Oxylipins in Aqueous Humor of Primary Open-Angle Glaucoma Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Ophthalmic Examinations and Blood Tests
2.3. Oxylipins Analysis
2.4. Statistical Analysis
3. Results
3.1. Demographics and Clinical Examinations of the Study Subjects
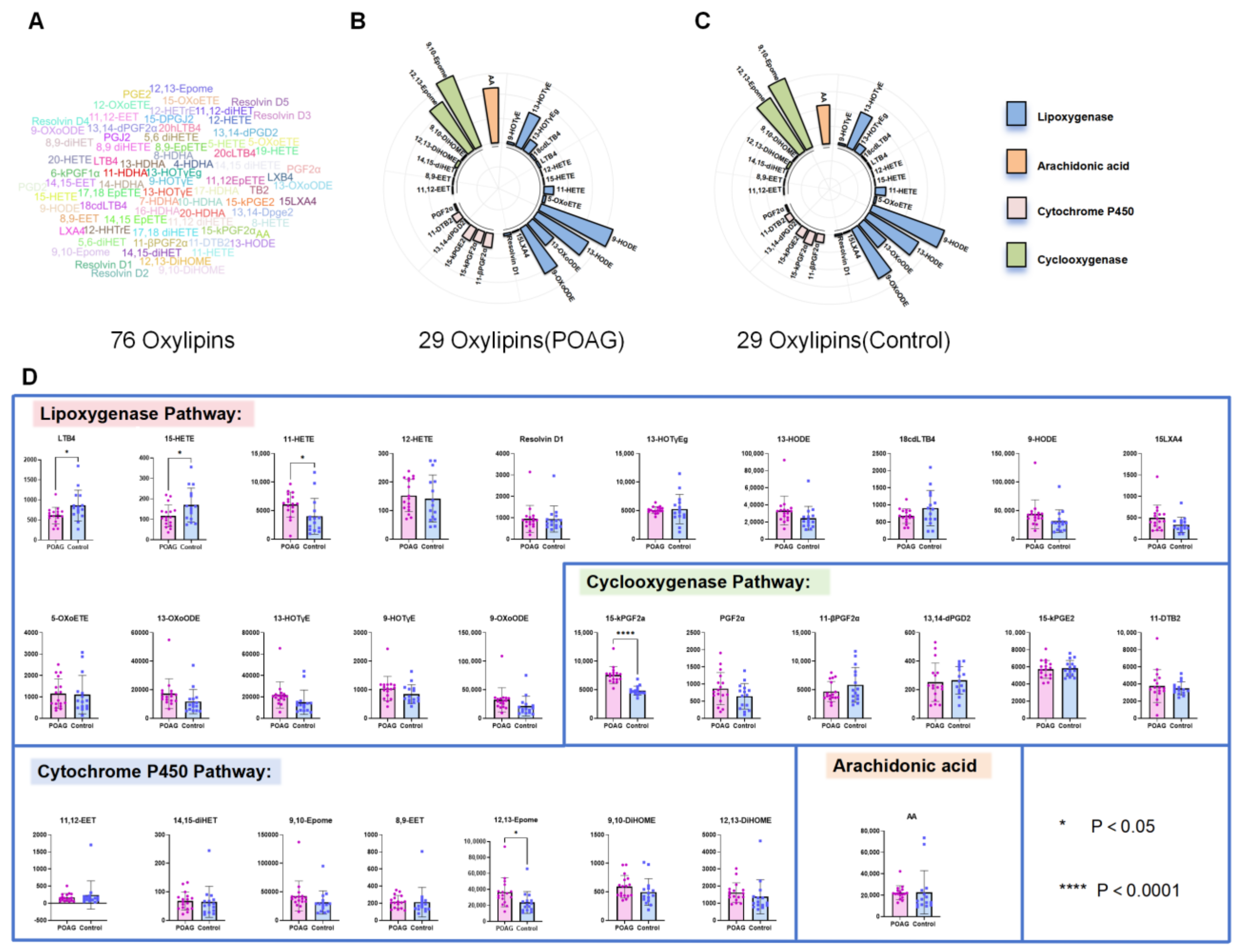
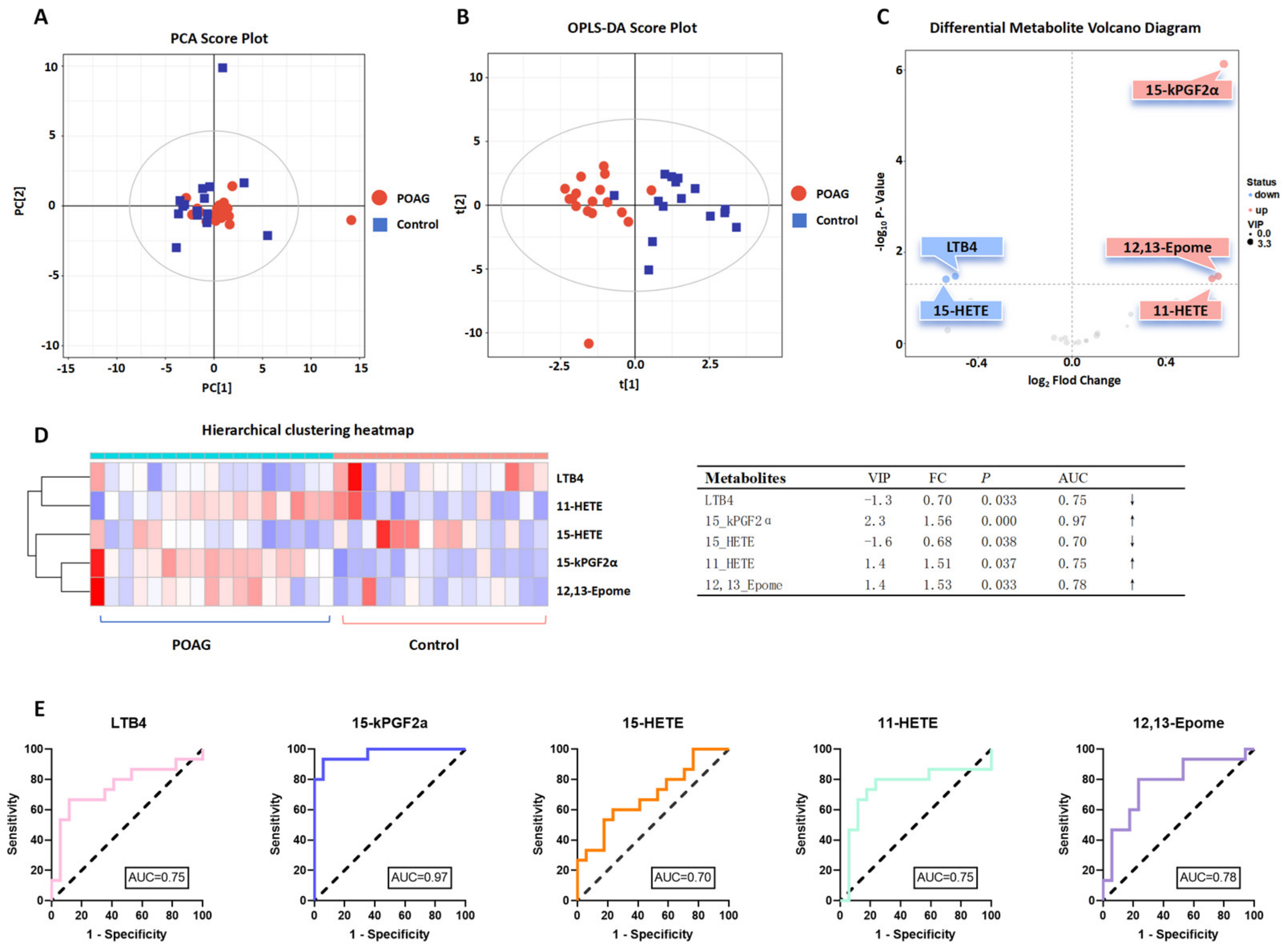
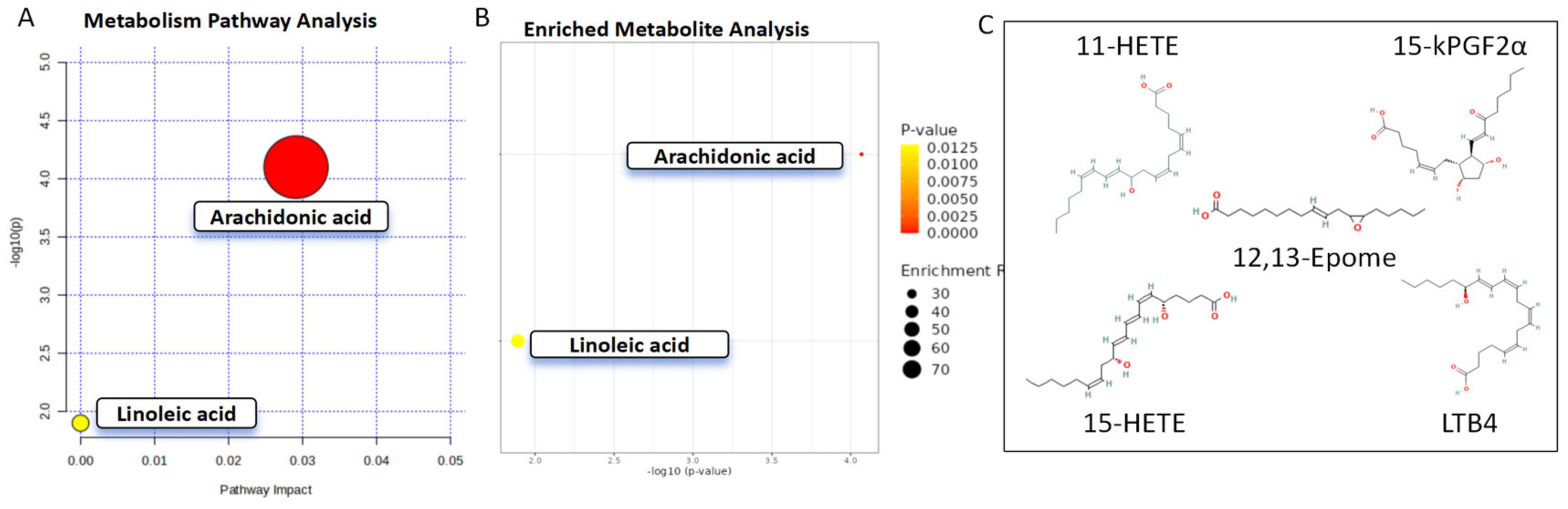
3.2. Identification of Oxylipins in Aqueous Humor of Primary Open-Angle Glaucoma Subjects
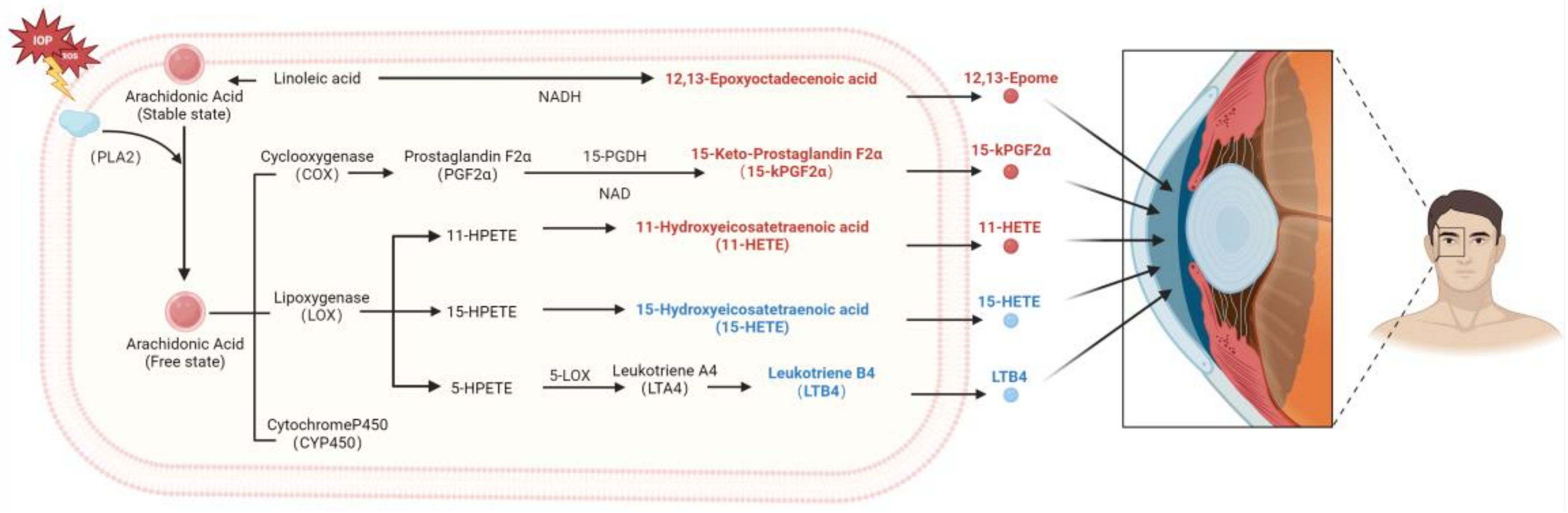
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayaram, H.; Kolko, M.; Friedman, D.S.; Gazzard, G. Glaucoma: Now and beyond. Lancet 2023, 402, 1788–1801. [Google Scholar] [CrossRef]
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in Adults-Screening, Diagnosis, and Management: A Review. JAMA 2021, 325, 164–174. [Google Scholar] [CrossRef]
- Jammal, A.A.; Thompson, A.C.; Mariottoni, E.B.; Estrela, T.; Shigueoka, L.S.; Berchuck, S.I.; Medeiros, F.A. Impact of Intraocular Pressure Control on Rates of Retinal Nerve Fiber Layer Loss in a Large Clinical Population. Ophthalmology 2021, 128, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Susanna, B.N.; Ogata, N.G.; Jammal, A.A.; Susanna, C.N.; Berchuck, S.I.; Medeiros, F.A. Corneal Biomechanics and Visual Field Progression in Eyes with Seemingly Well-Controlled Intraocular Pressure. Ophthalmology 2019, 126, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, Y.; Vithana, E.N.; Jia, L.; Zuo, X.; Wong, T.Y.; Chen, L.J.; Zhu, X.; Tam, P.O.S.; Gong, B.; et al. Common variants near ABCA1 and in PMM2 are associated with primary open-angle glaucoma. Nat. Genet. 2014, 46, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Nusinovici, S.; Li, H.; Thakur, S.; Baskaran, M.; Tham, Y.-C.; Zhou, L.; Sabanayagam, C.; Aung, T.; Silver, D.; Fan, Q.; et al. High-Density Lipoprotein 3 Cholesterol and Primary Open-Angle Glaucoma: Metabolomics and Mendelian Randomization Analyses. Ophthalmology 2022, 129, 285–294. [Google Scholar] [CrossRef]
- Thorleifsson, G.; Walters, G.B.; Hewitt, A.W.; Masson, G.; Helgason, A.; DeWan, A.; Sigurdsson, A.; Jonasdottir, A.; Gudjonsson, S.A.; Magnusson, K.P.; et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat. Genet. 2010, 42, 906–909. [Google Scholar] [CrossRef]
- Janssen, S.F.; Gorgels, T.G.M.F.; Ramdas, W.D.; Klaver, C.C.W.; van Duijn, C.M.; Jansonius, N.M.; Bergen, A.A.B. The vast complexity of primary open angle glaucoma: Disease genes, risks, molecular mechanisms and pathobiology. Prog. Retin. Eye Res. 2013, 37, 31–67. [Google Scholar] [CrossRef]
- Du, X.-M.; Kim, M.-J.; Hou, L.; Le Goff, W.; Chapman, M.J.; Van Eck, M.; Curtiss, L.K.; Burnett, J.R.; Cartland, S.P.; Quinn, C.M.; et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ. Res. 2015, 116, 1133–1142. [Google Scholar] [CrossRef]
- Zeleznik, O.A.; Kang, J.H.; Lasky-Su, J.; Eliassen, A.H.; Frueh, L.; Clish, C.B.; Rosner, B.A.; Elze, T.; Hysi, P.; Khawaja, A.; et al. Plasma metabolite profile for primary open-angle glaucoma in three US cohorts and the UK Biobank. Nat. Commun. 2023, 14, 2860. [Google Scholar] [CrossRef]
- Song, J.E.; Kim, J.M.; Lee, M.Y.; Jang, H.J.; Park, K.H. Effects of Consumption of Alcohol on Intraocular Pressure: Korea National Health and Nutrition Examination Survey 2010 to 2011. Nutrients 2020, 12, 2420. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Xu, J.; Chen, S.-L.; Chen, C.-B.; Liang, J.-J.; Liu, Z.; Huang, C.; Wu, Z.; Ng, T.K.; Zhang, M.; et al. Profile of Lipoprotein Subclasses in Chinese Primary Open-Angle Glaucoma Patients. Int. J. Mol. Sci. 2024, 25, 4544. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fu, C.; Sun, Y.; Wen, X.; Chen, C.-B.; Huang, C.; Ng, T.K.; Liu, Q.; Zhang, M. Untargeted and Oxylipin-Targeted Metabolomics Study on the Plasma Samples of Primary Open-Angle Glaucoma Patients. Biomolecules 2024, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Conwell, M.D.; Chen, X.; Kettenhofen, C.I.; Westlake, C.J.; Cantor, L.B.; Wells, C.D.; Weinreb, R.N.; Corson, T.W.; Spandau, D.F.; et al. Primary cilia signaling mediates intraocular pressure sensation. Proc. Natl. Acad. Sci. USA 2014, 111, 12871–12876. [Google Scholar] [CrossRef]
- Zeng, Q.; Gong, Y.; Zhu, N.; Shi, Y.; Zhang, C.; Qin, L. Lipids and lipid metabolism in cellular senescence: Emerging targets for age-related diseases. Ageing Res. Rev. 2024, 97, 102294. [Google Scholar] [CrossRef]
- Nayeem, M.A. Role of oxylipins in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1142–1154. [Google Scholar] [CrossRef]
- Azbukina, N.V.; Chistyakov, D.V.; Goriainov, S.V.; Kotelin, V.I.; Fedoseeva, E.V.; Petrov, S.Y.; Sergeeva, M.G.; Iomdina, E.N.; Zernii, E.Y. Targeted Lipidomic Analysis of Aqueous Humor Reveals Signaling Lipid-Mediated Pathways in Primary Open-Angle Glaucoma. Biology 2021, 10, 658. [Google Scholar] [CrossRef]
- Tang, Y.; Pan, Y.; Chen, Y.; Kong, X.; Chen, J.; Zhang, H.; Tang, G.; Wu, J.; Sun, X. Metabolomic Profiling of Aqueous Humor and Plasma in Primary Open Angle Glaucoma Patients Points Towards Novel Diagnostic and Therapeutic Strategy. Front. Pharmacol. 2021, 12, 621146. [Google Scholar] [CrossRef]
- Li, K.; Zhao, J.; Wang, M.; Niu, L.; Wang, Y.; Li, Y.; Zheng, Y. The Roles of Various Prostaglandins in Fibrosis: A Review. Biomolecules 2021, 11, 789. [Google Scholar] [CrossRef]
- Buisset, A.; Gohier, P.; Leruez, S.; Muller, J.; Amati-Bonneau, P.; Lenaers, G.; Bonneau, D.; Simard, G.; Procaccio, V.; Annweiler, C.; et al. Metabolomic Profiling of Aqueous Humor in Glaucoma Points to Taurine and Spermine Deficiency: Findings from the Eye-D Study. J. Proteome Res. 2019, 18, 1307–1315. [Google Scholar] [CrossRef]
- Riaposova, L.; Kim, S.H.; Hanyaloglu, A.C.; Sykes, L.; MacIntyre, D.A.; Bennett, P.R.; Terzidou, V. Prostaglandin F2α requires activation of calcium-dependent signalling to trigger inflammation in human myometrium. Front. Endocrinol. 2023, 14, 1150125. [Google Scholar] [CrossRef]
- Bremner, F.D. Putting raised intraocular pressure in context. Lancet 2015, 386, 102. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Xiao, H.-W.; Dong, J.-L.; Li, Y.; Wang, B.; Fan, S.-J.; Cui, M. Gut Microbiota-Derived PGF2α Fights against Radiation-Induced Lung Toxicity through the MAPK/NF-κB Pathway. Antioxidants 2021, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Lu, J.-W.; Zhang, C.-Y.; Wang, W.-S.; Ying, H.; Myatt, L.; Sun, K. PGE2 vs PGF2α in human parturition. Placenta 2021, 104, 208–219. [Google Scholar] [CrossRef]
- Smyth, E.M.; Grosser, T.; Wang, M.; Yu, Y.; FitzGerald, G.A. Prostanoids in health and disease. J. Lipid Res. 2009, 50 (Supplementary), S423–S428. [Google Scholar] [CrossRef] [PubMed]
- Helmersson-Karlqvist, J.; Ärnlöv, J.; Larsson, A.; Basu, S. Prostaglandin F2α formation is associated with mortality in a Swedish community-based cohort of older males. Eur. Heart J. 2015, 36, 238–243. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Chen, Y.; Cai, Q. The role of the LTB4-BLT1 axis in health and disease. Pharmacol. Res. 2020, 158, 104857. [Google Scholar] [CrossRef]
- Yokomizo, T.; Shimizu, T. The leukotriene B4 receptors BLT1 and BLT2 as potential therapeutic targets. Immunol. Rev. 2023, 317, 30–41. [Google Scholar] [CrossRef]
- Gong, M.; Duan, H.; Wu, F.; Ren, Y.; Gong, J.; Xu, L.; Lu, F.; Wang, D. Berberine Alleviates Insulin Resistance and Inflammation via Inhibiting the LTB4-BLT1 Axis. Front. Pharmacol. 2021, 12, 722360. [Google Scholar] [CrossRef]
- Gerstmeier, J.; Seegers, J.; Witt, F.; Waltenberger, B.; Temml, V.; Rollinger, J.M.; Stuppner, H.; Koeberle, A.; Schuster, D.; Werz, O. Ginkgolic Acid is a Multi-Target Inhibitor of Key Enzymes in Pro-Inflammatory Lipid Mediator Biosynthesis. Front. Pharmacol. 2019, 10, 797. [Google Scholar] [CrossRef]
- Subbarao, K.; Jala, V.R.; Mathis, S.; Suttles, J.; Zacharias, W.; Ahamed, J.; Ali, H.; Tseng, M.T.; Haribabu, B. Role of leukotriene B4 receptors in the development of atherosclerosis: Potential mechanisms. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Serezani, C.H.; Lewis, C.; Jancar, S.; Peters-Golden, M. Leukotriene B4 amplifies NF-κB activation in mouse macrophages by reducing SOCS1 inhibition of MyD88 expression. J. Clin. Investig. 2011, 121, 671–682. [Google Scholar] [CrossRef]
- Brandt, S.L.; Serezani, C.H. Too much of a good thing: How modulating LTB4 actions restore host defense in homeostasis or disease. Semin. Immunol. 2017, 33, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Anita, N.Z.; Kwan, F.; Ryoo, S.W.; Major-Orfao, C.; Lin, W.Z.; Noor, S.; Lanctôt, K.L.; Herrmann, N.; Oh, P.I.; Shah, B.R.; et al. Cytochrome P450-soluble epoxide hydrolase derived linoleic acid oxylipins and cognitive performance in type 2 diabetes. J. Lipid Res. 2023, 64, 100395. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Shanely, R.A.; Luo, B.; Meaney, M.P.; Dew, D.A.; Pappan, K.L. Metabolomics approach to assessing plasma 13- and 9-hydroxy-octadecadienoic acid and linoleic acid metabolite responses to 75-km cycling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R68–R74. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Edin, M.L.; Wang, Y.; Luo, Y.; Wan, D.; Yang, H.; Song, C.-Q.; Xue, W.; Sanidad, K.Z.; et al. Targeted Metabolomics Identifies the Cytochrome P450 Monooxygenase Eicosanoid Pathway as a Novel Therapeutic Target of Colon Tumorigenesis. Cancer Res. 2019, 79, 1822–1830. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Edin, M.L.; De Maeyer, R.P.H.; Bystrom, J.; Newson, J.; Lih, F.B.; Stables, M.; Zeldin, D.C.; Bishop-Bailey, D. CYP450-derived oxylipins mediate inflammatory resolution. Proc. Natl. Acad. Sci. USA 2016, 113, E3240–E3249. [Google Scholar] [CrossRef]
- Warner, D.; Vatsalya, V.; Zirnheld, K.H.; Warner, J.B.; Hardesty, J.E.; Umhau, J.C.; McClain, C.J.; Maddipati, K.; Kirpich, I.A. Linoleic Acid-Derived Oxylipins Differentiate Early Stage Alcoholic Hepatitis From Mild Alcohol-Associated Liver Injury. Hepatol. Commun. 2021, 5, 947–960. [Google Scholar] [CrossRef]
- Jian, W.; Lee, S.H.; Williams, M.V.; Blair, I.A. 5-Lipoxygenase-mediated endogenous DNA damage. J. Biol. Chem. 2009, 284, 16799–16807. [Google Scholar] [CrossRef]
- Badrani, J.H.; Cavagnero, K.; Eastman, J.J.; Kim, A.S.; Strohm, A.; Yan, C.; Deconde, A.; Zuraw, B.L.; White, A.A.; Christiansen, S.C.; et al. Lower serum 15-HETE level predicts nasal ILC2 accumulation during COX-1 inhibition in AERD. J. Allergy Clin. Immunol. 2023, 152, 1330–1335. [Google Scholar] [CrossRef]
- Li, J.; Rao, J.; Liu, Y.; Cao, Y.; Zhang, Y.; Zhang, Q.; Zhu, D. 15-Lipoxygenase promotes chronic hypoxia-induced pulmonary artery inflammation via positive interaction with nuclear factor-κB. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Ambaw, Y.A.; Fuchs, D.; Raida, M.; Mazengia, N.T.; Torta, F.; Wheelock, C.E.; Wenk, M.R.; Tong, L. Changes of tear lipid mediators after eyelid warming or thermopulsation treatment for meibomian gland dysfunction. Prostaglandins Other Lipid Mediat. 2020, 151, 106474. [Google Scholar] [CrossRef] [PubMed]
- Myer, C.; Perez, J.; Abdelrahman, L.; Mendez, R.; Khattri, R.B.; Junk, A.K.; Bhattacharya, S.K. Differentiation of soluble aqueous humor metabolites in primary open angle glaucoma and controls. Exp. Eye Res. 2020, 194, 108024. [Google Scholar] [CrossRef] [PubMed]
- Cabrerizo, J.; Urcola, J.A.; Vecino, E. Changes in the Lipidomic Profile of Aqueous Humor in Open-Angle Glaucoma. J. Glaucoma 2017, 26, 349–355. [Google Scholar]
- Pulukool, S.K.; Bhagavatham SK, S.; Kannan, V.; Sukumar, P.; Dandamudi, R.B.; Ghaisas, S.; Kunchala, H.; Saieesh, D.; Naik, A.A.; Pargaonkar, A.; et al. Elevated dimethylarginine, ATP, cytokines, metabolic remodeling involving tryptophan metabolism and potential microglial inflammation characterize primary open angle glaucoma. Sci. Rep. 2021, 11, 9766. [Google Scholar]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]

| POAG (n = 17) | Control (n = 15) | p | |
|---|---|---|---|
| Age (years) | 64.71 ± 10.31 | 66.47 ± 7.74 | 0.593 a |
| Sex (male/female) | 15/2 | 12/3 | 0.645 b |
| Height (m) | 1.61 ± 0.09 | 1.64 ± 0.05 | 0.307 a |
| Weight (kg) | 60.90 ± 8.22 | 64.20 ± 7.86 | 0.256 a |
| BMI (kg/m2) | 24.31 ± 2.50 | 23.51 ± 2.75 | 0.399 a |
| SBP (mmHg) | 137.88 ± 18.97 | 133.60 ± 15.56 | 0.494 a |
| DBP (mmHg) | 80.00 ± 12.83 | 85.73 ± 14.31 | 0.152 a |
| WBC (×109/L) | 7.48 ± 2.55 | 7.03 ± 2.09 | 0.738 c |
| Neu (×109/L) | 5.08 ± 1.57 | 4.65 ± 1.90 | 0.486 a |
| Lym (×109/L) | 1.95 ± 0.74 | 2.27 ± 0.68 | 0.225 a |
| Mono (×109/L) | 1.05 ± 1.91 | 0.54 ± 0.22 | 0.317 a |
| Eos (×109/L) | 0.13 ± 0.10 | 0.20 ± 0.14 | 0.178 a |
| Baso (×109/L) | 0.16 ± 0.24 | 0.07 ± 0.06 | 0.147 a |
| Glucose (mmol/L) | 6.59 ± 2.02 | 6.02 ± 0.94 | 0.992 a |
| TC (mmol/L) | 4.96 ± 1.08 | 5.52 ± 0.59 | 0.090 a |
| TG (mmol/L) | 1.18 ± 0.41 | 1.16 ± 0.50 | 0.942 a |
| HDL (mmol/L) | 1.41 ± 0.29 | 1.65 ± 0.36 | 0.041 a |
| LDL (mmol/L) | 3.14 ± 0.94 | 3.37 ± 0.71 | 0.442 a |
| Apo-A1 (g/L) | 1.36 ± 0.27 | - | - |
| Apo-B (g/L) | 1.03 ± 0.22 | - | - |
| Apo-A1/Apo-B | 0.77 ± 0.14 | - | - |
| LPa (mg/L) | 124.58 ± 93.28 | - | - |
| POAG (n = 12) | Control (n = 15) | p | |
|---|---|---|---|
| Laterality (R/L) | 6/6 | 10/5 | 0.452 b |
| BCVA (logMAR) | 0.65 ± 0.85 | 0.24 ± 0.24 | 0.085 c |
| IOP (mmHg) | 23.52 ± 8.46 | 13.53 ± 1.99 | < 0.001 a,* |
| AL (mm) | 23.31 ± 1.05 | 23.42 ± 0.79 | 0.751 a |
| CCT (µm) | 547.63 ± 46.01 | 536.46 ± 44.85 | 0.541 a |
| ACD (mm) | 3.10 ± 0.33 | 3.17 ± 0.27 | 0.557 a |
| Macular thickness (µm) | 237.40 ± 33.25 | - | - |
| RNFL thickness (µm) | 58.10 ± 7.99 | - | - |
| C/D | 0.78 ± 0.16 | - | - |
| S-pRNFL (µm) | 70.20 ± 12.24 | - | - |
| I-pRNFL (µm) | 60.50 ± 12.34 | - | - |
| N-pRNFL (µm) | 56.10 ± 8.56 | - | - |
| T-pRNFL (µm) | 48.40 ± 11.13 | - | - |
| Disk rim Area (mm2) | 0.69 ± 0.34 | - | - |
| Disk area (mm2) | 1.95 ± 0.35 | - | - |
| Cup volume (mm3) | 0.53 ± 0.43 | - | - |
| VF-MD (dB) | −23.18 ± 5.49 | - | - |
| VF-PSD (dB) | 8.17 ± 2.16 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhou, K.; Fu, C.; Chen, C.-B.; Sun, Y.; Wen, X.; Yang, L.; Ng, T.-K.; Liu, Q.; Zhang, M. Oxylipins in Aqueous Humor of Primary Open-Angle Glaucoma Patients. Biomolecules 2024, 14, 1127. https://doi.org/10.3390/biom14091127
Xu J, Zhou K, Fu C, Chen C-B, Sun Y, Wen X, Yang L, Ng T-K, Liu Q, Zhang M. Oxylipins in Aqueous Humor of Primary Open-Angle Glaucoma Patients. Biomolecules. 2024; 14(9):1127. https://doi.org/10.3390/biom14091127
Chicago/Turabian StyleXu, Jianming, Kewen Zhou, Changzhen Fu, Chong-Bo Chen, Yaru Sun, Xin Wen, Luxi Yang, Tsz-Kin Ng, Qingping Liu, and Mingzhi Zhang. 2024. "Oxylipins in Aqueous Humor of Primary Open-Angle Glaucoma Patients" Biomolecules 14, no. 9: 1127. https://doi.org/10.3390/biom14091127
APA StyleXu, J., Zhou, K., Fu, C., Chen, C.-B., Sun, Y., Wen, X., Yang, L., Ng, T.-K., Liu, Q., & Zhang, M. (2024). Oxylipins in Aqueous Humor of Primary Open-Angle Glaucoma Patients. Biomolecules, 14(9), 1127. https://doi.org/10.3390/biom14091127






