Hydrogen Sulfide: A Versatile Molecule and Therapeutic Target in Health and Diseases
Abstract
1. Introduction
2. Synthesis and Distribution of H2S
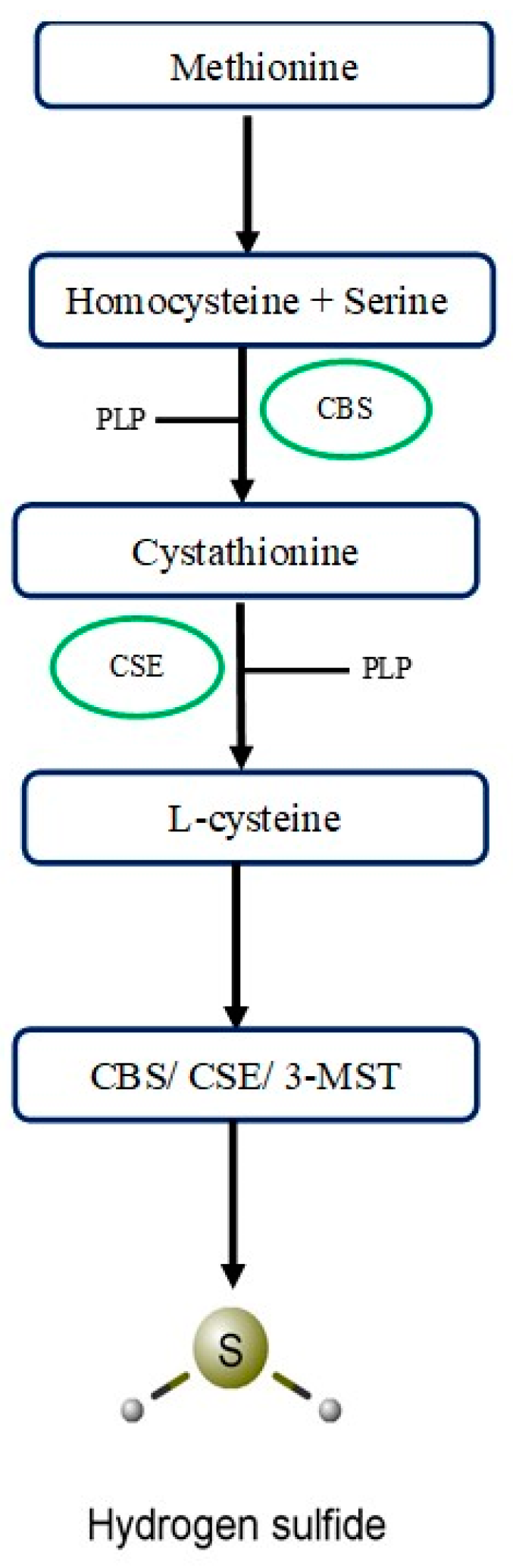
2.1. Cystathionine-β-Synthase
2.2. Cystathionine-γ-Lyase
2.3. 3-Mercaptopyruvate Sulfurtransferase
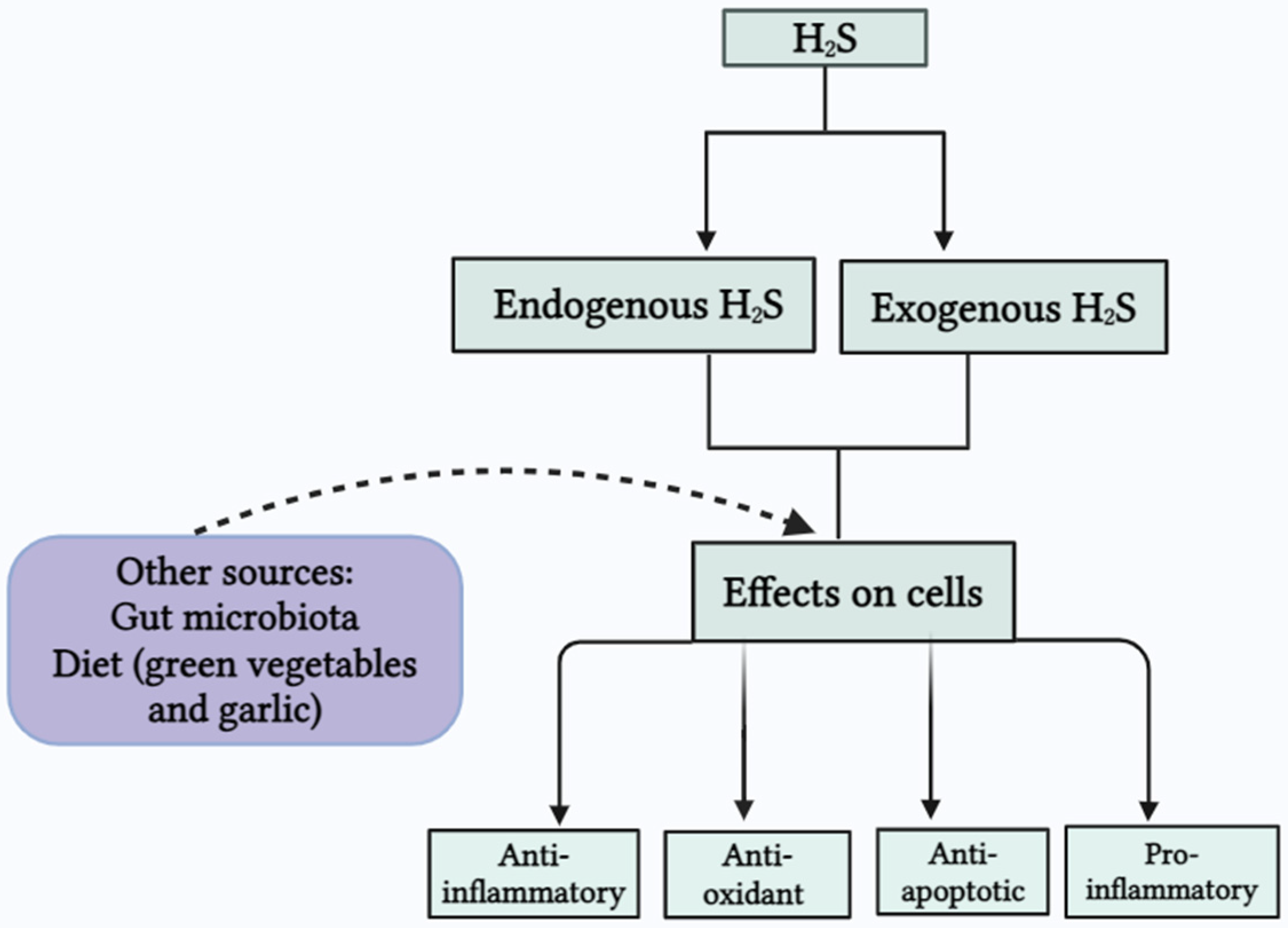
2.4. Other Sources of H2S
3. H2S Donors
4. H2S Synthesis Inhibitors
5. H2S in Inflammatory Conditions
5.1. H2S and Arthritis/Joint Inflammation
5.2. H2S and Sepsis
5.3. Acute Pancreatitis
6. H2S and Diseases of the Cardiovascular System
7. H2S and Viral Diseases
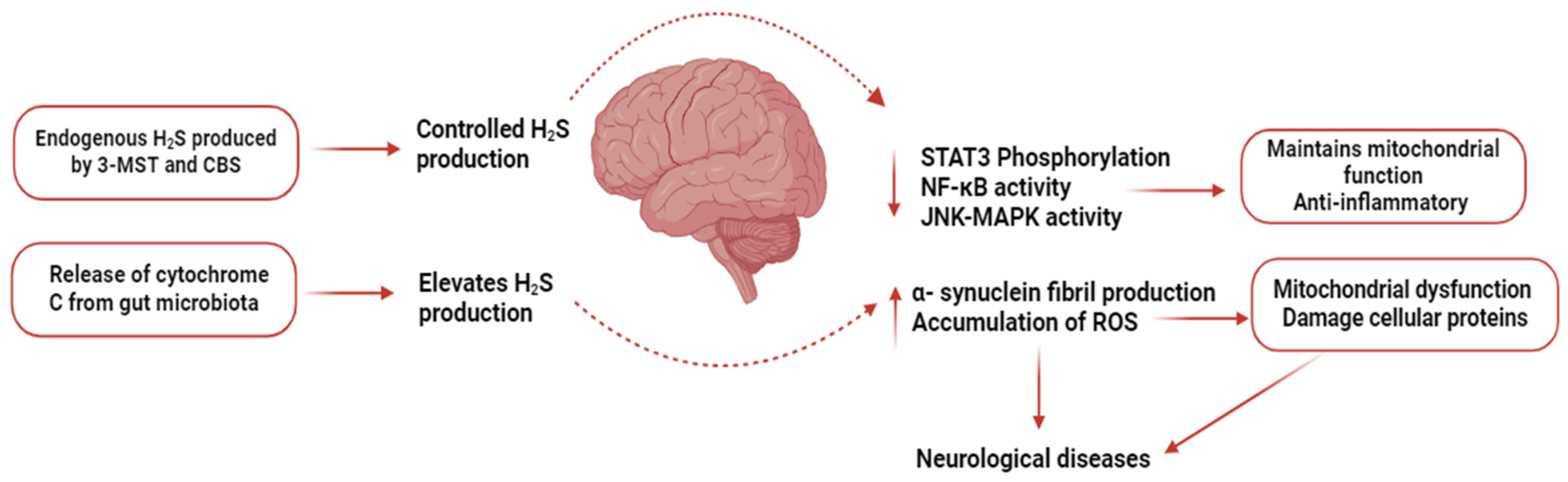
8. H2S and CNS Diseases
9. Uses of H2S Donors in the Clinical Setting
10. Gaps in Knowledge and the Way Forward
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodrigues, L.M.; Gregório, J.; Wehrwein, E. Contemporary views on the future of physiology—A report from the 2019 P-MIG focus group. Front. Physiol. 2023, 14, 1176146. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Rotariu, M.; Turnea, M.A.; Anghelescu, A.; Albadi, I.; Dogaru, G.; Silișteanu, S.C.; Ionescu, E.V.; Firan, F.C.; Ionescu, A.M.; et al. Topical Reappraisal of Molecular Pharmacological Approaches to Endothelial Dysfunction in Diabetes Mellitus Angiopathy. Curr. Issues Mol. Biol. 2022, 44, 3378–3397. [Google Scholar] [CrossRef] [PubMed]
- Huerta de la Cruz, S.; Medina-Terol, G.J.; Tapia-Martínez, J.A.; Silva-Velasco, D.L.; Beltran-Ornelas, J.H.; Sánchez-López, A.; Sancho, M.; Centurión, D. Hydrogen sulfide as a neuromodulator of the vascular tone. Eur. J. Pharmacol. 2023, 940, 175455. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, Q. Advances of H2S in Regulating Neurodegenerative Diseases by Preserving Mitochondria Function. Antioxidants 2023, 12, 652. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xia, H.; Sharp, T.E., 3rd; LaPenna, K.B.; Elrod, J.W.; Casin, K.M.; Liu, K.; Calvert, J.W.; Chau, V.Q.; Salloum, F.N.; et al. Mitochondrial H2S Regulates BCAA Catabolism in Heart Failure. Circ. Res. 2022, 131, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Zuhra, K.; Augsburger, F.; Majtan, T.; Szabo, C. Cystathionine-β-Synthase: Molecular Regulation and Pharmacological Inhibition. Biomolecules 2020, 10, 697. [Google Scholar] [CrossRef]
- Tamizhselvi, R.; Moore, P.K.; Bhatia, M. Hydrogen sulfide acts as a mediator of inflammation in acute pancreatitis: In vitro studies using isolated mouse pancreatic acinar cells. J. Cell. Mol. Med. 2007, 11, 315–326. [Google Scholar] [CrossRef]
- Szabo, C.; Coletta, C.; Chao, C.; Módis, K.; Szczesny, B.; Papapetropoulos, A.; Hellmich, M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 12474–12479. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Thomas, M.; Ghorpade, A.; Gendelman, H.E.; Banerjee, R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J. Biol. Chem. 2006, 281, 35785–35793. [Google Scholar] [CrossRef]
- Kondo, K.; Bhushan, S.; King, A.L.; Prabhu, S.D.; Hamid, T.; Koenig, S.; Murohara, T.; Predmore, B.L.; Gojon, G., Sr.; Gojon, G., Jr.; et al. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation 2013, 127, 1116–1127. [Google Scholar] [CrossRef]
- Dunham, I.; Shimizu, N.; Roe, B.A.; Chissoe, S.; Hunt, A.R.; Collins, J.E.; Bruskiewich, R.; Beare, D.M.; Clamp, M.; Smink, L.J.; et al. The DNA sequence of human chromosome 22. Nature 1999, 402, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Peleli, M.; Bibli, S.I.; Li, Z.; Chatzianastasiou, A.; Varela, A.; Katsouda, A.; Zukunft, S.; Bucci, M.; Vellecco, V.; Davos, C.H.; et al. Cardiovascular phenotype of mice lacking 3-mercaptopyruvate sulfurtransferase. Biochem. Pharmacol. 2020, 176, 113833. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, K.; Olson, K.R. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem. Pharmacol. 2013, 85, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Carbonero, F.; Benefiel, A.C.; Gaskins, H.R. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 504–518. [Google Scholar] [CrossRef]
- Rabus, R.; Venceslau, S.S.; Wöhlbrand, L.; Voordouw, G.; Wall, J.D.; Pereira, I.A. A post-genomic view of the ecophysiology, catabolism and biotechnological relevance of sulphate-reducing prokaryotes. Adv. Microb. Physiol. 2015, 66, 55–321. [Google Scholar] [CrossRef]
- Awano, N.; Wada, M.; Mori, H.; Nakamori, S.; Takagi, H. Identification and functional analysis of Escherichia coli cysteine desulfhydrases. Appl. Environ. Microbiol. 2005, 71, 4149–4152. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Marino, A.; C Breschi, M.; Da Settimo, F.; Calderone, V. Hydrogen sulphide: Biopharmacological roles in the cardiovascular system and pharmaceutical perspectives. Curr. Med. Chem. 2012, 19, 3325–3336. [Google Scholar] [CrossRef]
- Zhao, Y.; Biggs, T.D.; Xian, M. Hydrogen sulfide (H 2 S) releasing agents: Chemistry and biological applications. Chem. Commun. 2014, 50, 11788–11805. [Google Scholar] [CrossRef]
- Li, L.; Bhatia, M.; Zhu, Y.Z.; Zhu, Y.C.; Ramnath, R.D.; Wang, Z.J.; Anuar, F.B.M.; Whiteman, M.; Salto-Tellez, M.; Moore, P.K. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005, 19, 1196–1198. [Google Scholar] [CrossRef] [PubMed]
- Tamizhselvi, R.; Moore, P.K.; Bhatia, M. Inhibition of hydrogen sulfide synthesis attenuates chemokine production and protects mice against acute pancreatitis and associated lung injury. Pancreas 2008, 36, e24–e31. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, D.; Ottani, A.; Zaffe, D.; Galantucci, M.; Strinati, F.; Lodi, R.; Guarini, S. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol. Learn. Mem. 2013, 104, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.W.; Elston, M.; Nicholson, C.K.; Gundewar, S.; Jha, S.; Elrod, J.W.; Ramachandran, A.; Lefer, D.J. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation 2010, 122, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pacheco, A.; Xian, M. Medicinal Chemistry: Insights into the Development of Novel H2S Donors. Handb. Exp. Pharmacol. 2015, 230, 365–388. [Google Scholar] [CrossRef]
- Whiteman, M.; Li, L.; Rose, P.; Tan, C.H.; Parkinson, D.B.; Moore, P.K. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal. 2010, 12, 1147–1154. [Google Scholar] [CrossRef]
- Montanaro, R.; Vellecco, V.; Torregrossa, R.; Casillo, G.M.; Manzo, O.L.; Mitidieri, E.; Bucci, M.; Castaldo, S.; Sorrentino, R.; Whiteman, M.; et al. Hydrogen sulfide donor AP123 restores endothelial nitric oxide-dependent vascular function in hyperglycemia via a CREB-dependent pathway. Redox Biol. 2023, 62, 102657. [Google Scholar] [CrossRef]
- Xu, W.; Cui, C.; Cui, C.; Chen, Z.; Zhang, H.; Cui, Q.; Xu, G.; Fan, J.; Han, Y.; Tang, L. Hepatocellular cystathionine γ lyase/hydrogen sulfide attenuates nonalcoholic fatty liver disease by activating farnesoid X receptor. Hepatology 2022, 76, 1794–1810. [Google Scholar] [CrossRef]
- Dongó, E.; Beliczai-Marosi, G.; Dybvig, A.S.; Kiss, L. The mechanism of action and role of hydrogen sulfide in the control of vascular tone. Nitric Oxide 2018, 81, 75–87. [Google Scholar] [CrossRef]
- Yuan, S.; Shen, X.; Kevil, C.G. Beyond a gasotransmitter: Hydrogen sulfide and polysulfide in cardiovascular health and immune response. Antioxid. Redox Signal. 2017, 27, 634–653. [Google Scholar] [CrossRef]
- Wu, W.J.; Jia, W.W.; Liu, X.H.; Pan, L.L.; Zhang, Q.Y.; Yang, D.; Shen, X.Y.; Liu, L.; Zhu, Y.Z. S-propargyl-cysteine attenuates inflammatory response in rheumatoid arthritis by modulating the Nrf2-ARE signaling pathway. Redox Biol. 2016, 10, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Sidhapuriwala, J.N.; Hegde, A.; Ang, A.D.; Zhu, Y.Z.; Bhatia, M. Effects of S-propargyl-cysteine (SPRC) in caerulein-induced acute pancreatitis in mice. PLoS ONE 2012, 7, e32574. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Kan, J.; Liu, X.; Wang, S.; Zhu, Y. Cardioprotective effect of controlled release sprc to heart failure of rats after myocardial infarction and its possible mechanism. Heart 2012, 98, E232. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; An, G.; Lu, X. Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clin. Sci. 2015, 128, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, Y.; Escaffre, O.; Ivanciuc, T.; Komaravelli, N.; Kelley John, P.; Coletta, C.; Szabo, C.; Rockx, B.; Garofalo Roberto, P.; et al. Role of Hydrogen Sulfide in Paramyxovirus Infections. J. Virol. 2015, 89, 5557–5568. [Google Scholar] [CrossRef] [PubMed]
- Vaamonde-García, C.; Burguera, E.F.; Vela-Anero, Á.; Hermida-Gómez, T.; Filgueira-Fernández, P.; Fernández-Rodríguez, J.A.; Meijide-Faílde, R.; Blanco, F.J. Intraarticular Administration Effect of Hydrogen Sulfide on an In Vivo Rat Model of Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 7421. [Google Scholar] [CrossRef]
- Li, L.; Salto-Tellez, M.; Tan, C.-H.; Whiteman, M.; Moore, P.K. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free. Radic. Biol. Med. 2009, 47, 103–113. [Google Scholar] [CrossRef]
- Lazarević, M.; Battaglia, G.; Jevtić, B.; Đedović, N.; Bruno, V.; Cavalli, E.; Miljković, Đ.; Nicoletti, F.; Momčilović, M.; Fagone, P. Upregulation of Tolerogenic Pathways by the Hydrogen Sulfide Donor GYY4137 and Impaired Expression of H2S-Producing Enzymes in Multiple Sclerosis. Antioxidants 2020, 9, 608. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Liu, N.; Liu, B. AP39, a novel mitochondria-targeted hydrogen sulfide donor ameliorates doxorubicin-induced cardiotoxicity by regulating the AMPK/UCP2 pathway. PLoS ONE 2024, 19, e0300261. [Google Scholar] [CrossRef]
- Ahmad, A.; Szabo, C. Both the H2S biosynthesis inhibitor aminooxyacetic acid and the mitochondrially targeted H2S donor AP39 exert protective effects in a mouse model of burn injury. Pharmacol. Res. 2016, 113, 348–355. [Google Scholar] [CrossRef]
- Padiya, R.; Chowdhury, D.; Borkar, R.; Srinivas, R.; Pal Bhadra, M.; Banerjee, S.K. Garlic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLoS ONE 2014, 9, e94228. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, I.; Sorrentino, L.; Paoletti, A.; Marra, R.; Arbitrio, M. The state of the art on acetylcholinesterase inhibitors in the treatment of Alzheimer’s disease. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211029113. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Hai, P.; Yue, W. Effect of Allicin on the Expression of tau Protein in Transgenic Mice Brain with Alzheimer’s Disease. Nat. Prod. Res. Dev. 2016, 28, 685. [Google Scholar]
- Liu, Y.; Li, A.; Feng, X.; Sun, X.; Zhu, X.; Zhao, Z. Pharmacological Investigation of the Anti-Inflammation and Anti-Oxidation Activities of Diallyl Disulfide in a Rat Emphysema Model Induced by Cigarette Smoke Extract. Nutrients 2018, 10, 79. [Google Scholar] [CrossRef]
- Batallé, G.; Bai, X.; Pouso-Vázquez, E.; Roch, G.; Rodríguez, L.; Pol, O. The Recovery of Cognitive and Affective Deficiencies Linked with Chronic Osteoarthritis Pain and Implicated Pathways by Slow-Releasing Hydrogen Sulfide Treatment. Antioxidants 2021, 10, 1632. [Google Scholar] [CrossRef]
- Bhatia, M.; Sidhapuriwala, J.N.; Sparatore, A.; Moore, P.K. Treatment with H2S-releasing diclofenac protects mice against acute pancreatitis-associated lung injury. Shock 2008, 29, 84–88. [Google Scholar] [CrossRef]
- Sidhapuriwala, J.; Li, L.; Sparatore, A.; Bhatia, M.; Moore, P.K. Effect of S-diclofenac, a novel hydrogen sulfide releasing derivative, on carrageenan-induced hindpaw oedema formation in the rat. Eur. J. Pharmacol. 2007, 569, 149–154. [Google Scholar] [CrossRef]
- Zhang, H.; Zhi, L.; Moochhala, S.; Moore, P.K.; Bhatia, M. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L960–L971. [Google Scholar] [CrossRef]
- Tamizhselvi, R.; Koh, Y.H.; Sun, J.; Zhang, H.; Bhatia, M. Hydrogen sulfide induces ICAM-1 expression and neutrophil adhesion to caerulein-treated pancreatic acinar cells through NF-kappaB and Src-family kinases pathway. Exp. Cell Res. 2010, 316, 1625–1636. [Google Scholar] [CrossRef]
- Bibli, S.I.; Andreadou, I.; Chatzianastasiou, A.; Tzimas, C.; Sanoudou, D.; Kranias, E.; Brouckaert, P.; Coletta, C.; Szabo, C.; Kremastinos, D.T.; et al. Cardioprotection by H2S engages a cGMP-dependent protein kinase G/phospholamban pathway. Cardiovasc. Res. 2015, 106, 432–442. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Wang, H. Hydrogen sulfide alleviates oxidative stress injury and reduces apoptosis induced by MPP+ in Parkinson’s disease cell model. Mol. Cell. Biochem. 2020, 472, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Jin, H.; Wei, H.; Li, W.; Bu, D.; Tang, X.; Ren, Y.; Tang, C.; Du, J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Panopoulos, P.; Chasapis, C.T.; Coletta, C.; Zhou, Z.; Cirino, G.; Giannis, A.; Szabo, C.; Spyroulias, G.A.; Papapetropoulos, A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br. J. Pharmacol. 2013, 169, 922–932. [Google Scholar] [CrossRef]
- Maekawa, M.; Okamura, T.; Kasai, N.; Hori, Y.; Summer, K.H.; Konno, R. D-amino-acid oxidase is involved in D-serine-induced nephrotoxicity. Chem. Res. Toxicol. 2005, 18, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Deng, Y.; Pan, L.; Fu, H.; Han, X.; Li, Y.; Shi, H.; Wang, T. Therapeutic potential of endogenous hydrogen sulfide inhibition in breast cancer (Review). Oncol. Rep. 2021, 45, 68. [Google Scholar] [CrossRef]
- Badiei, A.; Chambers, S.T.; Gaddam, R.R.; Bhatia, M. Cystathionine-γ-lyase gene silencing with siRNA in monocytes/macrophages attenuates inflammation in cecal ligation and puncture-induced sepsis in the mouse. J. Biosci. 2016, 41, 87–95. [Google Scholar] [CrossRef]
- Banjac, A.; Perisic, T.; Sato, H.; Seiler, A.; Bannai, S.; Weiss, N.; Kölle, P.; Tschoep, K.; Issels, R.; Daniel, P. The cystine/cysteine cycle: A redox cycle regulating susceptibility versus resistance to cell death. Oncogene 2008, 27, 1618–1628. [Google Scholar] [CrossRef]
- Cramer, S.L.; Saha, A.; Liu, J.; Tadi, S.; Tiziani, S.; Yan, W.; Triplett, K.; Lamb, C.; Alters, S.E.; Rowlinson, S. Systemic depletion of L-cyst (e) ine with cyst (e) inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 2017, 23, 120–127. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Miliani, L.; Nielsen, O.; Andersen, P.; Girardin, S. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Han, N.-R.; Ko, S.-G.; Park, H.-J.; Moon, P.-D. Hydrogen Sulfide Downregulates Oncostatin M Expression via PI3K/Akt/NF-κB Signaling Processes in Neutrophil-like Differentiated HL-60 Cells. Antioxidants 2023, 12, 417. [Google Scholar] [CrossRef]
- Muniraj, N.; Stamp, L.K.; Badiei, A.; Hegde, A.; Cameron, V.; Bhatia, M. Hydrogen sulfide acts as a pro-inflammatory mediator in rheumatic disease. Int. J. Rheum. Dis. 2017, 20, 182–189. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Sidhapuriwala, J.; Moochhala, S.M.; Moore, P.K. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br. J. Pharmacol. 2005, 145, 141–144. [Google Scholar] [CrossRef]
- Brancaleone, V.; Esposito, I.; Gargiulo, A.; Vellecco, V.; Asimakopoulou, A.; Citi, V.; Calderone, V.; Gobbetti, T.; Perretti, M.; Papapetropoulos, A.; et al. D-Penicillamine modulates hydrogen sulfide (H2S) pathway through selective inhibition of cystathionine-γ-lyase. Br. J. Pharmacol. 2016, 173, 1556–1565. [Google Scholar] [CrossRef]
- Fox, B.; Schantz, J.T.; Haigh, R.; Wood, M.E.; Moore, P.K.; Viner, N.; Spencer, J.P.; Winyard, P.G.; Whiteman, M. Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: Is H2S a novel cytoprotective mediator in the inflamed joint? J. Cell. Mol. Med. 2012, 16, 896–910. [Google Scholar] [CrossRef]
- Kloesch, B.; Liszt, M.; Krehan, D.; Broell, J.; Kiener, H.; Steiner, G. High concentrations of hydrogen sulphide elevate the expression of a series of pro-inflammatory genes in fibroblast-like synoviocytes derived from rheumatoid and osteoarthritis patients. Immunol. Lett. 2012, 141, 197–203. [Google Scholar] [CrossRef]
- Burguera, E.F.; Vela-Anero, Á.; Gato-Calvo, L.; Vaamonde-García, C.; Meijide-Faílde, R.; Blanco, F.J. Hydrogen sulfide biosynthesis is impaired in the osteoarthritic joint. Int. J. Biometeorol. 2020, 64, 997–1010. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.-H.; Moore, P.K. Characterization of a Novel, Water-Soluble Hydrogen Sulfide–Releasing Molecule (GYY4137). Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fox, B.; Keeble, J.; Salto-Tellez, M.; Winyard, P.G.; Wood, M.E.; Moore, P.K.; Whiteman, M. The complex effects of the slow-releasing hydrogen sulfide donor GYY4137 in a model of acute joint inflammation and in human cartilage cells. J. Cell. Mol. Med. 2013, 17, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, H.; Fang, L.; Bian, J.; Gao, Y.; Li, C. H2S Donor and Bone Metabolism. Front. Pharmacol. 2021, 12, 661601. [Google Scholar] [CrossRef]
- Qian, Y.Q.; Feng, Z.H.; Li, X.B.; Hu, Z.C.; Xuan, J.W.; Wang, X.Y.; Xu, H.C.; Chen, J.X. Downregulating PI3K/Akt/NF-κB signaling with allicin for ameliorating the progression of osteoarthritis: In vitro and vivo studies. Food Funct. 2018, 9, 4865–4875. [Google Scholar] [CrossRef] [PubMed]
- Dief, A.E.; Mostafa, D.K.; Sharara, G.M.; Zeitoun, T.H. Hydrogen sulfide releasing naproxen offers better anti-inflammatory and chondroprotective effect relative to naproxen in a rat model of zymosan induced arthritis. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1537–1546. [Google Scholar]
- Szczesny, B.; Módis, K.; Yanagi, K.; Coletta, C.; Le Trionnaire, S.; Perry, A.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide 2014, 41, 120–130. [Google Scholar] [CrossRef]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef]
- Deutschman, C.S.; Tracey, K.J. Sepsis: Current Dogma and New Perspectives. Immunity 2014, 40, 463–475. [Google Scholar] [CrossRef]
- Opal, S.M.; van der Poll, T. Endothelial barrier dysfunction in septic shock. J. Intern. Med. 2015, 277, 277–293. [Google Scholar] [CrossRef]
- Rudd, K.E.; Kissoon, N.; Limmathurotsakul, D.; Bory, S.; Mutahunga, B.; Seymour, C.W.; Angus, D.C.; West, T.E. The global burden of sepsis: Barriers and potential solutions. Crit. Care 2018, 22, 232. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, C.; Jaimes, F. Organ Dysfunction in Sepsis: An Ominous Trajectory From Infection To Death. Yale J. Biol. Med. 2019, 92, 629–640. [Google Scholar] [PubMed]
- Zhang, H.; Zhi, L.; Moore, P.K.; Bhatia, M. Role of hydrogen sulfide in cecal ligation and puncture-induced sepsis in the mouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L1193–L1201. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Moore, P.K. Dexamethasone inhibits lipopolysaccharide-induced hydrogen sulphide biosynthesis in intact cells and in an animal model of endotoxic shock. J. Cell. Mol. Med. 2009, 13, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhi, L.; Moochhala, S.M.; Moore, P.K.; Bhatia, M. Endogenous hydrogen sulfide regulates leukocyte trafficking in cecal ligation and puncture-induced sepsis. J. Leukoc. Biol. 2007, 82, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Coletta, C.; Szabo, C. Potential role of hydrogen sulfide in the pathogenesis of vascular dysfunction in septic shock. Curr. Vasc. Pharmacol. 2013, 11, 208–221. [Google Scholar]
- Huang, C.W.; Feng, W.; Peh, M.T.; Peh, K.; Dymock, B.W.; Moore, P.K. A novel slow-releasing hydrogen sulfide donor, FW1256, exerts anti-inflammatory effects in mouse macrophages and in vivo. Pharmacol. Res. 2016, 113, 533–546. [Google Scholar] [CrossRef]
- Rios, E.C.; Szczesny, B.; Soriano, F.G.; Olah, G.; Szabo, C. Hydrogen sulfide attenuates cytokine production through the modulation of chromatin remodeling. Int. J. Mol. Med. 2015, 35, 1741–1746. [Google Scholar] [CrossRef]
- Badiei, A.; Rivers-Auty, J.; Ang, A.D.; Bhatia, M. Inhibition of hydrogen sulfide production by gene silencing attenuates inflammatory activity of LPS-activated RAW264. 7 cells. Appl. Microbiol. Biotechnol. 2013, 97, 7845–7852. [Google Scholar] [CrossRef]
- Collin, M.; Anuar, F.B.; Murch, O.; Bhatia, M.; Moore, P.K.; Thiemermann, C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br. J. Pharmacol. 2005, 146, 498–505. [Google Scholar] [CrossRef]
- Fiorucci, S.; Orlandi, S.; Mencarelli, A.; Caliendo, G.; Santagada, V.; Distrutti, E.; Santucci, L.; Cirino, G.; Wallace, J. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br. J. Pharmacol. 2007, 150, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rossoni, G.; Sparatore, A.; Lee, L.C.; Del Soldato, P.; Moore, P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free. Radic. Biol. Med. 2007, 42, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M. H2S and inflammation: An overview. Chem. Biochem. Pharmacol. Hydrog. Sulfide 2015, 230, 165–180. [Google Scholar]
- Merz, T.; McCook, O.; Brucker, C.; Waller, C.; Calzia, E.; Radermacher, P.; Datzmann, T. H2S in Critical Illness-A New Horizon for Sodium Thiosulfate? Biomolecules 2022, 12, 543. [Google Scholar] [CrossRef]
- Norris, E.J.; Feilen, N.; Nguyen, N.H.; Culberson, C.R.; Shin, M.C.; Fish, M.; Clemens, M.G. Hydrogen sulfide modulates sinusoidal constriction and contributes to hepatic micorcirculatory dysfunction during endotoxemia. Am. J. Physiol.—Gastrointest. Liver Physiol. 2013, 304, G1070–G1078. [Google Scholar] [CrossRef]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.-E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, R.R.; Fraser, R.; Badiei, A.; Chambers, S.; Cogger, V.C.; Le Couteur, D.G.; Ishii, I.; Bhatia, M. Cystathionine-gamma-lyase gene deletion protects mice against inflammation and liver sieve injury following polymicrobial sepsis. PLoS ONE 2016, 11, e0160521. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Chambers, S.; Fraser, R.; Cogger, V.C.; Le Couteur, D.G.; Ishii, I.; Bhatia, M. Cystathionine-Gamma-Lyase-Derived hydrogen sulfide-regulated substance P modulates liver sieve fenestrations in Caecal ligation and puncture-induced sepsis. Int. J. Mol. Sci. 2019, 20, 3191. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, R.R.; Chambers, S.; Murdoch, D.; Shaw, G.; Bhatia, M. Circulating levels of hydrogen sulfide and substance P in patients with sepsis. J. Infect. 2017, 75, 293–300. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Pan, L.; Wu, W.; Yang, D.; Qin, M.; Jia, W.; Xiao, C.; Long, F.; Ge, J. Endogenous hydrogen sulfide regulates histone demethylase JMJD3-mediated inflammatory response in LPS-stimulated macrophages and in a mouse model of LPS-induced septic shock. Biochem. Pharmacol. 2018, 149, 153–162. [Google Scholar] [CrossRef]
- Bee, N.; White, R.; Petros, A.J. Hydrogen sulfide in exhaled gases from ventilated septic neonates and children: A preliminary report. Pediatr. Crit. Care Med. 2017, 18, e327–e332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hegde, A.; Ng, S.W.; Adhikari, S.; Moochhala, S.M.; Bhatia, M. Hydrogen Sulfide Up-Regulates Substance P in Polymicrobial Sepsis-Associated Lung Injury1. J. Immunol. 2007, 179, 4153–4160. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.F.; Moochhala, S.M.; Bhatia, M. Hydrogen sulfide promotes transient receptor potential vanilloid 1-mediated neurogenic inflammation in polymicrobial sepsis. Crit. Care Med. 2010, 38, 619–628. [Google Scholar] [CrossRef]
- Ang, S.F.; Moochhala, S.M.; MacAry, P.A.; Bhatia, M. Hydrogen sulfide and neurogenic inflammation in polymicrobial sepsis: Involvement of substance P and ERK-NF-κB signaling. PLoS ONE 2011, 6, e24535. [Google Scholar] [CrossRef]
- Ang, S.-F.; Sio, S.W.S.; Moochhala, S.M.; MacAry, P.A.; Bhatia, M. Hydrogen Sulfide Upregulates Cyclooxygenase-2 and Prostaglandin E Metabolite in Sepsis-Evoked Acute Lung Injury via Transient Receptor Potential Vanilloid Type 1 Channel Activation. J. Immunol. 2011, 187, 4778–4787. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Zhi, L.; Zhang, H.; Ng, S.W.; Moore, P.K. Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L896–L904. [Google Scholar] [CrossRef]
- Fonseca Sepúlveda, E.V.; Guerrero-Lozano, R. Acute pancreatitis and recurrent acute pancreatitis: An exploration of clinical and etiologic factors and outcomes. J. Pediatr. Rio. J. 2019, 95, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, R.; Masso, L.; Kapadia, D.; Kulairi, Z.I. Medication as a cause of acute pancreatitis. Am. J. Case Rep. 2017, 18, 839. [Google Scholar] [CrossRef]
- Quinlan, C.; Gallagher, T.; Moran, N.; Dass, G.; Sweeney, B.; Conlon, K.; Ridgway, P. Acute pancreatitis in the paediatric population: Different disease, same management? Ir. J. Med. Sci. 2011, 180, S254–S255. [Google Scholar] [CrossRef]
- Zerem, E. Treatment of severe acute pancreatitis and its complications. World J. Gastroenterol. WJG 2014, 20, 13879. [Google Scholar] [CrossRef]
- Balthazar, E.J. Acute pancreatitis: Assessment of severity with clinical and CT evaluation. Radiology 2002, 223, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Wong, F.L.; Fu, D.; Lau, H.Y.; Moochhala, S.M.; Moore, P.K. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005, 19, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Ang, A.D.; Rivers-Auty, J.; Hegde, A.; Ishii, I.; Bhatia, M. The effect of CSE gene deletion in caerulein-induced acute pancreatitis in the mouse. Am. J. Physiol.—Gastrointest. Liver Physiol. 2013, 305, G712–G721. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liao, R.; Qiang, Z.; Zhang, C. Pro-inflammatory cytokine-driven PI3K/Akt/Sp1 signalling and H2S production facilitates the pathogenesis of severe acute pancreatitis. Biosci. Rep. 2017, 37, BSR20160483. [Google Scholar] [CrossRef]
- Shanmugam, M.; Jing, Z.; Bhatia, M. Aminooxyacetate inhibits hydrogen sulfide and ammonium synthesis and protects mice in acute pancreatitis. Int. J. Integr. Biol. 2009, 8, 7–14. [Google Scholar]
- Bhatia, M.; Sidhapuriwala, J.N.; Ng, S.W.; Tamizhselvi, R.; Moochhala, S.M. Pro-inflammatory effects of hydrogen sulphide on substance P in caerulein-induced acute pancreatitis. J. Cell. Mol. Med. 2008, 12, 580–590. [Google Scholar] [CrossRef]
- Tamizhselvi, R.; Shrivastava, P.; Koh, Y.H.; Zhang, H.; Bhatia, M. Preprotachykinin-A gene deletion regulates hydrogen sulfide-induced toll-like receptor 4 signaling pathway in cerulein-treated pancreatic acinar cells. Pancreas 2011, 40, 444–452. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef]
- Mani, S.; Li, H.; Untereiner, A.; Wu, L.; Yang, G.; Austin, R.C.; Dickhout, J.G.; Lhoták, Š.; Meng, Q.H.; Wang, R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 2013, 127, 2523–2534. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.B.; Feng, L.; Hodges, J.K.; Lechuga, T.J.; Zhang, H. Human trophoblast-derived hydrogen sulfide stimulates placental artery endothelial cell angiogenesis. Biol. Reprod. 2017, 97, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Zhou, Z.X.; Ren, Z.; Yang, S.; Liu, L.S.; Wang, Z.; Wei, D.H.; Ma, X.F.; Ma, Y.; Jiang, Z.S. EndMT: Potential Target of H2S against Atherosclerosis. Curr. Med. Chem. 2021, 28, 3666–3680. [Google Scholar] [CrossRef]
- Scammahorn, J.J.; Nguyen, I.T.N.; Bos, E.M.; Van Goor, H.; Joles, J.A. Fighting Oxidative Stress with Sulfur: Hydrogen Sulfide in the Renal and Cardiovascular Systems. Antioxidants 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Elmoselhi, A.B.; Hata, T.; Makino, N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc. Res. 2000, 47, 446–456. [Google Scholar] [CrossRef]
- Luan, H.F.; Zhao, Z.B.; Zhao, Q.H.; Zhu, P.; Xiu, M.Y.; Ji, Y. Hydrogen sulfide postconditioning protects isolated rat hearts against ischemia and reperfusion injury mediated by the JAK2/STAT3 survival pathway. Braz. J. Med. Biol. Res. 2012, 45, 898–905. [Google Scholar] [CrossRef]
- Jin, H.; Wang, Y.; Wang, X.; Sun, Y.; Tang, C.; Du, J. Sulfur dioxide preconditioning increases antioxidative capacity in rat with myocardial ischemia reperfusion (I/R) injury. Nitric Oxide 2013, 32, 56–61. [Google Scholar] [CrossRef]
- Gao, L.; Xu, Z.; Yin, Z.; Chen, K.; Wang, C.; Zhang, H. Association of hydrogen sulfide with alterations of monocyte chemokine receptors, CCR2 and CX3CR1 in patients with coronary artery disease. Inflamm. Res. 2015, 64, 627–635. [Google Scholar] [CrossRef]
- Feng, S.J.; Li, H.; Wang, S.X. Lower Hydrogen Sulfide Is Associated with Cardiovascular Mortality, Which Involves cPKCβII/Akt Pathway in Chronic Hemodialysis Patients. Blood Purif. 2015, 40, 260–269. [Google Scholar] [CrossRef]
- Leucker, T.M.; Nomura, Y.; Kim, J.H.; Bhatta, A.; Wang, V.; Wecker, A.; Jandu, S.; Santhanam, L.; Berkowitz, D.; Romer, L.; et al. Cystathionine γ-lyase protects vascular endothelium: A role for inhibition of histone deacetylase 6. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H711–H720. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, L.; Yu, X. Protective effect of hydrogen sulfide on rats with myocardial ischemia/reperfusion injury and its mechanism. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2015, 31, 316–320. [Google Scholar] [PubMed]
- Hu, M.Z.; Zhou, B.; Mao, H.Y.; Sheng, Q.; Du, B.; Chen, J.L.; Pang, Q.F.; Ji, Y. Exogenous Hydrogen Sulfide Postconditioning Protects Isolated Rat Hearts From Ischemia/Reperfusion Injury Through Sirt1/PGC-1α Signaling Pathway. Int. Heart J. 2016, 57, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Qipshidze, N.; Metreveli, N.; Mishra, P.K.; Lominadze, D.; Tyagi, S.C. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. Int. J. Biol. Sci. 2012, 8, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Barr, L.A.; Shimizu, Y.; Lambert, J.P.; Nicholson, C.K.; Calvert, J.W. Hydrogen sulfide attenuates high fat diet-induced cardiac dysfunction via the suppression of endoplasmic reticulum stress. Nitric Oxide 2015, 46, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Kesherwani, V.; Nandi, S.S.; Sharawat, S.K.; Shahshahan, H.R.; Mishra, P.K. Hydrogen sulfide mitigates homocysteine-mediated pathological remodeling by inducing miR-133a in cardiomyocytes. Mol. Cell. Biochem. 2015, 404, 241–250. [Google Scholar] [CrossRef]
- Nandi, S.S.; Mishra, P.K. H2S and homocysteine control a novel feedback regulation of cystathionine beta synthase and cystathionine gamma lyase in cardiomyocytes. Sci. Rep. 2017, 7, 3639. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, J.; Rong, J. Pharmacological Modulation of Cardiac Remodeling after Myocardial Infarction. Oxidative Med. Cell. Longev. 2020, 2020, 8815349. [Google Scholar] [CrossRef]
- Shindo, T.; Manabe, I.; Fukushima, Y.; Tobe, K.; Aizawa, K.; Miyamoto, S.; Kawai-Kowase, K.; Moriyama, N.; Imai, Y.; Kawakami, H.; et al. Krüppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat. Med. 2002, 8, 856–863. [Google Scholar] [CrossRef]
- Huang, J.; Wang, D.; Zheng, J.; Huang, X.; Jin, H. Hydrogen sulfide attenuates cardiac hypertrophy and fibrosis induced by abdominal aortic coarctation in rats. Mol. Med. Rep. 2012, 5, 923–928. [Google Scholar] [CrossRef]
- Meng, G.; Xiao, Y.; Ma, Y.; Tang, X.; Xie, L.; Liu, J.; Gu, Y.; Yu, Y.; Park, C.M.; Xian, M.; et al. Hydrogen Sulfide Regulates Krüppel-Like Factor 5 Transcription Activity via Specificity Protein 1 S-Sulfhydration at Cys664 to Prevent Myocardial Hypertrophy. J. Am. Heart Assoc. 2016, 5, e004160. [Google Scholar] [CrossRef]
- Su, H.; Su, H.; Liu, C.-H.; Hu, H.-J.; Zhao, J.-B.; Zou, T.; Tang, Y.-X. H2S inhibits atrial fibrillation-induced atrial fibrosis through miR-133a/CTGF axis. Cytokine 2021, 146, 155557. [Google Scholar] [CrossRef]
- Xiong, R.; Lu, X.; Song, J.; Li, H.; Wang, S. Molecular mechanisms of hydrogen sulfide against uremic accelerated atherosclerosis through cPKCβII/Akt signal pathway. BMC Nephrol. 2019, 20, 358. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, Q.; Li, X.; Wei, R.; Ge, T.; Zheng, X.; Li, B.; Liu, K.; Cui, R. Hydrogen sulfide: A new therapeutic target in vascular diseases. Front. Endocrinol. 2022, 13, 934231. [Google Scholar] [CrossRef] [PubMed]
- Roell, W.; Lewalter, T.; Sasse, P.; Tallini, Y.N.; Choi, B.R.; Breitbach, M.; Doran, R.; Becher, U.M.; Hwang, S.M.; Bostani, T.; et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature 2007, 450, 819–824. [Google Scholar] [CrossRef]
- Testai, L.; Marino, A.; Piano, I.; Brancaleone, V.; Tomita, K.; Di Cesare Mannelli, L.; Martelli, A.; Citi, V.; Breschi, M.C.; Levi, R.; et al. The novel H2S-donor 4-carboxyphenyl isothiocyanate promotes cardioprotective effects against ischemia/reperfusion injury through activation of mitoK(ATP) channels and reduction of oxidative stress. Pharmacol. Res. 2016, 113, 290–299. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Bornbaum, J.; Boengler, K.; Torregrossa, R.; Whiteman, M.; Wood, M.E.; Schulz, R.; Baxter, G.F. AP39, a mitochondria-targeting hydrogen sulfide (H2S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. Br. J. Pharmacol. 2017, 174, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Knutelska, J.; Bednarski, M.; Nowiński, L.; Zygmunt, M.; Bilska-Wilkosz, A.; Iciek, M.; Otto, M.; Żytka, I.; Sapa, J.; et al. Alpha lipoic acid protects the heart against myocardial post ischemia-reperfusion arrhythmias via KATP channel activation in isolated rat hearts. Pharmacol. Rep. 2014, 66, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, E.; Ali, M.J.; Rushing, A.M.; Scarborough, A.L.; Bradley, J.M.; Organ, C.L.; Islam, K.N.; Polhemus, D.J.; Evangelista, S.; Cirino, G.; et al. Zofenopril Protects Against Myocardial Ischemia-Reperfusion Injury by Increasing Nitric Oxide and Hydrogen Sulfide Bioavailability. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Nicholson, C.K.; Lambert, J.P.; Molkentin, J.D.; Sadoshima, J.; Calvert, J.W. Thioredoxin 1 is essential for sodium sulfide-mediated cardioprotection in the setting of heart failure. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 744–751. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.L.; Liu, H.R.; Rose, P.; Zhu, Y.Z. Protective effects of cysteine analogues on acute myocardial ischemia: Novel modulators of endogenous H2S production. Antioxid. Redox Signal. 2010, 12, 1155–1165. [Google Scholar] [CrossRef]
- Peake, B.F.; Nicholson, C.K.; Lambert, J.P.; Hood, R.L.; Amin, H.; Amin, S.; Calvert, J.W. Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1215–H1224. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.J.; Li, Z.; Pattillo, C.B.; Gojon Sr, G.; Gojon Jr, G.; Giordano, T.; Krum, H. A novel hydrogen sulfide prodrug, SG 1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc. Ther. 2015, 33, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.K.; Donnelly, E.; Donnarumma, E.; Hossain, F.; Gardner, J.D.; Islam, K.N. H2S Prodrug, SG-1002, Protects against Myocardial Oxidative Damage and Hypertrophy In Vitro via Induction of Cystathionine β-Synthase and Antioxidant Proteins. Biomedicines 2023, 11, 612. [Google Scholar] [CrossRef]
- de Koning, M.-S.L.; Assa, S.; Maagdenberg, C.G.; van Veldhuisen, D.J.; Pasch, A.; van Goor, H.; Lipsic, E.; van der Harst, P. Safety and Tolerability of Sodium Thiosulfate in Patients with an Acute Coronary Syndrome Undergoing Coronary Angiography: A Dose-Escalation Safety Pilot Study (SAFE-ACS). J. Interv. Cardiol. 2020, 2020, 6014915. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Murphy, B.; Mustafi, S.B.; Dey, A.; Xiong, X.; Rao, G.; Naz, S.; Zhang, M.; Yang, D.; Dhanasekaran, D.N. Cystathionine β-synthase regulates mitochondrial morphogenesis in ovarian cancer. FASEB J. 2018, 32, 4145. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, B.; Qin, Y.; Li, A.; Gao, M.; Liu, H.; Gong, G. Mitochondrial fusion protein Mfn2 and its role in heart failure. Front. Mol. Biosci. 2021, 8, 681237. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shackelford, R.E.; Shen, X.; Dominic, P.; Kevil, C.G. Sulfide regulation of cardiovascular function in health and disease. Nat. Rev. Cardiol. 2023, 20, 109–125. [Google Scholar] [CrossRef]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef]
- Escaffre, O.; Borisevich, V.; Rockx, B. Pathogenesis of Hendra and Nipah virus infection in humans. J. Infect. Dev. Ctries. 2013, 7, 308–311. [Google Scholar] [CrossRef]
- Bao, X.; Liu, T.; Spetch, L.; Kolli, D.; Garofalo, R.P.; Casola, A. Airway epithelial cell response to human metapneumovirus infection. Virology 2007, 368, 91–101. [Google Scholar] [CrossRef]
- Komaravelli, N.; Casola, A. Respiratory Viral Infections and Subversion of Cellular Antioxidant Defenses. J. Pharmacogenomics Pharmacoproteomics 2014, 5, 1000141. [Google Scholar] [CrossRef] [PubMed]
- Gojon, G.; Morales, G.A. SG1002 and Catenated Divalent Organic Sulfur Compounds as Promising Hydrogen Sulfide Prodrugs. Antioxid. Redox Signal. 2020, 33, 1010–1045. [Google Scholar] [CrossRef]
- Palamara, A.T.; Perno, C.F.; Ciriolo, M.R.; Dini, L.; Balestra, E.; D’Agostini, C.; Di Francesco, P.; Favalli, C.; Rotilio, G.; Garaci, E. Evidence for antiviral activity of glutathione: In vitro inhibition of herpes simplex virus type 1 replication. Antivir. Res. 1995, 27, 237–253. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.Y.; Yang, J.S.; Kim, J.W.; Kim, V.N.; Chang, H. The Architecture of SARS-CoV-2 Transcriptome. Cell 2020, 181, 914–921.e10. [Google Scholar] [CrossRef] [PubMed]
- Renieris, G.; Katrini, K.; Damoulari, C.; Akinosoglou, K.; Psarrakis, C.; Kyriakopoulou, M.; Dimopoulos, G.; Lada, M.; Koufargyris, P.; Giamarellos-Bourboulis, E.J. Serum hydrogen sulfide and outcome association in pneumonia by the SARS-CoV-2 coronavirus. Shock 2020, 54, 633–637. [Google Scholar] [CrossRef]
- Ivanciuc, T.; Sbrana, E.; Ansar, M.; Bazhanov, N.; Szabo, C.; Casola, A.; Garofalo, R.P. Hydrogen Sulfide Is an Antiviral and Antiinflammatory Endogenous Gasotransmitter in the Airways. Role in Respiratory Syncytial Virus Infection. Am. J. Respir. Cell Mol. Biol. 2016, 55, 684–696. [Google Scholar] [CrossRef]
- Cho, H.Y.; Miller-DeGraff, L.; Blankenship-Paris, T.; Wang, X.; Bell, D.A.; Lih, F.; Deterding, L.; Panduri, V.; Morgan, D.L.; Yamamoto, M.; et al. Sulforaphane enriched transcriptome of lung mitochondrial energy metabolism and provided pulmonary injury protection via Nrf2 in mice. Toxicol. Appl. Pharmacol. 2019, 364, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Bazhanov, N.; Ivanciuc, T.; Wu, H.; Garofalo, M.; Kang, J.; Xian, M.; Casola, A. Thiol-Activated Hydrogen Sulfide Donors Antiviral and Anti-Inflammatory Activity in Respiratory Syncytial Virus Infection. Viruses 2018, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A. Sulfur-Containing Compounds as Hydrogen Sulfide Donors and Broad-Spectrum Antiviral Agents; Washington State University: Washington, DC, USA, 2017. [Google Scholar]
- Khattak, S.; Zhang, Q.-Q.; Sarfraz, M.; Muhammad, P.; Ngowi, E.E.; Khan, N.H.; Rauf, S.; Wang, Y.-Z.; Qi, H.-W.; Wang, D.; et al. The Role of Hydrogen Sulfide in Respiratory Diseases. Biomolecules 2021, 11, 682. [Google Scholar] [CrossRef]
- Ge, X.; Sun, J.; Fei, A.; Gao, C.; Pan, S.; Wu, Z. Hydrogen sulfide treatment alleviated ventilator-induced lung injury through regulation of autophagy and endoplasmic reticulum stress. Int. J. Biol. Sci. 2019, 15, 2872–2884. [Google Scholar] [CrossRef]
- Han, W.; Dong, Z.; Dimitropoulou, C.; Su, Y. Hydrogen sulfide ameliorates tobacco smoke-induced oxidative stress and emphysema in mice. Antioxid. Redox Signal. 2011, 15, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Spassov, S.G.; Donus, R.; Ihle, P.M.; Engelstaedter, H.; Hoetzel, A.; Faller, S. Hydrogen Sulfide Prevents Formation of Reactive Oxygen Species through PI3K/Akt Signaling and Limits Ventilator-Induced Lung Injury. Oxid. Med. Cell. Longev. 2017, 2017, 3715037. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, G.; Wondimu, T.; Ross, B.; Wang, R. Hydrogen sulfide and asthma. Exp. Physiol. 2011, 96, 847–852. [Google Scholar] [CrossRef]
- Derwall, M.; Francis, R.C.; Kida, K.; Bougaki, M.; Crimi, E.; Adrie, C.; Zapol, W.M.; Ichinose, F. Administration of hydrogen sulfide via extracorporeal membrane lung ventilation in sheep with partial cardiopulmonary bypass perfusion: A proof of concept study on metabolic and vasomotor effects. Crit. Care 2011, 15, R51. [Google Scholar] [CrossRef]
- Perry, M.M.; Hui, C.K.; Whiteman, M.; Wood, M.E.; Adcock, I.; Kirkham, P.; Michaeloudes, C.; Chung, K.F. Hydrogen sulfide inhibits proliferation and release of IL-8 from human airway smooth muscle cells. Am. J. Respir. Cell. Mol. Biol. 2011, 45, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.J.; Wang, M.J.; Moore, P.K.; Jin, H.M.; Yao, T.; Zhu, Y.C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2007, 76, 29–40. [Google Scholar] [CrossRef]
- Dai, J.; Teng, X.; Jin, S.; Wu, Y. The Antiviral Roles of Hydrogen Sulfide by Blocking the Interaction between SARS-CoV-2 and Its Potential Cell Surface Receptors. Oxidative Med. Cell. Longev. 2021, 2021, 7866992. [Google Scholar] [CrossRef] [PubMed]
- Farese, S.; Stauffer, E.; Kalicki, R.; Hildebrandt, T.; Frey, B.M.; Frey, F.J.; Uehlinger, D.E.; Pasch, A. Sodium thiosulfate pharmacokinetics in hemodialysis patients and healthy volunteers. Clin. J. Am. Soc. Nephrol. 2011, 6, 1447–1455. [Google Scholar] [CrossRef]
- Evgen’ev, M.B.; Frenkel, A. Possible application of H2S-producing compounds in therapy of coronavirus (COVID-19) infection and pneumonia. Cell Stress Chaperones 2020, 25, 713–715. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Polhemus, D.J.; Lefer, D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014, 114, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, S.; Wen, J.-Y.; Chen, Z.-W. 3-Mercaptopyruvate sulfurtransferase/hydrogen sulfide protects cerebral endothelial cells against oxygen-glucose deprivation/reoxygenation-induced injury via mitoprotection and inhibition of the RhoA/ROCK pathway. Am. J. Physiol. Cell Physiol. 2020, 319, C720–C733. [Google Scholar] [CrossRef]
- Nicholson, C.K.; Calvert, J.W. Hydrogen sulfide and ischemia-reperfusion injury. Pharmacol. Res. 2010, 62, 289–297. [Google Scholar] [CrossRef]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, R.; Jeong, N.Y. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases. Int. J. Med. Sci. 2019, 16, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sandhir, R. Hydrogen Sulfide in Physiological and Pathological Mechanisms in Brain. CNS Neurol. Disord. Drug Targets 2018, 17, 654–670. [Google Scholar] [CrossRef]
- Aschner, M.; Skalny, A.V.; Ke, T.; da Rocha, J.B.; Paoliello, M.M.; Santamaria, A.; Bornhorst, J.; Rongzhu, L.; Svistunov, A.A.; Djordevic, A.B.; et al. Hydrogen Sulfide (H2S) Signaling as a Protective Mechanism against Endogenous and Exogenous Neurotoxicants. Curr. Neuropharmacol. 2022, 20, 1908–1924. [Google Scholar] [CrossRef]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Hodgson, N.; Trivedi, M.; Muratore, C.; Li, S.; Deth, R. Soluble oligomers of amyloid-β cause changes in redox state, DNA methylation, and gene transcription by inhibiting EAAT3 mediated cysteine uptake. J. Alzheimers Dis. 2013, 36, 197–209. [Google Scholar] [CrossRef]
- Aoyama, K.; Suh, S.W.; Hamby, A.M.; Liu, J.; Chan, W.Y.; Chen, Y.; Swanson, R.A. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat. Neurosci. 2006, 9, 119–126. [Google Scholar] [CrossRef]
- Liu, X.Q.; Liu, X.Q.; Jiang, P.; Huang, H.; Yan, Y. Plasma levels of endogenous hydrogen sulfide and homocysteine in patients with Alzheimer’s disease and vascular dementia and the significance thereof. Zhonghua Yi Xue Za Zhi 2008, 88, 2246–2249. [Google Scholar] [PubMed]
- Yan, Y.; Yan, Q.; Qian, L.; Jiang, Y.; Chen, X.; Zeng, S.; Xu, Z.; Gong, Z. S-adenosylmethionine administration inhibits levodopa-induced vascular endothelial growth factor-A expression. Aging 2020, 12, 21290–21307. [Google Scholar] [CrossRef] [PubMed]
- Eto, K.; Asada, T.; Arima, K.; Makifuchi, T.; Kimura, H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002, 293, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.Y.; Wu, X.; Lu, T.; Cui, G.; Chen, G. Research progress of hydrogen sulfide in Alzheimer’s disease from laboratory to hospital: A narrative review. Med. Gas. Res. 2020, 10, 125–129. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Bian, J.S. Hydrogen sulfide protects amyloid-β induced cell toxicity in microglia. J. Alzheimers Dis. 2010, 22, 1189–1200. [Google Scholar] [CrossRef]
- Cao, L.; Cao, X.; Zhou, Y.; Nagpure, B.V.; Wu, Z.Y.; Hu, L.F.; Yang, Y.; Sethi, G.; Moore, P.K.; Bian, J.S. Hydrogen sulfide inhibits ATP-induced neuroinflammation and Aβ(1-42) synthesis by suppressing the activation of STAT3 and cathepsin S. Brain Behav. Immun. 2018, 73, 603–614. [Google Scholar] [CrossRef]
- Rao, S.P.; Xie, W.; Kwon, Y.I.C.; Juckel, N.; Xie, J.; Dronamraju, V.R.; Vince, R.; Lee, M.K.; More, S.S. Sulfanegen stimulates 3-mercaptopyruvate sulfurtransferase activity and ameliorates Alzheimer’s disease pathology and oxidative stress in vivo. Redox Biol. 2022, 57, 102484. [Google Scholar] [CrossRef]
- Moore, D.J.; West, A.B.; Dawson, V.L.; Dawson, T.M. Molecular pathophysiology of Parkinson’s disease. Annu. Rev. Neurosci. 2005, 28, 57–87. [Google Scholar] [CrossRef]
- Parkinson, J. An essay on the shaking palsy. 1817. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 223–236; discussion 222. [Google Scholar] [CrossRef]
- Shefa, U.; Kim, M.S.; Jeong, N.Y.; Jung, J. Antioxidant and Cell-Signaling Functions of Hydrogen Sulfide in the Central Nervous System. Oxid. Med. Cell. Longev. 2018, 2018, 1873962. [Google Scholar] [CrossRef]
- Hu, L.F.; Lu, M.; Wu, Z.Y.; Wong, P.T.; Bian, J.S. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol. Pharmacol. 2009, 75, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Schwab, C.; Yu, S.; McGeer, E.; McGeer, P.L. Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol. Aging 2009, 30, 1523–1534. [Google Scholar] [CrossRef]
- Murros, K.E. Hydrogen Sulfide Produced by Gut Bacteria May Induce Parkinson’s Disease. Cells 2022, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Ross, C.A.; Aylward, E.H.; Wild, E.J.; Langbehn, D.R.; Long, J.D.; Warner, J.H.; Scahill, R.I.; Leavitt, B.R.; Stout, J.C.; Paulsen, J.S.; et al. Huntington disease: Natural history, biomarkers and prospects for therapeutics. Nat. Rev. Neurol. 2014, 10, 204–216. [Google Scholar] [CrossRef]
- Paul, B.D.; Sbodio, J.I.; Snyder, S.H. Mutant Huntingtin Derails Cysteine Metabolism in Huntington’s Disease at Both Transcriptional and Post-Translational Levels. Antioxidants 2022, 11, 1470. [Google Scholar] [CrossRef]
- Morozko, E.L.; Smith-Geater, C.; Monteys, A.M.; Pradhan, S.; Lim, R.G.; Langfelder, P.; Kachemov, M.; Kulkarni, J.A.; Zaifman, J.; Hill, A. PIAS1 modulates striatal transcription, DNA damage repair, and SUMOylation with relevance to Huntington’s disease. Proc. Natl. Acad. Sci. USA 2021, 118, e2021836118. [Google Scholar] [CrossRef] [PubMed]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Golgi stress response reprograms cysteine metabolism to confer cytoprotection in Huntington’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, 780–785. [Google Scholar] [CrossRef]
- Giovinazzo, D.; Bursac, B.; Sbodio, J.I.; Nalluru, S.; Vignane, T.; Snowman, A.M.; Albacarys, L.M.; Sedlak, T.W.; Torregrossa, R.; Whiteman, M. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA 2021, 118, e2017225118. [Google Scholar] [CrossRef]
- Vandini, E.; Ottani, A.; Zaffe, D.; Calevro, A.; Canalini, F.; Cavallini, G.M.; Rossi, R.; Guarini, S.; Giuliani, D. Mechanisms of Hydrogen Sulfide against the Progression of Severe Alzheimer’s Disease in Transgenic Mice at Different Ages. Pharmacology 2019, 103, 50–60. [Google Scholar] [CrossRef]
- Zhao, F.L.; Fang, F.; Qiao, P.F.; Yan, N.; Gao, D.; Yan, Y. AP39, a Mitochondria-Targeted Hydrogen Sulfide Donor, Supports Cellular Bioenergetics and Protects against Alzheimer’s Disease by Preserving Mitochondrial Function in APP/PS1 Mice and Neurons. Oxid. Med. Cell. Longev. 2016, 2016, 8360738. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.L.; Wright, J.M.; Hansen, S.; Allan, B.M.; Baird, P.A.; MacLeod, P.M. Failure of aminooxyacetic acid therapy in Huntington disease. Neurology 1980, 30, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Guth, P.S.; Blair, P.; Norris, C.; Risey, J.; Reed, H.T.; Housley, G.; Briner, W.; Bryant, G.; Miller, R. Evaluation of amino-oxyacetic acid as a palliative in tinnitus. Ann. Otol. Rhinol. Laryngol. 1990, 99, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Calvert, J.W.; Duranski, M.R.; Ramachandran, A.; Lefer, D.J. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: Role of antioxidant and antiapoptotic signaling. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H801–H806. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Zhuo, L.; Wang, Y.; Jun, M.; Li, G.; Wang, L.; Hong, D. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology 2018, 23, 669–675. [Google Scholar] [CrossRef]
- Borghi, C.; Omboni, S.; Novo, S.; Vinereanu, D.; Ambrosio, G.; Ambrosioni, E. Efficacy and safety of zofenopril versus ramipril in the treatment of myocardial infarction and heart failure: A review of the published and unpublished data of the randomized double-blind SMILE-4 study. Adv. Ther. 2018, 35, 604–618. [Google Scholar] [CrossRef]
- Ambrosioni, E.; Borghi, C.; Magnani, B. The effect of the angiotensin-converting–enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. New Engl. J. Med. 1995, 332, 80–85. [Google Scholar] [CrossRef]
- Paquette, J.M.; Rufiange, M.; Iovu Niculita, M.; Massicotte, J.; Lefebvre, M.; Colin, P.; Telmat, A.; Ranger, M. Safety, tolerability and pharmacokinetics of trimebutine 3-thiocarbamoylbenzenesulfonate (GIC-1001) in a randomized phase I integrated design study: Single and multiple ascending doses and effect of food in healthy volunteers. Clin. Ther. 2014, 36, 1650–1664. [Google Scholar] [CrossRef]
- Wallace, J.L.; Nagy, P.; Feener, T.D.; Allain, T.; Ditrói, T.; Vaughan, D.J.; Muscara, M.N.; De Nucci, G.; Buret, A.G. A proof-of-concept, Phase 2 clinical trial of the gastrointestinal safety of a hydrogen sulfide-releasing anti-inflammatory drug. Br. J. Pharmacol. 2020, 177, 769–777. [Google Scholar] [CrossRef]
- Vajdi, M.; Noshadi, N.; Hassanizadeh, S.; Bonyadian, A.; Seyedhosseini-Ghaheh, H.; Askari, G. The effects of alpha lipoic acid (ALA) supplementation on blood pressure in adults: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2023, 10. [Google Scholar] [CrossRef]
- Pavlovskiy, Y.; Yashchenko, A.; Zayachkivska, O. H2S donors reverse age-related gastric malfunction impaired due to fructose-induced injury via CBS, CSE, and TST expression. Front. Pharmacol. 2020, 11, 1134. [Google Scholar] [CrossRef] [PubMed]



| H2S Donor | Mechanism of Action | Disease Models | Effects on Cells | References |
|---|---|---|---|---|
| S-propargyl-cysteine (SPRC) | Ameliorates Nrf2-ARE pathway | In rheumatoid arthritis | Anti-inflammatory | [31] |
| Suppression of pro-inflammatory cytokines | In pancreatitis-induced acute lung injury | Anti-inflammatory | [32] | |
| Decreases the ROS formation, Bax caspase 3 and 9, increase Bcl-2 expression | Myocardial infarction and heart failure | Antioxidant Promotes angiogenesis | [33] | |
| Morpholin-4-ium 4-methoxyphenyl-morp holino-phosphinodithioate (GYY4137) | Inhibition of P13/Akt/TLR4 pathway | Atherosclerosis | Maintain mitochondrial function | [34] |
| Suppression of pro-inflammatory cytokines and chemokines | RSV-infected cells | Anti-inflammatory | [35] | |
| Activation of Nrf2-ARE pathway and suppression of NOS2 and COX-2 expression. | In osteoarthritis | Antioxidant | [36] | |
| Decreased upregulation of iNOS, COX2, NF-кB, and STAT 3 | Endotoxemia | Anti-inflammatory | [37] | |
| Increased TGF-β expression and decreased IFN-γ and IL-17 production | Multiple sclerosis | Anti-inflammatory | [38] | |
| AP-39 | Modulation of AMPK/UCP2 pathway and decrease in ROS | Ischemia–reperfusion injury and cardiotoxicity | Antioxidant Maintain mitochondrial function | [39] |
| Decrease in MPO and IL-6 levels, increase in IL-10 levels | Acute lung injury | Anti-inflammatory | [40] | |
| Allicin and Diallyl disulfide (DADS) | Activates Nrf2/ARE pathway | Cardiac hypertrophy and Alzheimer’s disease | Antioxidant | [41,42,43] |
| Inhibition of pro-inflammatory cytokines | In viral-associated lung injury | Anti-inflammatory | [44] | |
| Suppression of p-AKT, NOS2, and PI3K levels | In osteoarthritis | Mitigate pain | [45] | |
| S-diclofenac | Suppression of NF-κB pathway | Pancreatic and acute lung injury in acute pancreatitis | Anti-inflammatory | [46] |
| Stabilizes P53, P21, P53AIPI, and Bax | Atherosclerosis | Antiproliferative | [36] | |
| Inhibition of COX enzymes, NO production, and decreased MPO activity | Carrageenan induced hind-paw edema | Anti-inflammatory | [47] | |
| NaHS | Activation of ERK and NF-κB signaling pathways | Sepsis-induced organ damage | Pro-inflammatory | [48] |
| Activation of NF-κB and Src-family kinase signaling pathways | Acute pancreatitis | Pro-inflammatory | [49] | |
| Activation of cGMP/PKG signaling pathway | Myocardial infarction | Antioxidant | [50] | |
| Inhibition of mitochondrial transmembrane potential loss | Parkinson’s disease | Relieve mitochondrial dysfunction | [51] | |
| Inhibits IkB-a degradation and NF-κB nuclear translocation | Atherosclerosis | Decreases expression of adhesion molecules (specifically ICAM-1) | [52] |
| Therapeutic Compound | Pharmacological Profile | Targeted Condition | Clinical Trial Phase | References |
|---|---|---|---|---|
| IK-1001 | Injection of Na2S (stabilized form) | Mitigation of cardiac complications during coronary artery bypass surgery | Discontinued after phase II | [215] |
| Na2S2O3 | Sodium salt (inorganic) with thiosulfate ions | Cyanide intoxication, cisplatin toxicities, and calciphylaxis in end-stage renal disease | Approved | [179,216] |
| Zofenopril | ACE inhibitor in conjunction with a H2S donor | Hypertension | Approved | [217,218] |
| GIC-1001 | Analgesic effect of trimebutine with H2S release | Visceral pain during sedation-free, full colonoscopy | Clinical phase II (pending) | [219] |
| SG1002 | H2S-releasing prodrug | Heart failure | Phase I | [152] |
| ATB-346 | H2S-releasing naproxen-derived compound | Inflammatory conditions | Phase II | [220] |
| Alpha lipoic acid | Organosulfur compound | Hypertension, cardioprotection, diabetes, and obesity | Phase II | [221] |
| ATB-340 | H2S releasing aspirin-derived compound | Anti-coagulant for CVD | Discontinued | [222] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, A.; Bhatia, M. Hydrogen Sulfide: A Versatile Molecule and Therapeutic Target in Health and Diseases. Biomolecules 2024, 14, 1145. https://doi.org/10.3390/biom14091145
Shahid A, Bhatia M. Hydrogen Sulfide: A Versatile Molecule and Therapeutic Target in Health and Diseases. Biomolecules. 2024; 14(9):1145. https://doi.org/10.3390/biom14091145
Chicago/Turabian StyleShahid, Aqsa, and Madhav Bhatia. 2024. "Hydrogen Sulfide: A Versatile Molecule and Therapeutic Target in Health and Diseases" Biomolecules 14, no. 9: 1145. https://doi.org/10.3390/biom14091145
APA StyleShahid, A., & Bhatia, M. (2024). Hydrogen Sulfide: A Versatile Molecule and Therapeutic Target in Health and Diseases. Biomolecules, 14(9), 1145. https://doi.org/10.3390/biom14091145






