Application of CoLD-CoP to Detecting Competitively and Cooperatively Binding Ligands
Abstract
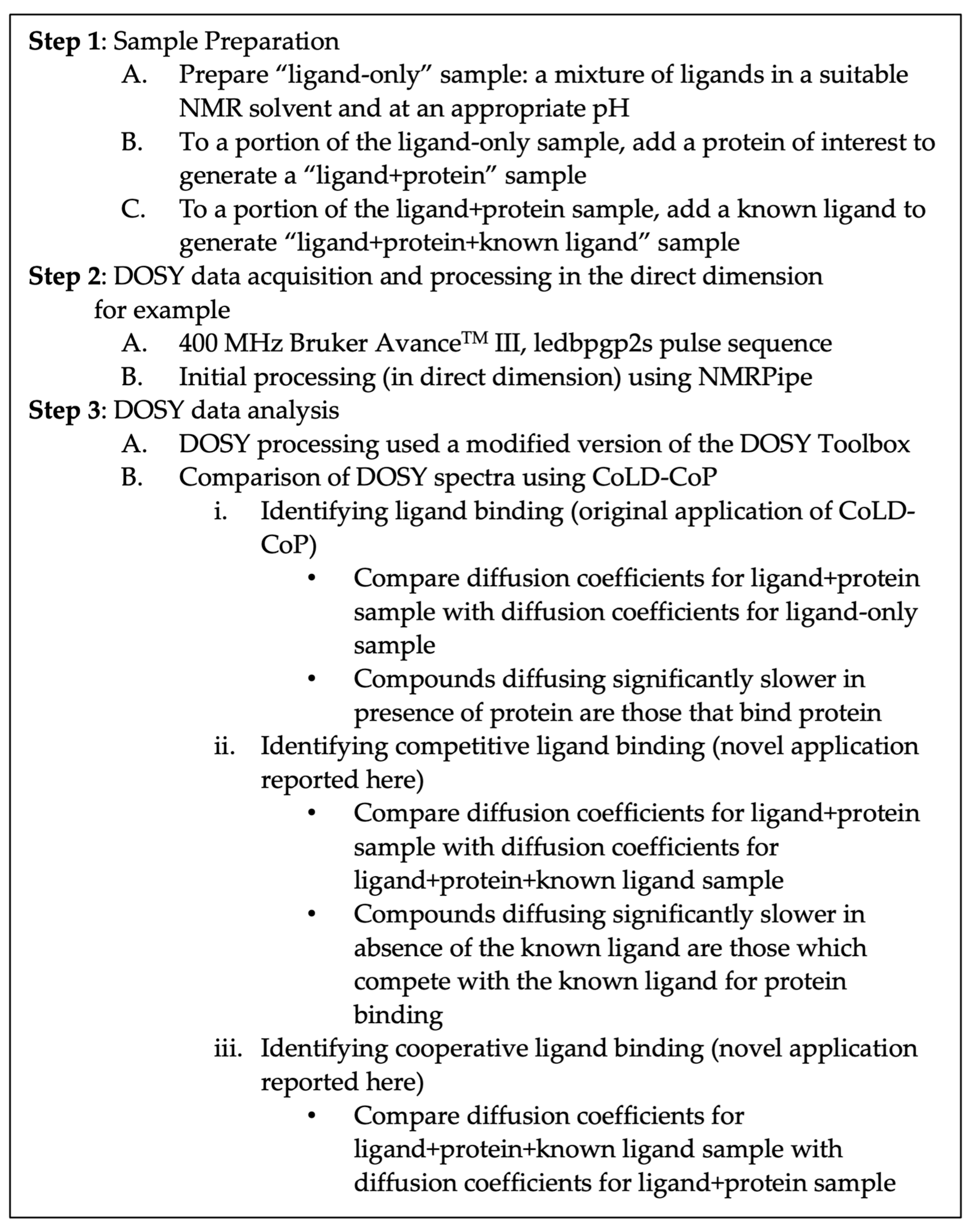
:1. Introduction
2. Materials and Methods
3. Results
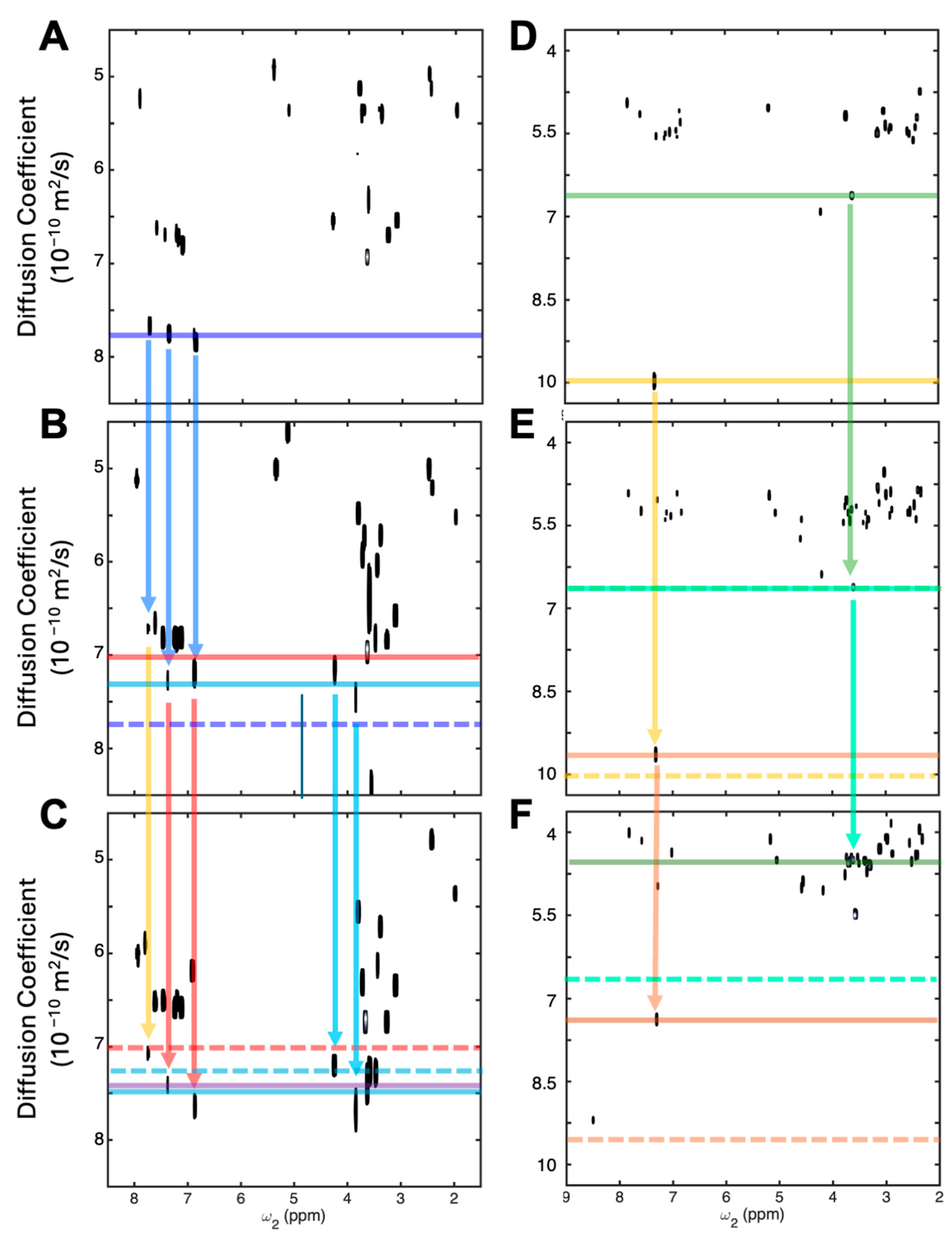
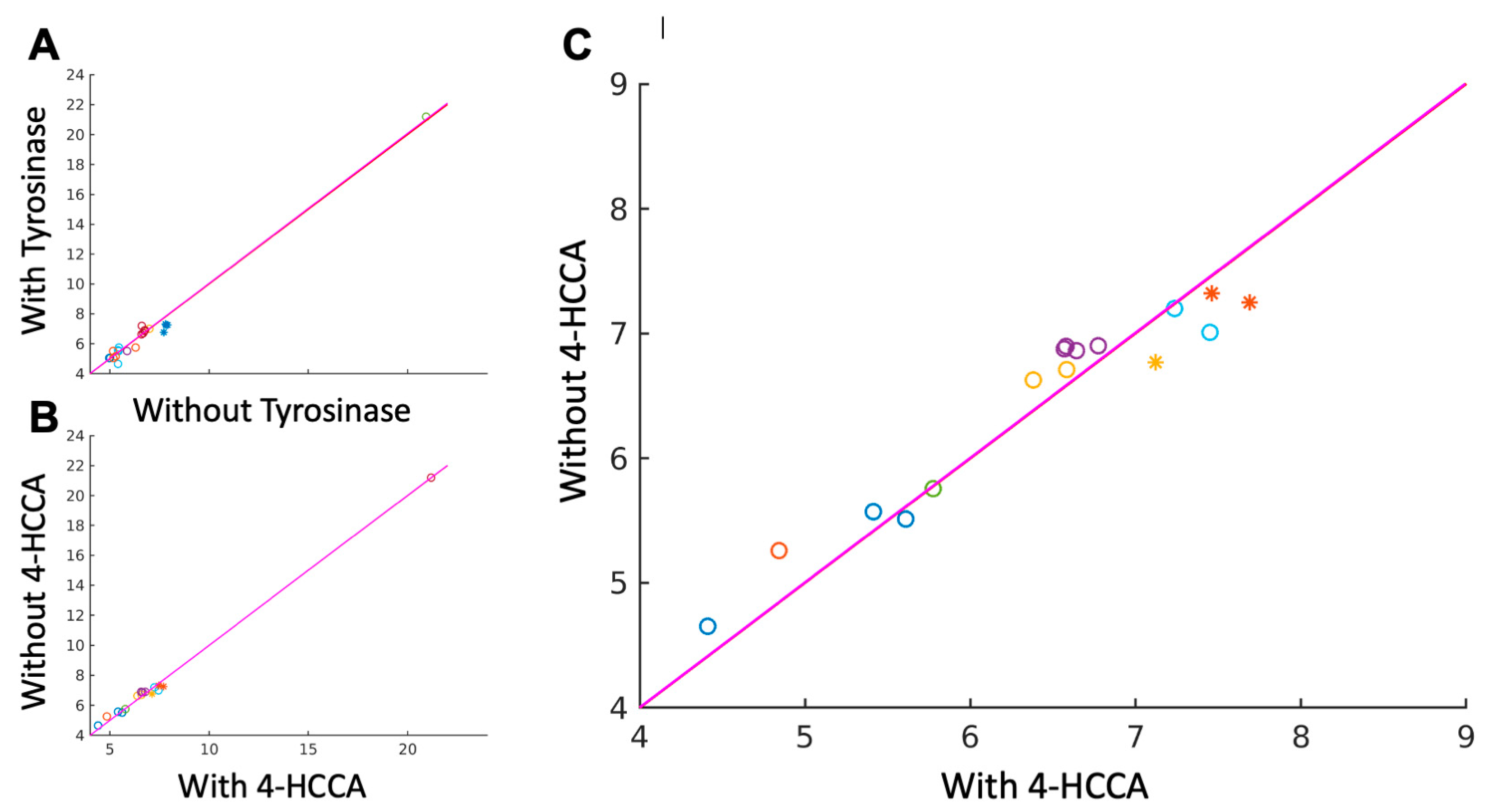
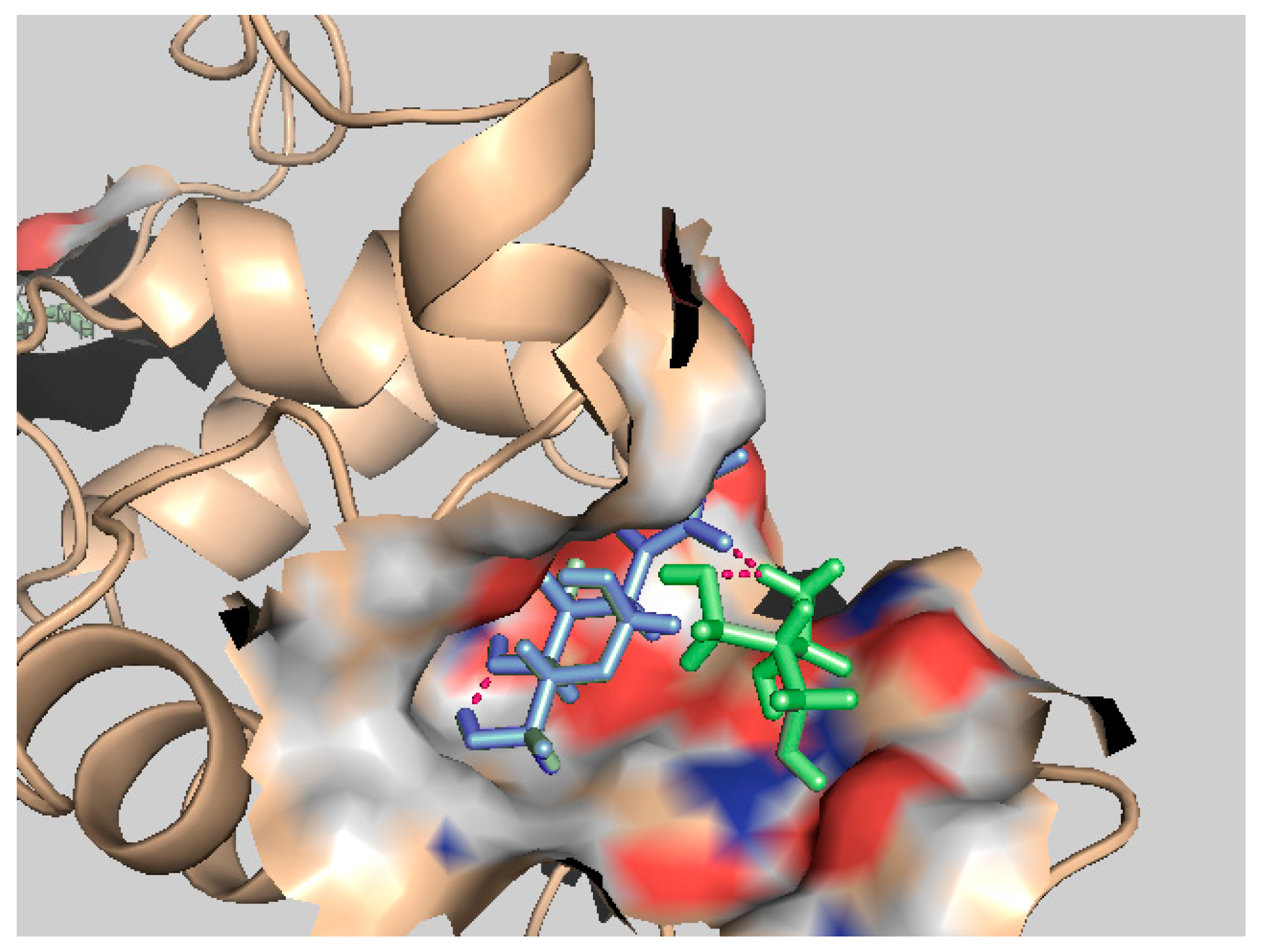
3.1. Detecting Competitive Binding to Tyrosinase
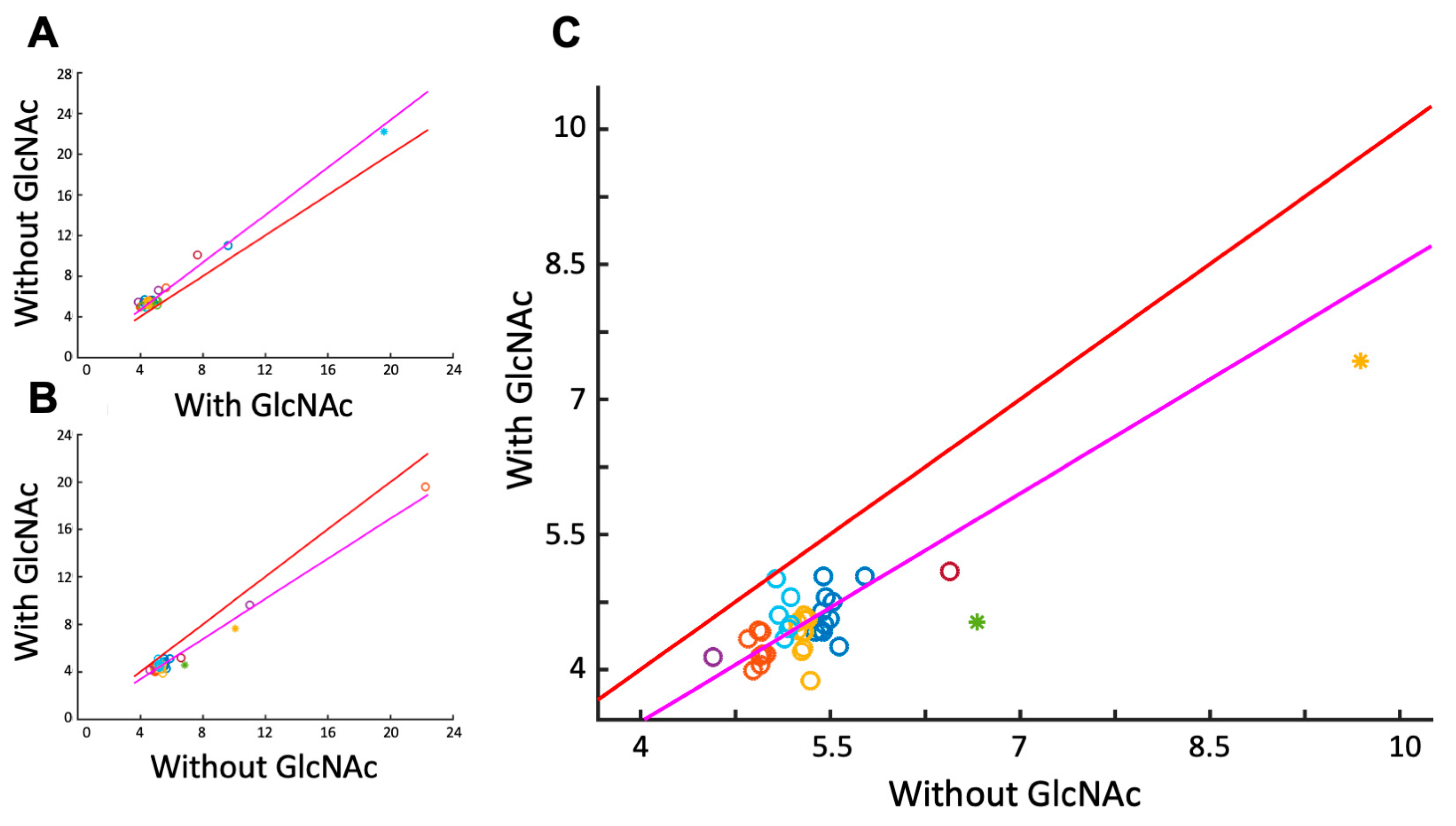
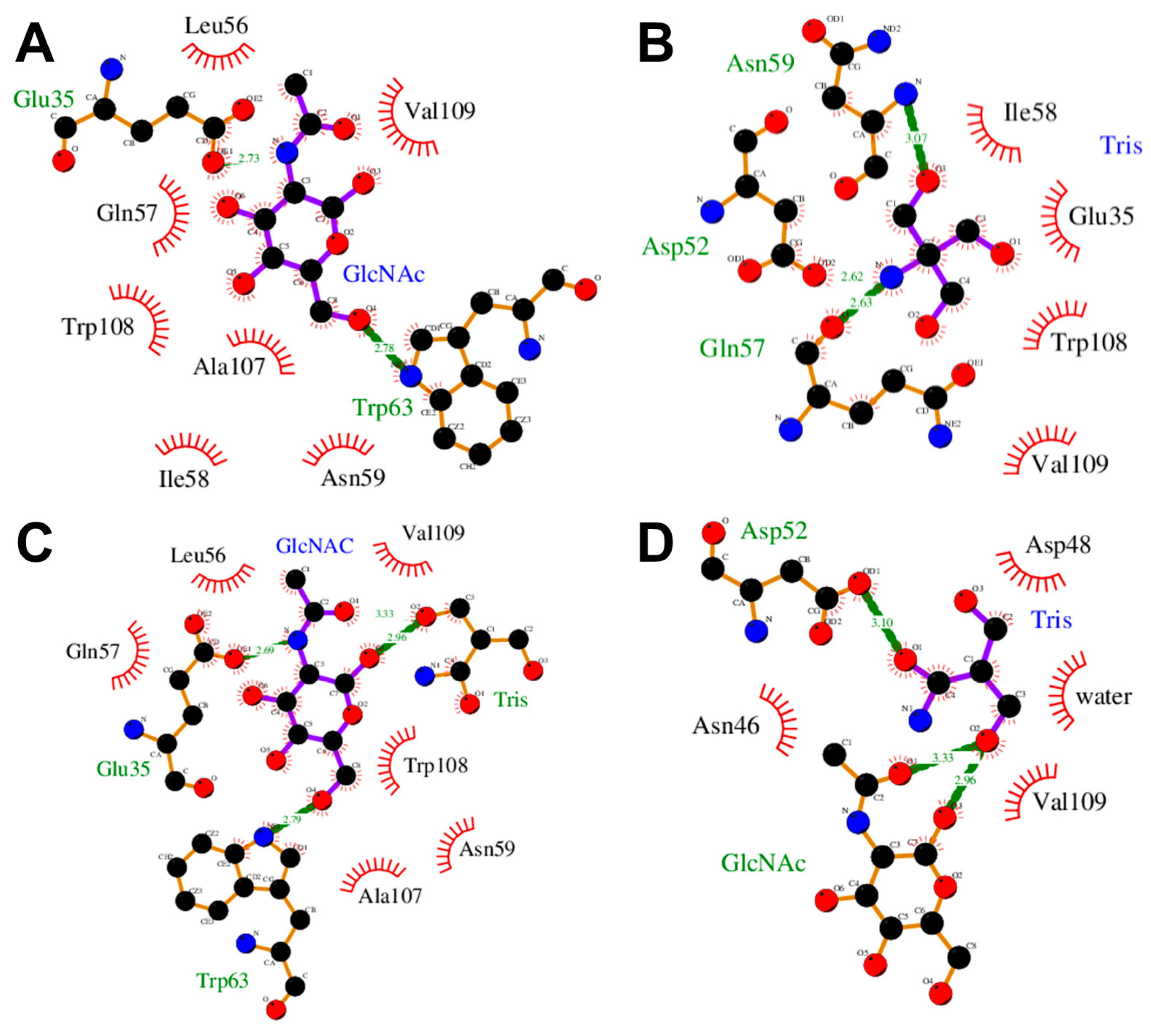
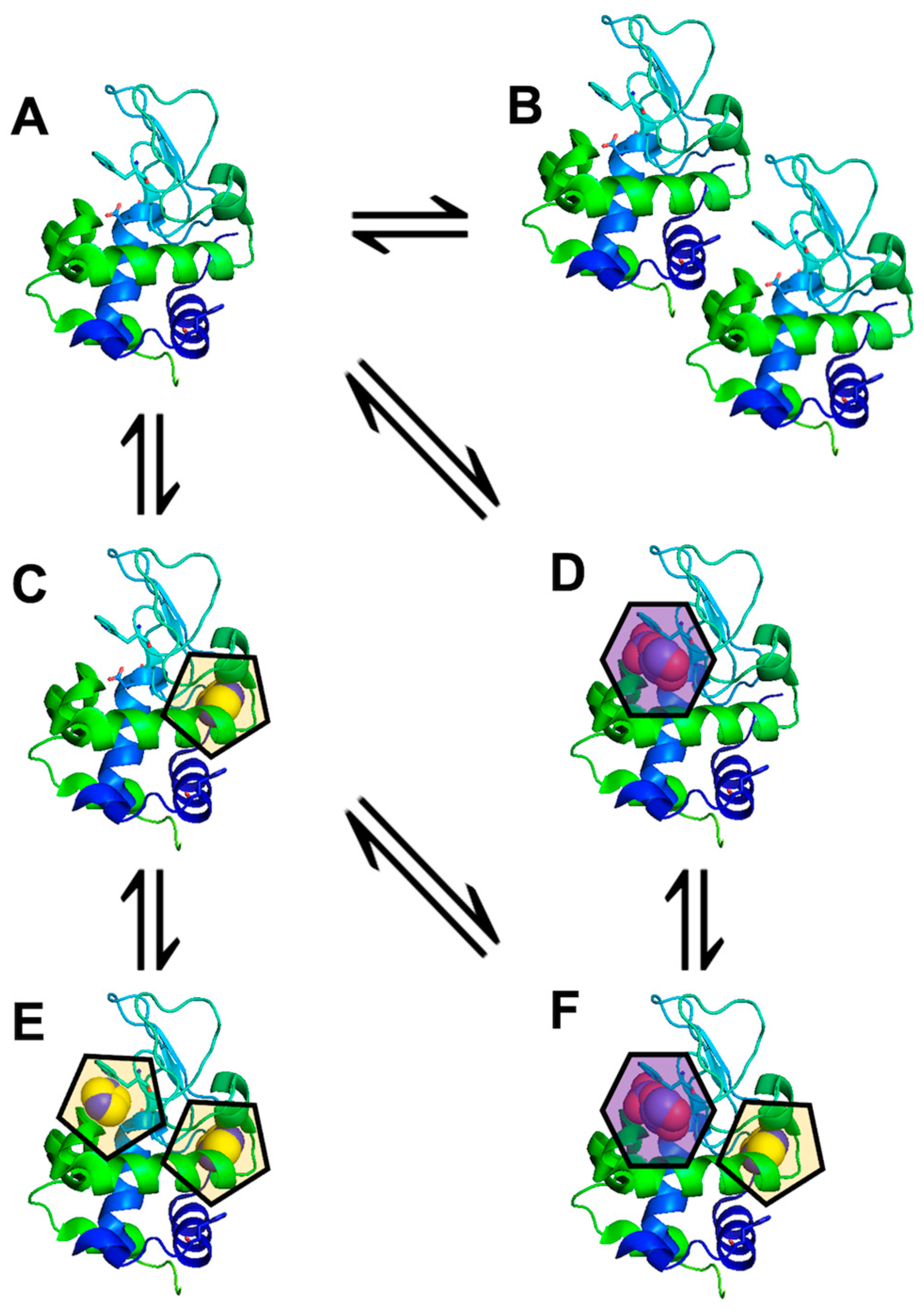
3.2. Detecting Cooperative Binding to Lysozyme
3.3. Validation of Cooperative Binding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rees, D.C.; Congreve, M.; Murray, C.W.; Carr, R. Fragment-Based Lead Discovery. Nat. Rev. Drug Discov. 2004, 3, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Austin, D.; Hayford, T. Research and Development in the Pharmaceutical Industry; Congressional Budget Office: Washington, DC, USA, 2021. [Google Scholar]
- Lamoree, B.; Hubbard, R.E. Current Perspectives in Fragment-Based Lead Discovery (FBLD). Essays Biochem. 2017, 61, 453–464. [Google Scholar]
- Emwas, A.-H.; Szczepski, K.; Poulson, B.G.; Chandra, K.; McKay, R.T.; Dhahri, M.; Alahmari, F.; Jaremko, L.; Lachowicz, J.I.; Jaremko, M. NMR as a “Gold Standard” Method in Drug Design and Discovery. Molecules 2020, 25, 4597. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, P.; Hartman, A.M.; Hirsch, A.K.; Empting, M. Concepts and Core Principles of Fragment-Based Drug Design. Molecules 2019, 24, 4309. [Google Scholar] [CrossRef]
- Ouertani, A.; Neifar, M.; Ouertani, R.; Masmoudi, A.S.; Mosbah, A.; Cherif, A. Effectiveness of Enzyme Inhibitors in Biomedicine and Pharmacotherapy. Adv. Tissue Eng. Regen. Med. Open Access 2019, 5, 85–90. [Google Scholar] [CrossRef]
- Anderson, J.W.; Vaisar, D.; Jones, D.N.; Pegram, L.M.; Vigers, G.P.; Chen, H.; Moffat, J.G.; Ahn, N.G. Conformation Selection by ATP-Competitive Inhibitors and Allosteric Communication in ERK2. eLife 2024, 12, RP91507. [Google Scholar] [CrossRef]
- Kerber, P.J.; Nuñez, R.; Jensen, D.R.; Zhou, A.L.; Peterson, F.C.; Hill, R.B.; Volkman, B.F.; Smith, B.C. Chapter Nine-Fragment-Based Screening by Protein-Detected NMR Spectroscopy. In Methods in Enzymology; Lloyd, M., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 690, pp. 285–310. ISBN 0076-6879. [Google Scholar]
- Fielding, L. NMR Methods for the Determination of Protein–Ligand Dissociation Constants. Prog. Nucl. Magn. Reson. Spectrosc. 2007, 51, 219–242. [Google Scholar] [CrossRef]
- Jayalakshmi, V.; Krishna, N.R. CORCEMA Refinement of the Bound Ligand Conformation within the Protein Binding Pocket in Reversibly Forming Weak Complexes Using STD-NMR Intensities. J. Magn. Reson. 2004, 168, 36–45. [Google Scholar] [CrossRef]
- Meyer, B.; Peters, T. NMR Spectroscopy Techniques for Screening and Identifying Ligand Binding to Protein Receptors. Angew. Chem. Int. Ed. 2003, 42, 864–890. [Google Scholar] [CrossRef]
- Carr, R.A.; Congreve, M.; Murray, C.W.; Rees, D.C. Fragment-Based Lead Discovery: Leads by Design. Drug Discov. Today 2005, 10, 987–992. [Google Scholar] [CrossRef]
- Di Carluccio, C.; Forgione, M.C.; Martini, S.; Berti, F.; Molinaro, A.; Marchetti, R.; Silipo, A. Investigation of Protein-Ligand Complexes by Ligand-Based NMR Methods. Carbohydr. Res. 2021, 503, 108313. [Google Scholar] [CrossRef] [PubMed]
- Delsuc, M.; Malliavin, T. Maximum Entropy Processing of DOSY NMR Spectra. Anal. Chem. 1998, 70, 2146–2148. [Google Scholar] [CrossRef]
- Guest, J.; Kiraly, P.; Nilsson, M.; Morris, G.A. Signal-to-Noise Ratio in Diffusion-Ordered Spectroscopy: How Good Is Good Enough? Magn Reson 2021, 2, 733–739. [Google Scholar] [CrossRef]
- Snyder, D.A.; Chantova, M.; Chaudhry, S. Analysis of Ligand–Protein Exchange by Clustering of Ligand Diffusion Coefficient Pairs (CoLD-CoP). J. Magn. Reson. 2015, 255, 44–50. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, Q.-H.; Zhuang, J.-X.; Zhong, X.; Zhou, J.-J.; Guo, Y.-J.; Chen, Q.-X. Inhibitory Effects of α-Cyano-4-Hydroxycinnamic Acid on the Activity of Mushroom Tyrosinase. Food Chem. 2009, 112, 609–613. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Chen, Q.-X.; Song, K.-K.; Xie, J.-J. Inhibitory Effects of Salicylic Acid Family Compounds on the Diphenolase Activity of Mushroom Tyrosinase. Food Chem. 2006, 95, 579–584. [Google Scholar] [CrossRef]
- Swan, I.D. The Inhibition of Hen Egg-White Lysozyme by Imidazole and Indole Derivatives. J. Mol. Biol. 1972, 65, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Hampe, O.G.; Tondo, C.V.; Hasson-Voloch, A. A Biophysical Model of Lysozyme Self-Association. Biophys. J. 1982, 40, 77–82. [Google Scholar] [CrossRef]
- Kang, T.; Hao, W.; Niu, Y.; Luo, Z.; Jin, G. Kinetic Analysis of the Weak Affinity Interaction between Tris and Lysozyme. Biochem. Biophys. Res. Commun. 2015, 457, 659–663. [Google Scholar] [CrossRef]
- Quan, L.; Wei, D.; Jiang, X.; Liu, Y.; Li, Z.; Li, N.; Li, K.; Liu, F.; Lai, L. Resurveying the Tris Buffer Solution: The Specific Interaction between Tris (Hydroxymethyl) Aminomethane and Lysozyme. Anal. Biochem. 2008, 378, 144–150. [Google Scholar] [CrossRef]
- Von Dreele, R. Binding of N-Acetylglucosamine to Chicken Egg Lysozyme: A Powder Diffraction Study. Acta Crystallogr. Sect. D Biol. Crystallogr. 2001, 57, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Manchenko, G.P. Handbook of Detection of Enzymes on Electrophoretic Gels; CRC Press: Boca Raton, FL, USA, 2002; ISBN 0-429-12800-2. [Google Scholar]
- Tuccinardi, T. What Is the Current Value of MM/PBSA and MM/GBSA Methods in Drug Discovery? Expert Opin. Drug Discov. 2021, 16, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. NMRPipe: A Multidimensional Spectral Processing System Based on UNIX Pipes. J. Biomol. NMR 1995, 6, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M. The DOSY Toolbox: A New Tool for Processing PFG NMR Diffusion Data. J. Magn. Reson. 2009, 200, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Short, T.; Alzapiedi, L.; Brüschweiler, R.; Snyder, D. A Covariance NMR Toolbox for MATLAB and OCTAVE. J. Magn. Reson. 2011, 209, 75–78. [Google Scholar] [CrossRef]
- The MathWorks. Matlab; The MathWorks: Natick, MA, USA, 2012. [Google Scholar]
- Hsu, K.-C.; Chen, Y.-F.; Lin, S.-R.; Yang, J.-M. iGEMDOCK: A Graphical Environment of Enhancing GEMDOCK Using Pharmacological Interactions and Post-Screening Analysis. BMC Bioinform. 2011, 12, S33. [Google Scholar] [CrossRef]
- Schrodinger, L. Schrodinger Software Suite; Schrödinger, LLC.: New York, NY, USA, 2011; Volume 670. [Google Scholar]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In SC ‘06: Proceedings of the 2006 ACM/IEEE conference on Supercomputing; Association for Computing Machinery: New York, NY, USA, 2006; p. 84-es. [Google Scholar]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A Program to Generate Schematic Diagrams of Protein-Ligand Interactions. Protein Eng. Des. Sel. 1995, 8, 127–134. [Google Scholar] [CrossRef]
- DeLano, W. MacPyMOL: A PyMOL-Based Molecular Graphics Application for MacOS X; DeLano Scientific LLC.: Palo Alto, CA, USA, 2007. [Google Scholar]
- Wang, Z.; Pan, H.; Sun, H.; Kang, Y.; Liu, H.; Cao, D.; Hou, T. fastDRH: A Webserver to Predict and Analyze Protein–Ligand Complexes Based on Molecular Docking and MM/PB (GB) SA Computation. Brief. Bioinform. 2022, 23, bbac201. [Google Scholar] [CrossRef]
- Onuma, K.; Inaka, K. Lysozyme Dimer Association: Similarities and Differences Compared with Lysozyme Monomer Association. J. Cryst. Growth 2008, 310, 1174–1181. [Google Scholar] [CrossRef]
- Klein Herenbrink, C.; Sykes, D.A.; Donthamsetti, P.; Canals, M.; Coudrat, T.; Shonberg, J.; Scammells, P.J.; Capuano, B.; Sexton, P.M.; Charlton, S.J. The Role of Kinetic Context in Apparent Biased Agonism at GPCRs. Nat. Commun. 2016, 7, 10842. [Google Scholar] [CrossRef] [PubMed]

| Mixture | Compound | Concentration (mM 1) |
|---|---|---|
| Tyrosinase Ligand Only | GlcNAc | 2 |
| (TL) | Salicylic Acid | 3 |
| Tartrate | 2 | |
| Thiamine | 2 | |
| Tris 2 | 10 | |
| Tryptamine | 3 | |
| ligand+protein | TL—Salicylic Acid 3 | (as above) |
| (TLP) | Salicylic Acid | 1 |
| Mushroom Tyrosinase | 4 μM | |
| ligand+protein+known ligand | TLP | (as above) |
| (TLPK) | 4-HCCA 4 | 8 |
| Lysozyme ligand only | Caffeine | 50 |
| (LL) | Citrate | 50 |
| Imidazole | 100 | |
| Tartrate | 50 | |
| Tris 2 | 50 | |
| Tryptamine | 50 | |
| ligand+protein | LL | (as above) |
| (LLP) | Hen Egg White Lysozyme | 1 |
| ligand+protein+known ligand | LLP | (as above) |
| (LLPK) | GlcNAc | 300 |
| Parameters | GlcNAc 1 | Tris 1 | Ternary Complex 1 | Ternary Complex— (GlcNAc + Tris) 1 |
|---|---|---|---|---|
| PB1 | 5.41 | −5.32 | −5.34 | −5.43 |
| PB3 | −6.89 | −16.99 | −27.33 | −3.45 |
| PB4 | −17.16 | −25.67 | −42.35 | 0.48 |
| GB1 | −23.13 | −28.59 | −49.64 | 2.08 |
| GB2 | −25.42 | −27.1 | −55.25 | −2.73 |
| GB5 2 | −29 | −28.05 | −59.96 | −2.91 |
| GB6 | −20.25 | −26.19 | −50.8 | −4.36 |
| GB7 2 | −34.67 | −26.31 | −68.03 | −7.05 |
| GB8 2,3 | −21.77 | −20.27 | −45.05 | −3.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patnala, S.V.; Robles, R.; Snyder, D.A. Application of CoLD-CoP to Detecting Competitively and Cooperatively Binding Ligands. Biomolecules 2024, 14, 1136. https://doi.org/10.3390/biom14091136
Patnala SV, Robles R, Snyder DA. Application of CoLD-CoP to Detecting Competitively and Cooperatively Binding Ligands. Biomolecules. 2024; 14(9):1136. https://doi.org/10.3390/biom14091136
Chicago/Turabian StylePatnala, Shiva V., Roberto Robles, and David A. Snyder. 2024. "Application of CoLD-CoP to Detecting Competitively and Cooperatively Binding Ligands" Biomolecules 14, no. 9: 1136. https://doi.org/10.3390/biom14091136







