Membrane Activity of Melittin and Magainin-I at Low Peptide-to-Lipid Ratio: Different Types of Pores and Translocation Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ζ-Potential Measurements
2.3. Patch-Clamp Experiments
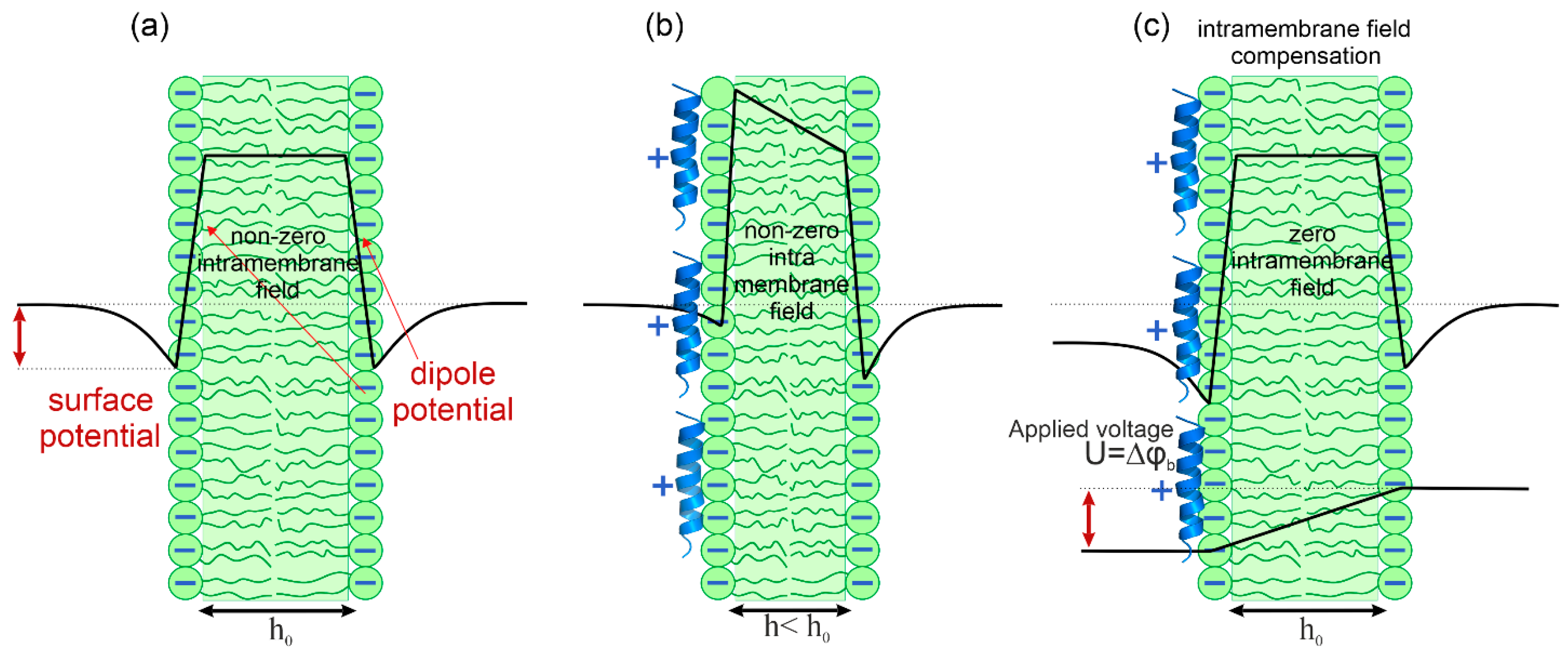
2.4. Intramembrane Field Compensation Method
3. Results
3.1. ζ-Potential Measurements on Liposomes
3.2. Patch-Clamp Experiments on Melittin and Magainin Pore Formation
3.3. Intramembrane Field Compensation Measurements of the Melittin and Magainin Adsorption and Translocation across the Membrane
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef]
- Gold, H.S.; Moellering, R.C. Antimicrobial-Drug Resistance. N. Engl. J. Med. 1996, 335, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. The Drug-Resistant Bacteria That Pose the Greatest Health Threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef]
- Petty, N.K.; Ben Zakour, N.L.; Stanton-Cook, M.; Skippington, E.; Totsika, M.; Forde, B.M.; Phan, M.-D.; Gomes Moriel, D.; Peters, K.M.; Davies, M.; et al. Global Dissemination of a Multidrug Resistant Escherichia Coli Clone. Proc. Natl. Acad. Sci. USA 2014, 111, 5694–5699. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.I.; Mergani, A.; Aklilu, E.; Kamaruzzaman, N.F. Antimicrobial Peptides: Bringing Solution to the Rising Threats of Antimicrobial Resistance in Livestock. Front. Vet. Sci. 2022, 9, 851052. [Google Scholar] [CrossRef]
- Alanis, A.J. Resistance to Antibiotics: Are We in the Post-Antibiotic Era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Memariani, H.; Memariani, M.; Moravvej, H.; Shahidi-Dadras, M. Melittin: A Venom-Derived Peptide with Promising Anti-Viral Properties. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 5–17. [Google Scholar] [CrossRef]
- Fennell, J.F.; Shipman, W.H.; Cole, L.J. Antibacterial Action of a Bee Venom Fraction (Melittin) against a Penicillin-Resistant Staphylococcus and Other Microorganisms. Res. Dev. Tech. Rep. 1967, 5, 1–13. [Google Scholar]
- Socarras, K.; Theophilus, P.; Torres, J.; Gupta, K.; Sapi, E. Antimicrobial Activity of Bee Venom and Melittin against Borrelia Burgdorferi. Antibiotics 2017, 6, 31. [Google Scholar] [CrossRef]
- Guha, S.; Ferrie, R.P.; Ghimire, J.; Ventura, C.R.; Wu, E.; Sun, L.; Kim, S.Y.; Wiedman, G.R.; Hristova, K.; Wimley, W.C. Applications and Evolution of Melittin, the Quintessential Membrane Active Peptide. Biochem. Pharmacol. 2021, 193, 114769. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zietz, C.M.; Mudgapalli, A.; Wang, S.; Wang, Z. The Evolution of the Antimicrobial Peptide Database over 18 Years: Milestones and New Features. Protein Sci. 2022, 31, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Ghimire, J.; Wu, E.; Wimley, W.C. Mechanistic Landscape of Membrane-Permeabilizing Peptides. Chem. Rev. 2019, 119, 6040–6085. [Google Scholar] [CrossRef]
- Starr, C.G.; Wimley, W.C. Antimicrobial Peptides Are Degraded by the Cytosolic Proteases of Human Erythrocytes. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2319–2326. [Google Scholar] [CrossRef]
- Munk, J.K.; Ritz, C.; Fliedner, F.P.; Frimodt-Møller, N.; Hansen, P.R. Novel Method To Identify the Optimal Antimicrobial Peptide in a Combination Matrix, Using Anoplin as an Example. Antimicrob. Agents Chemother. 2014, 58, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hamill, P.; Hancock, R.E.W. Peptide Antimicrobial Agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef]
- Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies, 2nd ed.; Wang, G. (Ed.) CABI: Wallingford, UK; Boston, MA, USA, 2017; ISBN 978-1-78639-040-0. [Google Scholar]
- Huang, H.W. Molecular Mechanism of Antimicrobial Peptides: The Origin of Cooperativity. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1292–1302. [Google Scholar] [CrossRef]
- Zemel, A.; Ben-Shaul, A.; May, S. Perturbation of a Lipid Membrane by Amphipathic Peptides and Its Role in Pore Formation. Eur. Biophys. J. 2005, 34, 230–242. [Google Scholar] [CrossRef]
- Frey, S.; Tamm, L.K. Orientation of Melittin in Phospholipid Bilayers. A Polarized Attenuated Total Reflection Infrared Study. Biophys. J. 1991, 60, 922–930. [Google Scholar] [CrossRef]
- Dempsey, C.E.; Butler, G.S. Helical Structure and Orientation of Melittin in Dispersed Phospholipid Membranes from Amide Exchange Analysis in Situ. Biochemistry 1992, 31, 11973–11977. [Google Scholar] [CrossRef]
- Yang, L.; Harroun, T.A.; Weiss, T.M.; Ding, L.; Huang, H.W. Barrel-Stave Model or Toroidal Model? A Case Study on Melittin Pores. Biophys. J. 2001, 81, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lu, X.; Deng, Z.; Xiao, S.; Yuan, B.; Yang, K. How Melittin Inserts into Cell Membrane: Conformational Changes, Inter-Peptide Cooperation, and Disturbance on the Membrane. Molecules 2019, 24, 1775. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; Del Vecchio, P.; Grimaldi, A.; Notomista, E.; Cafaro, V.; Pane, K.; Schuabb, V.; Winter, R.; Petraccone, L. Membrane Disintegration by the Antimicrobial Peptide (P)GKY20: Lipid Segregation and Domain Formation. Phys. Chem. Chem. Phys. 2019, 21, 3989–3998. [Google Scholar] [CrossRef]
- Qian, S.; Wang, W.; Yang, L.; Huang, H.W. Structure of the Alamethicin Pore Reconstructed by X-ray Diffraction Analysis. Biophys. J. 2008, 94, 3512–3522. [Google Scholar] [CrossRef]
- Sekiya, Y.; Sakashita, S.; Shimizu, K.; Usui, K.; Kawano, R. Channel Current Analysis Estimates the Pore-Formation and the Penetration of Transmembrane Peptides. Analyst 2018, 143, 3540–3543. [Google Scholar] [CrossRef]
- Priyadarshini, D.; Ivica, J.; Separovic, F.; De Planque, M.R.R. Characterisation of Cell Membrane Interaction Mechanisms of Antimicrobial Peptides by Electrical Bilayer Recording. Biophys. Chem. 2022, 281, 106721. [Google Scholar] [CrossRef]
- Watanabe, H.; Kawano, R. Channel Current Analysis for Pore-Forming Properties of an Antimicrobial Peptide, Magainin 1, Using the Droplet Contact Method. Anal. Sci. 2016, 32, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Chui, J.K.W.; Fyles, T.M. Ionic Conductance of Synthetic Channels: Analysis, Lessons, and Recommendations. Chem. Soc. Rev. 2012, 41, 148–175. [Google Scholar] [CrossRef]
- Wheaten, S.A.; Ablan, F.D.O.; Spaller, B.L.; Trieu, J.M.; Almeida, P.F. Translocation of Cationic Amphipathic Peptides across the Membranes of Pure Phospholipid Giant Vesicles. J. Am. Chem. Soc. 2013, 135, 16517–16525. [Google Scholar] [CrossRef]
- Kondrashov, O.V.; Galimzyanov, T.R.; Jiménez-Munguía, I.; Batishchev, O.V.; Akimov, S.A. Membrane-Mediated Interaction of Amphipathic Peptides Can Be Described by a One-Dimensional Approach. Phys. Rev. 2019, 99, 22401. [Google Scholar] [CrossRef]
- Schwarz, G.; Zong, R.; Popescu, T. Kinetics of Melittin Induced Pore Formation in the Membrane of Lipid Vesicles. Biochim. Biophys. Acta Biomembr. 1992, 1110, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Grant, E., Jr.; Beeler, T.J.; Taylor, K.M.P.; Gable, K.; Roseman, M.A. Mechanism of Magainin 2a Induced Permeabilization of Phospholipid Vesicles. Biochemistry 1992, 31, 9912–9918. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Murase, O.; Fujii, N.; Miyajima, K. Translocation of a Channel-Forming Antimicrobial Peptide, Magainin 2, across Lipid Bilayers by Forming a Pore. Biochemistry 1995, 34, 6521–6526. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Yoneyama, S.; Miyajima, K. Pore Formation and Translocation of Melittin. Biophys. J. 1997, 73, 831–838. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Murase, O.; Fujii, N.; Miyajima, K. An Antimicrobial Peptide, Magainin 2, Induced Rapid Flip-Flop of Phospholipids Coupled with Pore Formation and Peptide Translocation. Biochemistry 1996, 35, 11361–11368. [Google Scholar] [CrossRef]
- Marks, J.R.; Placone, J.; Hristova, K.; Wimley, W.C. Spontaneous Membrane-Translocating Peptides by Orthogonal High-Throughput Screening. J. Am. Chem. Soc. 2011, 133, 8995–9004. [Google Scholar] [CrossRef]
- Rashid, M.d.M.O.; Moghal, M.d.M.R.; Billah, M.d.M.; Hasan, M.; Yamazaki, M. Effect of Membrane Potential on Pore Formation by the Antimicrobial Peptide Magainin 2 in Lipid Bilayers. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183381. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Xue, Z.; Jia, Y.; Li, R.; He, C.; Chen, H. The Structure-Mechanism Relationship and Mode of Actions of Antimicrobial Peptides: A Review. Trends Food Sci. Technol. 2021, 109, 103–115. [Google Scholar] [CrossRef]
- Moghal, M.d.M.R.; Hossain, F.; Yamazaki, M. Action of Antimicrobial Peptides and Cell-Penetrating Peptides on Membrane Potential Revealed by the Single GUV Method. Biophys. Rev. 2020, 12, 339–348. [Google Scholar] [CrossRef]
- Magainins, Z.M. A Class of Antimicrobial Peptides from Xenopus Skin: Isolation, Characterization of Two Active Forms, and Partial cDNA Sequence of a Precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5443–5449. [Google Scholar]
- Dempsey, C.E. The Actions of Melittin on Membranes. Biochim. Biophys. Acta Rev. Biomembr. 1990, 1031, 143–161. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Nakamura, A.; Murase, O.; Sugishita, K.; Fujii, N.; Miyajima, K. Modulation of Magainin 2—Lipid Bilayer Interactions by Peptide Charge. Biochemistry 1997, 36, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Santo, K.P.; Berkowitz, M.L. Difference between Magainin-2 and Melittin Assemblies in Phosphatidylcholine Bilayers: Results from Coarse-Grained Simulations. J. Phys. Chem. 2012, 116, 3021–3030. [Google Scholar] [CrossRef] [PubMed]
- Ladokhin, A.S.; White, S.H. Folding of Amphipathic α-Helices on Membranes: Energetics of Helix Formation by Melittin. J. Mol. Biol. 1999, 285, 1363–1369. [Google Scholar] [CrossRef]
- Woo, S.Y.; Lee, H. Aggregation and Insertion of Melittin and Its Analogue MelP5 into Lipid Bilayers at Different Concentrations: Effects on Pore Size, Bilayer Thickness and Dynamics. Phys. Chem. Chem. Phys. 2017, 19, 7195–7203. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Takeda, A.; Tachi, T.; Matsuzaki, K. Dimer Structure of Magainin 2 Bound to Phospholipid Vesicles. Biopolymers 2002, 64, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Murase, O.; Tokuda, H.; Funakoshi, S.; Fujii, N.; Miyajima, K. Orientational and Aggregational States of Magainin 2 in Phospholipid Bilayers. Biochemistry 1994, 33, 3342–3349. [Google Scholar] [CrossRef] [PubMed]
- Goliaei, A.; Santo, K.P.; Berkowitz, M.L. Local Pressure Changes in Lipid Bilayers Due to Adsorption of Melittin and Magainin-H2 Antimicrobial Peptides: Results from Computer Simulations. J. Phys. Chem. 2014, 118, 12673–12679. [Google Scholar] [CrossRef]
- Ermakov, Y.A. Boundary Potential of Lipid Bilayers: Methods, Interpretations and Biological Applications. J. Phys. Conf. Ser. 2017, 794, 12007. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Colloid science; Academic Press: London, UK; New York, NY, USA, 1981; ISBN 978-0-12-361960-0. [Google Scholar]
- Mueller, P.; Rudin, D.O.; Ti Tien, H.; Wescott, W.C. Reconstitution of Cell Membrane Structure In Vitro and Its Transformation into an Excitable System. Nature 1962, 194, 979–980. [Google Scholar] [CrossRef]
- Jiménez-Munguía, I.; Volynsky, P.E.; Batishchev, O.V.; Akimov, S.A.; Korshunova, G.A.; Smirnova, E.A.; Knorre, D.A.; Sokolov, S.S.; Severin, F.F. Effects of Sterols on the Interaction of SDS, Benzalkonium Chloride, and A Novel Compound, Kor105, with Membranes. Biomolecules 2019, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Ultrathin Electrochemical Chemo- and Biosensors; Mirsky, V.M. (Ed.) Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2004; Volume 2, ISBN 978-3-642-05961-2. [Google Scholar]
- Batishchev, O.V.; Shilova, L.A.; Kachala, M.V.; Tashkin, V.Y.; Sokolov, V.S.; Fedorova, N.V.; Baratova, L.A.; Knyazev, D.G.; Zimmerberg, J.; Chizmadzhev, Y.A. pH-Dependent Formation and Disintegration of the Influenza A Virus Protein Scaffold To Provide Tension for Membrane Fusion. J. Virol. 2016, 90, 575–585. [Google Scholar] [CrossRef]
- Marukovich, N.; McMurray, M.; Finogenova, O.; Nesterenko, A.; Batishchev, O.; Ermakov, Y. Interaction of Polylysines with the Surface of Lipid Membranes. In Advances in Planar Lipid Bilayers and Liposomes; Elsevier: Amsterdam, The Netherlands, 2013; Volume 17, pp. 139–166. ISBN 978-0-12-411516-3. [Google Scholar]
- Carey, A.B.; Ashenden, A.; Köper, I. Model Architectures for Bacterial Membranes. Biophys. Rev. 2022, 14, 111–143. [Google Scholar] [CrossRef]
- Teixeira, V.; Feio, M.J.; Bastos, M. Role of Lipids in the Interaction of Antimicrobial Peptides with Membranes. Progress Lipid Res. 2012, 51, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.; Gresalfi, T.; Riccio, T.; McLaughlin, S. Adsorption of Monovalent Cations to Bilayer Membranes Containing Negative Phospholipids. Biochemistry 1979, 18, 5213–5223. [Google Scholar] [CrossRef]
- McLaughlin, S.; Mulrine, N.; Gresalfi, T.; Vaio, G.; McLaughlin, A. Adsorption of Divalent Cations to Bilayer Membranes Containing Phosphatidylserine. J. Gen. Physiol. 1981, 77, 445–473. [Google Scholar] [CrossRef]
- Tamba, Y.; Ariyama, H.; Levadny, V.; Yamazaki, M. Kinetic Pathway of Antimicrobial Peptide Magainin 2-Induced Pore Formation in Lipid Membranes. J. Phys. Chem. 2010, 114, 12018–12026. [Google Scholar] [CrossRef] [PubMed]
- Beschiaschvili, G.; Seelig, J. Melittin Binding to Mixed Phosphatidylglycerol/Phosphatidylcholine Membranes. Biochemistry 1990, 29, 52–58. [Google Scholar] [CrossRef]
- Dathe, M.; Nikolenko, H.; Meyer, J.; Beyermann, M.; Bienert, M. Optimization of the Antimicrobial Activity of Magainin Peptides by Modification of Charge. FEBS Lett. 2001, 501, 146–150. [Google Scholar] [CrossRef]
- Wenk, M.R.; Seelig, J. Magainin 2 Amide Interaction with Lipid Membranes: Calorimetric Detection of Peptide Binding and Pore Formation. Biochemistry 1998, 37, 3909–3916. [Google Scholar] [CrossRef]
- Melo, M.N.; Ferre, R.; Castanho, M.A.R.B. Antimicrobial Peptides: Linking Partition, Activity and High Membrane-Bound Concentrations. Nat. Rev. Microbiol. 2009, 7, 245–250. [Google Scholar] [CrossRef]
- Kondrashov, O.V.; Akimov, S.A. The Possibility of Pore Formation in Lipid Membranes by Several Molecules of Amphipathic Peptides. Biochem. Mosc. Suppl. Ser. 2022, 16, 338–350. [Google Scholar] [CrossRef]
- Lee, M.-T.; Sun, T.-L.; Hung, W.-C.; Huang, H.W. Process of Inducing Pores in Membranes by Melittin. Proc. Natl. Acad. Sci. USA 2013, 110, 14243–14248. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C.; Hristova, K. Antimicrobial Peptides: Successes, Challenges and Unanswered Questions. J. Membr. Biol. 2011, 239, 27–34. [Google Scholar] [CrossRef]
- Terwilliger, T.C.; Weissman, L.; Eisenberg, D. The Structure of Melittin in the Form I Crystals and Its Implication for Melittin’s Lytic and Surface Activities. Biophys. J. 1982, 37, 353–361. [Google Scholar] [CrossRef]
- Kondrashov, O.V.; Akimov, S.A. Alteration of Average Thickness of Lipid Bilayer by Membrane-Deforming Inclusions. Biomolecules 2023, 13, 1731. [Google Scholar] [CrossRef] [PubMed]




| Melittin | Magainin | ||||
|---|---|---|---|---|---|
| Concentration, nM | Δφb Change at Low Ionic Strength, mV | Δφb Change at High Ionic Strength, mV | Concentration, nM | Δφb Change at Low Ionic Strength, mV | Δφb Change at High Ionic Strength, mV |
| 70 | 9 ± 2 | 10 ± 3 | 80 | 16 ± 3 | 8 ± 3 |
| 140 | 5 ± 2 | 8 ± 3 | 160 | 11 ± 2 | 7 ± 2 |
| Characteristic time of the Δφb decrease, min | - | 70 ± 7 | - | 9 ± 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volovik, M.V.; Batishchev, O.V. Membrane Activity of Melittin and Magainin-I at Low Peptide-to-Lipid Ratio: Different Types of Pores and Translocation Mechanisms. Biomolecules 2024, 14, 1118. https://doi.org/10.3390/biom14091118
Volovik MV, Batishchev OV. Membrane Activity of Melittin and Magainin-I at Low Peptide-to-Lipid Ratio: Different Types of Pores and Translocation Mechanisms. Biomolecules. 2024; 14(9):1118. https://doi.org/10.3390/biom14091118
Chicago/Turabian StyleVolovik, Marta V., and Oleg V. Batishchev. 2024. "Membrane Activity of Melittin and Magainin-I at Low Peptide-to-Lipid Ratio: Different Types of Pores and Translocation Mechanisms" Biomolecules 14, no. 9: 1118. https://doi.org/10.3390/biom14091118





