A Nanobody of PEDV S1 Protein: Screening and Expression in Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Phage Library Rescue
2.2.2. Screening of Nanobody Library
2.2.3. Recombinant Nanobody Crude Extraction and Acquisition of Nucleotide Sequences
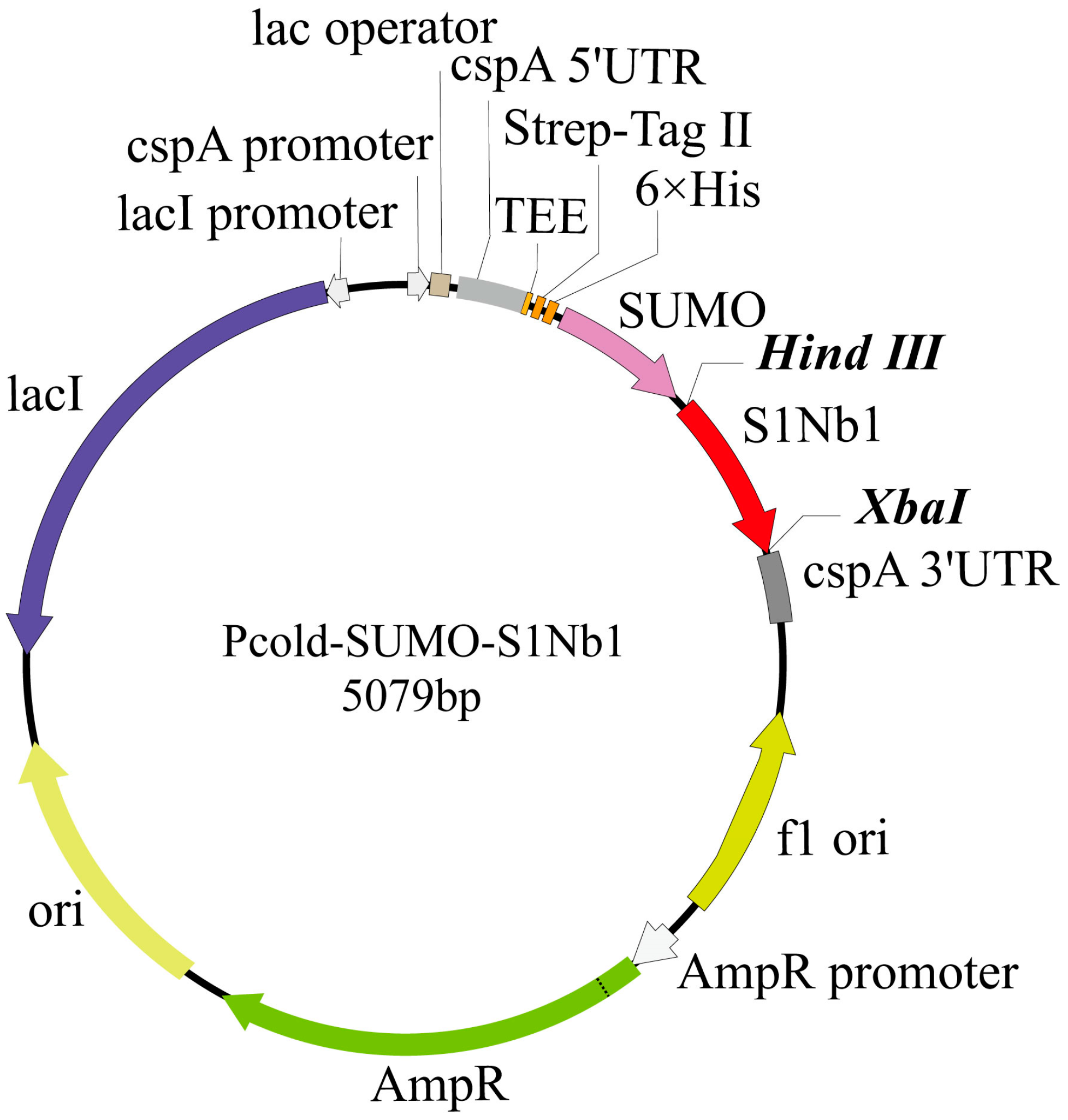
2.2.4. Construction of Nanobody Recombinant Vector
2.2.5. Expression and Purification of Recombinant Nanobodies
2.2.6. Identification of Affinity of Recombinant Nanobodies
3. Results
3.1. Phage Library Rescue
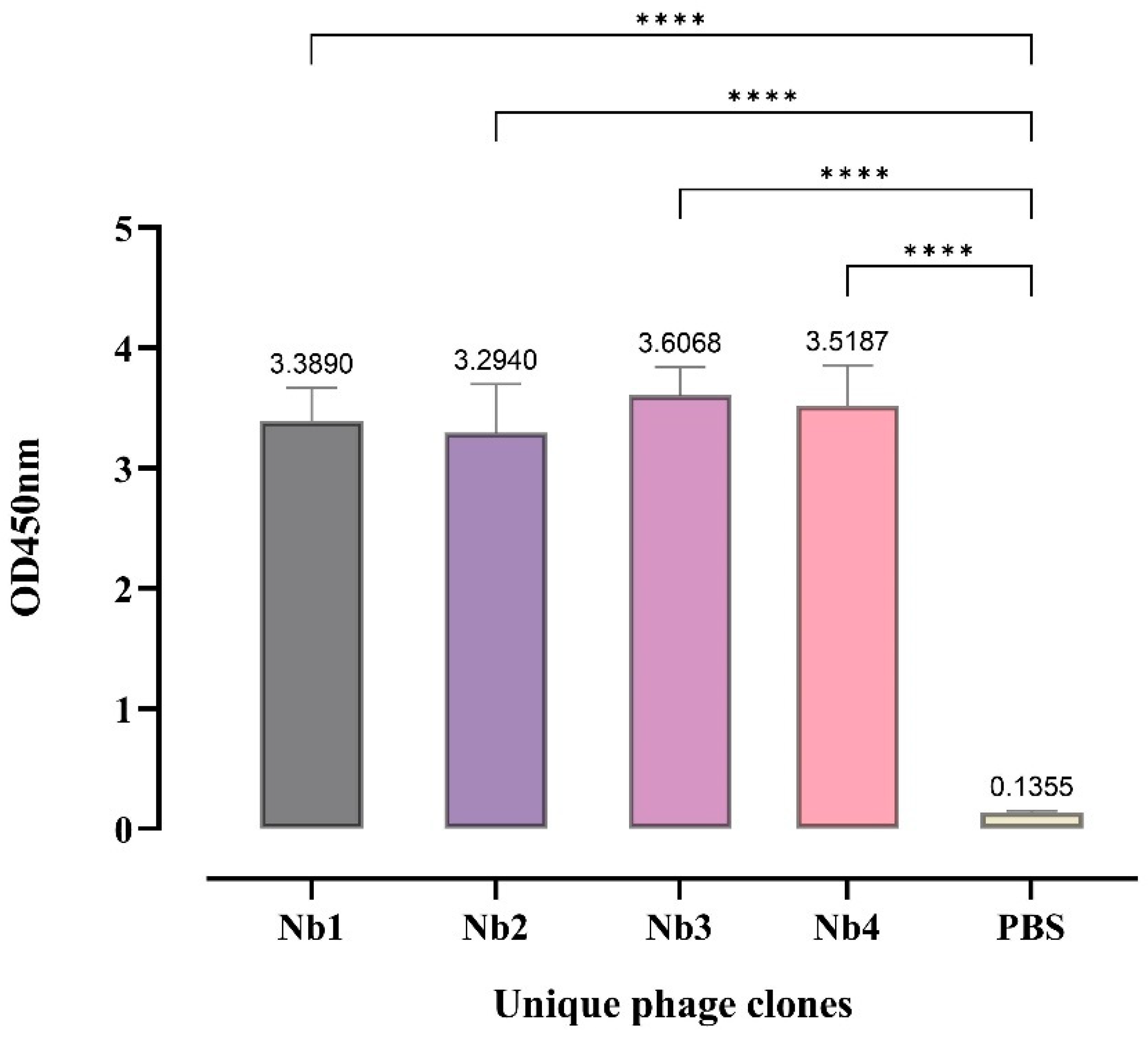
3.2. Screening of Nanobody Library
3.3. Acquisition of Nanobody Nucleotide Sequences
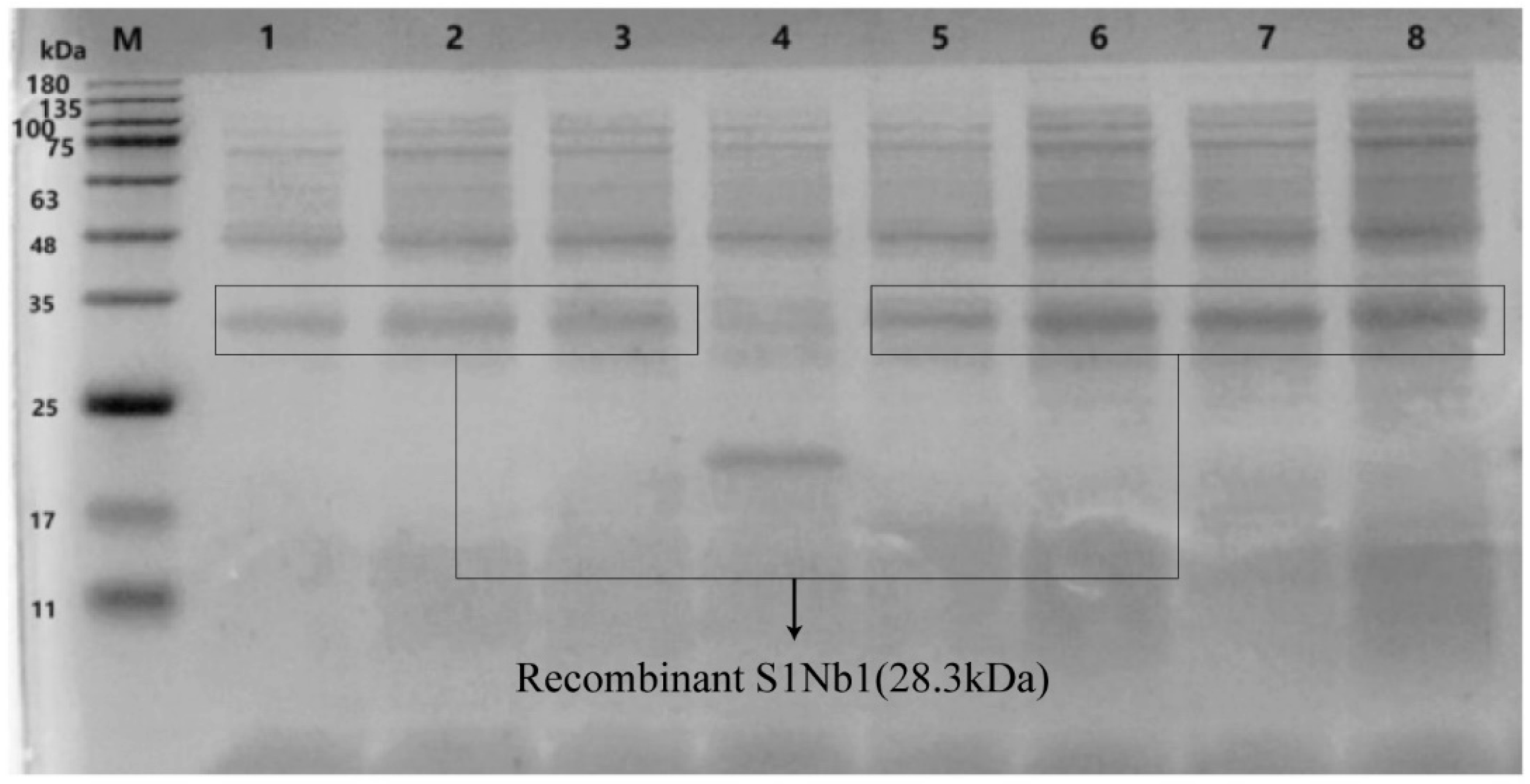
3.4. Expression and Purification of Recombinant Nanobodies
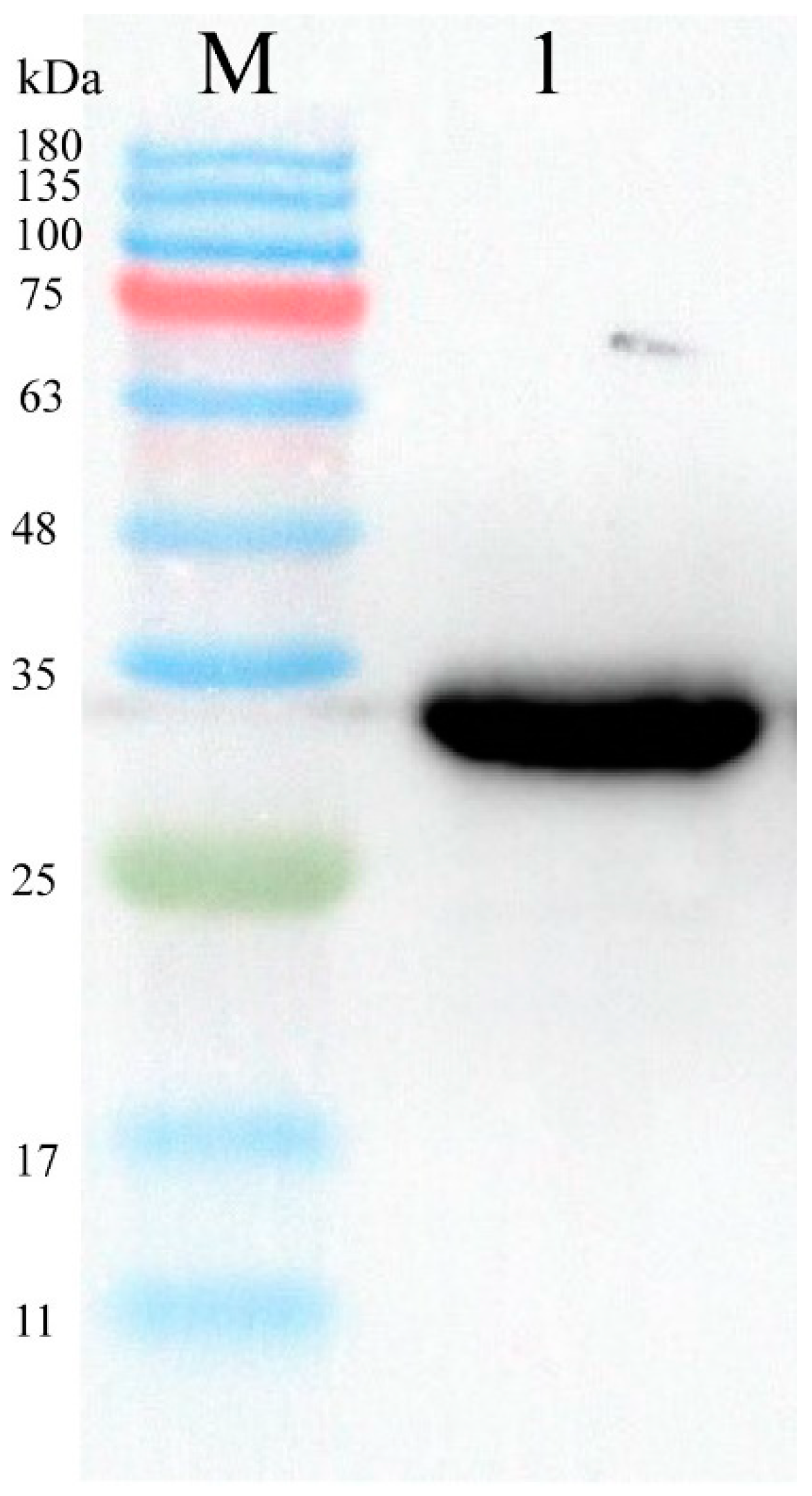
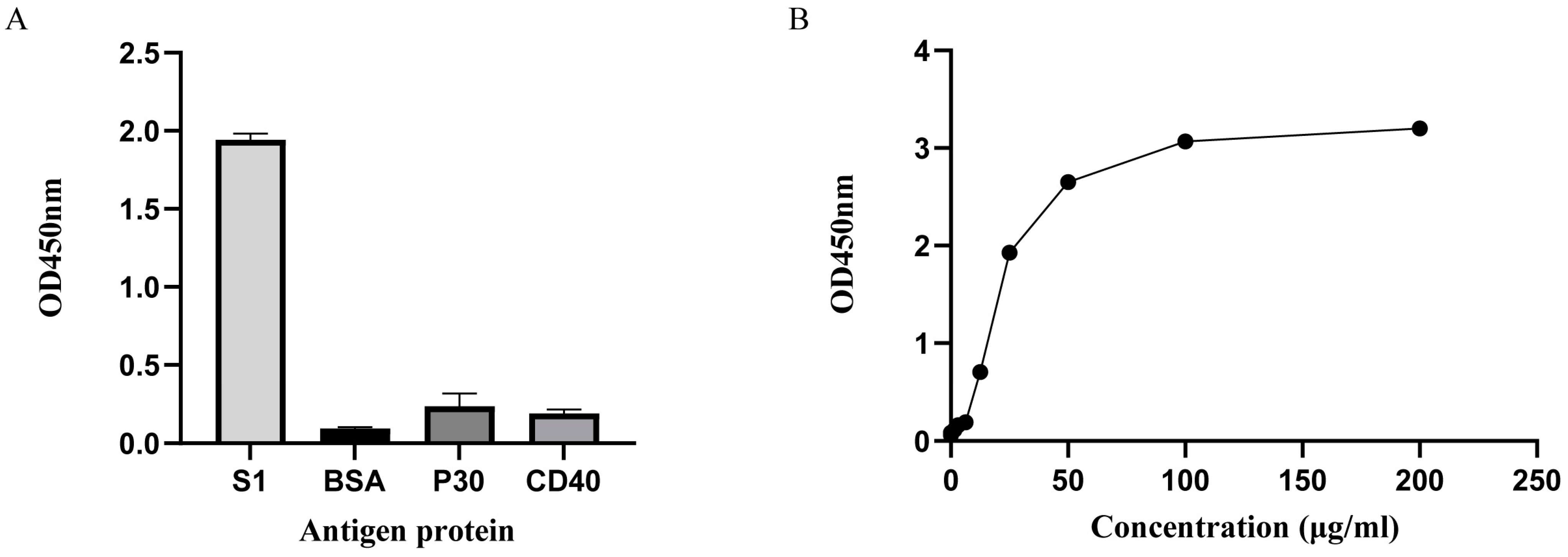
3.5. Identification of Nanobody
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pensaert, M.B.; de Bouck, P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Pijpers, A.; van Nieuwstadt, A.P.; Terpstra, C.; Verheijden, J.H. Porcine epidemic diarrhoea virus as a cause of persistent diarrhoea in a herd of breeding and finishing pigs. Vet. Rec. 1993, 132, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef]
- Lin, C.M.; Saif, L.J.; Marthaler, D.; Wang, Q. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 2016, 226, 20–39. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Huang, J.; Yao, Y.; Zhao, W.; Zhang, Y.; Qing, J.; Ren, J.; Yan, Z.; Wang, Z.; et al. Isolation and oral immunogenicity assessment of porcine epidemic diarrhea virus NH-TA2020 strain: One of the predominant strains circulating in China from 2017 to 2021. Virol. Sin. 2022, 37, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, H.; Qian, Y.; Wang, Z.; Tang, Q.; Cao, W. Study on the transmissible gastroenteritis coronavirus. Agric. Sci. Technol. Shanghai 1980, 2, 42–45. [Google Scholar]
- Xuan, H.; Xing, D.-k.; Wang, D.-y.; Zhu, W.; Zhao, F.; Gong, H.; Fei, S. Study on the culture of porcine epidemic diarrhea virus adapted to fetal porcine intestine primary cell monolayer. Chin. J. Vet. Sci. 1984, 4, 202–208. [Google Scholar]
- Rosas-Murrieta, N.H.; Rodriguez-Enriquez, A.; Herrera-Camacho, I.; Millan-Perez-Pena, L.; Santos-Lopez, G.; Rivera-Benitez, J.F. Comparative Review of the State of the Art in Research on the Porcine Epidemic Diarrhea Virus and SARS-CoV-2, Scope of Knowledge between Coronaviruses. Viruses 2024, 16, 238. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, Y.; Zhao, J.; Geng, N.; Liu, K.; Zhao, Y.; Wang, F.; Liu, S.; Li, N.; Meng, F.; et al. Molecular epidemiological survey of porcine epidemic diarrhea in some areas of Shandong and genetic evolutionary analysis of S gene. Front. Vet. Sci. 2022, 9, 1015717. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Lucio de Esesarte, E.; Guo, H.; van den Elzen, P.; Aarts, E.; van den Born, E.; Rottier, P.J.M.; Bosch, B.J. Cell Attachment Domains of the Porcine Epidemic Diarrhea Virus Spike Protein Are Key Targets of Neutralizing Antibodies. J. Virol. 2017, 91, e00273-17. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Shao, C.; Ma, Y.; He, H.; Jiang, S.; Zhou, Y.; Wu, Y.; Ba, S.; Shi, L.; et al. Isolation and characterization of Chinese porcine epidemic diarrhea virus with novel mutations and deletions in the S gene. Vet. Microbiol. 2018, 221, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Bosch, B.J.; van der Zee, R.; de Haan, C.A.; Rottier, P.J. The coronavirus spike protein is a class I virus fusion protein: Structural and functional characterization of the fusion core complex. J. Virol. 2003, 77, 8801–8811. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Bae, J.L.; Kang, T.J.; Kim, J.; Chung, G.H.; Lim, C.W.; Laude, H.; Yang, M.S.; Jang, Y.S. Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Mol. Cells 2002, 14, 295–299. [Google Scholar] [CrossRef]
- Godet, M.; Grosclaude, J.; Delmas, B.; Laude, H. Major receptor-binding and neutralization determinants are located within the same domain of the transmissible gastroenteritis virus (coronavirus) spike protein. J. Virol. 1994, 68, 8008–8016. [Google Scholar] [CrossRef]
- Liu, C.; Tang, J.; Ma, Y.; Liang, X.; Yang, Y.; Peng, G.; Qi, Q.; Jiang, S.; Li, J.; Du, L.; et al. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J. Virol. 2015, 89, 6121–6125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Zhou, J.; Wang, X.; Ma, L.; Li, J.; Yang, L.; Yuan, H.; Pang, D.; Ouyang, H. Porcine Epidemic Diarrhea Virus: An Updated Overview of Virus Epidemiology, Virulence Variation Patterns and Virus-Host Interactions. Viruses 2022, 14, 2434. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Meng, Q.; Jiao, Y.; Wu, Y.; Zuo, Y.; Wang, H.; Sun, D.; Dong, S.; Zhai, H.; Tong, W.; et al. Identification of a novel B-cell epitope in the spike protein of porcine epidemic diarrhea virus. Virol. J. 2020, 17, 46. [Google Scholar] [CrossRef]
- Shi, W.; Hao, H.; Li, M.; Niu, J.; Hu, Y.; Zhao, X.; Li, Q. Expression and Purification of a PEDV-Neutralizing Antibody and Its Functional Verification. Viruses 2021, 13, 472. [Google Scholar] [CrossRef]
- Shirato, K.; Maejima, M.; Islam, M.T.; Miyazaki, A.; Kawase, M.; Matsuyama, S.; Taguchi, F. Porcine aminopeptidase N is not a cellular receptor of porcine epidemic diarrhea virus, but promotes its infectivity via aminopeptidase activity. J. Gen. Virol. 2016, 97, 2528–2539. [Google Scholar] [CrossRef]
- Li, W.; Luo, R.; He, Q.; van Kuppeveld, F.J.M.; Rottier, P.J.M.; Bosch, B.J. Aminopeptidase N is not required for porcine epidemic diarrhea virus cell entry. Virus Res. 2017, 235, 6–13. [Google Scholar] [CrossRef]
- Whitworth, K.M.; Rowland, R.R.R.; Petrovan, V.; Sheahan, M.; Cino-Ozuna, A.G.; Fang, Y.; Hesse, R.; Mileham, A.; Samuel, M.S.; Wells, K.D.; et al. Resistance to coronavirus infection in amino peptidase N-deficient pigs. Transgenic Res. 2019, 28, 21–32. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Z.; Yang, H. Aminopeptidase N Knockout Pigs Are Not Resistant to Porcine Epidemic Diarrhea Virus Infection. Virol. Sin. 2019, 34, 592–595. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Goel, S.; Cai, W. Nanobody: The “magic bullet” for molecular imaging? Theranostics 2014, 4, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. A guide to: Generation and design of nanobodies. FEBS J. 2021, 288, 2084–2102. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh-Ghassabeh, G.; Devoogdt, N.; De Pauw, P.; Vincke, C.; Muyldermans, S. Nanobodies and their potential applications. Nanomedicine 2013, 8, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Van Heeke, G.; Allosery, K.; De Brabandere, V.; De Smedt, T.; Detalle, L.; de Fougerolles, A. Nanobodies(R) as inhaled biotherapeutics for lung diseases. Pharmacol. Ther. 2017, 169, 47–56. [Google Scholar] [CrossRef]
- Mitchell, L.S.; Colwell, L.J. Comparative analysis of nanobody sequence and structure data. Proteins 2018, 86, 697–706. [Google Scholar] [CrossRef]
- Harmsen, M.M.; De Haard, H.J. Properties, production, and applications of camelid single-domain antibody fragments. Appl. Microbiol. Biotechnol. 2007, 77, 13–22. [Google Scholar] [CrossRef]
- Li, M.; Fan, X.; Liu, J.; Hu, Y.; Huang, H. Selection by phage display of nanobodies directed against hypoxia inducible factor-1alpha (HIF-1alpha). Biotechnol. Appl. Biochem. 2015, 62, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Liu, Q.; Huang, G.; Liu, J.; Wei, W. Engineering nanobodies for next-generation molecular imaging. Drug Discov. Today 2022, 27, 1622–1638. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, M.; Conrath, K.; Van Meirhaeghe, A.; Meersman, F.; Heremans, K.; Frenken, L.G.; Muyldermans, S.; Wyns, L.; Matagne, A. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002, 11, 500–515. [Google Scholar] [CrossRef]
- Kaur, H. Stability testing in monoclonal antibodies. Crit. Rev. Biotechnol. 2021, 41, 692–714. [Google Scholar] [CrossRef]
- Goldman, E.R.; Liu, J.L.; Zabetakis, D.; Anderson, G.P. Enhancing Stability of Camelid and Shark Single Domain Antibodies: An Overview. Front. Immunol. 2017, 8, 865. [Google Scholar] [CrossRef]
- Peyron, I.; Kizlik-Masson, C.; Dubois, M.D.; Atsou, S.; Ferriere, S.; Denis, C.V.; Lenting, P.J.; Casari, C.; Christophe, O.D. Camelid-derived single-chain antibodies in hemostasis: Mechanistic, diagnostic, and therapeutic applications. Res. Pract. Thromb. Haemost. 2020, 4, 1087–1110. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.P.; Vilaplana, L.; Marco, M.P. Nanobody: Outstanding features for diagnostic and therapeutic applications. Anal. Bioanal. Chem. 2019, 411, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Bates, A.; Power, C.A. David vs. Goliath: The Structure, Function, and Clinical Prospects of Antibody Fragments. Antibodies 2019, 8, 28. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, L.; Li, Y.; Xiong, Y.; Tu, Z.; Fu, J.; Chen, B. Anti-idiotypic nanobody as citrinin mimotope from a naive alpaca heavy chain single domain antibody library. Anal. Bioanal. Chem. 2015, 407, 5333–5341. [Google Scholar] [CrossRef]
- Yang, S.; Li, L.; Yin, S.; Shang, Y.; Khan, M.U.Z.; He, X.; Yuan, L.; Gao, X.; Liu, X.; Cai, J. Single-domain antibodies as promising experimental tools in imaging and isolation of porcine epidemic diarrhea virus. Appl. Microbiol. Biotechnol. 2018, 102, 8931–8942. [Google Scholar] [CrossRef]
- Tokuhara, D.; Álvarez, B.; Mejima, M.; Hiroiwa, T.; Takahashi, Y.; Kurokawa, S.; Kuroda, M.; Oyama, M.; Kozuka-Hata, H.; Nochi, T.; et al. Rice-based oral antibody fragment prophylaxis and therapy against rotavirus infection. J. Clin. Investig. 2013, 123, 3829–3838. [Google Scholar] [CrossRef] [PubMed]
- Panikar, S.S.; Banu, N.; Haramati, J.; Del Toro-Arreola, S.; Riera Leal, A.; Salas, P. Nanobodies as efficient drug-carriers: Progress and trends in chemotherapy. J. Control. Release Off. J. Control. Release Soc. 2021, 334, 389–412. [Google Scholar] [CrossRef]
- Morrison, C. Nanobody approval gives domain antibodies a boost. Nat. Rev. Drug Discov. 2019, 18, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Schrama, D.; Reisfeld, R.A.; Becker, J.C. Antibody targeted drugs as cancer therapeutics. Nat. Rev. Drug Discov. 2006, 5, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Arce, L.P.; Pavan, M.F.; Bok, M.; Gutiérrez, S.E.; Estein, S.M.; Santos, A.T.; Condorí, W.E.; Uhart, M.M.; Parreño, V.; Vizoso-Pinto, M.G.; et al. A multispecies competitive nanobody-based ELISA for the detection of antibodies against hepatitis E virus. Sci. Rep. 2023, 13, 15448. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, J.; Wu, S.; Guo, H.; Du, Y.; Wan, B.; Ji, P.; Wu, Y.; Zhuang, G.; Zhang, A.; et al. HRP-conjugated-nanobody-based cELISA for rapid and sensitive clinical detection of ASFV antibodies. Appl. Microbiol. Biotechnol. 2022, 106, 4269–4285. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yu, Y.; Xin, C.; Jin, G. Comparative study of optical fiber immunosensors based on traditional antibody or nanobody for detecting HER2. Talanta 2024, 277, 126317. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Wang, L.; Zhao, X.; Lu, T.; Na, A.M.; Wang, X.; Cao, J.; Du, Y. Preparation and characterization of a single-domain antibody specific for the porcine epidemic diarrhea virus spike protein. AMB Express 2019, 9, 104. [Google Scholar] [CrossRef]
- Marblestone, J.G.; Edavettal, S.C.; Lim, Y.; Lim, P.; Zuo, X.; Butt, T.R. Comparison of SUMO fusion technology with traditional gene fusion systems: Enhanced expression and solubility with SUMO. Protein Sci. 2006, 15, 182–189. [Google Scholar] [CrossRef]
- Bird, L.E. High throughput construction and small scale expression screening of multi-tag vectors in Escherichia coli. Methods 2011, 55, 29–37. [Google Scholar] [CrossRef]
- Singhvi, P.; Panda, A.K. Solubilization and Refolding of Inclusion Body Proteins. Methods Mol. Biol. 2022, 2406, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.M.; Sharma, A.; Upadhyay, A.K.; Singh, A.; Garg, L.C.; Panda, A.K. Solubilization of inclusion body proteins using n-propanol and its refolding into bioactive form. Protein Expr. Purif. 2012, 81, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Berryman, M.A.; Triplett, E.W.; Ludvigsson, J. Human leukocyte antigen-dependent colonization of Lactobacillus in the early-life gut. Front. Microbiomes 2023, 2, 1192773. [Google Scholar] [CrossRef]
- Pusch, O.; Boden, D.; Hannify, S.; Lee, F.; Tucker, L.D.; Boyd, M.R.; Wells, J.M.; Ramratnam, B. Bioengineering lactic acid bacteria to secrete the HIV-1 virucide cyanovirin. J. Acquir. Immune Defic. Syndr. 2005, 40, 512–520. [Google Scholar] [CrossRef]
- Giomarelli, B.; Provvedi, R.; Meacci, F.; Maggi, T.; Medaglini, D.; Pozzi, G.; Mori, T.; McMahon, J.B.; Gardella, R.; Boyd, M.R. The microbicide cyanovirin-N expressed on the surface of commensal bacterium Streptococcus gordonii captures HIV-1. AIDS 2002, 16, 1351–1356. [Google Scholar] [CrossRef]


| Panning Rounds | I | II | III |
|---|---|---|---|
| Input (PFU/mL) | 5 × 1011 | 5 × 1011 | 5 × 1011 |
| Eluted (PFU/mL) | 3 × 104 | 3.5 × 109 | 1.24 × 1010 |
| E/I | 6 × 10−8 | 7 × 10−3 | 2.48 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Dong, X.; Zhang, Z.; Qin, Z. A Nanobody of PEDV S1 Protein: Screening and Expression in Escherichia coli. Biomolecules 2024, 14, 1116. https://doi.org/10.3390/biom14091116
Hao Z, Dong X, Zhang Z, Qin Z. A Nanobody of PEDV S1 Protein: Screening and Expression in Escherichia coli. Biomolecules. 2024; 14(9):1116. https://doi.org/10.3390/biom14091116
Chicago/Turabian StyleHao, Zhipeng, Xufeng Dong, Zhongtao Zhang, and Zhihua Qin. 2024. "A Nanobody of PEDV S1 Protein: Screening and Expression in Escherichia coli" Biomolecules 14, no. 9: 1116. https://doi.org/10.3390/biom14091116





