A New Rat Model of Sacral Cord Injury Producing a Neurogenic Bladder and Its Functional and Mechanistic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. SSCI Model
2.4. Urodynamics
2.5. Ultrasound
2.6. Immunohistochemistry
2.7. Immunofluorescent Staining
2.8. RNA-Seq
2.9. Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
2.10. Protein Extraction and Western Blot
2.11. Statistical Analysis
3. Results
3.1. Changes in Bladder Shape in the SSCI Rat Model
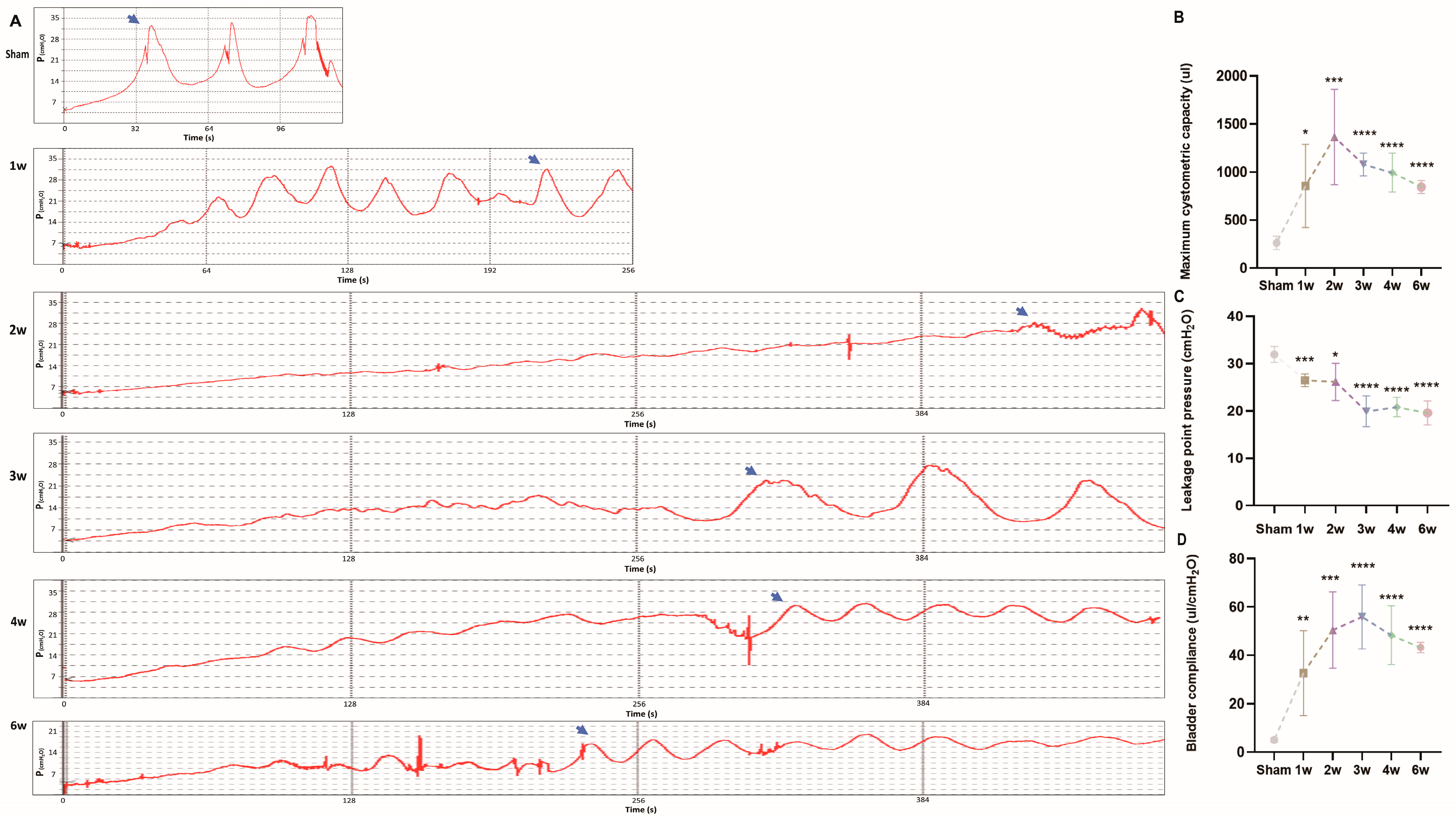
3.2. Urodynamics of the SSCI Rat Model
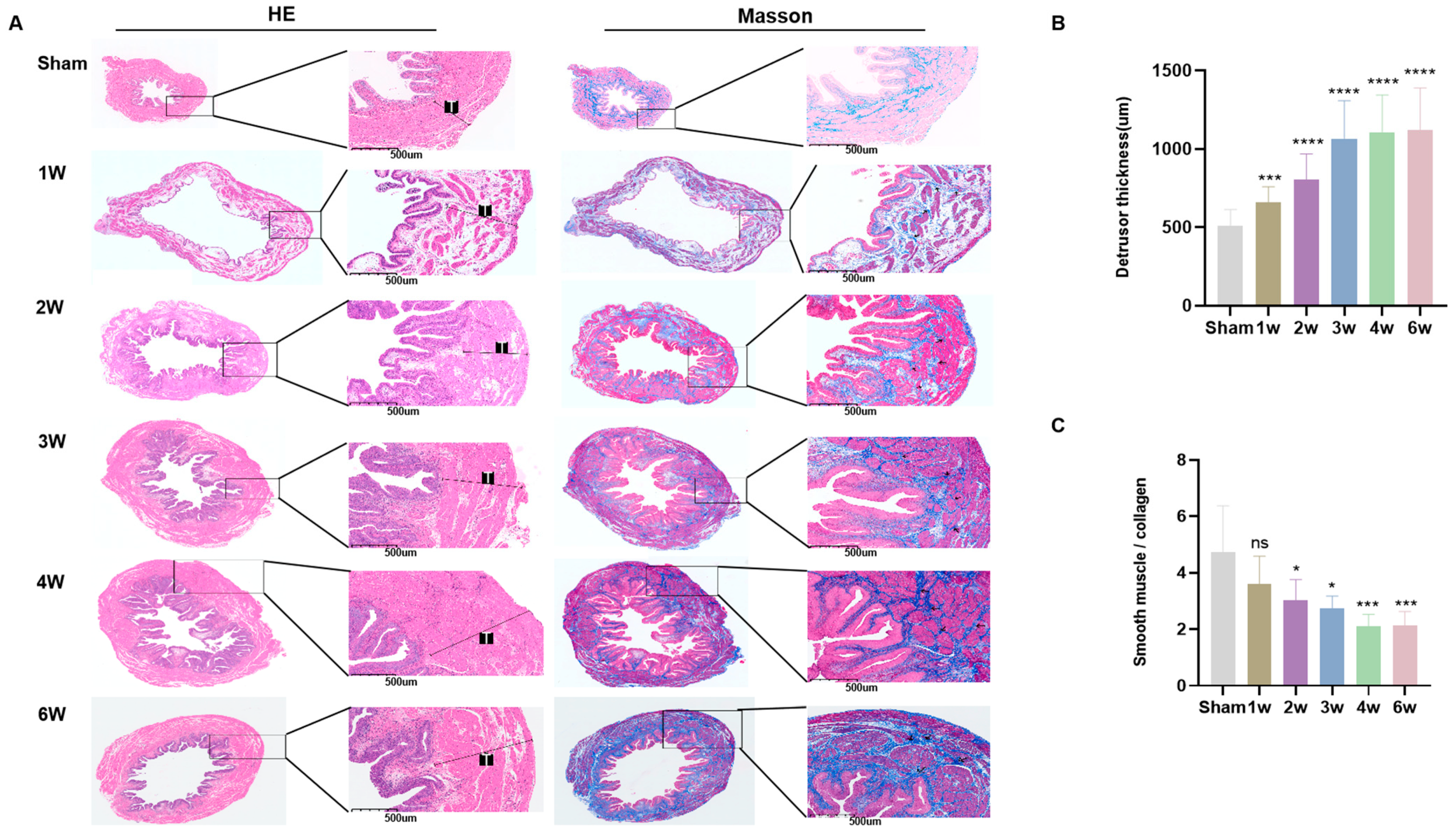
3.3. Postoperative Pathological Features of SSCI
3.4. Postoperative Immunofluorescent Staining of SSCI
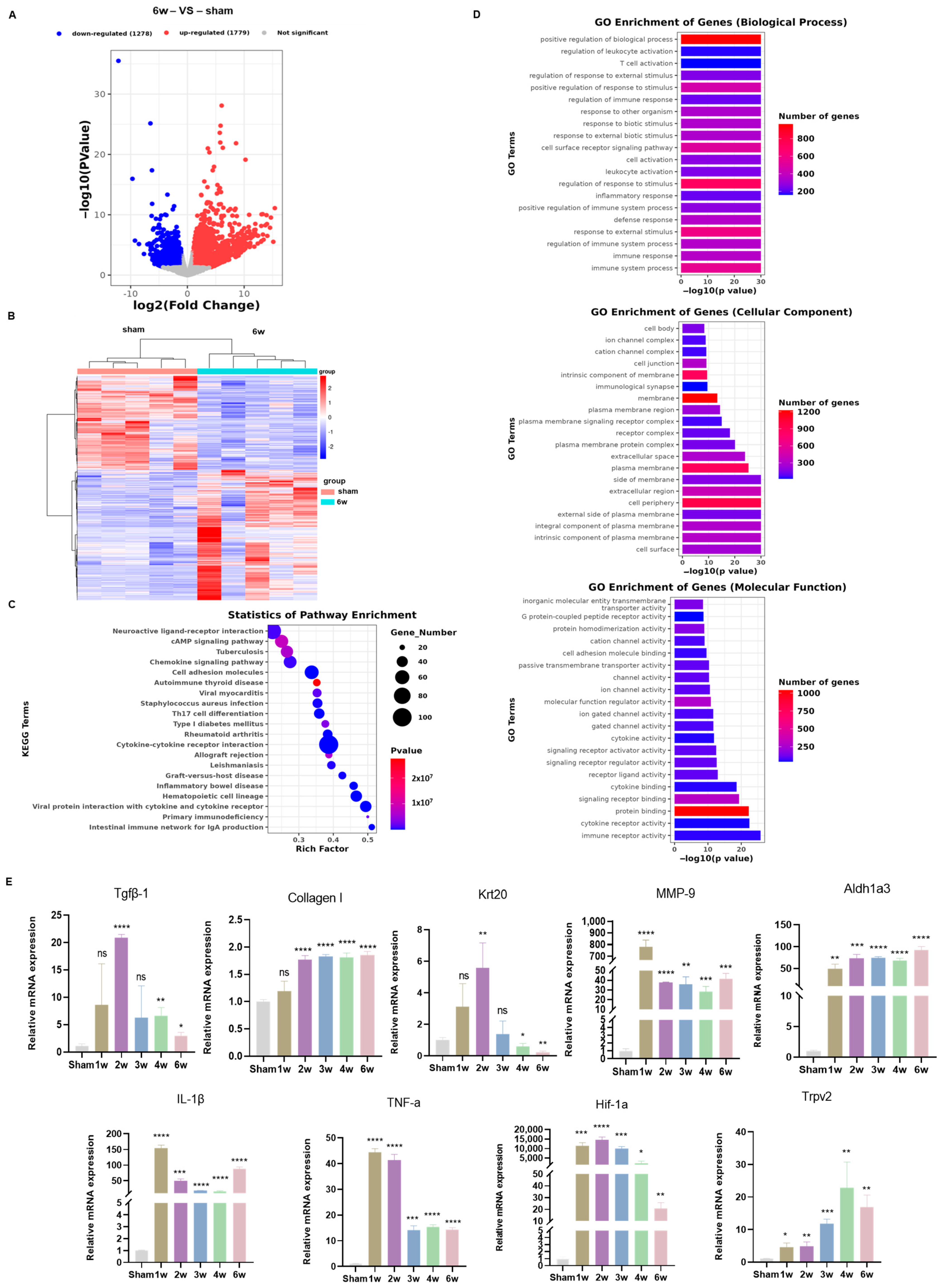
3.5. Transcriptome Analysis Uncovered Different Signaling Pathways in the SSCI Sham Group and the 6-Week Postoperative Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stein, R.; Bogaert, G.; Dogan, H.S.; Hoen, L.; Kocvara, R.; Nijman, R.J.M.; Quadackers, J.S.L.T.; Rawashdeh, Y.F.; Silay, M.S.; Tekgul, S.; et al. EAU/ESPU guidelines on the management of neurogenic bladder in children and adolescent part I diagnostics and conservative treatment. Neurourol. Urodyn. 2020, 39, 45–57. [Google Scholar] [CrossRef]
- Sripathi, V.; Mitra, A. Management of Neurogenic Bladder. Indian J. Pediatr. 2017, 84, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, M.; Lurvink, M.; A Barf, H.; Post, M.W.M.; A van Asbeck, F.W.; Gooskens, R.H.J.M.; Prevo, A.J.H. High prevalence of incontinence among young adults with spina bifida: Description, prediction and problem perception. Spinal Cord 2005, 43, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.P.; Rajappa, M.; Wadwekar, V.; Narayan, S.K.; Barathi, D.; Madhugiri, V.S. Tethered Cord Syndrome–A Study of the Short-Term Effects of Surgical Detethering on Markers of Neuronal Injury and Electrophysiologic Parameters. World Neurosurg. 2016, 94, 239–247. [Google Scholar] [CrossRef] [PubMed]
- López Pereira, P.; Moneo, J.; Martínez Urrutia, M.J.; Jaureguizar, E. Neuro-urologic impact of spinal cord untethering in patients with myelomeningocele and tethered cord. Acta Urol. Esp. 1995, 19, 383–388. [Google Scholar]
- Stein, R.; Assion, C.; Beetz, R.; Bürst, M.; Cremer, R.; Ermert, A.; Goepel, M.; Kuwertz-Bröking, E.; Ludwikowski, B.; Michael, T.; et al. Neurogenic bladder function disorders in patients with meningomyelocele: S2k guidelines on diagnostics and therapy. Der Urol. 2015, 54, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Schmidek, H.H.; Smith, D.A.; Kristiansen, T.K. Sacral Fractures. Neurosurgery 1984, 15, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, K.J.; Soloniuk, D.S.; Razack, N. Neurological injury and patterns of sacral fractures. J. Neurosurg. 1990, 72, 889–893. [Google Scholar] [CrossRef]
- Rizkalla, J.M.; Lines, T.; Nimmons, S. Classifications in Brief: The Denis Classification of Sacral Fractures. Clin. Orthop. Relat. Res. 2019, 477, 2178–2181. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Wang, Y.; Shang, A. Functional, morphological and molecular characteristics in a novel rat model of spinal sacral nerve injury-surgical approach, pathological process and clinical relevance. Sci. Rep. 2022, 12, 10026. [Google Scholar] [CrossRef]
- Li, Q.; Hong, Y.; Chen, J.; Zhou, X.; Tian, X.; Yu, Y.; Shen, L.; Long, C.; Cai, M.; Wu, S.; et al. Hypoxia-Induced HIF-1α Expression Promotes Neurogenic Bladder Fibrosis via EMT and Pyroptosis. Cells 2022, 11, 3836. [Google Scholar] [CrossRef]
- Dorsher, P.T.; McIntosh, P.M. Neurogenic bladder. Adv. Urol. 2012, 2012, 816274. [Google Scholar] [CrossRef]
- Pang, D. Total Resection of Complex Spinal Cord Lipomas: How, Why, and When to Operate? Neurol. Med. -Chir. 2015, 55, 695–721. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.-W.; Oh, H.-M.; Lee, J.-E.; Kim, J.-H.; Hwang, J.-M.; Park, E.; Jung, T.-D. An unusual complication of sacral nerve root injury following bone marrow harvesting: A case report. BMC Cancer 2019, 19, 347. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.W.; Thomas, J.C.; Baskin, L.S.; Zderic, S.A.; Thom, E.A.; Burrows, P.K.; Lee, H.; Houtrow, A.J.; MacPherson, C.; Adzick, N.S.; et al. Effect of Prenatal Repair of Myelomeningocele on Urological Outcomes at School Age. J. Urol. 2019, 202, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Luca, F.; Valvo, M.; Ghezzi, T.L.; Zuccaro, M.; Cenciarelli, S.; Trovato, C.; Sonzogni, A.; Biffi, R. Impact of robotic surgery on sexual and urinary functions after fully robotic nerve-sparing total mesorectal excision for rectal cancer. Ann. Surg. 2013, 257, 672–678. [Google Scholar] [CrossRef]
- Arlandis, S.; Bø, K.; Cobussen-Boekhorst, H.; Costantini, E.; de Heide, M.; Farag, F.; Groen, J.; Karavitakis, M.; Lapitan, M.C.; Manso, M.; et al. European Association of Urology Guidelines on the Management of Female Non-neurogenic Lower Urinary Tract Symptoms. Part 2: Underactive Bladder, Bladder Outlet Obstruction, and Nocturia. Eur. Urol. 2022, 82, 60–70. [Google Scholar] [CrossRef]
- Osman, N.I.; Chapple, C.R. Are There Pharmacotherapeutic Options for Underactive Bladder? Eur. Urol. Focus 2018, 4, 6–7. [Google Scholar] [CrossRef]
- Deng, H.; Liao, L.; Li, X.; Liu, Q.; Wang, X.; Zhou, Z. Effects of Intravesical Electrical Stimulation on Urinary Adenosine Triphosphate and Nitric Oxide in Rats With Detrusor Underactivity Induced By Bilateral Pelvic Nerve Crush Injury: The Possible Underlying Mechanism. Int. Neurourol. J. 2022, 26, 288–298. [Google Scholar] [CrossRef]
- Deruyver, Y.; Weyne, E.; Dewulf, K.; Rietjens, R.; Pinto, S.; Van Ranst, N.; Franken, J.; Vanneste, M.; Albersen, M.; Gevaert, T.; et al. Intravesical Activation of the Cation Channel TRPV4 Improves Bladder Function in a Rat Model for Detrusor Underactivity. Eur. Urol. 2018, 74, 336–345. [Google Scholar] [CrossRef]
- Dewulf, K.; Weyne, E.; Gevaert, T.; Deruyver, Y.; Voets, T.; De Ridder, D.; Everaerts, W.; Albersen, M. Functional and molecular characterisation of the bilateral pelvic nerve crush injury rat model for neurogenic detrusor underactivity. BJU Int. 2019, 123, E86–E96. [Google Scholar] [CrossRef]
- Doelman, A.W.; Streijger, F.; Majerus, S.J.A.; Damaser, M.S.; Kwon, B.K. Assessing Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury: Animal Models in Preclinical Neuro-Urology Research. Biomedicines 2023, 11, 1539. [Google Scholar] [CrossRef]
- Sekido, N.; Jyoraku, A.; Okada, H.; Wakamatsu, D.; Matsuya, H.; Nishiyama, H. A novel animal model of underactive bladder: Analysis of lower urinary tract function in a rat lumbar canal stenosis model. Neurourol. Urodyn. 2012, 31, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.S.; Youssef, H.A.; Saleh, A.S.; Bollen, P.; Zvara, P. Anesthetic protocols for urodynamic studies of the lower urinary tract in small rodents—A systematic review. PLoS ONE 2021, 16, e0253192. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, S.; Downie, J. Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourol. Urodyn. 2000, 19, 87–99. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, M.; Song, J.; Zhao, J. Detrusor Underactivity Model in Rats by Conus Medullaris Transection. J. Vis. Exp. 2020, 162, e61576. [Google Scholar] [CrossRef]
- Shimizu, S.; Nagao, Y.; Kurabayashi, A.; Shimizu, T.; Higashi, Y.; Karashima, T.; Saito, M. Aging-related severe hypertension induces detrusor underactivity in rats. Life Sci. 2021, 283, 119855. [Google Scholar] [CrossRef] [PubMed]
- Pannek, J.; Pannek-Rademacher, S. Usefulness of Hydrastis for the prevention of encrustation of long-term indwelling catheters in persons with neurogenic bladder dysfunction: A case series. Spinal Cord Ser. Cases 2021, 7, 66. [Google Scholar] [CrossRef]
- Amarenco, G.; Ismaël, S.S.; Chesnel, C.; Charlanes, A.; Le Breton, F. Diagnosis and clinical evaluation of neurogenic bladder. Eur. J. Phys. Rehabil. Med. 2017, 53, 975–980. [Google Scholar] [CrossRef]
- Li, Y.L.; Wen, Y.B.; He, X.F.; Wu, J.W.; Li, Y.W.; Han, Z.J.; Feng, J.J.; Yan, S.H.; Li, S.L.; Heesakkers, J.P.; et al. Reconstruction of bladder function and prevention of renal deterioration by means of end-to-side neurorrhaphy in rats with neurogenic bladder. Neurourol. Urodyn. 2018, 37, 1272–1280. [Google Scholar] [CrossRef]
- Tateshita, T.; Ueda, K.; Kajikawa, A. End-to-end and end-to-side neurorrhaphy between thick donor nerves and thin recipient nerves: An axon regeneration study in a rat model. Neural Regen. Res. 2018, 13, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, Y.; He, Y.; Xing, D.; Liu, E.; Yang, X.; Zhu, W.; Wang, Q.; Wen, J.G. The TGF-β1 pathway is early involved in neurogenic bladder fibrosis of juvenile rats. Pediatr. Res. 2021, 90, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Shaker, H.; Mourad, M.S.; Elbialy, M.H.; Elhilali, M. Urinary bladder hyperreflexia: A rat animal model. Neurourol. Urodyn. 2003, 22, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Pourmehr, H.; Rahbarghazi, R.; Mahmoudi, J.; Roshangar, L.; Chapple, C.R.; Hajebrahimi, S.; Abolhasanpour, N.; Azghani, M.-R. Intra-bladder wall transplantation of bone marrow mesenchymal stem cells improved urinary bladder dysfunction following spinal cord injury. Life Sci. 2019, 221, 20–28. [Google Scholar] [CrossRef]
- Fowler, C.J.; Griffiths, D.; de Groat, W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef]
- Wang, J.; Ren, L.; Liu, X.; Xu, W.; Liu, M.; Hu, P.; Wang, T.; Liu, J.; Ling, Q. Transcriptomics Reveals Molecular Features of the Bilateral Pelvic Nerve Injury Rat Model of Detrusor Underactivity. Biomolecules 2023, 13, 1260. [Google Scholar] [CrossRef]
- Rana, S.; Alom, F.; Martinez, R.C.; Fuller, D.D.; Mickle, A.D. Acute ampakines increase voiding function and coordination in a rat model of SCI. eLife 2024, 12, RP89767. [Google Scholar] [CrossRef]
- Saito, T.; Gotoh, D.; Wada, N.; Tyagi, P.; Minagawa, T.; Ogawa, T.; Ishizuka, O.; Yoshimura, N. Time-dependent progression of neurogenic lower urinary tract dysfunction after spinal cord injury in the mouse model. Am. J. Physiol. Physiol. 2021, 321, F26–F32. [Google Scholar] [CrossRef]
- Stover, J.; Nagatomi, J. Cyclic pressure stimulates DNA synthesis through the PI3K/Akt signaling pathway in rat bladder smooth muscle cells. Ann. Biomed. Eng. 2007, 35, 1585–1594. [Google Scholar] [CrossRef]
- Guo, W.; Shapiro, K.; Wang, Z.; Armann, K.; Shen, B.; Wang, J.; Roppolo, J.R.; de Groat, W.C.; Tai, C. Restoring both continence and micturition after chronic spinal cord injury by pudendal neuromodulation. Exp. Neurol. 2021, 340, 113658. [Google Scholar] [CrossRef]
- Podolsky, M.J.; Kheyfets, B.; Pandey, M.; Beigh, A.H.; Yang, C.D.; Lizama, C.O.; Datta, R.; Lin, L.L.; Wang, Z.; Wolters, P.J.; et al. Genome-wide screens identify SEL1L as an intracellular rheostat controlling collagen turnover. Nat. Commun. 2024, 15, 1531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Meng, H.F.; Xu, H.Q.; Guo, Z.X.; Yan, J.F.; Gao, J.L.; Niu, L.N.; Wang, S.L.; Jiao, K. DPSCs regulate epithelial-T cell interactions in oral submucous fibrosis. Stem Cell Res. Ther. 2024, 15, 113. [Google Scholar] [CrossRef]
- Deveaud, C.M.; Macarak, E.J.; Kucich, U.; Ewalt, D.H.; Abrams, W.R.; Howard, P.S. Molecular analysis of collagens in bladder fibrosis. J. Urol. 1998, 160, 1518–1527. [Google Scholar] [CrossRef]
- Chen, J.; Peng, L.; Chen, G.; Chen, Y.; Zeng, X.; Zhang, J.; Zhang, C.; Shen, H.; Liao, B.; Luo, D. Single-cell transcriptomics reveal the remodeling landscape of bladder in patients with obstruction-induced detrusor underactivity. Medcomm 2024, 5, e490. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.T.; Tingskov, S.J.; Djurhuus, J.C.; Nørregaard, R.; Olsen, L.H. Can bladder fibrosis in congenital urinary tract obstruction be reversed? J. Pediatr. Urol. 2017, 13, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Şekerci, A.; Işbilen, B.; Işman, F.; Akbal, C.; Şimşek, F.; Tarcan, T. Urinary NGF, TGF-β1, TIMP-2 and bladder wall thickness predict neurourological findings in children with myelodysplasia. J. Urol. 2014, 191, 199–205. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiao, X.; Tan, T.K.; Zhao, H.; Zhang, Y.; Liu, L.; Zhang, J.; Wang, L.; Cao, Q.; Wang, Y.; et al. Matrix metalloproteinase 9-dependent Notch signaling contributes to kidney fibrosis through peritubular endothelial–mesenchymal transition. Nephrol. Dial. Transplant. 2017, 32, 781–791. [Google Scholar] [CrossRef]
- Feng, M.; Ding, J.; Wang, M.; Zhang, J.; Zhu, X.; Guan, W. Kupffer-derived matrix metalloproteinase-9 contributes to liver fibrosis resolution. Int. J. Biol. Sci. 2018, 14, 1033–1040. [Google Scholar] [CrossRef]
- Zuo, C.; Li, X.; Huang, J.; Chen, D.; Ji, K.; Yang, Y.; Xu, T.; Zhu, D.; Yan, C.; Gao, P. Osteoglycin attenuates cardiac fibrosis by suppressing cardiac myofibroblast proliferation and migration through antagonizing lysophosphatidic acid 3/matrix metalloproteinase 2/epidermal growth factor receptor signalling. Cardiovasc. Res. 2018, 114, 703–712. [Google Scholar] [CrossRef]
- Tan, T.K.; Zheng, G.; Hsu, T.-T.; Wang, Y.; Lee, V.W.; Tian, X.; Wang, Y.; Cao, Q.; Wang, Y.; Harris, D.C. Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells. Am. J. Pathol. 2010, 176, 1256–1270. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, L.; Qiang, C.; Chen, C.; Shen, Z.; Ding, F.; Lv, L.; Zhu, T.; Lu, Y.; Cui, X. The role of matrix metalloproteinase 9 in fibrosis diseases and its molecular mechanisms. Biomed. Pharmacother. 2024, 171, 116116. [Google Scholar] [CrossRef]
- Moll, R.; Schiller, D.L.; Franke, W.W. Identification of protein IT of the intestinal cytoskeleton as a novel type I cytokeratin with unusual properties and expression patterns. J. Cell Biol. 1990, 111, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Matsumoto, Y.; Ochi, T.; Koga, H.; Hattori, N.; Yamataka, A.; Nakamura, T. Distinct effects of Fgf7 and Fgf10 on the terminal differentiation of murine bladder urothelium revealed using an organoid culture system. BMC Urol. 2023, 23, 169. [Google Scholar] [CrossRef]
- Poturnajova, M.; Kozovska, Z.; Matuskova, M. Aldehyde dehydrogenase 1A1 and 1A3 isoforms—Mechanism of activation and regulation in cancer. Cell. Signal. 2021, 87, 110120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Myojin, T.; Li, K.; Kurita, A.; Seto, M.; Motoyama, A.; Liu, X.; Satoh, A.; Munemasa, S.; Murata, Y.; et al. A Major Intestinal Catabolite of Quercetin Glycosides, 3-Hydroxyphenylacetic Acid, Protects the Hepatocytes from the Acetaldehyde-Induced Cytotoxicity through the Enhancement of the Total Aldehyde Dehydrogenase Activity. Int. J. Mol. Sci. 2022, 23, 1762. [Google Scholar] [CrossRef]
- Shao, C.; Sullivan, J.P.; Girard, L.; Augustyn, A.; Yenerall, P.; Rodriguez-Canales, J.; Liu, H.; Behrens, C.; Shay, J.W.; Wistuba, I.I.; et al. Essential role of aldehyde dehydrogenase 1A3 for the maintenance of non–small cell lung cancer stem cells is associated with the STAT3 pathway. Clin. Cancer Res. 2014, 20, 4154–4166. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, N.; Carrasco, A.; Xie, A.X.; Pineda, R.H.; Malykhina, A.P.; Wilcox, D.T. Functional constipation induces bladder overactivity associated with upregulations of Htr2 and Trpv2 pathways. Sci. Rep. 2021, 11, 1149. [Google Scholar] [CrossRef]
- Andersson, K.-E. Agents in early development for treatment of bladder dysfunction—Promise of drugs acting at TRP channels? Expert Opin. Investig. Drugs 2019, 28, 749–755. [Google Scholar] [CrossRef]
- Lepiarczyk, E.; Paukszto, Ł.; Wiszpolska, M.; Łopieńska-Biernat, E.; Bossowska, A.; Majewski, M.K.; Majewska, M. Molecular Influence of Resiniferatoxin on the Urinary Bladder Wall Based on Differential Gene Expression Profiling. Cells 2023, 12, 462. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Hong, Y.; Zhou, X.; Yu, C.; Tian, X.; Zhao, J.; Long, C.; Shen, L.; Wu, S.; et al. Inhibition of the NF-κB Signaling Pathway Alleviates Pyroptosis in Bladder Epithelial Cells and Neurogenic Bladder Fibrosis. Int. J. Mol. Sci. 2023, 24, 11160. [Google Scholar] [CrossRef]
- Metcalfe, P.D.; Wang, J.; Jiao, H.; Huang, Y.; Hori, K.; Moore, R.B.; Tredget, E.E. Bladder outlet obstruction: Progression from inflammation to fibrosis. BJU Int. 2010, 106, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Lieb, J.I.; Chichester, P.; Kogan, B.; DAS, A.K.; Leggett, R.E.; SCHRÖDER, A.; Levin, R.M. Rabbit urinary bladder blood flow changes during the initial stage of partial outlet obstruction. J. Urol. 2000, 164, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Tarcan, T.; Choi, H.-P.; Azadzoi, K.M. Molecular Regulation of Concomitant Lower Urinary Tract Symptoms and Erectile Dysfunction in Pelvic Ischemia. Int. J. Mol. Sci. 2022, 23, 15988. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequence (5′ to 3′) | Number of Bases | |
|---|---|---|---|
| Krt20 | Forward: | TGTCAACGTGGAGGTGGATG | 20 |
| Reverse: | TTCAGAGGACACGACCTTGC | 20 | |
| Aldh1a3 | Forward: | ATGTGAGGTGGAAGAAGGCG | 20 |
| Reverse: | GGCACAATGTTCACCACACC | 20 | |
| Mmp9 | Forward: | CTGAGGCCCCTACAGAGTCT | 20 |
| Reverse: | GTTGTGGAAACTCACACGCC | 20 | |
| Trpv2 | Forward: | ACTACACACGGGGCTTTCAG | 20 |
| Reverse: | CGCTCATCAGGGGTACCATC | 20 | |
| Tgfβ-1 | Forward: | GACTCTCCACCTGCAAGACC | 20 |
| Reverse: | AGCCCTGTATTCCGTCTCCT | 20 | |
| Hif-1a | Forward: | CGATGCCCTGACTCTGCTAG | 20 |
| Reverse: | GGAGGGCTTGGAGAATTGCT | 20 | |
| IL-1β | Forward: | CTCACAGCAGCATCTCGACAAGAG | 24 |
| Reverse: | CACACTAGCAGGTCGTCATCATCC | 24 | |
| TNF-α | Forward: | GCATGATCCGAGATGTGGAACTGG | 24 |
| Reverse: | CGCCACGAGCAGGAATGAGAAG | 22 | |
| Collagen I | Forward: | CACTGCAAGAACAGCGTAGC | 20 |
| Reverse: | AAGTTCCGGTGTGACTCGTG | 20 | |
| GAPDH | Forward: | TGACTCTACCCACGGCAAGTTCAA | 24 |
| Reverse: | ACGACATACTCAGCACCAGCATCA | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, K.; Hou, Y.; Zhang, Z.; Yuan, F.; Huang, X.; Liu, P.; Zou, X.; Sun, J. A New Rat Model of Sacral Cord Injury Producing a Neurogenic Bladder and Its Functional and Mechanistic Studies. Biomolecules 2024, 14, 1141. https://doi.org/10.3390/biom14091141
Bai K, Hou Y, Zhang Z, Yuan F, Huang X, Liu P, Zou X, Sun J. A New Rat Model of Sacral Cord Injury Producing a Neurogenic Bladder and Its Functional and Mechanistic Studies. Biomolecules. 2024; 14(9):1141. https://doi.org/10.3390/biom14091141
Chicago/Turabian StyleBai, Kaiping, Yanping Hou, Zhiyuan Zhang, Fei Yuan, Xiaoling Huang, Pengtao Liu, Xiangyu Zou, and Jie Sun. 2024. "A New Rat Model of Sacral Cord Injury Producing a Neurogenic Bladder and Its Functional and Mechanistic Studies" Biomolecules 14, no. 9: 1141. https://doi.org/10.3390/biom14091141






