, C.J.; Gong, Z.L. MMP-1/TIMP-1 expressions in rectal submucosa of females with obstructed defecation syndrome associated with internal rectal prolapse. Histol. Histopathol. 2019, 34, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Guler, Z.; Roovers, J.P. Role of Fibroblasts and Myofibroblasts on the Pathogenesis and Treatment of Pelvic Organ Prolapse. Biomolecules 2022, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Zhou, Y.; Peng, C.; Chen, H.; Lu, Y. Mitofusin2 regulates the proliferation and function of fibroblasts: The possible mechanisms underlying pelvic organ prolapse development. Mol. Med. Rep. 2019, 20, 2859–2866. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, Y.; Qi, Y.; Bai, W.; Liao, Q. Relationship between the expressions of mitofusin-2 and procollagen in uterosacral ligament fibroblasts of postmenopausal patients with pelvic organ prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 174, 141–145. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Wang, X.-J.; Feng, W.; Hua, K.-Q. Advanced glycation end products decrease collagen I levels in fibroblasts from the vaginal wall of patients with POP via the RAGE, MAPK and NF-κB pathways. Int. J. Mol. Med. 2017, 40, 987–998. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; Li, R.; Wei, X.; Luan, W.; Liu, P.; Zhao, J. Hypoxia-Inducible Factor 1-α (HIF-1α) Induces Apoptosis of Human Uterosacral Ligament Fibroblasts through the Death Receptor and Mitochondrial Pathways. Med. Sci. Monit. 2018, 24, 8722–8733. [Google Scholar] [CrossRef]
- Sun, M.-J.; Cheng, Y.-S.; Liu, C.-S.; Sun, R. Changes in the PGC-1α and mtDNA copy number may play a role in the development of pelvic organ prolapse in pre-menopausal patients. Taiwan. J. Obstet. Gynecol. 2019, 58, 526–530. [Google Scholar] [CrossRef]
- ZONG, W.; JIANG, Y.; ZHAO, J.; ZHANG, J.; GAO, J. Estradiol plays a role in regulating the expression of lysyl oxidase family genes in mouse urogenital tissues and human Ishikawa cells. J. Zhejiang Univ. Sci. B 2015, 16, 857–864. [Google Scholar] [CrossRef]
- Sflomos, G.; Battista, L.; Aouad, P.; De Martino, F.; Scabia, V.; Stravodimou, A.; Ayyanan, A.; Ifticene-Treboux, A.; Bucher, P.; Fiche, M.; et al. Intraductal xenografts show lobular carcinoma cells rely on their own extracellular matrix and LOXL1. EMBO Mol. Med. 2021, 13, e13180. [Google Scholar] [CrossRef]
- Trackman, P.C. Lysyl Oxidase Isoforms and Potential Therapeutic Opportunities for Fibrosis and Cancer. Expert. Opin. Ther. Targets 2016, 20, 935–945. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, G.; He, Y.; Zuo, X.; Han, W.; Li, H. Estrogen inhibits the differentiation of fibroblasts induced by high stiffness matrix by enhancing DNMT1 expression. Tissue Cell 2023, 85, 102207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Choy, K.W.; Lui, W.T.; Pang, M.W.; Wong, Y.F.; Yip, S.K. 17β-Estradiol suppresses proliferation of fibroblasts derived from cardinal ligaments in patients with or without pelvic organ prolapse. Hum. Reprod. 2006, 21, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Rahn, D.D.; Richter, H.E.; Sung, V.W.; Pruszynski, J.E.; Hynan, L.S. Perioperative Vaginal Estrogen as Adjunct to Native Tissue Vaginal Apical Prolapse Repair: A Randomized Clinical Trial. JAMA 2023, 330, 615. [Google Scholar] [CrossRef]
- Reddy, R.A.; Cortessis, V.; Dancz, C.; Klutke, J.; Stanczyk, F.Z. Role of sex steroid hormones in pelvic organ prolapse. Menopause 2020, 27, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Fuermetz, A. Change of steroid receptor expression in the posterior vaginal wall after local estrogen therapy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 187, 45–50. [Google Scholar] [CrossRef]
- He, K.; Niu, G.; Gao, J.; Liu, J.-X.; Qu, H. MicroRNA-92 expression may be associated with reduced estrogen receptor β1 mRNA levels in cervical portion of uterosacral ligaments in women with pelvic organ prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 198, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Meyn, L.A.; Moalli, P.A. The Amount and Activity of Active Matrix Metalloproteinase 13 Is Suppressed by Estradiol and Progesterone in Human Pelvic Floor Fibroblasts. Biol. Reprod. 2009, 80, 367–374. [Google Scholar] [CrossRef]
- Zong, W.; Jallah, Z.C.; Moalli, P.A. Repetitive Mechanical Stretch Increases Extracellular Collagenase Activity in Vaginal Fibroblasts. Female Pelvic Med. Reconstr. Surg. 2010, 16, 257–262. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.; Wan, S.; Lou, L.; Gu, S.; Peng, J.; Zhao, S.; Hua, X. Exosomes isolated from TNF -α-treated bone marrow mesenchymal stem cells ameliorate pelvic floor dysfunction in rats. J. Cell. Mol. Med. 2024, 28, e18451. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, Q.; Fan, Y.; Hu, X.; Li, L.; Wang, J.; Cui, S. Transplantation of bone marrow-derived mesenchymal stem cells with silencing of microRNA-138 relieves pelvic organ prolapse through the FBLN5/IL-1β/elastin pathway. Aging 2021, 13, 3045–3059. [Google Scholar] [CrossRef]
- Vashaghian, M. Gentle cyclic straining of human fibroblasts on electrospun scaffolds enhances their regenerative potential. Acta Biomater. 2019, 84, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, E.L.; Rortveit, G.; Brown, J.S.; Creasman, J.M.; Thom, D.H.; Van Den Eeden, S.K.; Subak, L.L. Racial Differences in Pelvic Organ Prolapse. Obstet. Gynecol. 2009, 114, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Jack, G.S.; Nikolova, G.; Vilain, E.; Raz, S.; Rodríguez, L.V. Familial tranmission of genitovaginal prolapse. Int. Urogynecol. J. 2006, 17, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Samimi, P.; Jones, S.H.; Giri, A. Family history and pelvic organ prolapse: A systematic review and meta-analysis. Int. Urogynecol. J. 2021, 32, 759–774. [Google Scholar] [CrossRef]
- Altman, D.; Forsman, M.; Falconer, C.; Lichtenstein, P. Genetic Influence on Stress Urinary Incontinence and Pelvic Organ Prolapse. Eur. Urol. 2008, 54, 918–923. [Google Scholar] [CrossRef]
- Alperin, M.; Debes, K.; Abramowitch, S.; Meyn, L.; Moalli, P.A. LOXL1 deficiency negatively impacts the biomechanical properties of the mouse vagina and supportive tissues. Int. Urogynecol. J. 2008, 19, 977–986. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Gao, J.; Pawlyk, B.; Starcher, B.; Spencer, J.A.; Yanagisawa, H.; Zuo, J.; Li, T. Elastic fiber homeostasis requires lysyl oxidase–like 1 protein. Nat. Genet. 2004, 36, 178–182. [Google Scholar] [CrossRef]
- Budatha, M.; Roshanravan, S.; Zheng, Q.; Weislander, C.; Chapman, S.L.; Davis, E.C.; Starcher, B.; Word, R.A.; Yanagisawa, H. Extracellular matrix proteases contribute to progression of pelvic organ prolapse in mice and humans. J. Clin. Investig. 2011, 121, 2048–2059. [Google Scholar] [CrossRef]
- Clark-Patterson, G.L.; Roy, S.; Desrosiers, L.; Knoepp, L.R.; Sen, A.; Miller, K.S. Role of fibulin-5 insufficiency and prolapse progression on murine vaginal biomechanical function. Sci. Rep. 2021, 11, 20956. [Google Scholar] [CrossRef]
- Ott, J.; Wang, J.; Leal, S.M. Genetic linkage analysis in the age of whole-genome sequencing. Nat. Rev. Genet. 2015, 16, 275–284. [Google Scholar] [CrossRef]
- Nikolova, G.; Lee, H.; Berkovitz, S.; Nelson, S.; Sinsheimer, J.; Vilain, E.; Rodríguez, L.V. Sequence variant in the laminin γ1 (LAMC1) gene associated with familial pelvic organ prolapse. Hum. Genet. 2007, 120, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Allen-Brady, K.; Norton, P.A.; Farnham, J.M.; Teerlink, C.; Cannon-Albright, L.A. Significant Linkage Evidence for a Predisposition Gene for Pelvic Floor Disorders on Chromosome 9q21. Am. J. Hum. Genet. 2009, 84, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Allen-Brady, K.; Cannon-Albright, L.A.; Farnham, J.M.; Norton, P.A. Evidence for pelvic organ prolapse predisposition genes on chromosomes 10 and 17. Am. J. Obstet. Gynecol. 2015, 212, 771.e1–771.e7. [Google Scholar] [CrossRef] [PubMed]
- Ashikari, A.; Suda, T.; Miyazato, M. Collagen type 1A1, type 3A1, and LOXL1/4 polymorphisms as risk factors of pelvic organ prolapse. BMC Res. Notes 2021, 14, 15. [Google Scholar] [CrossRef]
- Batista, N.C.; Bortolini, M.A.T.; Silva, R.S.P.; Teixeira, J.B.; Melo, N.C.; Santos, R.G.M.; Pepicelli, F.A.A.; Castro, R.A. Collagen I and collagen III polymorphisms in women with pelvic organ prolapse. Neurourol. Urodyn. 2020, 39, 1977–1984. [Google Scholar] [CrossRef]
- Li, L.; Sun, Z.; Chen, J.; Zhang, Y.; Shi, H.; Zhu, L. Genetic polymorphisms in collagen-related genes are associated with pelvic organ prolapse. Menopause 2020, 27, 223–229. [Google Scholar] [CrossRef]
- Feiner, B.; Fares, F.; Azam, N.; Auslender, R.; David, M.; Abramov, Y. Does COLIA1 SP1-binding site polymorphism predispose women to pelvic organ prolapse? Int. Urogynecol. J. 2009, 20, 1061–1065. [Google Scholar] [CrossRef]
- Ferrari, M.M.; Rossi, G.; Biondi, M.L.; Viganò, P.; Dell’Utri, C.; Meschia, M. Type I collagen and matrix metalloproteinase 1, 3 and 9 gene polymorphisms in the predisposition to pelvic organ prolapse. Arch. Gynecol. Obstet. 2012, 285, 1581–1586. [Google Scholar] [CrossRef]
- Cho, H.J.; Jung, H.J.; Kim, S.K.; Choi, J.R.; Cho, N.H.; Bai, S.W. Polymorphism of a COLIA1 Gene Sp1 Binding Site in Korean Women with Pelvic Organ Prolapse. Yonsei Med. J. 2009, 50, 564–568. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Girão, M.J.B.C.; da Silva, I.D.C.G.; Sartori, M.G.F.; Martins, K.d.F.; Castro, R.d.A. COL1A1 Sp1-binding site polymorphism as a risk factor for genital prolapse. Int. Urogynecol. J. 2008, 19, 1471–1475. [Google Scholar] [CrossRef]
- Teixeira, F.H.; Fernandes, C.E.; do Souto, R.P.; de Oliveira, E. Polymorphism rs1800255 from COL3A1 gene and the risk for pelvic organ prolapse. Int. Urogynecol. J. 2020, 31, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Lince, S.L.; van Kempen, L.C.; Dijkstra, J.R.; IntHout, J.; Vierhout, M.E.; Kluivers, K.B. Collagen type III alpha 1 polymorphism (rs1800255, COL3A1 2209 G>A) assessed with high-resolution melting analysis is not associated with pelvic organ prolapse in the Dutch population. Int. Urogynecol. J. 2014, 25, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Kluivers, K.B.; Dijkstra, J.R.; Hendriks, J.C.M.; Lince, S.L.; Vierhout, M.E.; van Kempen, L.C.L. COL3A1 2209G>A is a predictor of pelvic organ prolapse. Int. Urogynecol. J. 2009, 20, 1113–1118. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chung, Y.-W.; Lin, W.-Y.; Wang, J.-C.; Tsai, F.-J.; Tsai, C.-H. Collagen type 3 alpha 1 polymorphism and risk of pelvic organ prolapse. Int. J. Gynecol. Obstet. 2008, 103, 55–58. [Google Scholar] [CrossRef]
- Martins, K.d.F.; de Jármy-DiBella, Z.I.K.; da Fonseca, A.M.R.M.; Castro, R.A.; da Silva, I.D.C.G.; Girão, M.J.B.C.; Sartori, M.G.F. Evaluation of demographic, clinical characteristics, and genetic polymorphism as risk factors for pelvic organ prolapse in Brazilian women. Neurourol. Urodyn. 2011, 30, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.J.; Chung, S.M.; Choi, J.R.; Jung, H.J.; Kim, S.K.; Bai, S.W. The relationship between COL3A1 exon 31 polymorphism and pelvic organ prolapse. J. Urol. 2009, 181, 1213–1216. [Google Scholar] [CrossRef]
- Wu, J.M.; Visco, A.G.; Grass, E.A.; Craig, D.M.; Fulton, R.G.; Haynes, C.; Amundsen, C.L.; Shah, S.H. Comprehensive analysis of LAMC1 genetic variants in advanced pelvic organ prolapse. Am. J. Obstet. Gynecol. 2012, 206, 447.e1–447.e6. [Google Scholar] [CrossRef]
- Chen, C.; Hill, L.D.; Schubert, C.M.; Strauss, J.F.; Matthews, C.A. Is laminin gamma-1 a candidate gene for advanced pelvic organ prolapse? Am. J. Obstet. Gynecol. 2010, 202, e505.e1–e505.e5. [Google Scholar] [CrossRef]
- Costa E Silva, C.L.; Bortolini, M.a.T.; Batista, N.C.; Silva, R.S.P.; Teixeira, J.B.; Oliveira, É.; Souto, R.P.; Castro, R.A. The rs2165241 polymorphism of the Loxl1 gene in postmenopausal women with pelvic organ prolapse. Climacteric 2022, 25, 407–412. [Google Scholar] [CrossRef]
- Dos Santos, R.G.M.; Pepicelli, F.C.A.; Batista, N.C.; de Carvalho, C.V.; Bortolini, M.A.T.; Castro, R.A. Collagen XVIII and LOXL-4 polymorphisms in women with and without advanced pelvic organ prolapse. Int. Urogynecol. J. 2018, 29, 893–898. [Google Scholar] [CrossRef]
- Khadzhieva, M.B.; Kamoeva, S.V.; Chumachenko, A.G.; Ivanova, A.V.; Volodin, I.V.; Vladimirov, I.S.; Abilev, S.K.; Salnikova, L.E. Fibulin-5 (FBLN5) gene polymorphism is associated with pelvic organ prolapse. Maturitas 2014, 78, 287–292. [Google Scholar] [CrossRef]
- Paula, M.V.B.d.; Lira Júnior, M.A.d.F.; Monteiro, V.C.e.S.C.; Souto, R.P.; Fernandes, C.E.; Oliveira, E.d.e. Evaluation of the fibulin 5 gene polymorphism as a factor related to the occurrence of pelvic organ prolapse. Rev. Assoc. Med. Bras. 2020, 66, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Skorupski, P.; Jankiewicz, K.; Miotła, P.; Marczak, M.; Kulik-Rechberger, B.; Rechberger, T. The polymorphisms of the MMP-1 and the MMP-3 genes and the risk of pelvic organ prolapse. Int. Urogynecol. J. 2013, 24, 1033–1038. [Google Scholar] [CrossRef]
- Maeda, P.M.; Bicudo, A.P.S.L.; Watanabe, R.T.M.; Fonseca, T.S.M.; do Souto, R.P.; Fernandes, C.E.; de Oliveira, E. Study of the polymorphism rs3025058 of the MMP-3 gene and risk of pelvic organ prolapse in Brazilian women. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 3, 100031. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Visco, A.G.; Grass, E.A.; Craig, D.M.; Fulton, R.G.; Haynes, C.; Weidner, A.C.; Shah, S.H. Matrix Metalloproteinase-9 Genetic Polymorphisms and the Risk for Advanced Pelvic Organ Prolapse. Obstet. Gynecol. 2012, 120, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Lin, W.-Y.; Chen, Y.-H.; Chen, W.-C.; Tsai, F.-J.; Tsai, C.-H. Matrix metalloproteinase-9 polymorphism and risk of pelvic organ prolapse in Taiwanese women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 149, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.-Q.; Wang, S.-Z.; Lu, J.-L.; Wang, X.-L.; Zhang, Z.-Y. Association of matrix metalloproteinase-10 polymorphisms with susceptibility to pelvic organ prolapse: Genetic mutations of MMP-10 in POP. J. Obstet. Gynaecol. Res. 2015, 41, 1972–1981. [Google Scholar] [CrossRef]
- Abulaizi, A.; Abula, A.; Ababaikeli, G.; Wan, X.; Du, R.; Zhakeer, A. Identification of pelvic organ prolapse risk susceptibility gene SNP locus in Xinjiang women. Int. Urogynecol. J. 2020, 31, 123–130. [Google Scholar] [CrossRef]
- Nakad, B.; Fares, F.; Azzam, N.; Feiner, B.; Zilberlicht, A.; Abramov, Y. Estrogen receptor and laminin genetic polymorphism among women with pelvic organ prolapse. Taiwan. J. Obstet. Gynecol. 2017, 56, 750–754. [Google Scholar] [CrossRef]
- Li, Y.; Nie, N.; Gong, L.; Bao, F.; An, C.; Cai, H.; Yao, X.; Liu, Y.; Yang, C.; Wu, B.; et al. Structural, functional and molecular pathogenesis of pelvic organ prolapse in patient and Loxl1 deficient mice. Aging 2021, 13, 25886–25902. [Google Scholar] [CrossRef]
- Skorupski, P.; Król, J.; Staręga, J.; Adamiak, A.; Jankiewicz, K.; Rechberger, T. An α-1 chain of type I collagen Sp1-binding site polymorphism in women suffering from stress urinary incontinence. Am. J. Obstet. Gynecol. 2006, 194, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, R.; Kirby, A.C.; Tikkinen, K.A.O.; Mangera, A.; Thiagamoorthy, G.; Rajan, P.; Pesonen, J.; Ambrose, C.; Gonzalez-Maffe, J.; Bennett, P.; et al. Systematic review and metaanalysis of genetic association studies of urinary symptoms and prolapse in women. Am. J. Obstet. Gynecol. 2015, 212, 199.e1–199.e24. [Google Scholar] [CrossRef] [PubMed]
- Allen-Brady, K.; Chua, J.W.F.; Cuffolo, R.; Koch, M.; Sorrentino, F.; Cartwright, R. Systematic review and meta-analysis of genetic association studies of pelvic organ prolapse. Int. Urogynecol. J. 2021, 33, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, M.H.; Ruiz-Zapata, A.M.; Bril, H.; Bleeker, M.C.G.; Belien, J.A.M.; Stoop, R.; Helder, M.N. Changes in tissue composition of the vaginal wall of premenopausal women with prolapse. Am. J. Obstet. Gynecol. 2014, 210, 168.e1–168.e9. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.M.; Velez Edwards, D.R.; Edwards, T.; Giri, A.; Jerome, R.N.; Wu, J.M. Genetic epidemiology of pelvic organ prolapse: A systematic review. Am. J. Obstet. Gynecol. 2014, 211, 326–335. [Google Scholar] [CrossRef]
- Niu, K.; Chen, X.; Lu, Y. COL3A1 rs1800255 polymorphism is associated with pelvic organ prolapse susceptibility in Caucasian individuals: Evidence from a meta-analysis. PLoS ONE 2021, 16, e0250943. [Google Scholar] [CrossRef]
- Chen, B.; Wen, Y.; Zhang, Z.; Wang, H.; Warrington, J.A.; Polan, M.L. Menstrual phase-dependent gene expression differences in periurethral vaginal tissue from women with stress incontinence. Am. J. Obstet. Gynecol. 2003, 189, 89–97. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Chen, J.; Guo, X.; Guan, H.; Li, C. Differential expression profiling of matrix metalloproteinases and tissue inhibitors of metalloproteinases in females with or without pelvic organ prolapse. Mol. Med. Rep. 2014, 10, 2004–2008. [Google Scholar] [CrossRef]
- Dviri, M.; Leron, E.; Dreiher, J.; Mazor, M.; Shaco-Levy, R. Increased matrix metalloproteinases-1,-9 in the uterosacral ligaments and vaginal tissue from women with pelvic organ prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 156, 113–117. [Google Scholar] [CrossRef]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.-Å. Estrogen receptor alpha and beta in health and disease. Best. Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef]
- Lang, J.H.; Zhu, L.; Sun, Z.J.; Chen, J. Estrogen levels and estrogen receptors in patients with stress urinary incontinence and pelvic organ prolapse. Int. J. Gynecol. Obstet. 2003, 80, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Wan, L.; Chung, Y.-W.; Chen, W.-C.; Tsai, F.-J.; Tsai, C.-H. Estrogen receptor beta gene haplotype is associated with pelvic organ prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 138, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Lang, J.; Zhu, L.; Chen, J. Exome Sequencing Identifies a Novel Gene, WNK1, for Susceptibility to Pelvic Organ Prolapse (POP). PLoS ONE 2015, 10, e0119482. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef]
- Allen-Brady, K.; Cannon-Albright, L.; Farnham, J.M.; Teerlink, C.; Vierhout, M.E.; van Kempen, L.C.L.; Kluivers, K.B.; Norton, P.A. Identification of six loci associated with pelvic organ prolapse using genome-wide association analysis. Obstet. Gynecol. 2011, 118, 1345–1353. [Google Scholar] [CrossRef]
- Giri, A.; Wu, J.M.; Ward, R.M.; Hartmann, K.E.; Park, A.J.; North, K.E.; Graff, M.; Wallace, R.B.; Bareh, G.; Qi, L.; et al. Genetic Determinants of Pelvic Organ Prolapse among African American and Hispanic Women in the Women’s Health Initiative. PLoS ONE 2015, 10, e0141647. [Google Scholar] [CrossRef]
- Olafsdottir, T.; Thorleifsson, G.; Sulem, P.; Stefansson, O.A.; Medek, H.; Olafsson, K.; Ingthorsson, O.; Gudmundsson, V.; Jonsdottir, I.; Halldorsson, G.H.; et al. Genome-wide association identifies seven loci for pelvic organ prolapse in Iceland and the UK Biobank. Commun. Biol. 2020, 3, 129. [Google Scholar] [CrossRef]
- Cox, C.K.; Pandit, A.; Zawistowski, M.; Dutta, D.; Narla, G.; Swenson, C.W. Genome-Wide Association Study of Pelvic Organ Prolapse Using the Michigan Genomics Initiative. Female Pelvic Med. Reconstr. Surg. 2021, 27, 502–506. [Google Scholar] [CrossRef]
- Pujol-Gualdo, N.; Läll, K.; Lepamets, M.; Estonian Biobank Research Team; Rossi, H.-R.; Arffman, R.K.; Piltonen, T.T.; Mägi, R.; Laisk, T. Advancing our understanding of genetic risk factors and potential personalized strategies for pelvic organ prolapse. Nat. Commun. 2022, 13, 3584. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, P.; Duan, A.; Hao, Y.; Lu, C.; Lu, D. Genome-wide DNA methylation analysis of uterosacral ligaments in women with pelvic organ prolapse. Mol. Med. Rep. 2019, 19, 391–399. [Google Scholar] [CrossRef]
- Klutke, J.; Stanczyk, F.Z.; Ji, Q.; Campeau, J.D.; Klutke, C.G. Suppression of lysyl oxidase gene expression by methylation in pelvic organ prolapse. Int. Urogynecol. J. 2010, 21, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, T.; Zhang, L.; Zhao, J.; Gong, J.; Zhao, C. Increased microRNA-221/222 and decreased estrogen receptor α in the cervical portion of the uterosacral ligaments from women with pelvic organ prolapse. Int. Urogynecol. J. 2012, 23, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.J.; Kim, E.J.; Lee, M.; Kim, H.; Choi, J.R.; Chae, H.D.; Moon, Y.J.; Kim, S.K.; Bai, S.W. MicroRNA-30d and microRNA-181a regulate HOXA11 expression in the uterosacral ligaments and are overexpressed in pelvic organ prolapse. J. Cell. Mol. Med. 2015, 19, 501–509. [Google Scholar] [CrossRef] [PubMed]
| Gene | SNPs | Sample Size (Case/Control) | Population | Correlation to POP | Year | Reference |
|---|---|---|---|---|---|---|
| COL1A1 | rs1800012 | 52/28 | Japanese | No | 2021 | [54] |
| 348/286 | Brazilian | No | 2020 | [55] | ||
| 48/48 | Chinese | No | 2019 | [56] | ||
| 36/36 | Caucasian, Ashkenazi–Jewish Israeli | No | 2009 | [57] | ||
| Sp1 G>T | 137/96 | Italian | No | 2012 | [58] | |
| 15/15 | Korean | No | 2009 | [59] | ||
| 107/209 | Brazilian | No | 2008 | [60] | ||
| COL3A1 | rs1800255 | 52/28 | Japanese | No | 2021 | [54] |
| 348/286 | Brazilian | No | 2020 | [55] | ||
| 48/48 | Chinese | No | 2019 | [54] | ||
| 112/180 | Brazilian | No | 2019 | [61] | ||
| 272/82 | Dutch | No | 2014 | [62] | ||
| 202/102 | Netherland | Positive | 2009 | [63] | ||
| 84/147 | Taiwan | Negative | 2008 | [64] | ||
| Exon 31, 2092G>A | 107/209 | Brazilian | No | 2011 | [65] | |
| 36/36 | Korean | Positive | 2008 | [66] | ||
| rs76425569 | 48/48 | Chinese | Positive | 2019 | [56] | |
| rs388222 | 48/48 | Chinese | Positive | 2019 | [56] | |
| rs2281968 | 48/48 | Chinese | Positive | 2019 | [56] | |
| LAMC1 | rs10911193(C/T) | 239/197 | Non-Hispanic white women | No | 2012 | [67] |
| 165/246 | Caucasian/African–American | No | 2010 | [68] | ||
| family pedigree | American | Positive | 2007 | [51] | ||
| rs20563 (A/G) | 165/246 239/197 | Caucasian/African–American non-Hispanic white women | No | 2010 | [68] | |
| rs20558 (T/C) | 165/246 | Caucasian/African–American | No | 2010 | [68] | |
| LOXL1 | rs2165241 | 426/410 | Brazilian | No | 2022 | [69] |
| 52/28 | Japanese | No | 2021 | [54] | ||
| LOXL4 | rs2862296 | 52/28 | Japanese | Positive | 2021 | [54] |
| 285/247 | Brazilian | No | 2018 | [70] | ||
| FBLN5 | rs2018736 | 210/292 | Russian | Positive | 2014 | [71] |
| rs12589592 | 210/292 | Russian | Positive | 2014 | [71] | |
| rs12586948 | 112/180 | Brazilian | No | 2020 | [72] | |
| MMP1 | −1607/−1608,1G/2G | 133/132 | Poland | No | 2013 | [73] |
| 137/96 | Italian | Positive | 2012 | [58] | ||
| MMP3 | −1612/−1617,5A/6A | 133/132 | Poland | No | 2013 | [73] |
| −1171 5A/6A | 137/96 | Italian | No | 2012 | [58] | |
| −1171 5A/6A rs3025058 | 112/180 | Brazilian | No | 2019 | [74] | |
| MMP9 | −1562 C/T | 137/96 | Italian | No | 2012 | [58] |
| rs17576 | 239/197 | non-Hispanic white women | No | 2012 | [75] | |
| 92/152 | Taiwanese | Positive | 2010 | [76] | ||
| MMP10 | rs17435959 | 91/172 | Chinese | Positive | 2015 | [77] |
| ESR1 | rs2234693 | 88/108 | Chinese | Positive | 2019 | [78] |
| rs17847075 | 88/108 | Chinese | Positive | 2019 | [78] | |
| rs2228480 G/A | 26/26 | Israel | Positive | 2017 | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, Z.; Li, Y.; Gao, X.; Wang, T.; Huang, Y.; Wu, M. Genetics of Female Pelvic Organ Prolapse: Up to Date. Biomolecules 2024, 14, 1097. https://doi.org/10.3390/biom14091097
Li Y, Li Z, Li Y, Gao X, Wang T, Huang Y, Wu M. Genetics of Female Pelvic Organ Prolapse: Up to Date. Biomolecules. 2024; 14(9):1097. https://doi.org/10.3390/biom14091097
Chicago/Turabian StyleLi, Yuting, Zihan Li, Yinuo Li, Xiaofan Gao, Tian Wang, Yibao Huang, and Mingfu Wu. 2024. "Genetics of Female Pelvic Organ Prolapse: Up to Date" Biomolecules 14, no. 9: 1097. https://doi.org/10.3390/biom14091097





