Prognostic Value of Circulating Fibrosis Biomarkers in Dilated Cardiomyopathy (DCM): Insights into Clinical Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment, Follow-Up, and End Points
2.2. Biomarker Measurement
2.3. Statistical Analysis
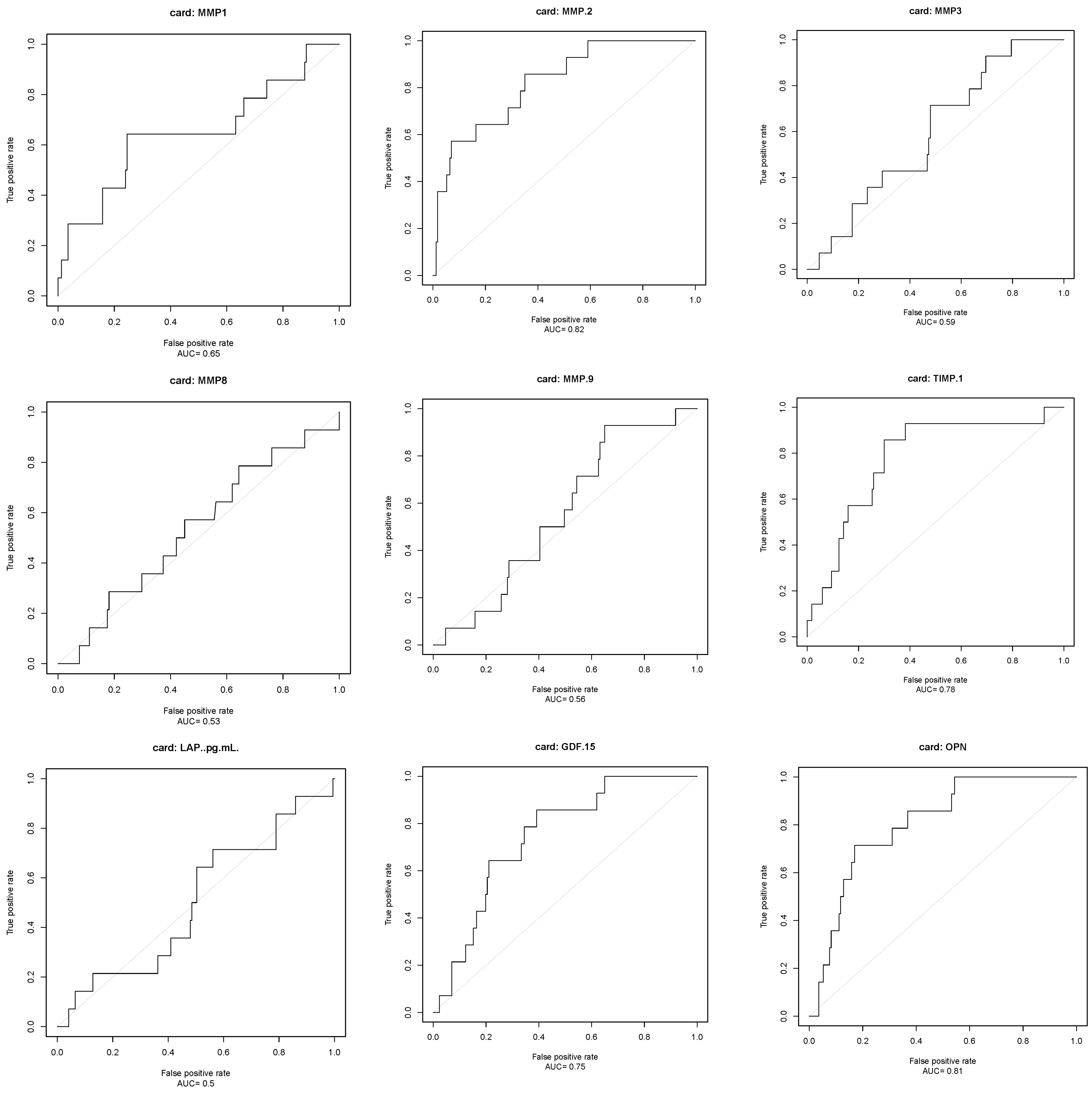
3. Result
3.1. Patients’ Characteristics
3.2. Differences in Patient Characteristics by Biomarker Cut-Off Levels
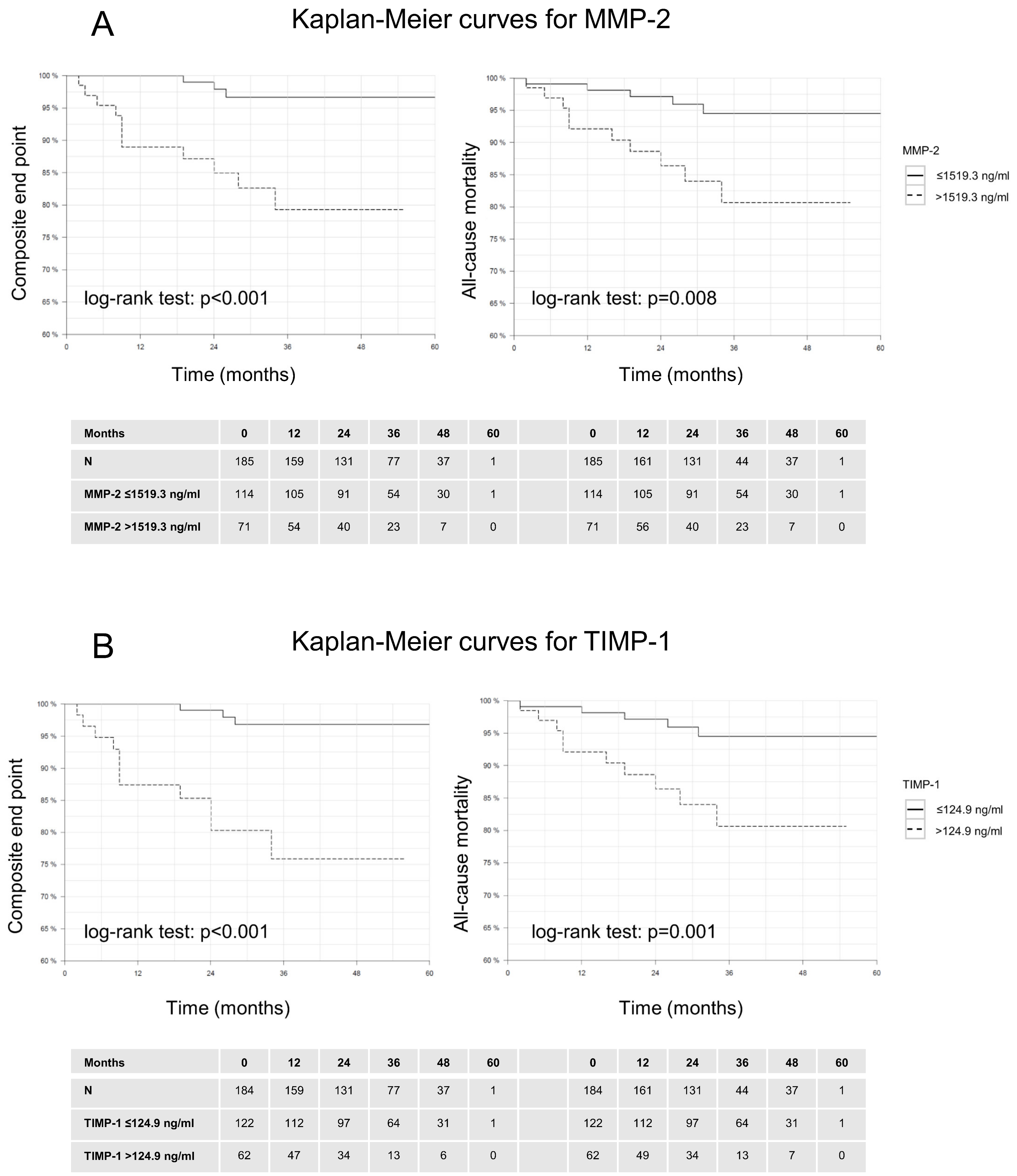
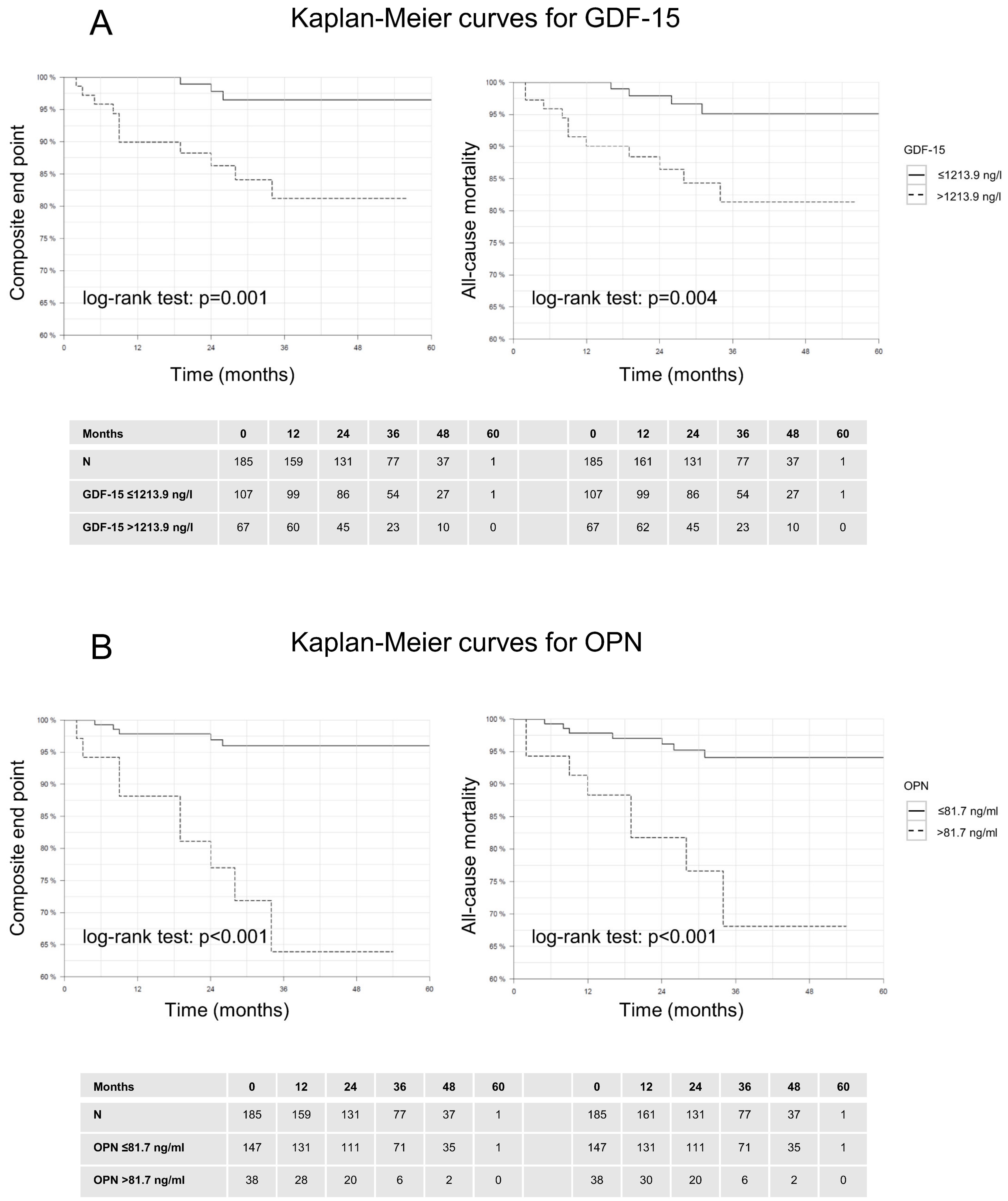
3.3. Outcome Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.L.; Mann, C.J.; Vidal, B.; Ardite, E.; Perdiguero, E.; Munoz-Canoves, P. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr. Top. Dev. Biol. 2011, 96, 167–201. [Google Scholar] [PubMed]
- Creemers, E.E.; Pinto, Y.M. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc. Res. 2011, 89, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Krenning, G.; Zeisberg, E.M.; Kalluri, R. The origin of fibroblasts and mechanism of cardiac fibrosis. J. Cell Physiol. 2010, 225, 631–637. [Google Scholar] [CrossRef]
- Kayvanpour, E.; Sedaghat-Hamedani, F.; Amr, A.; Lai, A.; Haas, J.; Holzer, D.B.; Frese, K.S.; Keller, A.; Jensen, K.; Katus, H.A.; et al. Genotype-phenotype associations in dilated cardiomyopathy: Meta-analysis on more than 8000 individuals. Clin. Res. Cardiol. 2017, 106, 127–139. [Google Scholar] [CrossRef]
- Pescariu, S.A.; Sosdean, R.; Tudoran, C.; Ionac, A.; Pop, G.N.; Timar, R.Z.; Pescariu, S.; Tudoran, M. Echocardiographic Parameters as Predictors for the Efficiency of Resynchronization Therapy in Patients with Dilated Cardiomyopathy and HFrEF. Diagnostics 2021, 12, 35. [Google Scholar] [CrossRef]
- Lau, C.; Gul, U.; Liu, B.; Captur, G.; Hothi, S.S. Cardiovascular Magnetic Resonance Imaging in Familial Dilated Cardiomyopathy. Medicina 2023, 59, 439. [Google Scholar] [CrossRef] [PubMed]
- Grasso, M.; Bondavalli, D.; Vilardo, V.; Cavaliere, C.; Gatti, I.; Di Toro, A.; Giuliani, L.; Urtis, M.; Ferrari, M.; Cattadori, B.; et al. The new 2023 ESC guidelines for the management of cardiomyopathies: A guiding path for cardiologist decisions. Eur. Heart J. Suppl. 2024, 26 (Suppl. 1), i1–i5. [Google Scholar] [CrossRef]
- Lopez, B.; Gonzalez, A.; Ravassa, S.; Beaumont, J.; Moreno, M.U.; San Jose, G.; Querejeta, R.; Díez, J. Circulating Biomarkers of Myocardial Fibrosis: The Need for a Reappraisal. J. Am. Coll. Cardiol. 2015, 65, 2449–2456. [Google Scholar] [CrossRef]
- Woulfe, K.C.; Siomos, A.K.; Nguyen, H.; SooHoo, M.; Galambo, C.; Stauffer, B.L.; Sucharov, C.; Miyamoto, S. Fibrosis and Fibrotic Gene Expression in Pediatric and Adult Patients with Idiopathic Dilated Cardiomyopathy. J. Card. Fail. 2016. [CrossRef]
- Polyakova, V.; Hein, S.; Kostin, S.; Ziegelhoeffer, T.; Schaper, J. Matrix metalloproteinases and their tissue inhibitors in pressure-overloaded human myocardium during heart failure progression. J. Am. Coll. Cardiol. 2004, 44, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Polyakova, V.; Loeffler, I.; Hein, S.; Miyagawa, S.; Piotrowska, I.; Dammer, S.; Risteli, J.; Schaper, J.; Kostin, S. Fibrosis in endstage human heart failure: Severe changes in collagen metabolism and MMP/TIMP profiles. Int. J. Cardiol. 2011, 151, 18–33. [Google Scholar] [CrossRef]
- Li, J.; Schwimmbeck, P.L.; Tschope, C.; Leschka, S.; Husmann, L.; Rutschow, S.; Reichenbach, F.; Noutsias, M.; Kobalz, U.; Poller, W.; et al. Collagen degradation in a murine myocarditis model: Relevance of matrix metalloproteinase in association with inflammatory induction. Cardiovasc. Res. 2002, 56, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; Coker, M.L.; Heung, L.J.; Bond, B.R.; Gunasinghe, H.R.; Etoh, T.; Goldberg, A.T.; Zellner, J.L.; Crumbley, A.J. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation 2000, 102, 1944–1949. [Google Scholar] [CrossRef]
- Spinale, F.G.; Coker, M.L.; Krombach, S.R.; Mukherjee, R.; Hallak, H.; Houck, W.V.; Clair, M.J.; Kribbs, S.B.; Johnson, L.L.; Peterson, J.T.; et al. Matrix metalloproteinase inhibition during the development of congestive heart failure: Effects on left ventricular dimensions and function. Circ. Res. 1999, 85, 364–376. [Google Scholar] [CrossRef]
- Banfi, C.; Cavalca, V.; Veglia, F.; Brioschi, M.; Barcella, S.; Mussoni, L.; Boccotti, L.; Tremoli, E.; Biglioli, P.; Agostoni, P. Neurohormonal activation is associated with increased levels of plasma matrix metalloproteinase-2 in human heart failure. Eur. Heart J. 2005, 26, 481–488. [Google Scholar] [CrossRef]
- Wilson, E.M.; Gunasinghe, H.R.; Coker, M.L.; Sprunger, P.; Lee-Jackson, D.; Bozkurt, B.; Deswal, A.; Mann, D.L.; Spinale, F.G. Plasma matrix metalloproteinase and inhibitor profiles in patients with heart failure. J. Card. Fail. 2002, 8, 390–398. [Google Scholar] [CrossRef]
- George, J.; Patal, S.; Wexler, D.; Roth, A.; Sheps, D.; Keren, G. Circulating matrix metalloproteinase-2 but not matrix metalloproteinase-3, matrix metalloproteinase-9, or tissue inhibitor of metalloproteinase-1 predicts outcome in patients with congestive heart failure. Am. Heart J. 2005, 150, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Brehm, M.; Fassbach, M.; Pelzer, B.; Scheuring, S.; Kury, P.; Strauer, B.E.; Schwartzkopff, B. Increased cardiac mRNA expression of matrix metalloproteinase-1 (MMP-1) and its inhibitor (TIMP-1) in DCM patients. Clin. Res. Cardiol. 2006, 95, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Feldman, A.M.; Sun, Y.; McTiernan, C.F. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation 1998, 98, 1728–1734. [Google Scholar] [CrossRef]
- Schwartzkopff, B.; Fassbach, M.; Pelzer, B.; Brehm, M.; Strauer, B.E. Elevated serum markers of collagen degradation in patients with mild to moderate dilated cardiomyopathy. Eur. J. Heart Fail. 2002, 4, 439–444. [Google Scholar] [CrossRef]
- Kempf, T.; von Haehling, S.; Peter, T.; Allhoff, T.; Cicoira, M.; Doehner, W.; Ponikowski, P.; Filippatos, G.S.; Rozentryt, P.; Drexler, H.; et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Kempf, T.; Zarbock, A.; Widera, C.; Butz, S.; Stadtmann, A.; Rossaint, J.; Bolomini-Vittori, M.; Korf-Klingebiel, M.; Napp, L.C.; Hansen, B.; et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat. Med. 2011, 17, 581–588. [Google Scholar] [CrossRef]
- Lok, S.I.; Winkens, B.; Goldschmeding, R.; van Geffen, A.J.; Nous, F.M.; van Kuik, J.; Van Der Weide, P.; Klöpping, C.; Kirkels, J.H.; Lahpor, J.R.; et al. Circulating growth differentiation factor-15 correlates with myocardial fibrosis in patients with non-ischaemic dilated cardiomyopathy and decreases rapidly after left ventricular assist device support. Eur. J. Heart Fail. 2012, 14, 1249–1256. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Matricellular proteins in cardiac adaptation and disease. Physiol. Rev. 2012, 92, 635–688. [Google Scholar] [CrossRef]
- Engebretsen, K.V.; Skardal, K.; Bjornstad, S.; Marstein, H.S.; Skrbic, B.; Sjaastad, I.; Christensen, G.; Bjørnstad, J.L.; Tønnessen, T. Attenuated development of cardiac fibrosis in left ventricular pressure overload by SM16, an orally active inhibitor of ALK5. J. Mol. Cell Cardiol. 2014, 76, 148–157. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Peterson, J.T.; Subramanian, V.; Singh, M.; Singh, K. Inhibition of matrix metalloproteinases improves left ventricular function in mice lacking osteopontin after myocardial infarction. Mol. Cell Biochem. 2009, 322, 53–62. [Google Scholar] [CrossRef]
- Wang, J.; Gong, X.; Chen, H.; Qin, S.; Zhou, N.; Su, Y.; Ge, J. Effect of Cardiac Resynchronization Therapy on Myocardial Fibrosis and Relevant Cytokines in a Canine Model with Experimental Heart Failure. J. Cardiovasc. Electrophysiol. 2017, 28, 438–445. [Google Scholar]
- Singh, M.; Foster, C.R.; Dalal, S.; Singh, K. Osteopontin: Role in extracellular matrix deposition and myocardial remodeling post-MI. J. Mol. Cell Cardiol. 2010, 48, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Dalal, S.; Singh, K. Osteopontin: At the cross-roads of myocyte survival and myocardial function. Life Sci. 2014, 118, 1–6. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | All Patients (Max. N = 185) |
|---|---|
| Age, mean (SD), years | 55.0 (13.1) |
| Males, n (%) | 117 (63.2) |
| BMI, mean (SD), kg/m2 | 27.6 (6.2) |
| Arterial hypertension, n (%) | 122 (84.1) |
| Diabetes, n (%) | 20 (14.3) |
| Tobacco smoking | |
| Current smoker, n (%) | 26 (27.4) |
| Never smoker, (%) | 40 (42.1) |
| Quit smoking, n (%) | 29 (30.5) |
| Alcohol excess *, n (%) | 0 (0.0) |
| Family history of CV disease, n (%) | 19 (14.7) |
| Lipid profile | |
| Total cholesterol, mean (SD), mg/dL | 189.0 (42.7) |
| High-density lipoprotein, mean (SD), mg/dL | 48.2 (14.9) |
| Low-density lipoprotein, mean (SD), mg/dL | 113.1 (30.0) |
| Triglyceride, mean (SD), mg/dL | 174.1 (110.8) |
| White blood cell count, mean (SD),/nL | 8.3 (3.5) |
| Hemoglobin, mean (SD), g/dL | 13.7 (1.8) |
| Serum creatinine, mean (SD), mg/dL | 1.1 (0.9) |
| NT-proBNP, median (IQR), ng/L | 1027.0 [278.0; 3588.0] |
| hs-TNT, median (IQR), pg/mL | 16.0 [9.0; 36.0] |
| Heart rate, mean (SD), beats/min | 79.2 (19.5) |
| Left bundle-branch block, n (%) | 5 (25.0) |
| Atrial fibrillation, n (%) | 42 (22.8) |
| Blood pressure, mean (SD), mmHg | |
| Systolic | 125.1 (16.9) |
| Diastolic | 74.1 (14.3) |
| Dyspnoea, n (%) | |
| NYHA I | 30 (54.5) |
| NYHA II | 9 (16.4) |
| NYHA III | 7 (12.7) |
| NYHA IV | 9 (16.4) |
| 6MWT, mean (SD), m | 395.5 (185.4) |
| VO2 max, mean (SD), mL/(kg·min) | 16.0 (6.1) |
| Medication at first visit | |
| Beta blocker | 137 (75.3) |
| RAS inhibitor | 140 (76.9) |
| Echocardiography | |
| LV ejection fraction, mean (SD) | 31.6 (12.5) |
| Cardiac MRI data | |
| LV ejection fraction, mean (SD) | 40.3 (13.2) |
| LV stroke volume, mean (SD), mL | 92.5 (29.1) |
| LV-ESV index, mean (SD), mL/m2 | 76.9 (41.4) |
| LV-EDV index, mean (SD), mL/m2 | 121.1 (40.6) |
| LV-ESD index, mean (SD), mm/m2 | 24.1 (6.4) |
| LV-EDD index, mean (SD), mm/m2 | 30.5 (5.3) |
| LV mass index, mean (SD), g/m2 | 59.9 (19.3) |
| Septum wall thickness, mean (SD), mm | 9.6 (2.2) |
| RV-EDD index, mean (SD), mm/m2 | 23.7 (4.3) |
| LA diameter, mean (SD), mm | 39.5 (8.8) |
| MAPSE, mean (SD), mm | 8.9 (3.2) |
| TAPSE, mean (SD), mm | 18.6 (5.0) |
| Extent of late gadolinium enhancement, %, SD | 4.5 (3.0) |
| Biomarker | All Patients (Max. N = 185) |
|---|---|
| MMP-1, median (IQR), ng/L | 566.71 [328.2, 960.6] |
| MMP-2, median (IQR),ng/mL | 1385.0 [1119.9, 1785.5] |
| MMP-3, median (IQR), ng/L | 6617.4 [4417.1, 8957.4] |
| MMP-8, median (IQR), ng/L | 3756.6 [1736.6, 7914.2] |
| MMP-9, median (IQR), ng/mL | 116.8 [59.4, 226.7] |
| TIMP-1, median (IQR), ng/mL | 104.5 [78.2, 144.0] |
| LAP, median (IQR), ng/L | 10362.0 [6457.0, 15039.5] |
| GDF-15, median (IQR), ng/L | 1049.0 [708.2, 2041.1] |
| OPN, median (IQR), ng/mL | 52.8 [35.9, 75.2] |
| Syndecan-1, median (IQR), ng/L | 4038.7 [2735.2, 5430.4] |
| Syndecan-4, median (IQR), ng/L | 2198.09 [1508.0, 2919.7] |
| Soluble ST2, median (IQR), ng/mL | 13.4 [10.1, 19.2] |
| Galectin-3, median (IQR), ng/mL | 11.6 [9.3, 15.7] |
| Variable | Cut-Off | Sensitivity (%) | Specificity (%) | PPV | NPV |
|---|---|---|---|---|---|
| MMP-2 | >1519.3 ng/mL | 86 | 65 | 17 | 98 |
| TIMP-1 | >124.9 ng/mL | 86 | 70 | 19 | 98 |
| GDF-15 | >1213.9 ng/L | 86 | 61 | 15 | 98 |
| OPN | >81.7 ng/mL | 71 | 83 | 26 | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayvanpour, E.; Sedaghat-Hamedani, F.; Li, D.T.; Miersch, T.; Weis, T.; Hoefer, I.; Frey, N.; Meder, B. Prognostic Value of Circulating Fibrosis Biomarkers in Dilated Cardiomyopathy (DCM): Insights into Clinical Outcomes. Biomolecules 2024, 14, 1137. https://doi.org/10.3390/biom14091137
Kayvanpour E, Sedaghat-Hamedani F, Li DT, Miersch T, Weis T, Hoefer I, Frey N, Meder B. Prognostic Value of Circulating Fibrosis Biomarkers in Dilated Cardiomyopathy (DCM): Insights into Clinical Outcomes. Biomolecules. 2024; 14(9):1137. https://doi.org/10.3390/biom14091137
Chicago/Turabian StyleKayvanpour, Elham, Farbod Sedaghat-Hamedani, Daniel Tian Li, Tobias Miersch, Tanja Weis, Imo Hoefer, Norbert Frey, and Benjamin Meder. 2024. "Prognostic Value of Circulating Fibrosis Biomarkers in Dilated Cardiomyopathy (DCM): Insights into Clinical Outcomes" Biomolecules 14, no. 9: 1137. https://doi.org/10.3390/biom14091137








