Sheng Xue Ning as a Novel Agent that Promotes SCF-Driven Hematopoietic Stem/Progenitor Cell Proliferation to Promote Erythropoiesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Animals
2.3. Hemanalysis
2.4. Flow Cytometry Analysis
2.5. iTraq Quantitative Proteomics
2.6. Immunofluorescence Staining
2.7. Histopathological Analysis
2.8. Cell Culture
2.9. Cell Viability Assay
2.10. Cell Morphological Observations
2.11. Cell Differentiation
2.12. Colony Formation Assay
2.13. Western Blot
2.14. Statistical Analysis
3. Results
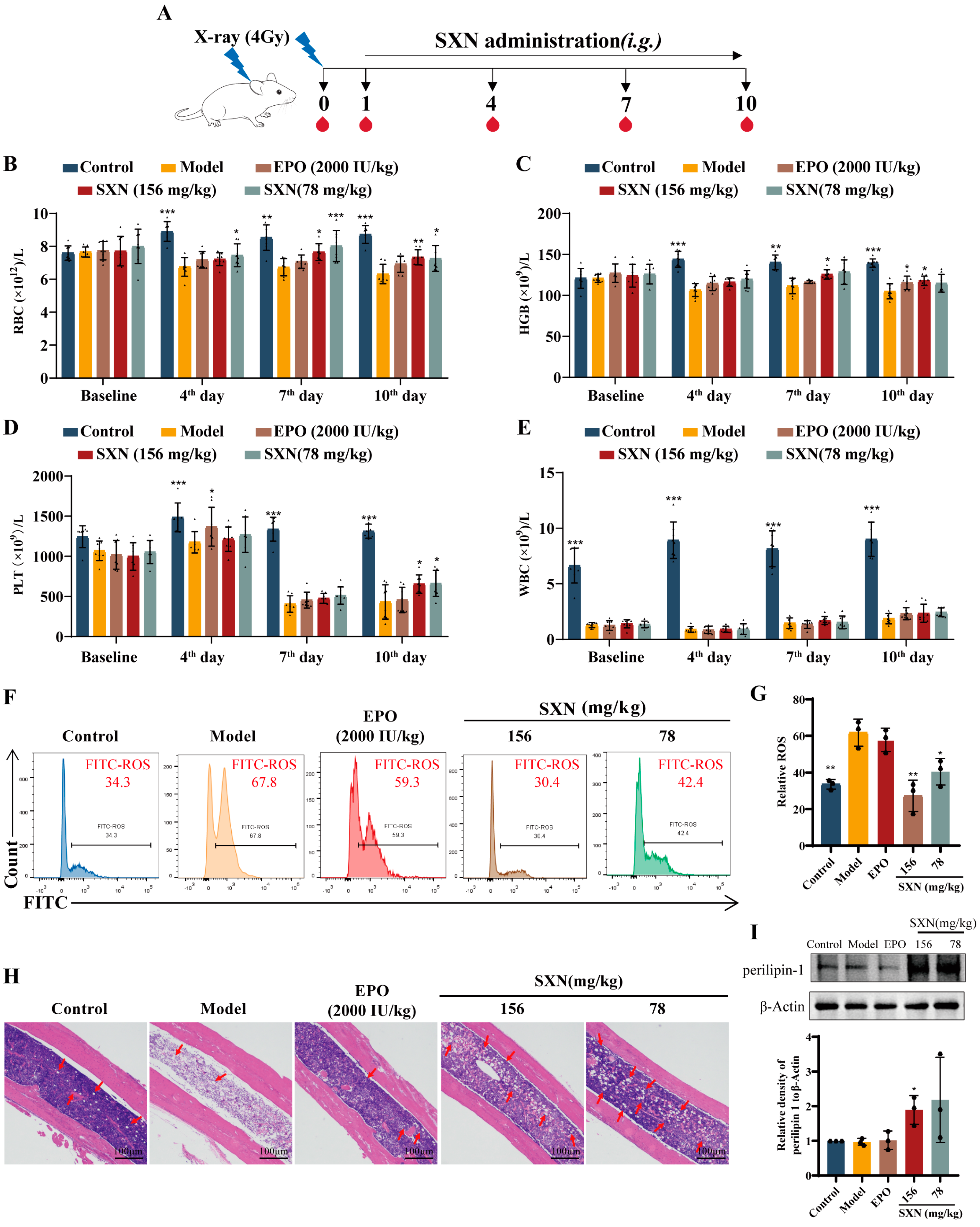
3.1. SXN Facilitated the Recovery of the Peripheral Blood Cell Count in X-ray Irradiated Mice
3.2. SXN Enhanced the Proliferation and Differentiation of HSPC in X-ray Irradiated Mice
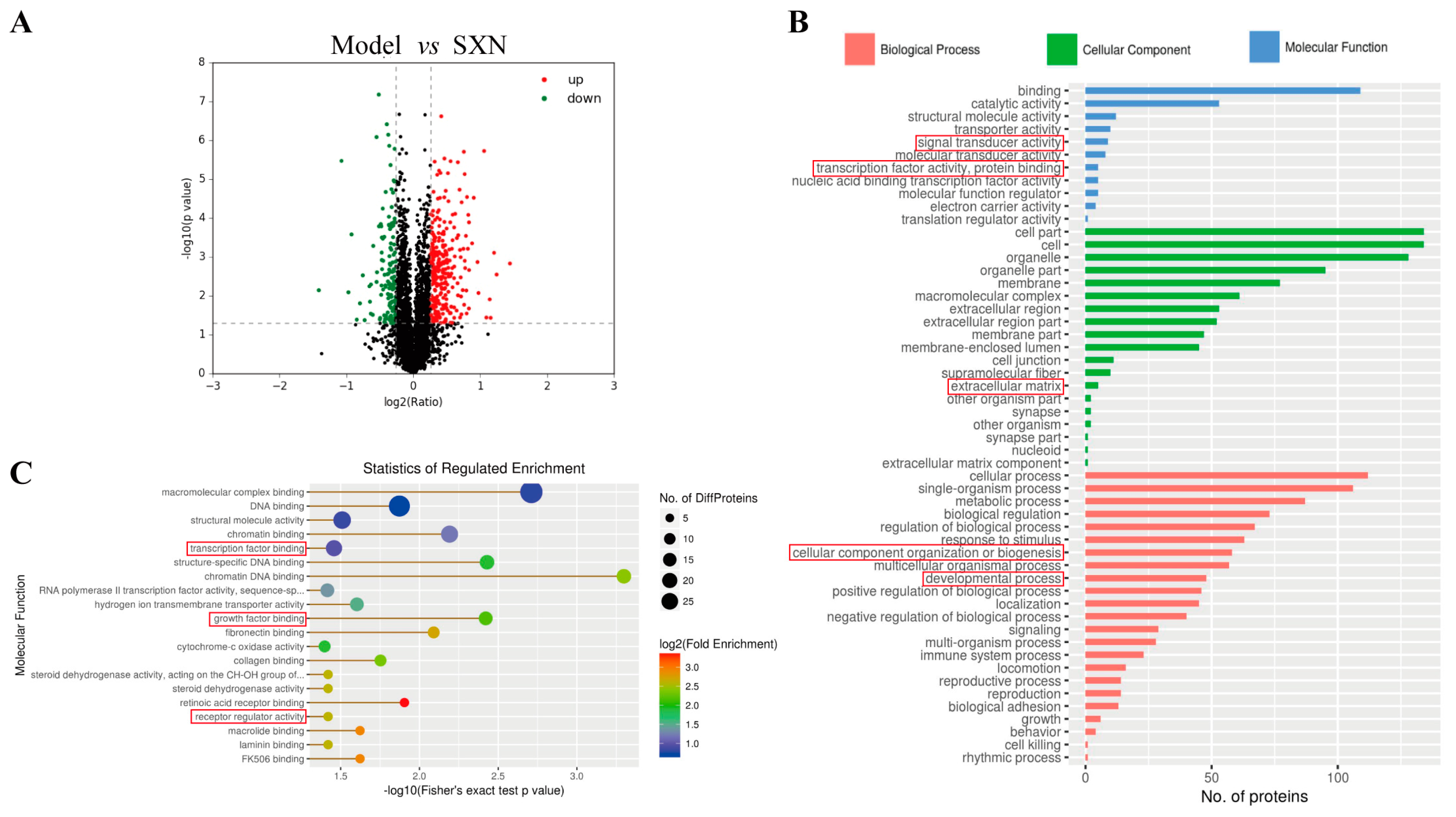
3.3. Proteomic Analysis of the Mechanism of SXN in Enhancing HSPC Proliferation and Differentiation after X-ray Irradiation
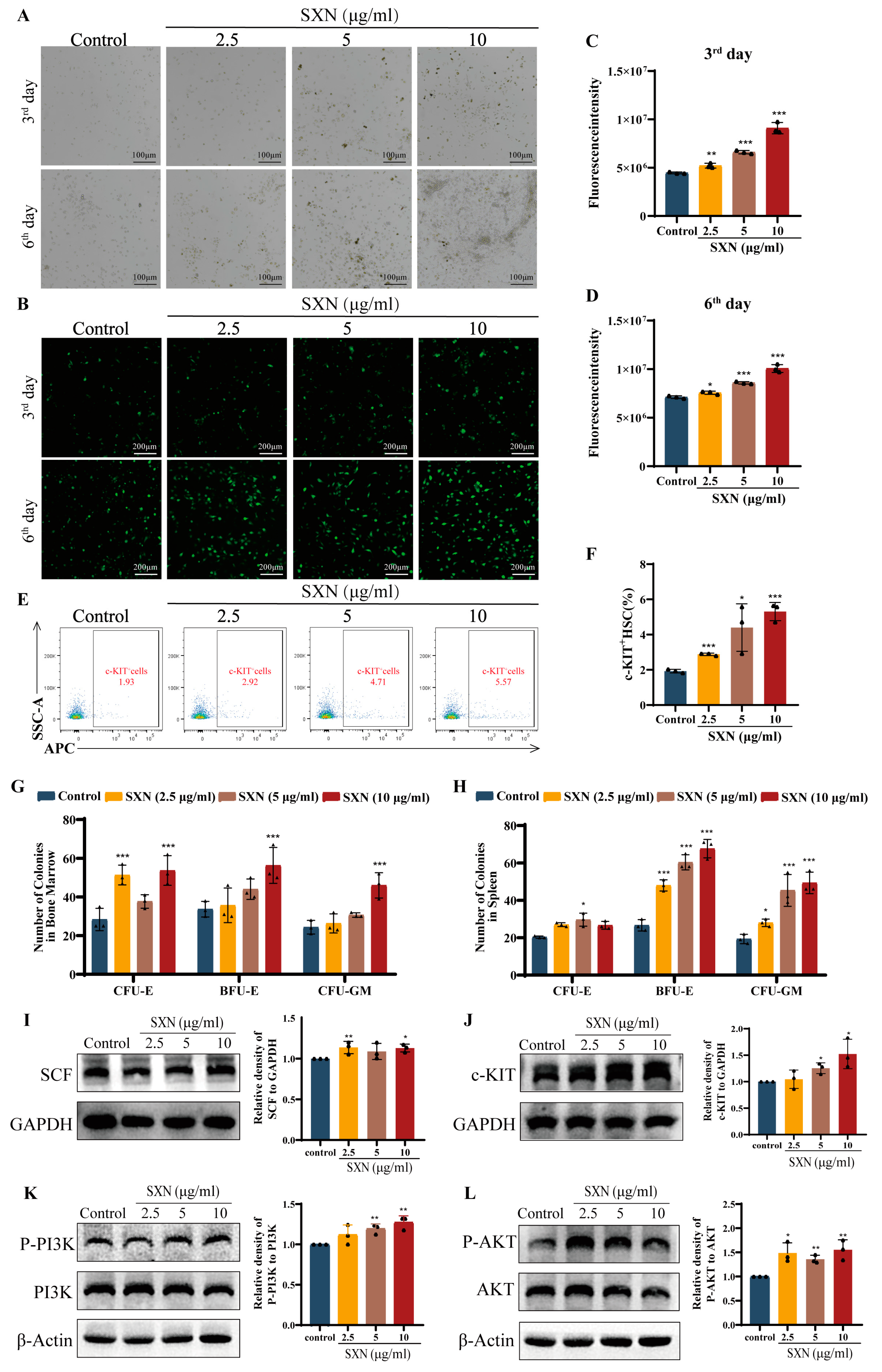
3.4. SXN Enhanced HSPC Proliferation via the Expression of SCF and the Activation of the c-KIT PI3K/Akt Signaling Pathway
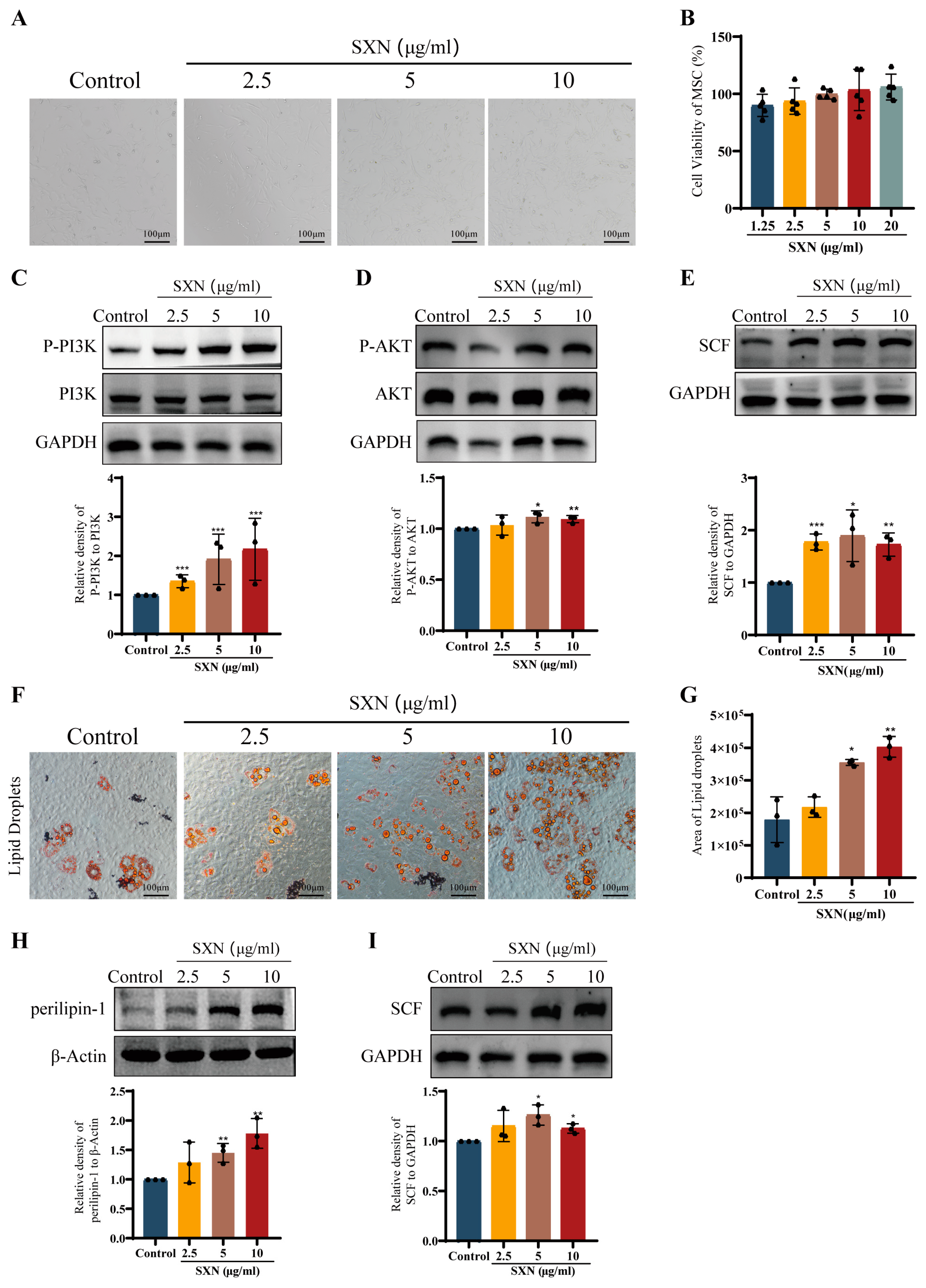
3.5. SXN Enhanced the Proliferation and Differentiation of HSPC In Vitro
3.6. SXN Upregulated the Expression of SCF by Promoting MSC Proliferation and Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
References
- Alexis, L.C.; Vijay, G.S. Molecular and cellular mechanisms that regulate human erythropoiesis. Blood 2022, 139, 2450–2459. [Google Scholar]
- Zon, L.I. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature 2008, 453, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.P.; Alexander, W.S. Haematopoietic stem cells: Past, present and future. Cell Death Discov. 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Frenette, P.S. HSC contribution in making steady-state blood. Immunity 2016, 45, 464–466. [Google Scholar] [CrossRef]
- Pinho, S.; Frenette, P.S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 2019, 20, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Birbrair, A.; Frenette, P.S. Niche heterogeneity in the bone marrow. Ann. N. Y. Acad. Sci. 2016, 1370, 82–96. [Google Scholar] [CrossRef]
- Crane, G.M.; Jeffery, E.; Morrison, S.J. Adult haematopoietic stem cell niches. Nat. Rev. Immunol. 2017, 17, 573–590. [Google Scholar] [CrossRef]
- Anthony, B.A.; Link, D.C. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014, 35, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, P.; Zhou, Y.; Sheng, Y.; Hong, Y.; Xiang, D.; Qian, Z.; Mosenson, J.; Wu, W.S. A novel slug-containing negative-feedback loop regulates SCF/c-Kit-mediated hematopoietic stem cell self-renewal. Leukemia 2017, 31, 403–413. [Google Scholar] [CrossRef]
- Sui, X.; Krantz, S.B.; Zhao, Z.J. Stem cell factor and erythropoietin inhibit apoptosis of human erythroid progenitor cells through different signalling pathways. Br. J. Haematol. 2000, 110, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.O.; Yu, H.; Yue, R.; Zhao, Z.; Rios, J.J.; Naveiras, O.; Morrison, S.J. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat. Cell Biol. 2017, 19, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Zsebo, K.M.; Williams, D.A.; Geissler, E.N.; Broudy, V.C.; Martin, F.H.; Atkins, H.L.; Hsu, R.Y.; Birkett, N.C.; Okino, K.H.; Murdock, D.C.; et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell 1990, 63, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Rönnstrand, L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol. Life Sci. 2004, 61, 2535–2548. [Google Scholar] [CrossRef]
- Prósper, F.; Solá, C.; Hornedo, J.; Arbona, C.; Menéndez, P.; Orfao, A.; Lluch, A.; Cortés-Funes, H.; López, J.J.; García-Conde, J. Mobilization of peripheral blood progenitor cells with a combination of cyclophosphamide, r-metHuSCF and filgrastim in patients with breast cancer previously treated with chemotherapy. Leukemia 2003, 17, 437–441. [Google Scholar] [CrossRef]
- Bozhilov, Y.K.; Hsu, I.; Brown, E.J.; Wilkinson, A.C. In vitro human haematopoietic stem cell expansion and differentiation. Cells 2023, 12, 896. [Google Scholar] [CrossRef]
- Nie, J.; Hu, H.S.; Chen, X.Y.; Liu, J.J.; Li, K.; Luo, J. Study on fingerprint of shengxuening tablets. Zhongguo Zhong Yao Za Zhi 2013, 38, 3502–3506. [Google Scholar] [PubMed]
- Suryavanshi, S.; Sharma, D.; Checker, R.; Thoh, M.; Gota, V.; Sandur, S.K.; Sainis, K.B. Amelioration of radiation-induced hematopoietic syndrome by an antioxidant chlorophyllin through increased stem cell activity and modulation of hematopoiesis. Free Radic. Biol. Med. 2015, 85, 56–70. [Google Scholar] [CrossRef]
- West, A.R.; Oates, P.S. Mechanisms of heme iron absorption: Current questions and controversies. World J. Gastroenterol. 2008, 14, 4101–4110. [Google Scholar] [CrossRef]
- Stevenson, D.K.; Vreman, H.J.; Wong, R.J. Heme, heme oxygenase-1, statins, and SARS-CoV-2. Antioxidants 2023, 12, 614. [Google Scholar] [CrossRef]
- Tang, X.J.; Rong, S.; Mei, C.L.; Ni, Z.H.; Jiang, G.R.; Yuan, W.J.; Wang, N.S.; Guo, Z.Y.; Ma, J.; Yan, H.D.; et al. Effect of sheng xue ning tablets on renal anemia in patients subject to maintenance hemodialysis and safety evaluation: A multi-setting prospective randomized study. Curr. Med. Sci. 2020, 40, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lv, Y.; Sun, Y.; Li, Y.; Wang, D.; Niu, J.; Zhao, P.; Zhang, M.; Wang, M.; Liu, W.; et al. The efficiency and safety of Shengxuening tablet on treating and preventing iron deficiency anemia: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 1029641. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Wu, N.; Huang, X.; Cui, Z.; Xu, J.; Yang, X.; Zeng, F.; Wang, K. Possible mechanisms by which silkworm faeces extract ameliorates adenine-induced renal anaemia in rats. J. Ethnopharmacol. 2021, 266, 113448. [Google Scholar] [CrossRef]
- Huang, F.H.; Zhu, L.J.; Li, D.D. Effect of Shengxuening on mice with cyclophosphamide-induced anemia and its mechanism. Chin. Tradit. Pat. Med. 2016, 38, 1205–1210. [Google Scholar]
- Wang, L.; Zhang, T.; Liu, S.; Mo, Q.; Jiang, N.; Chen, Q.; Yang, J.; Han, Y.W.; Chen, J.P.; Huang, F.H.; et al. Discovery of a novel megakaryopoiesis enhancer, ingenol, promoting thrombopoiesis through PI3K-Akt signaling independent of thrombopoietin. Pharmacol. Res. 2022, 177, 106096. [Google Scholar] [CrossRef]
- Xiang, L.; Zheng, J.; Zhang, M.; Ai, T.; Cai, B. FOXQ1 promotes the osteogenic differentiation of bone mesenchymal stem cells via Wnt/β-catenin signaling by binding with ANXA2. Stem Cell Res. Ther. 2020, 11, 403. [Google Scholar] [CrossRef]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, S.; Luo, J.; Mo, Q.; Ran, M.; Zhang, T.; Li, X.; Zou, W.; Mei, Q.; Chen, J.; et al. Targeting a thrombopoietin-independent strategy in the discovery of a novel inducer of megakaryocytopoiesis, DMAG, for the treatment of thrombocytopenia. Haematologica 2023, 108, 1394–1411. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Schulte, B.A.; LaRue, A.C.; Ogawa, M.; Zhou, D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood 2006, 107, 358–366. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, T.; Dong, Q.; Nice, E.C.; Huang, C.; Wei, Y. Redox homeostasis: The linchpin in stem cell self-renewal and differentiation. Cell Death Dis. 2013, 4, e537. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, A.; Frenette, P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014, 20, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Feng, W.; Li, H.; Gardner, D.; Luo, Y.; Wang, Y.; Liu, L.; Meng, A.; Sharpless, N.E.; Zhou, D. Total body irradiation causes long-term mouse BM injury via induction of HSC premature senescence in an Ink4a- and Arf-independent manner. Blood 2014, 123, 3105–3115. [Google Scholar] [CrossRef] [PubMed]
- Dzierzak, E.; Bigas, A. Blood development: Hematopoietic stem cell dependence and independence. Cell Stem Cell 2018, 22, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Oguro, H.; Ding, L.; Morrison, S.J. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell 2013, 13, 102–116. [Google Scholar] [CrossRef]
- Kent, D.G.; Copley, M.R.; Benz, C.; Wöhrer, S.; Dykstra, B.J.; Ma, E.; Cheyne, J.; Zhao, Y.; Bowie, M.B.; Zhao, Y.; et al. Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood 2009, 113, 6342–6350. [Google Scholar] [CrossRef]
- Challen, G.A.; Boles, N.; Lin, K.K.; Goodell, M.A. Mouse hematopoietic stem cell identification and analysis. Cytomet. Part A 2009, 75, 14–24. [Google Scholar] [CrossRef]
- Cenariu, D.; Iluta, S.; Zimta, A.A.; Petrushev, B.; Qian, L.; Dirzu, N.; Tomuleasa, C.; Bumbea, H.; Zaharie, F. Extramedullary hematopoiesis of the liver and spleen. J. Clin. Med. 2021, 10, 5831. [Google Scholar] [CrossRef]
- Inra, C.N.; Zhou, B.O.; Acar, M.; Murphy, M.M.; Richardson, J.; Zhao, Z.; Morrison, S.J. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature 2015, 527, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Short, C.; Lim, H.K.; Tan, J.; O’Neill, H.C. Targeting the spleen as an alternative site for hematopoiesis. Bioessays 2019, 41, e1800234. [Google Scholar] [CrossRef]
- Ginzburg, Y.; An, X.; Rivella, S.; Goldfarb, A. Normal and dysregulated crosstalk between iron metabolism and erythropoiesis. Elife 2023, 12, e90189. [Google Scholar] [CrossRef] [PubMed]
- Khodadi, E.; Shahrabi, S.; Shahjahani, M.; Azandeh, S.; Saki, N. Role of stem cell factor in the placental niche. Cell Tissue Res. 2016, 366, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef]
- Saidi, N.; Ghalavand, M.; Hashemzadeh, M.S.; Dorostkar, R.; Mohammadi, H.; Mahdian-Shakib, A. Dynamic changes of epigenetic signatures during chondrogenic and adipogenic differentiation of mesenchymal stem cells. Biomed. Pharmacother. 2017, 89, 719–731. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef]
- Dominici, M.; Rasini, V.; Bussolari, R.; Chen, X.; Hofmann, T.J.; Spano, C.; Bernabei, D.; Veronesi, E.; Bertoni, F.; Paolucci, P.; et al. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood 2009, 114, 2333–2343. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Y.; He, L.; Chen, M.; Li, W.; Xiao, T.; Guan, J.; Qi, Z.; Wang, Q.; Li, S.; et al. Haptoglobin is an early indicator of survival after radiation-induced severe injury and bone marrow transplantation in mice. Stem Cell Res. Ther. 2022, 13, 461. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.S.; Collino, M.; Yaqoob, M.M.; Thiemermann, C. Erythropoietin in the intensive care unit: Beyond treatment of anemia. Ann. Intensive Care 2011, 1, 40. [Google Scholar] [CrossRef]
- Grover, A.; Mancini, E.; Moore, S.; Mead, A.J.; Atkinson, D.; Rasmussen, K.D.; O’Carroll, D.; Jacobsen, S.E.; Nerlov, C. Erythropoietin guides multipotent hematopoietic progenitor cells toward an erythroid fate. J. Exp. Med. 2014, 211, 181–188. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, W.; He, X.; Chen, C.; Li, W.; Zhao, L.; Liu, L.; Liu, J.; Xie, L.; Zhang, Y.; et al. Endothelial cell-derived angiopoietin-like protein 2 supports hematopoietic stem cell activities in bone marrow niches. Blood 2022, 139, 1529–1540. [Google Scholar] [CrossRef]
- Le Blanc, K.; Samuelsson, H.; Gustafsson, B.; Remberger, M.; Sundberg, B.; Arvidson, J.; Ljungman, P.; Lönnies, H.; Nava, S.; Ringdén, O. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia 2007, 21, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Frenette, P.S. Niches for hematopoietic stem cells and their progeny. Immunity 2018, 48, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Takematsu, E.; Massidda, M.; Auster, J.; Chen, P.C.; Im, B.; Srinath, S.; Canga, S.; Singh, A.; Majid, M.; Sherman, M.; et al. Transmembrane stem cell factor protein therapeutics enhance revascularization in ischemia without mast cell activation. Nat. Commun. 2022, 13, 2497. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, X.; Wu, Q.; Jiang, L.; Chen, L.; Yu, Y.; Zhang, P.; Huang, X.; Wang, J.; Ju, Z.; et al. Transferrin receptor 1-mediated iron uptake plays an essential role in hematopoiesis. Haematologica 2020, 105, 2071–2082. [Google Scholar] [CrossRef]
- Dutt, S.; Hamza, I.; Bartnikas, T.B. Molecular Mechanisms of Iron and Heme Metabolism. Annu. Rev. Nutr. 2022, 42, 311–335. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Li, C.; Yang, F.; Zhang, L.; Xu, W.; Wang, L.; Wu, A.; Zou, W.; Wu, J.; Huang, F. Sheng Xue Ning as a Novel Agent that Promotes SCF-Driven Hematopoietic Stem/Progenitor Cell Proliferation to Promote Erythropoiesis. Biomolecules 2024, 14, 1147. https://doi.org/10.3390/biom14091147
Zeng Y, Li C, Yang F, Zhang L, Xu W, Wang L, Wu A, Zou W, Wu J, Huang F. Sheng Xue Ning as a Novel Agent that Promotes SCF-Driven Hematopoietic Stem/Progenitor Cell Proliferation to Promote Erythropoiesis. Biomolecules. 2024; 14(9):1147. https://doi.org/10.3390/biom14091147
Chicago/Turabian StyleZeng, Yueying, Chunlu Li, Fei Yang, Ling Zhang, Wanqi Xu, Long Wang, Anguo Wu, Wenjun Zou, Jianming Wu, and Feihong Huang. 2024. "Sheng Xue Ning as a Novel Agent that Promotes SCF-Driven Hematopoietic Stem/Progenitor Cell Proliferation to Promote Erythropoiesis" Biomolecules 14, no. 9: 1147. https://doi.org/10.3390/biom14091147
APA StyleZeng, Y., Li, C., Yang, F., Zhang, L., Xu, W., Wang, L., Wu, A., Zou, W., Wu, J., & Huang, F. (2024). Sheng Xue Ning as a Novel Agent that Promotes SCF-Driven Hematopoietic Stem/Progenitor Cell Proliferation to Promote Erythropoiesis. Biomolecules, 14(9), 1147. https://doi.org/10.3390/biom14091147





