Inhibition of MAT2A Impairs Skeletal Muscle Repair Function
Abstract
:1. Introduction
2. Material and Methods
2.1. Cell Culture and Treatment
2.2. Mouse Models
2.3. siRNA Transfection
2.4. Total RNA Extraction
2.5. Real-Time Quantitative PCR (qPCR)
2.6. RNA-Sequencing Analysis
2.7. Western Blot Analysis
2.8. Histochemistry and Immunofluorescence
2.9. Measurement of SAM Production
2.10. Apoptosis Analysis
2.11. MTT Assay
2.12. Statistical Analysis
3. Results
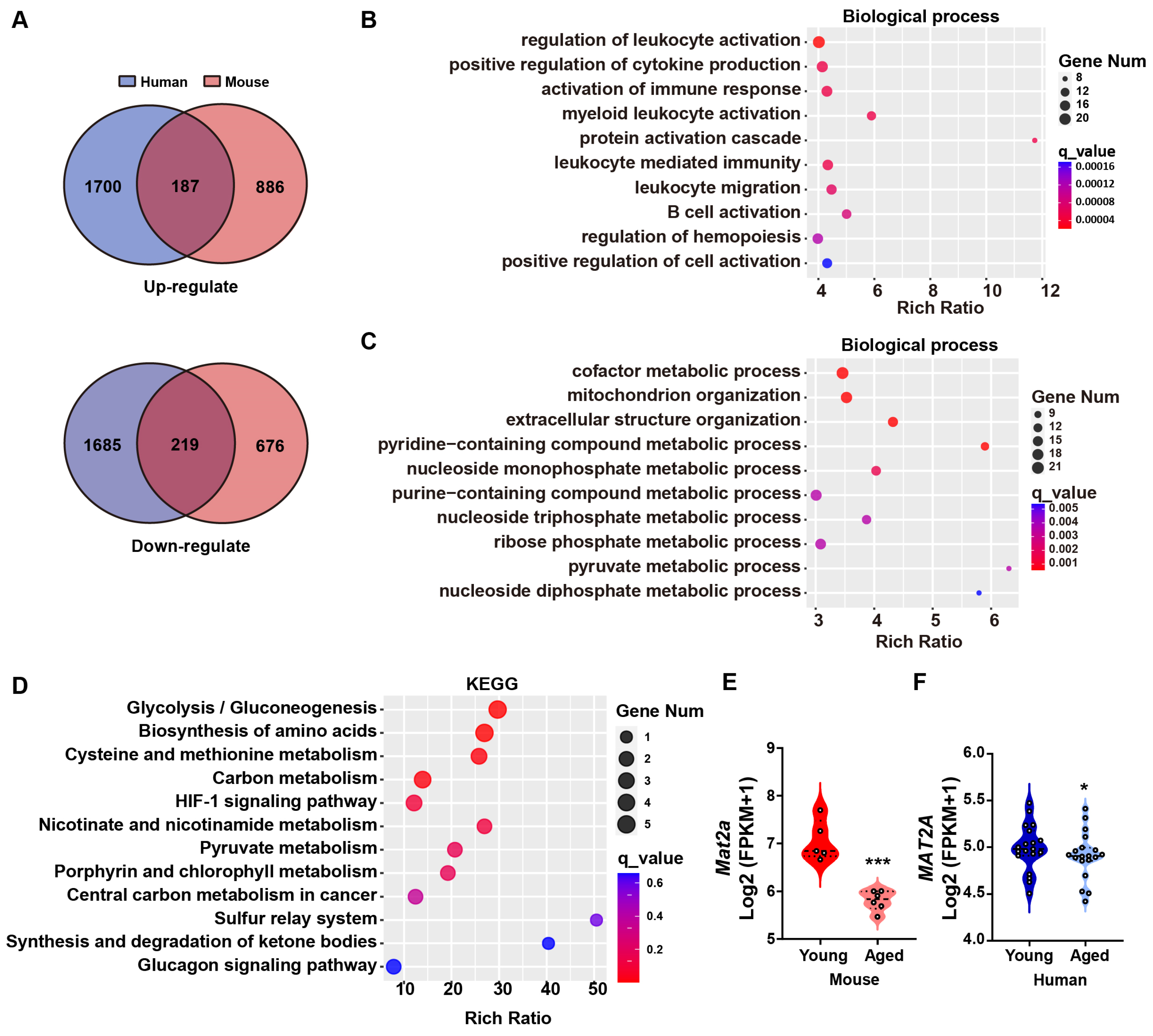
3.1. MAT2A Is Downregulated in the Muscle Tissue of Elderly Humans and Mice
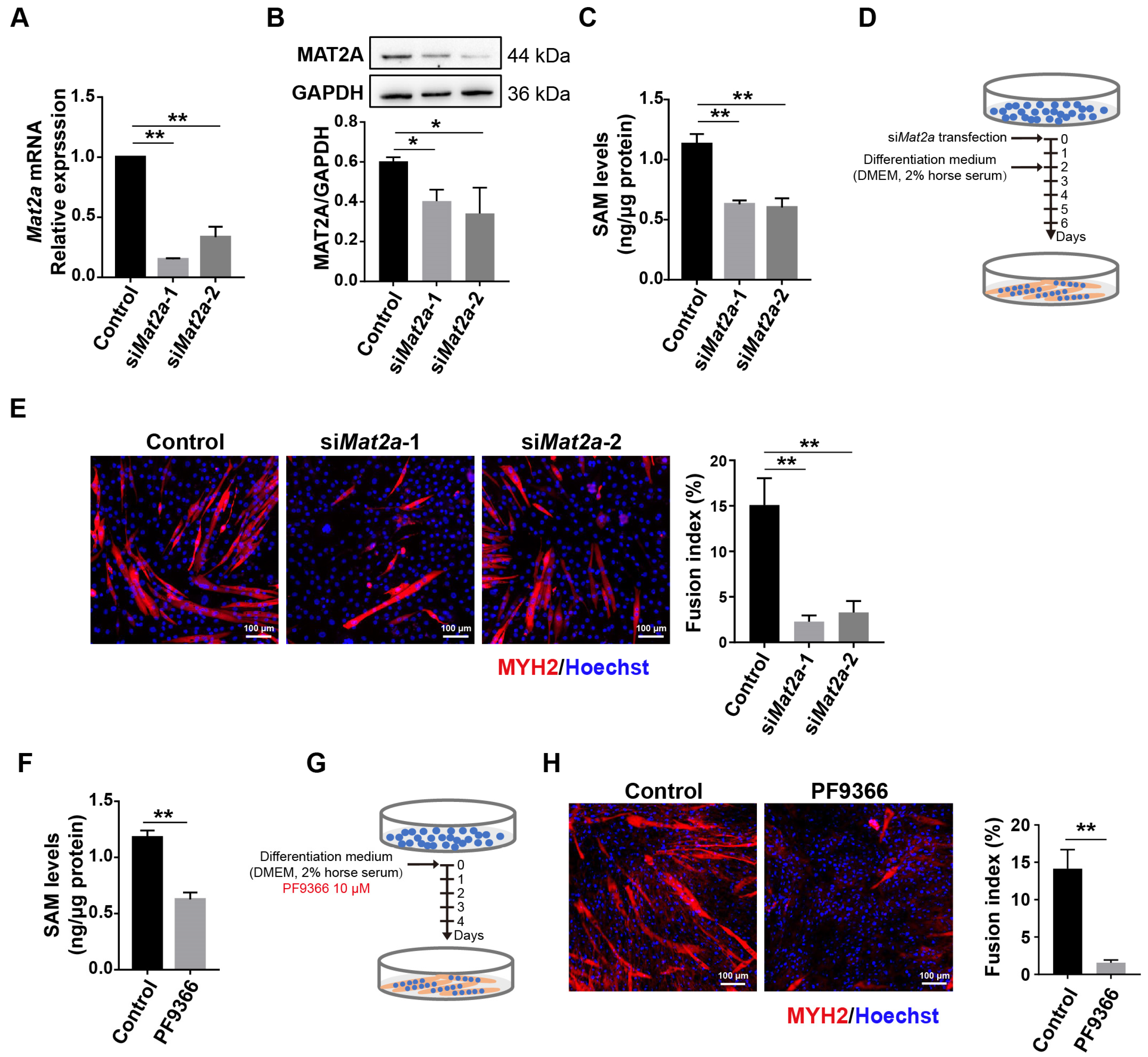
3.2. Knockdown of Mat2a Inhibited the Differentiation of Myoblasts
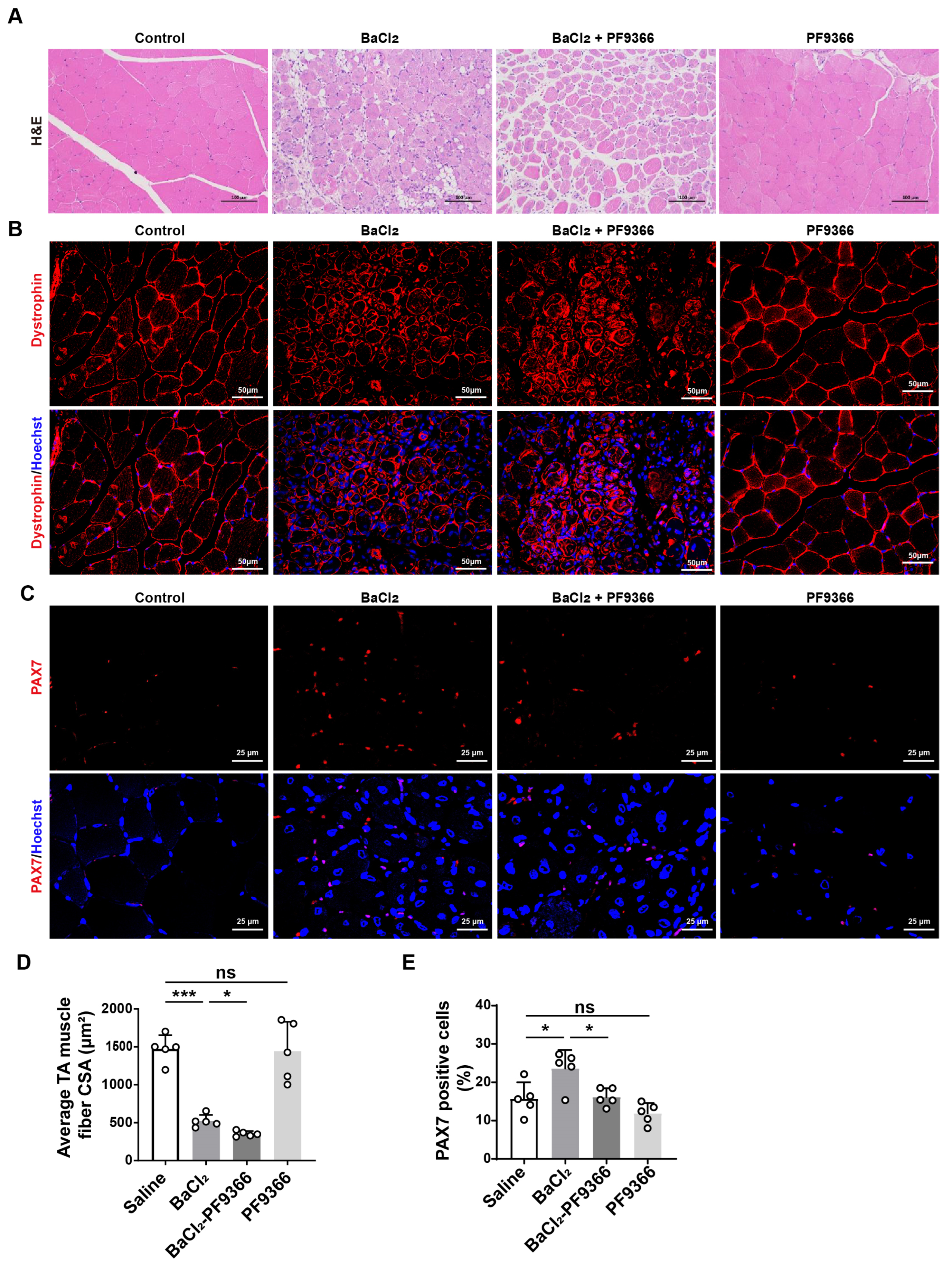
3.3. PF9366 Inhibited Skeletal Muscle Repair after BaCl2-Induced Injury in Mice
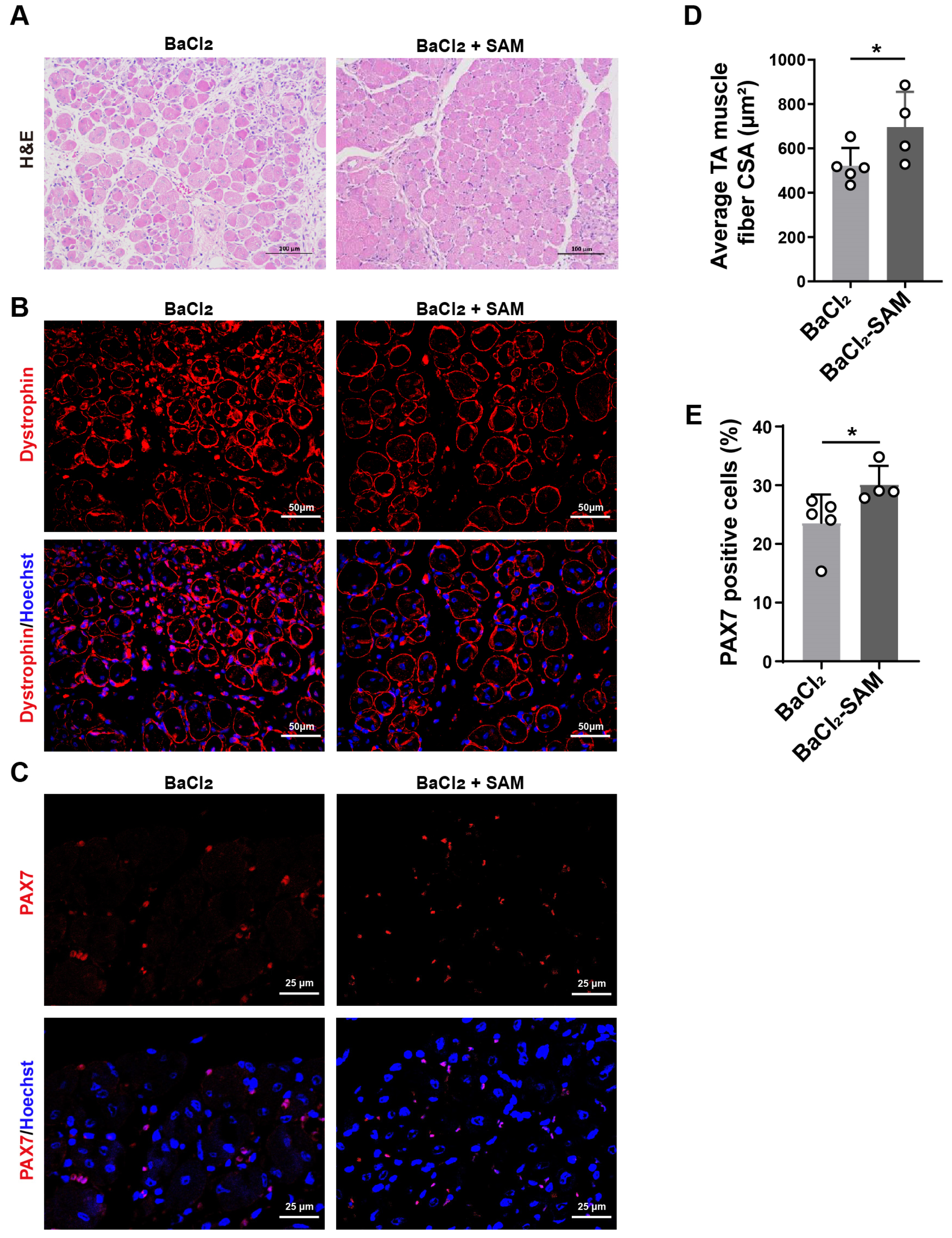
3.4. SAM Supplementation Promotes Skeletal Muscle Repair after BaCl2-Induced Injury in Mice
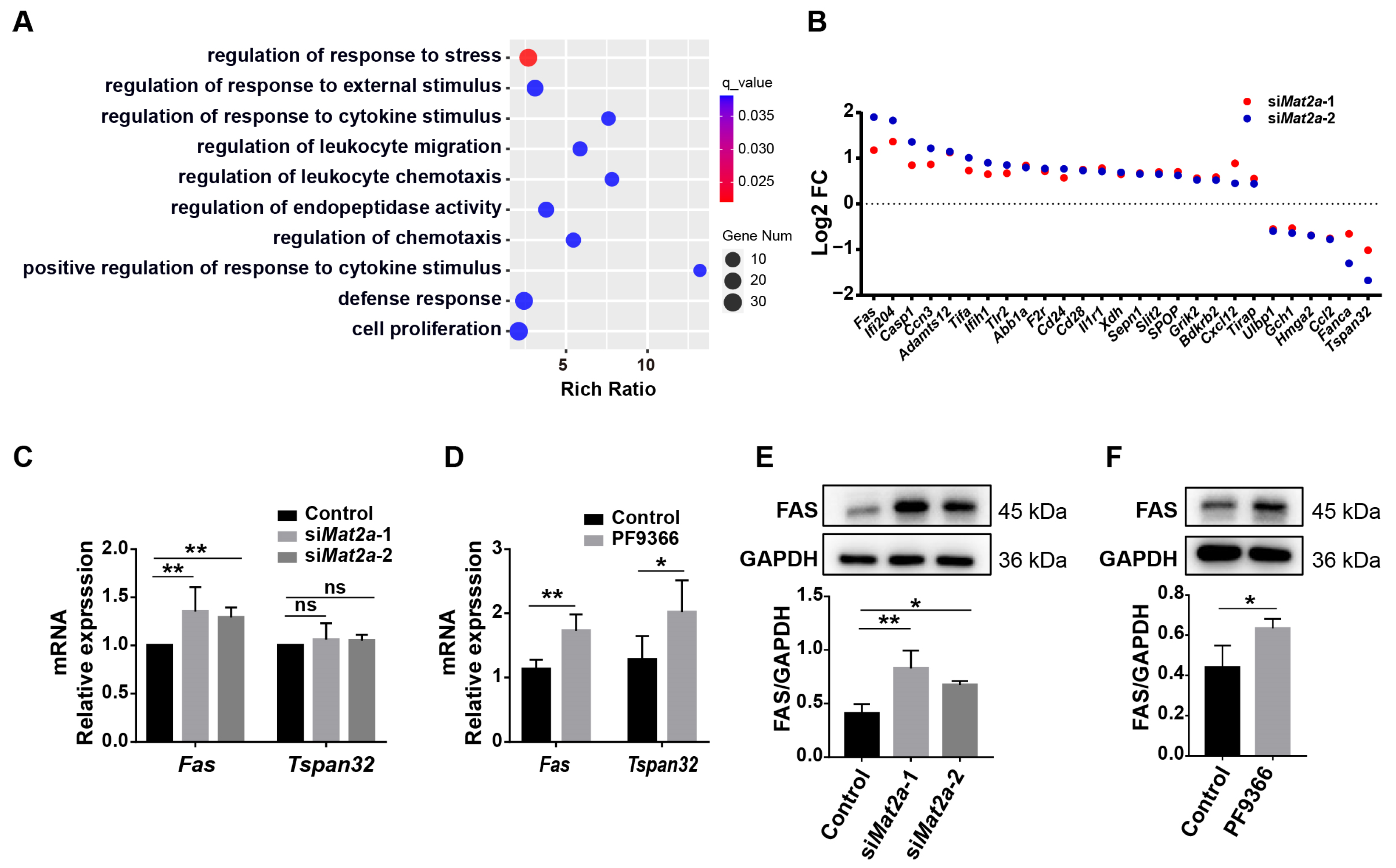
3.5. Inhibition of MAT2A Activity Promotes Fas Expression
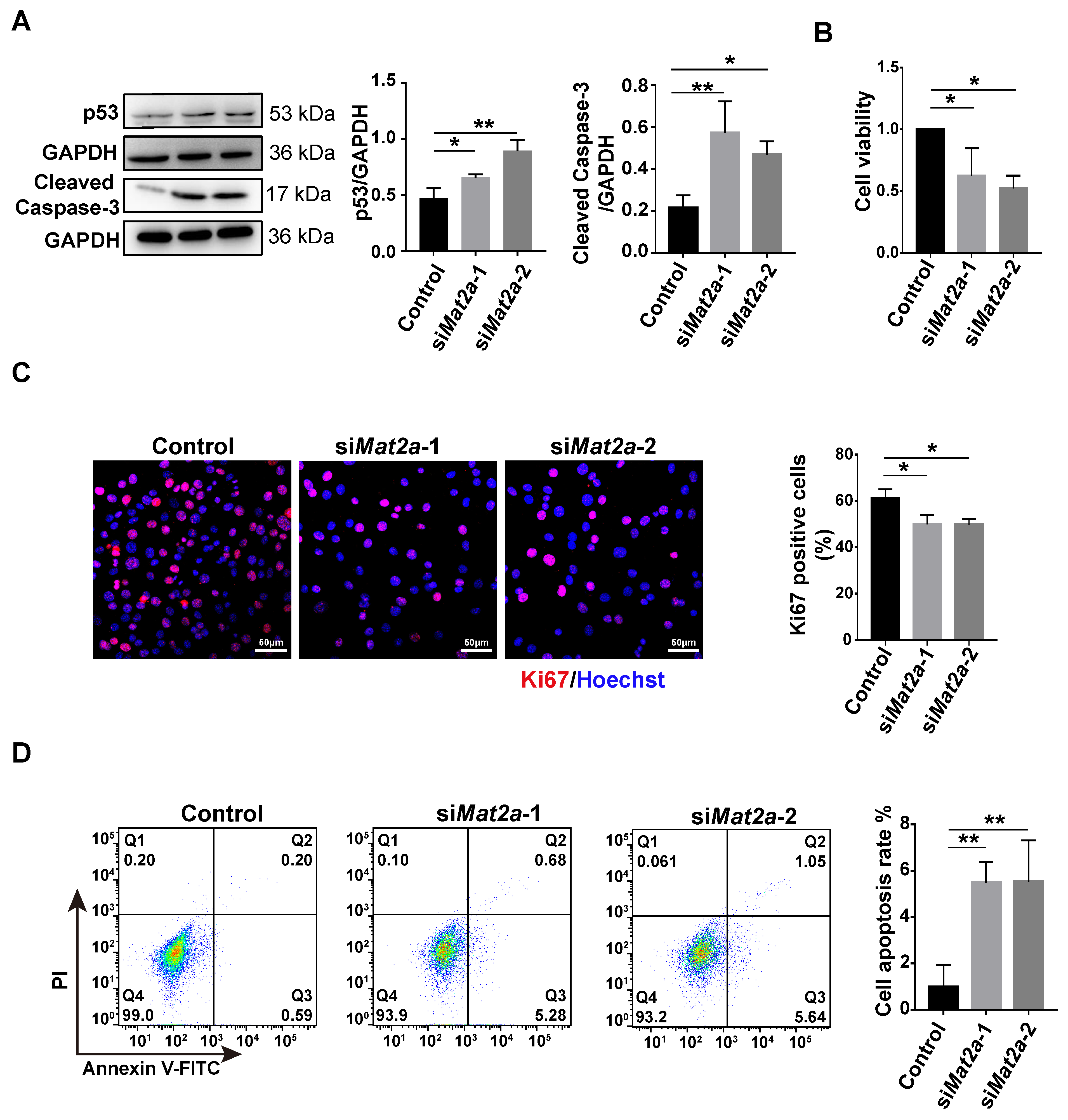
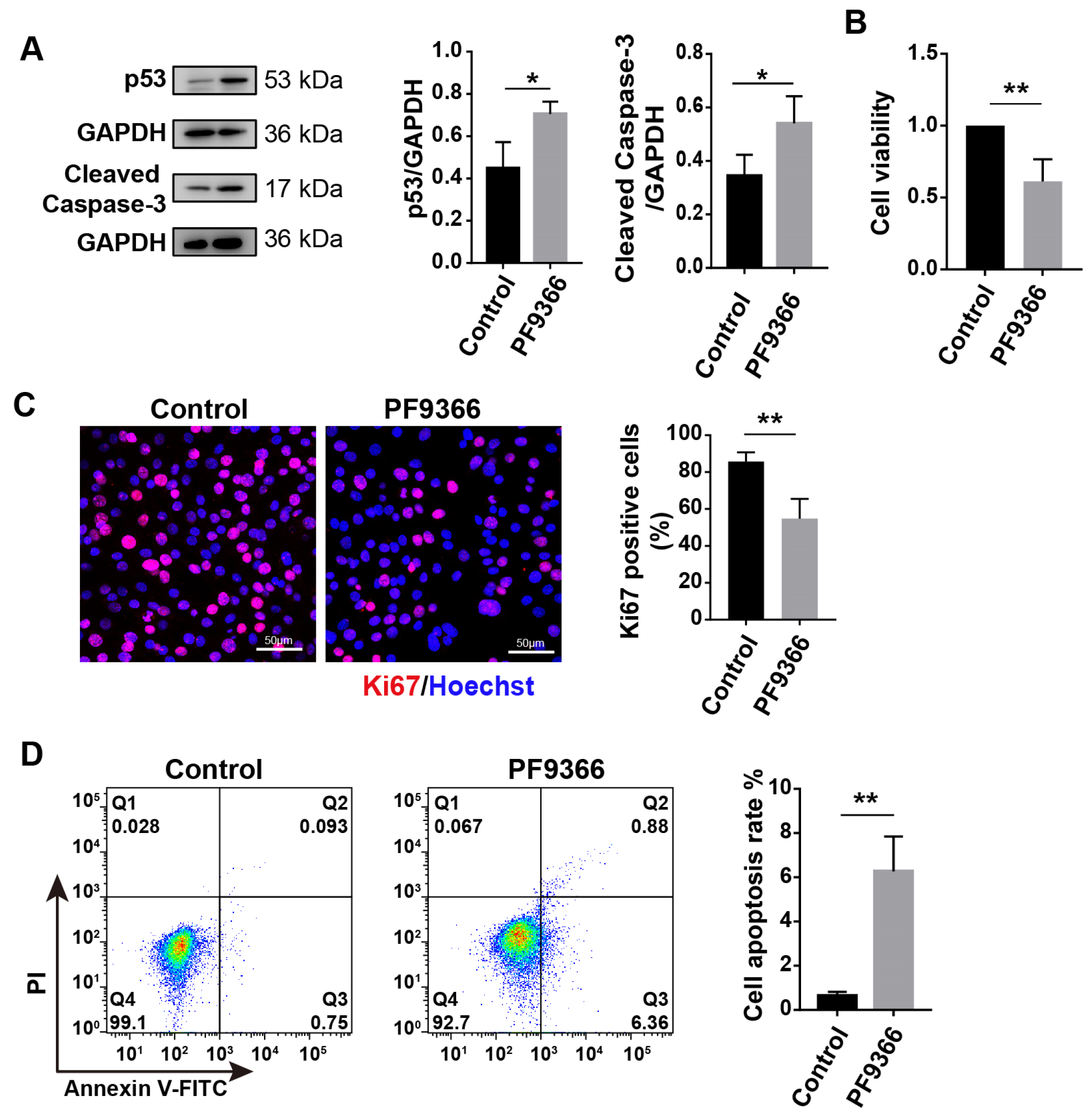
3.6. Inhibition of MAT2A Expression or Activity Suppressed Proliferation and Promoted Apoptosis of Myoblasts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trombetti, A.; Reid, K.F.; Hars, M.; Herrmann, F.R.; Pasha, E.; Phillips, E.M.; Fielding, R.A. Age-associated declines in muscle mass, strength, power, and physical performance: Impact on fear of falling and quality of life. Osteoporos. Int. 2016, 27, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Singer, B.D.; Vaughan, D.E. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 2017, 16, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.C.; Sinha, M.; Cerletti, M.; Dall’Osso, C.; Wagers, A.J. Skeletal muscle stem cells: Effects of aging and metabolism on muscle regenerative function. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.I.; Brett, J.O.; Both, P.; Benjamin, J.S.; Ishak, H.L.; Kang, J.; Kim, S.; Chung, M.; Arjona, M.; Nutter, C.W.; et al. Multiomics reveals glutathione metabolism as a driver of bimodality during stem cell aging. Cell Metab. 2023, 35, 472–486.e6. [Google Scholar] [CrossRef]
- Tezze, C.; Sandri, M.; Tessari, P. Anabolic Resistance in the Pathogenesis of Sarcopenia in the Elderly: Role of Nutrition and Exercise in Young and Old People. Nutrients 2023, 15, 4073. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, B.H.; Wang, X.; Huang, C.P.; Xu, S.M.; Wang, J.L.; Huang, T.E.; Xiao, W.L.; Tian, X.L.; Lan, X.Q.; et al. Handelin alleviates cachexia- and aging-induced skeletal muscle atrophy by improving protein homeostasis and inhibiting inflammation. J. Cachex- Sarcopenia Muscle 2023, 15, 173–188. [Google Scholar] [CrossRef]
- Joanisse, S.; Nederveen, J.P.; Snijders, T.; McKay, B.R.; Parise, G. Skeletal Muscle Regeneration, Repair and Remodelling in Aging: The Importance of Muscle Stem Cells and Vascularization. Gerontology 2017, 63, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Datzkiw, D.; Rudnicki, M.A. Satellite cells in ageing: Use it or lose it. Open Biol. 2020, 10, 200048. [Google Scholar] [CrossRef]
- Moiseeva, V.; Cisneros, A.; Sica, V.; Deryagin, O.; Lai, Y.; Jung, S.; Andres, E.; An, J.; Segales, J.; Ortet, L.; et al. Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature 2023, 613, 169–178. [Google Scholar] [CrossRef]
- Alway, S.E.; Paez, H.G.; Pitzer, C.R.; Ferrandi, P.J.; Khan, M.M.; Mohamed, J.S.; Carson, J.A.; Deschenes, M.R. Mitochondria transplant therapy improves regeneration and restoration of injured skeletal muscle. J. Cachex- Sarcopenia Muscle 2023, 14, 493–507. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Liu, Y.; Wang, X. Autophagy in muscle regeneration: Potential therapies for myopathies. J. Cachex- Sarcopenia Muscle 2022, 13, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Le Moal, E.; Pialoux, V.; Juban, G.; Groussard, C.; Zouhal, H.; Chazaud, B.; Mounier, R. Redox Control of Skeletal Muscle Regeneration. Antioxidants Redox Signal. 2017, 27, 276–310. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Argmann, C.; Houten, S.M.; Canto, C.; Jeninga, E.H.; Andreux, P.A.; Thomas, C.; Doenlen, R.; Schoonjans, K.; Auwerx, J. The metabolic footprint of aging in mice. Sci. Rep. 2011, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, J.; Moller, G.; Artati, A.; Huber, S.; Zeigerer, A.; Blaauw, B.; Adamski, J.; Dyar, K.A. Common Muscle Metabolic Signatures Highlight Arginine and Lysine Metabolism as Potential Therapeutic Targets to Combat Unhealthy Aging. Int. J. Mol. Sci. 2021, 22, 7958. [Google Scholar] [CrossRef] [PubMed]
- Fazelzadeh, P.; Hangelbroek, R.W.; Tieland, M.; de Groot, L.C.; Verdijk, L.B.; van Loon, L.J.; Smilde, A.K.; Alves, R.D.; Vervoort, J.; Muller, M.; et al. The Muscle Metabolome Differs between Healthy and Frail Older Adults. J. Proteome Res. 2016, 15, 499–509. [Google Scholar] [CrossRef]
- Flakoll, P.; Sharp, R.; Baier, S.; Levenhagen, D.; Carr, C.; Nissen, S. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 2004, 20, 445–451. [Google Scholar] [CrossRef]
- Maykish, A.; Sikalidis, A.K. Utilization of Hydroxyl-Methyl Butyrate, Leucine, Glutamine and Arginine Supplementation in Nutritional Management of Sarcopenia-Implications and Clinical Considerations for Type 2 Diabetes Mellitus Risk Modulation. J. Pers. Med. 2020, 10, 19. [Google Scholar] [CrossRef]
- Borsheim, E.; Bui, Q.U.; Tissier, S.; Kobayashi, H.; Ferrando, A.A.; Wolfe, R.R. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin. Nutr. 2008, 27, 189–195. [Google Scholar] [CrossRef]
- Wone, B.W.M.; Kinchen, J.M.; Kaup, E.R.; Wone, B. A procession of metabolic alterations accompanying muscle senescence in Manduca sexta. Sci. Rep. 2018, 8, 1006. [Google Scholar] [CrossRef]
- Kang, J.; Benjamin, D.I.; Kim, S.; Salvi, J.S.; Dhaliwal, G.; Lam, R.; Goshayeshi, A.; Brett, J.O.; Liu, L.; Rando, T.A. Depletion of SAM leading to loss of heterochromatin drives muscle stem cell ageing. Nat. Metab. 2024, 6, 153–168. [Google Scholar] [CrossRef]
- Lu, S.C.; Mato, J.M. S-adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 2012, 92, 1515–1542. [Google Scholar] [CrossRef] [PubMed]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.M.; Simile, M.M.; Calvisi, D.F.; Feo, C.F.; Feo, F. S-Adenosylmethionine: From the Discovery of Its Inhibition of Tumorigenesis to Its Use as a Therapeutic Agent. Cells 2022, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.M.; Feo, C.F.; Calvisi, D.F.; Feo, F. Deregulation of methionine metabolism as determinant of progression and prognosis of hepatocellular carcinoma. Transl. Gastroenterol. Hepatol. 2018, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, K.; Zhu, Y.; He, Y.; Wu, J.; Liu, Z. Silencing MAT2A gene by RNA interference inhibited cell growth and induced apoptosis in human hepatoma cells. Hepatol. Res. 2007, 37, 376–388. [Google Scholar] [CrossRef]
- Kalev, P.; Hyer, M.L.; Gross, S.; Konteatis, Z.; Chen, C.C.; Fletcher, M.; Lein, M.; Aguado-Fraile, E.; Frank, V.; Barnett, A.; et al. MAT2A Inhibition Blocks the Growth of MTAP-Deleted Cancer Cells by Reducing PRMT5-Dependent mRNA Splicing and Inducing DNA Damage. Cancer Cell 2021, 39, 209–224.e11. [Google Scholar] [CrossRef] [PubMed]
- Klimentova, E.A.; Suchkov, I.A.; Egorov, A.A.; Kalinin, R.E. Apoptosis and Cell Proliferation Markers in Inflammatory-Fibroproliferative Diseases of the Vessel Wall (Review). Sovrem. Tekhnologii Med. 2021, 12, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Tian, X.; Shi, Q. Fas, Caspase-8, and Caspase-9 pathway-mediated bile acid-induced fetal cardiomyocyte apoptosis in intrahepatic cholestasis pregnant rat models. J. Obstet. Gynaecol. Res. 2021, 47, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Munsch, D.; Watanabe-Fukunaga, R.; Bourdon, J.C.; Nagata, S.; May, E.; Yonish-Rouach, E.; Reisdorf, P. Human and mouse Fas (APO-1/CD95) death receptor genes each contain a p53-responsive element that is activated by p53 mutants unable to induce apoptosis. J. Biol. Chem. 2000, 275, 3867–3872. [Google Scholar] [CrossRef]
- Hernandez Borrero, L.J.; El-Deiry, W.S. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Kaiser, S.E.; Bolanos, B.; Nowlin, D.; Grantner, R.; Karlicek-Bryant, S.; Feng, J.L.; Jenkinson, S.; Freeman-Cook, K.; Dann, S.G.; et al. Targeting S-adenosylmethionine biosynthesis with a novel allosteric inhibitor of Mat2A. Nat. Chem. Biol. 2017, 13, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S.; Relaix, F.; Nagata, Y.; Ruiz, A.P.; Collins, C.A.; Partridge, T.A.; Beauchamp, J.R. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 2006, 119 Pt 9, 1824–1832. [Google Scholar] [CrossRef]
- Yoo, Y.M.; Jung, E.M.; Jeung, E.B. Rapamycin-induced autophagy decreases Myf5 and MyoD proteins in C2C12 myoblast cells. Toxicol. Vitr. 2019, 58, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Akima, H.; Maeda, K.; Shima, N. Neuromuscular activation of the quadriceps femoris, including the vastus intermedius, during isokinetic knee extensions. Sci. Rep. 2023, 13, 7674. [Google Scholar] [CrossRef] [PubMed]
- Kedlian, V.R.; Wang, Y.; Liu, T.; Chen, X.; Bolt, L.; Tudor, C.; Shen, Z.; Fasouli, E.S.; Prigmore, E.; Kleshchevnikov, V.; et al. Human skeletal muscle aging atlas. Nat. Aging 2024, 4, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.D.; Kacar, B. Cofactors are Remnants of Life’s Origin and Early Evolution. J. Mol. Evol. 2021, 89, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hindi, L.; McMillan, J.D.; Afroze, D.; Hindi, S.M.; Kumar, A. Isolation, Culturing, and Differentiation of Primary Myoblasts from Skeletal Muscle of Adult Mice. Bio-Protocol 2017, 7, e2248. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; Garcia-Prat, L.; Munoz-Canoves, P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat. Rev. Mol. Cell Biol. 2022, 23, 204–226. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, D.; Li, Y.; Dai, T.; Liu, F.; Yan, C. TGM2 positively regulates myoblast differentiation via enhancing the mTOR signaling. Biochim. Biophys. Acta (BBA)- Mol. Cell Res. 2022, 1869, 119173. [Google Scholar] [CrossRef]
- Morton, A.B.; Norton, C.E.; Jacobsen, N.L.; Fernando, C.A.; Cornelison, D.D.W.; Segal, S.S. Barium chloride injures myofibers through calcium-induced proteolysis with fragmentation of motor nerves and microvessels. Skelet. Muscle 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Jo, J.; Lee, M.; Park, J.; Yao, T.P.; Park, Y. Cathelicidin-related antimicrobial peptide mediates skeletal muscle degeneration caused by injury and Duchenne muscular dystrophy in mice. J. Cachex- Sarcopenia Muscle 2022, 13, 3091–3105. [Google Scholar] [CrossRef] [PubMed]
- Relaix, F.; Bencze, M.; Borok, M.J.; Der Vartanian, A.; Gattazzo, F.; Mademtzoglou, D.; Perez-Diaz, S.; Prola, A.; Reyes-Fernandez, P.C.; Rotini, A.; et al. Perspectives on skeletal muscle stem cells. Nat. Commun. 2021, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Lawson, J.A.; Mathew, S.J.; Hutcheson, D.A.; Kardon, G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 2011, 138, 3625–3637. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef] [PubMed]
- Deer, R.R.; Volpi, E. Protein intake and muscle function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Wilkinson, D.J.; Phillips, B.E.; Lund, J.N.; Smith, K.; Atherton, P.J. Human Skeletal Muscle Protein Metabolism Responses to Amino Acid Nutrition. Adv. Nutr. 2016, 7, 828S–838S. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Chen, H.; Guo, C.; Yuan, Z.; Zhou, X.; Zhang, Y.; Zhang, X.; Mo, D.; Chen, Y. Palmdelphin promotes myoblast differentiation and muscle regeneration. Sci. Rep. 2017, 7, 41608. [Google Scholar] [CrossRef] [PubMed]
- Laumonier, T.; Menetrey, J. Muscle injuries and strategies for improving their repair. J. Exp. Orthop. 2016, 3, 15. [Google Scholar] [CrossRef]
- Blanc, R.S.; Kallenbach, J.G.; Bachman, J.F.; Mitchell, A.; Paris, N.D.; Chakkalakal, J.V. Inhibition of inflammatory CCR2 signaling promotes aged muscle regeneration and strength recovery after injury. Nat. Commun. 2020, 11, 4167. [Google Scholar] [CrossRef]
- Southard, S.; Kim, J.R.; Low, S.; Tsika, R.W.; Lepper, C. Myofiber-specific TEAD1 overexpression drives satellite cell hyperplasia and counters pathological effects of dystrophin deficiency. Elife 2016, 5, e15461. [Google Scholar] [CrossRef]
- Jejurikar, S.S.; Henkelman, E.A.; Cederna, P.S.; Marcelo, C.L.; Urbanchek, M.G.; Kuzon, W.M., Jr. Aging increases the susceptibility of skeletal muscle derived satellite cells to apoptosis. Exp. Gerontol. 2006, 41, 828–836. [Google Scholar] [CrossRef]
- Gehring, S.; Rottmann, S.; Menkel, A.R.; Mertsching, J.; Krippner-Heidenreich, A.; Luscher, B. Inhibition of proliferation and apoptosis by the transcriptional repressor Mad1. Repression of Fas-induced caspase-8 activation. J. Biol. Chem. 2000, 275, 10413–10420. [Google Scholar] [CrossRef]
- Colleluori, G.; Aguirre, L.; Phadnis, U.; Fowler, K.; Armamento-Villareal, R.; Sun, Z.; Brunetti, L.; Hyoung Park, J.; Kaipparettu, B.A.; Putluri, N.; et al. Aerobic Plus Resistance Exercise in Obese Older Adults Improves Muscle Protein Synthesis and Preserves Myocellular Quality Despite Weight Loss. Cell Metab. 2019, 30, 261–273.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, X.; Wu, Y.; Tang, J.; Yu, Q.; Lv, X.; Zha, Z.; Hu, B.; Li, X.; Chen, J.; et al. The SESAME complex regulates cell senescence through the generation of acetyl-CoA. Nat. Metab. 2021, 3, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Rajabian, N.; Ikhapoh, I.; Shahini, S.; Choudhury, D.; Thiyagarajan, R.; Shahini, A.; Kulczyk, J.; Breed, K.; Saha, S.; Mohamed, M.A.; et al. Methionine adenosyltransferase2A inhibition restores metabolism to improve regenerative capacity and strength of aged skeletal muscle. Nat. Commun. 2023, 14, 886. [Google Scholar] [CrossRef] [PubMed]
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Moran Losada, P.; Berdnik, D.; Keller, A.; Verghese, J.; et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 2019, 25, 1843–1850. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, W.; Huang, T.-E.; Zhou, J.; Wang, B.; Wang, X.; Zeng, W.; Wang, Q.; Lan, X.; Xiang, Y. Inhibition of MAT2A Impairs Skeletal Muscle Repair Function. Biomolecules 2024, 14, 1098. https://doi.org/10.3390/biom14091098
Xiao W, Huang T-E, Zhou J, Wang B, Wang X, Zeng W, Wang Q, Lan X, Xiang Y. Inhibition of MAT2A Impairs Skeletal Muscle Repair Function. Biomolecules. 2024; 14(9):1098. https://doi.org/10.3390/biom14091098
Chicago/Turabian StyleXiao, Wanli, Tian-E Huang, Jing Zhou, Benhui Wang, Xiang Wang, Weirong Zeng, Qiquan Wang, Xinqiang Lan, and Yang Xiang. 2024. "Inhibition of MAT2A Impairs Skeletal Muscle Repair Function" Biomolecules 14, no. 9: 1098. https://doi.org/10.3390/biom14091098





