Oxyresveratrol Enhances the Anti-Cancer Effect of Cisplatin against Epithelial Ovarian Cancer Cells through Suppressing the Activation of Protein Kinase B (AKT)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. Cell Apoptosis Analysis
2.4. Cell Cycle Analysis
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
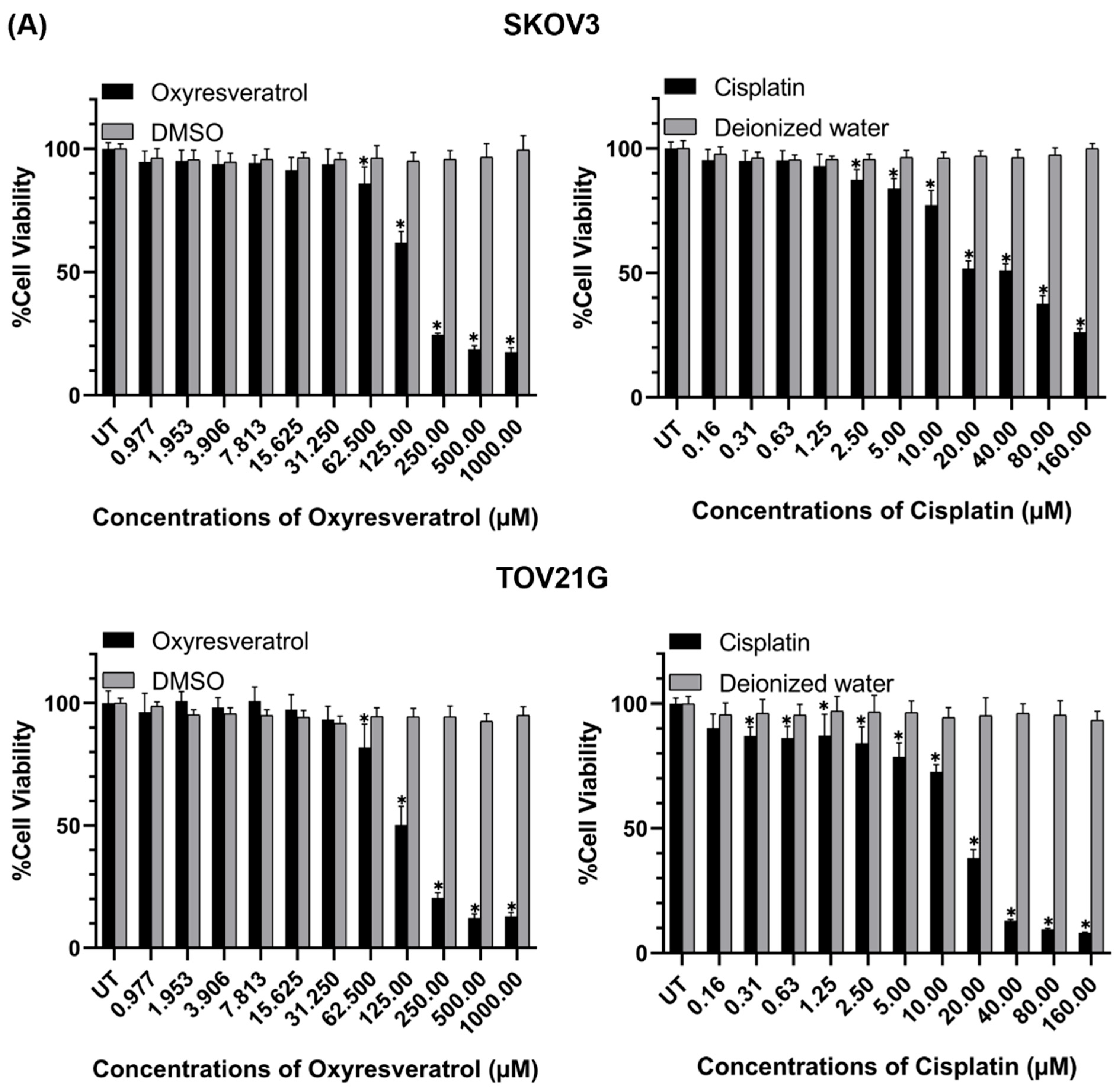
3.1. Cytotoxic Effects of Oxyresveratrol (OXY) and Its Combination with Cisplatin on Ovarian Cancer Cells
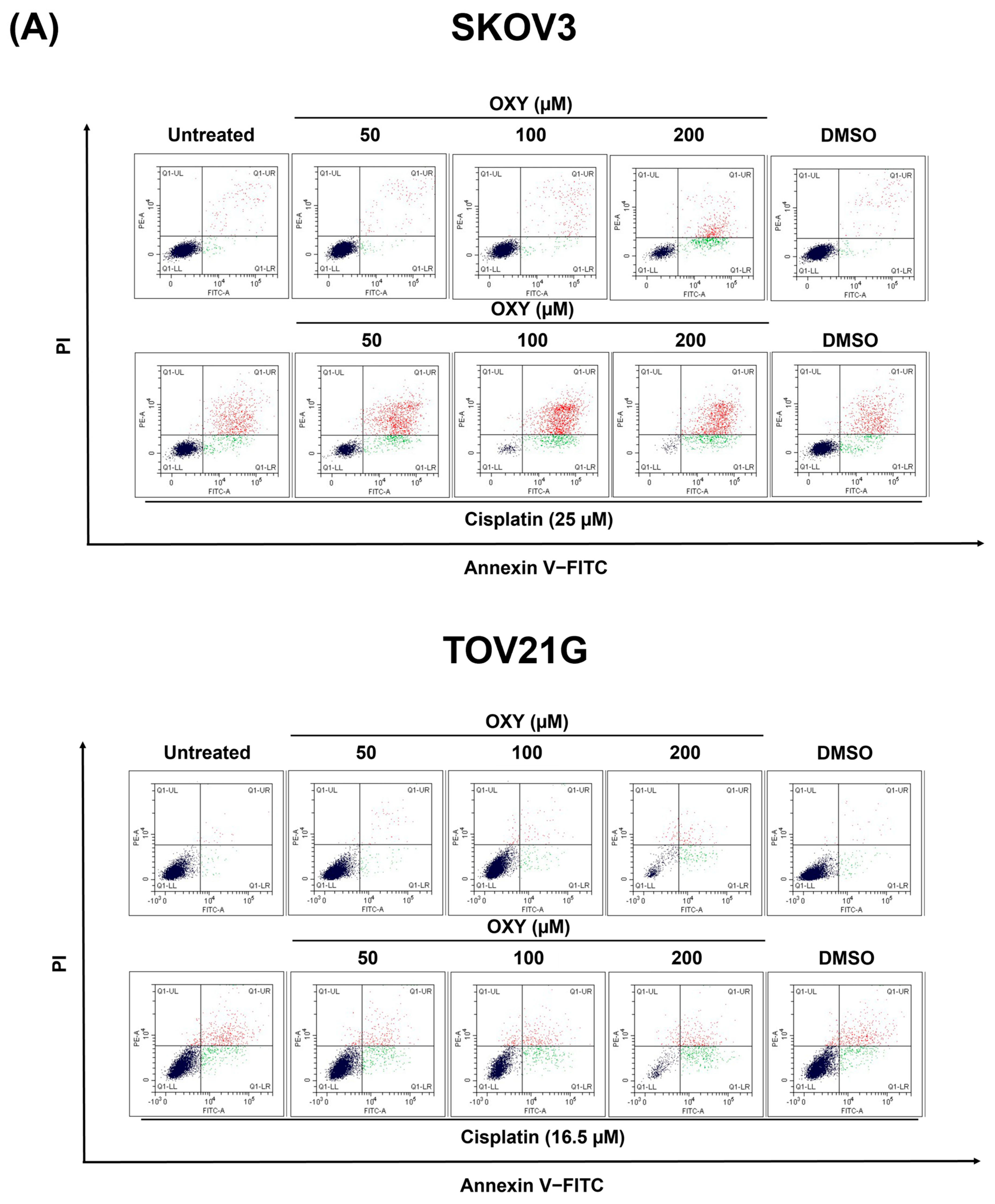
3.2. The Effects of Oxyresveratrol on Ovarian Cancer Cell Apoptosis Induction
3.3. The Effects of Oxyresveratrol on Ovarian Cancer Cell Cycle Arrest
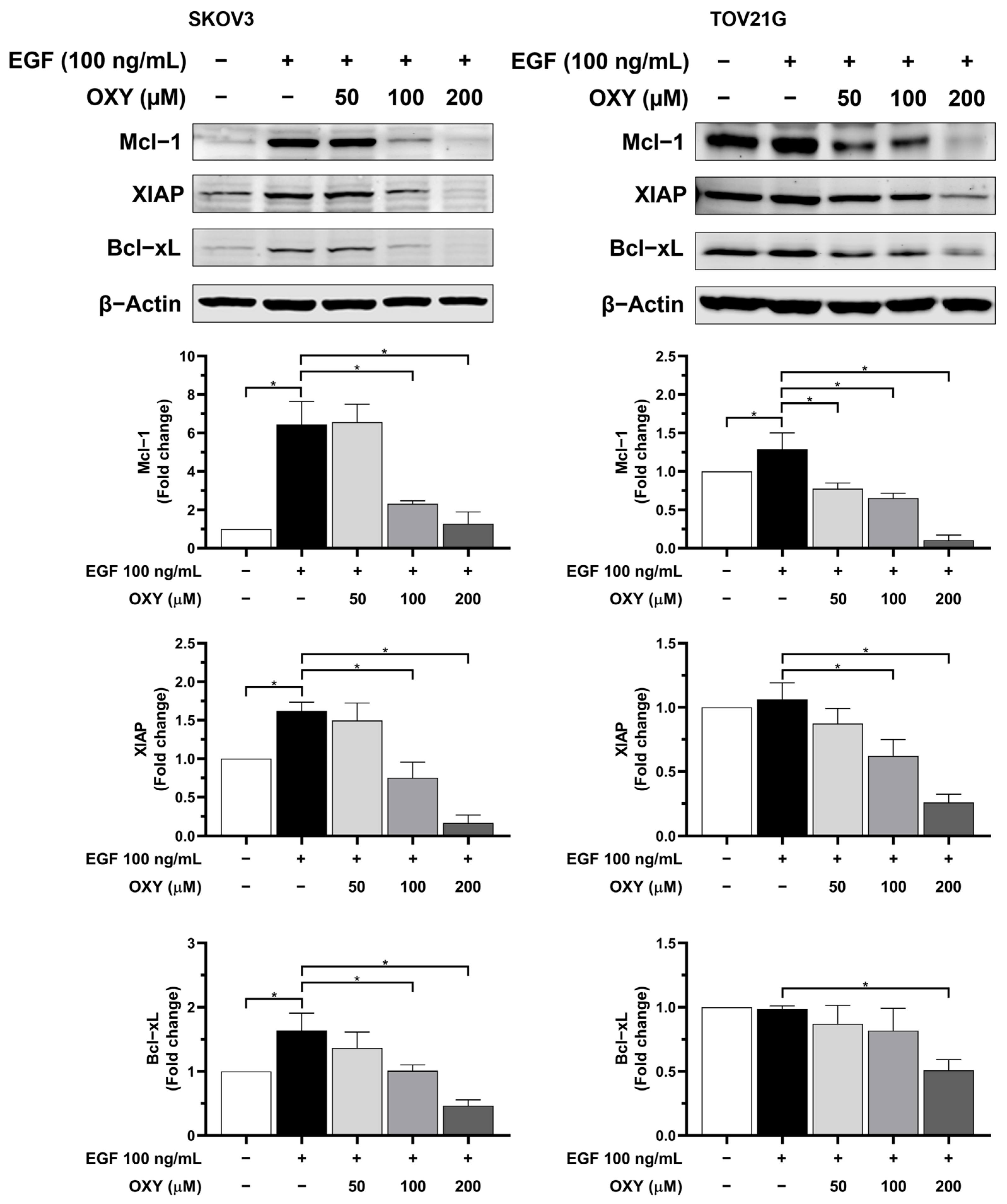
3.4. The Effects of Oxyresveratrol on Anti-Apoptotic Protein Production
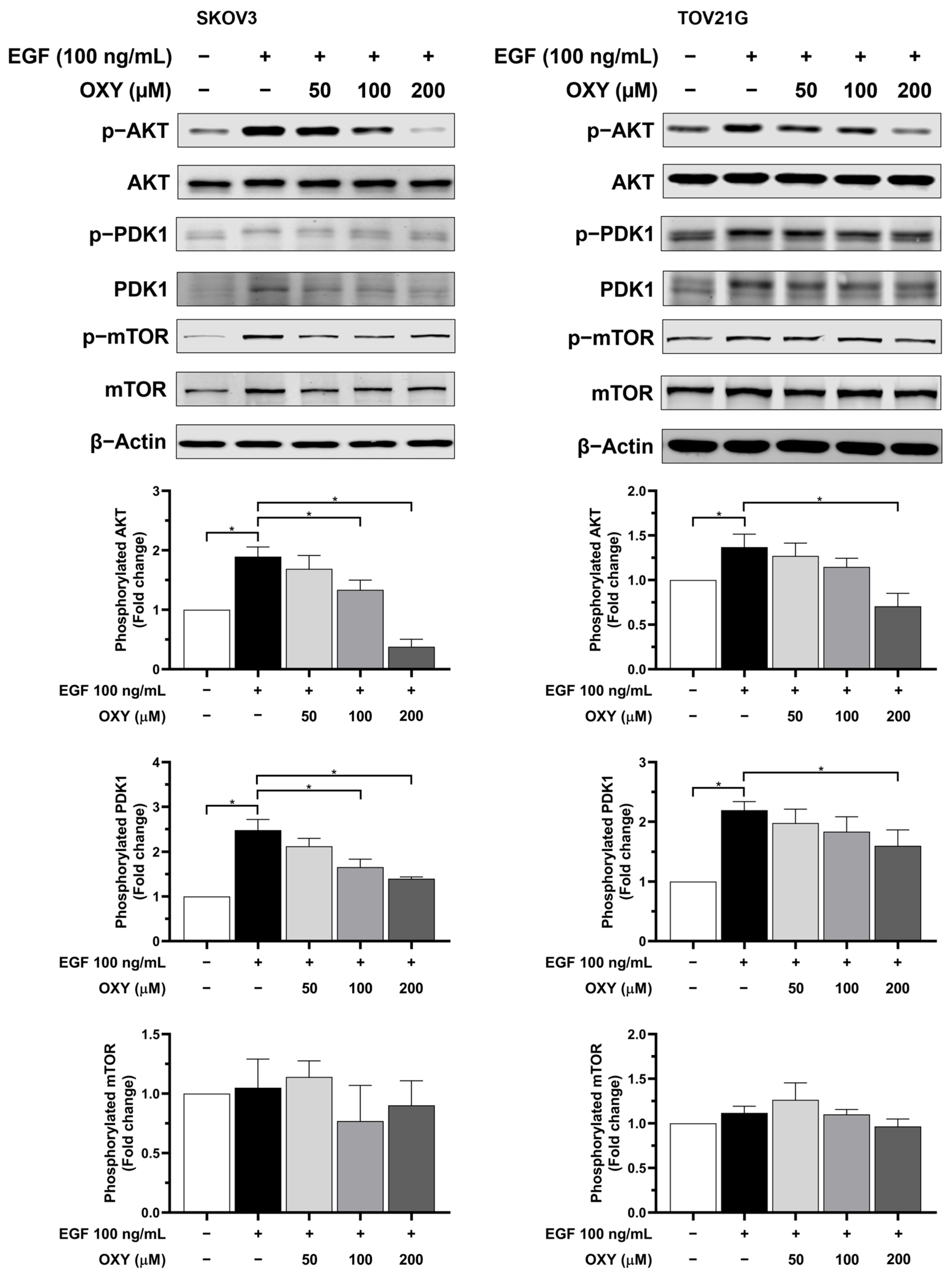
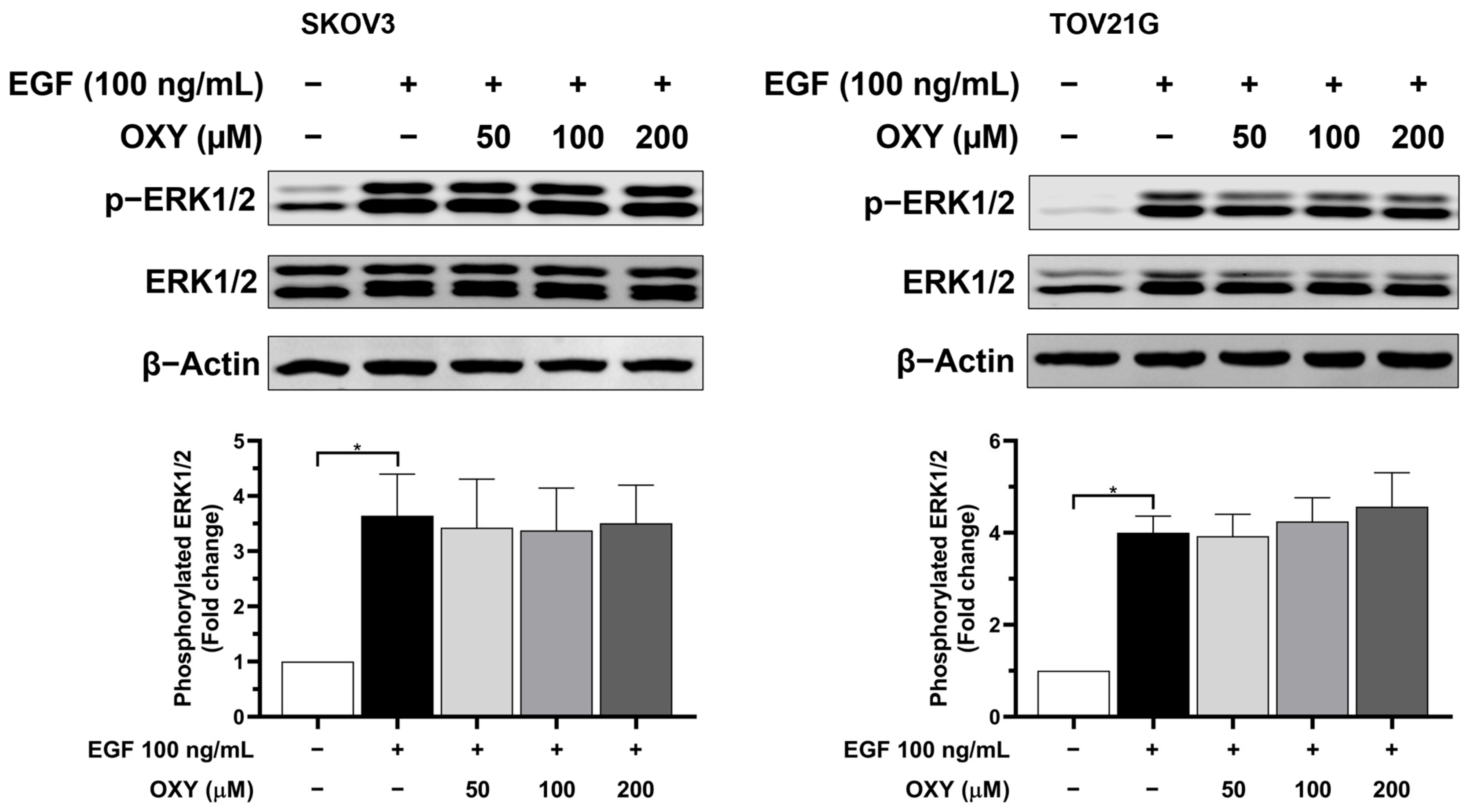
3.5. The Effects of Oxyresveratrol on Inhibiting Cell Growth and Survival Signaling Pathways
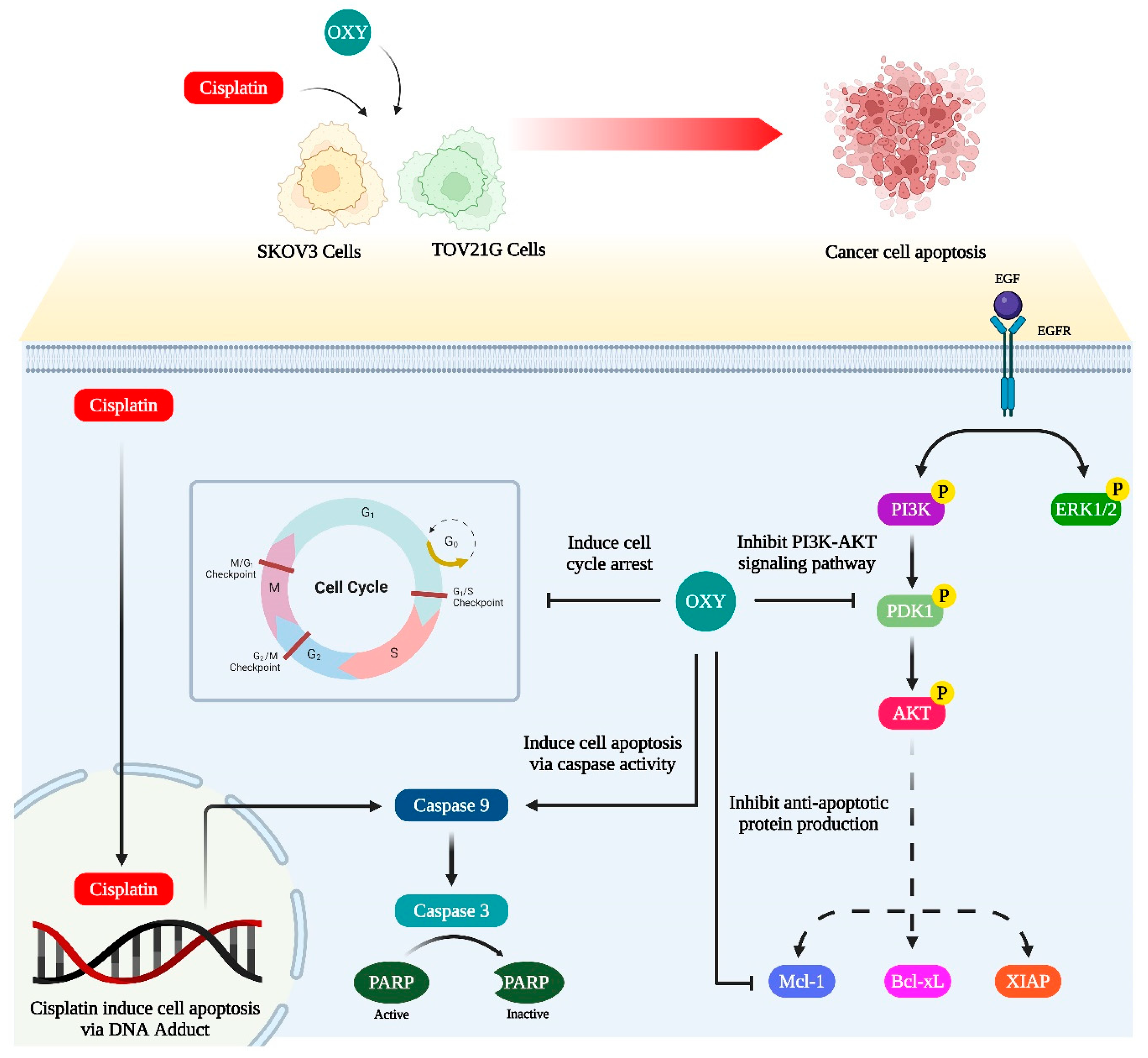
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Chan, W.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.-J.; Elcarte, E.; Withers, M.; et al. Worldwide Burden, Risk Factors, and Temporal Trends of Ovarian Cancer: A Global Study. Cancers 2022, 14, 2230. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Ovarian Cancer: Estimated New Cases and Death in 2023. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 6 December 2023).
- Li, S.S.; Ma, J.; Wong, A.S.T. Chemoresistance in ovarian cancer: Exploiting cancer stem cell metabolism. J. Gynecol. Oncol. 2018, 29, e32. [Google Scholar] [CrossRef]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomark. Cancer 2019, 11, 1179299x19860815. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.K.; Lin, Y.H.; Chang, H.A.; Lai, Y.S.; Chen, Y.C.; Huang, S.C.; Chou, C.Y.; Chiu, W.T. Chemoresistant ovarian cancer enhances its migration abilities by increasing store-operated Ca2+ entry-mediated turnover of focal adhesions. J. Biomed. Sci. 2020, 27, 36. [Google Scholar] [CrossRef]
- Ramus, S.J.; Gayther, S.A. The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol. Oncol. 2009, 3, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.R.; Davidoff, A.M.; Kerns, B.J.; Humphrey, P.A.; Pence, J.C.; Dodge, R.K.; Clarke-Pearson, D.L.; Iglehart, J.D.; Bast, R.C., Jr.; Berchuck, A. Overexpression and mutation of p53 in epithelial ovarian cancer. Cancer Res. 1991, 51, 2979–2984. [Google Scholar]
- Zhang, Y.; Cao, L.; Nguyen, D.; Lu, H. TP53 mutations in epithelial ovarian cancer. Transl. Cancer Res. 2016, 5, 650–663. [Google Scholar] [CrossRef]
- Thorstenson, Y.R.; Roxas, A.; Kroiss, R.; Jenkins, M.A.; Yu, K.M.; Bachrich, T.; Muhr, D.; Wayne, T.L.; Chu, G.; Davis, R.W.; et al. Contributions of ATM mutations to familial breast and ovarian cancer. Cancer Res. 2003, 63, 3325–3333. [Google Scholar]
- Hall, M.J.; Bernhisel, R.; Hughes, E.; Larson, K.; Rosenthal, E.T.; Singh, N.A.; Lancaster, J.M.; Kurian, A.W. Germline Pathogenic Variants in the Ataxia Telangiectasia Mutated (ATM) Gene are Associated with High and Moderate Risks for Multiple Cancers. Cancer Prev. Res. 2021, 14, 433–440. [Google Scholar] [CrossRef]
- Dobrzycka, B.; Terlikowski, S.J.; Kowalczuk, O.; Niklińska, W.; Chyczewski, L.; Kulikowski, M. Mutations in the KRAS gene in ovarian tumors. Folia Histochem. Cytobiol. 2009, 47, 221–224. [Google Scholar] [CrossRef]
- Therachiyil, L.; Anand, A.; Azmi, A.; Bhat, A.; Korashy, H.M.; Uddin, S. Role of RAS signaling in ovarian cancer. F1000Research 2022, 11, 1253. [Google Scholar] [CrossRef]
- Fresno Vara, J.A.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, A.; Said, N. PI3K-AKT-mTOR and NFκB Pathways in Ovarian Cancer: Implications for Targeted Therapeutics. Cancers 2019, 11, 949. [Google Scholar] [CrossRef] [PubMed]
- Cheaib, B.; Auguste, A.; Leary, A. The PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities and challenges. Chin. J. Cancer 2015, 34, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.C.; Couturier, D.L.; Paterson, A.; Karnezis, A.N.; Chow, C.; Nazeran, T.M.; Odunsi, A.; Gentry-Maharaj, A.; Vrvilo, A.; Hein, A.; et al. Clinical and pathological associations of PTEN expression in ovarian cancer: A multicentre study from the Ovarian Tumour Tissue Analysis Consortium. Br. J. Cancer 2020, 123, 793–802. [Google Scholar] [CrossRef]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef]
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S.; Basha, R. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef]
- Zoń, A.; Bednarek, I. Cisplatin in Ovarian Cancer Treatment-Known Limitations in Therapy Force New Solutions. Int. J. Mol. Sci. 2023, 24, 7585. [Google Scholar] [CrossRef]
- Perego, P.; Romanelli, S.; Carenini, N.; Magnani, I.; Leone, R.; Bonetti, A.; Paolicchi, A.; Zunino, F. Ovarian cancer cisplatin-resistant cell lines: Multiple changes including collateral sensitivity to Taxol. Ann. Oncol. 1998, 9, 423–430. [Google Scholar] [CrossRef]
- Sakai, W.; Swisher, E.M.; Karlan, B.Y.; Agarwal, M.K.; Higgins, J.; Friedman, C.; Villegas, E.; Jacquemont, C.; Farrugia, D.J.; Couch, F.J.; et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 2008, 451, 1116–1120. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Alzahrani, A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019, 59, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K. Oxyresveratrol: Sources, Productions, Biological Activities, Pharmacokinetics, and Delivery Systems. Molecules 2021, 26, 4212. [Google Scholar] [CrossRef]
- Ferlinahayati; Syah, Y.M.; Juliawaty, L.D.; Achmad, S.A.; Hakim, E.H.; Takayama, H.; Said, I.M.; Latip, J. Phenolic constituents from the wood of Morus australis with cytotoxic activity. Z. Naturforschung C J. Biosci. 2008, 63, 35–39. [Google Scholar] [CrossRef]
- Abdel Bar, F.M.; Abbas, G.M.; Gohar, A.A.; Lahloub, M.I. Antiproliferative activity of stilbene derivatives and other constituents from the stem bark of Morus nigra L. Nat. Prod. Res. 2020, 34, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Nam, K.A.; Hoe, Y.H.; Min, H.Y.; Kim, E.Y.; Ko, H.; Song, S.; Lee, T.; Kim, S. Synthesis and evaluation of cytotoxicity of stilbene analogues. Arch. Pharmacal Res. 2003, 26, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Sunilkumar, D.; Drishya, G.; Chandrasekharan, A.; Shaji, S.K.; Bose, C.; Jossart, J.; Perry, J.J.P.; Mishra, N.; Kumar, G.B.; Nair, B.G. Oxyresveratrol drives caspase-independent apoptosis-like cell death in MDA-MB-231 breast cancer cells through the induction of ROS. Biochem. Pharmacol 2020, 173, 113724. [Google Scholar] [CrossRef]
- Lv, T.; Jian, Z.; Li, D.; Ao, R.; Zhang, X.; Yu, B. Oxyresveratrol induces apoptosis and inhibits cell viability via inhibition of the STAT3 signaling pathway in Saos-2 cells. Mol. Med. Rep. 2020, 22, 5191–5198. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, C.; Chen, R. Phenolic constituents from stem bark of Morus wittiorum and their anti-inflammation and cytotoxicity. China J. Chin. Mater. Medica 2010, 35, 2700–2703. [Google Scholar]
- Sintuyanon, N.; Phoolcharoen, W.; Pavasant, P.; Sooampon, S. Resveratrol Demonstrated Higher Antiproliferative and Antiangiogenic Efficacy Compared with Oxyresveratrol on Head and Neck Squamous Cell Carcinoma Cell Lines. Nat. Prod. Commun. 2017, 12, 1934578X1701201134. [Google Scholar] [CrossRef]
- Hankittichai, P.; Lou, H.J.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Oxyresveratrol Inhibits IL-1β-Induced Inflammation via Suppressing AKT and ERK1/2 Activation in Human Microglia, HMC3. Int J Mol Sci 2020, 21, 6054. [Google Scholar] [CrossRef] [PubMed]
- Wikan, N.; Hankittichai, P.; Thaklaewphan, P.; Potikanond, S.; Nimlamool, W. Oxyresveratrol Inhibits TNF-α-Stimulated Cell Proliferation in Human Immortalized Keratinocytes (HaCaT) by Suppressing AKT Activation. Pharmaceutics 2021, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Wikan, N.; Lin, S.; Thaklaewphan, P.; Potikanond, S.; Nimlamool, W. Inhibitory actions of oxyresveratrol on the PI3K/AKT signaling cascade in cervical cancer cells. Biomed. Pharmacother. 2024, 170, 115982. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef]
- Chang, L.C.; Huang, C.F.; Lai, M.S.; Shen, L.J.; Wu, F.L.; Cheng, W.F. Prognostic factors in epithelial ovarian cancer: A population-based study. PLoS ONE 2018, 13, e0194993. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Wang, Y.; Zhao, X.; Qi, X. Hormone therapy for ovarian cancer: Emphasis on mechanisms and applications (Review). Oncol. Rep. 2021, 46, 223. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Singh, H. Bevacizumab and ovarian cancer. Ther. Adv. Med. Oncol. 2013, 5, 133–141. [Google Scholar] [CrossRef]
- Nan, Y.; Su, H.; Zhou, B.; Liu, S. The function of natural compounds in important anticancer mechanisms. Front Oncol. 2023, 12, 1049888. [Google Scholar] [CrossRef]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, G.; Li, C.; Wang, S.; Zhu, M.; Wang, J.; Yue, H.; Ma, X.; Zhen, Y.; Shu, X. Metabolic profile and structure-activity relationship of resveratrol and its analogs in human bladder cancer cells. Cancer Manag. Res. 2019, 11, 4631–4642. [Google Scholar] [CrossRef]
- Radapong, S.; Chan, K.; Sarker, S.D.; Ritchie, K.J. Oxyresveratrol Modulates Genes Associated with Apoptosis, Cell Cycle Control and DNA Repair in MCF-7 Cells. Front. Pharmacol. 2021, 12, 694562. [Google Scholar] [CrossRef] [PubMed]
- Amorntaveechai, A.; Osathanon, T.; Pavasant, P.; Sooampon, S. Effect of resveratrol and oxyresveratrol on deferoxamine-induced cancer stem cell marker expression in human head and neck squamous cell carcinoma. J. Oral. Biol. Craniofac. Res. 2022, 12, 253–257. [Google Scholar] [CrossRef]
- Lin, T.-A.; Lin, W.-S.; Chou, Y.-C.; Nagabhushanam, K.; Ho, C.-T.; Pan, M.-H. Oxyresveratrol inhibits human colon cancer cell migration through regulating epithelial–mesenchymal transition and microRNA. Food Funct. 2021, 12, 9658–9668. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Ye, X.; Jian, Z.; Zhong, Z.; Zhang, C.; Yi, C.; Yu, B. Oxyresveratrol Reduces the Migration of Human Osteosarcoma Cell U2OS via Attenuating STAT3 Activation. Nat. Prod. Commun. 2022, 17, 1934578X221102031. [Google Scholar] [CrossRef]
- Passos, C.L.; Ferreira, C.; Carvalho, A.G.; Silva, J.L.; Garrett, R.; Fialho, E. Oxyresveratrol in Breast Cancer Cells: Synergistic Effect with Chemotherapeutics Doxorubicin or Melphalan on Proliferation, Cell Cycle Arrest, and Cell Death. Pharmaceutics 2024, 16, 873. [Google Scholar] [CrossRef]
- Yahayo, W.; Pongkittiphan, V.; Supabphol, R.; Saiyudthong, S. Comparative Studies of Resveratrol, Oxyresveratrol and Dihydrooxyresveratrol on Doxorubicin-Treated Lung Cancer Cells. Asian Pac. J. Cancer Prev. 2024, 25, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Lee, D.G.; Joo, Y.H.; Chung, N. Synergistic inhibitory effects of the oxyresveratrol and dacarbazine combination against melanoma cells. Oncol. Lett. 2021, 22, 667. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Tan, K.-T.; Tung, Y.-T.; Lim, C.C. Oxyresveratrol inhibits the growth of human lung squamous cell carcinoma cells by triggering S-phase arrest and apoptosis. J. Food Bioact. 2019, 6, 131–139. [Google Scholar] [CrossRef]
- Chen, C.; Nimlamool, W.; Miller, C.J.; Lou, H.J.; Turk, B.E. Rational Redesign of a Functional Protein Kinase-Substrate Interaction. ACS Chem. Biol. 2017, 12, 1194–1198. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thaklaewphan, P.; Wikan, N.; Potikanond, S.; Nimlamool, W. Oxyresveratrol Enhances the Anti-Cancer Effect of Cisplatin against Epithelial Ovarian Cancer Cells through Suppressing the Activation of Protein Kinase B (AKT). Biomolecules 2024, 14, 1140. https://doi.org/10.3390/biom14091140
Thaklaewphan P, Wikan N, Potikanond S, Nimlamool W. Oxyresveratrol Enhances the Anti-Cancer Effect of Cisplatin against Epithelial Ovarian Cancer Cells through Suppressing the Activation of Protein Kinase B (AKT). Biomolecules. 2024; 14(9):1140. https://doi.org/10.3390/biom14091140
Chicago/Turabian StyleThaklaewphan, Phatarawat, Nitwara Wikan, Saranyapin Potikanond, and Wutigri Nimlamool. 2024. "Oxyresveratrol Enhances the Anti-Cancer Effect of Cisplatin against Epithelial Ovarian Cancer Cells through Suppressing the Activation of Protein Kinase B (AKT)" Biomolecules 14, no. 9: 1140. https://doi.org/10.3390/biom14091140





