Advances in Microbial Exopolysaccharides: Present and Future Applications
Abstract
1. Introduction
2. Microbial EPS
2.1. Chemical Structure, Composition, and Types of EPS
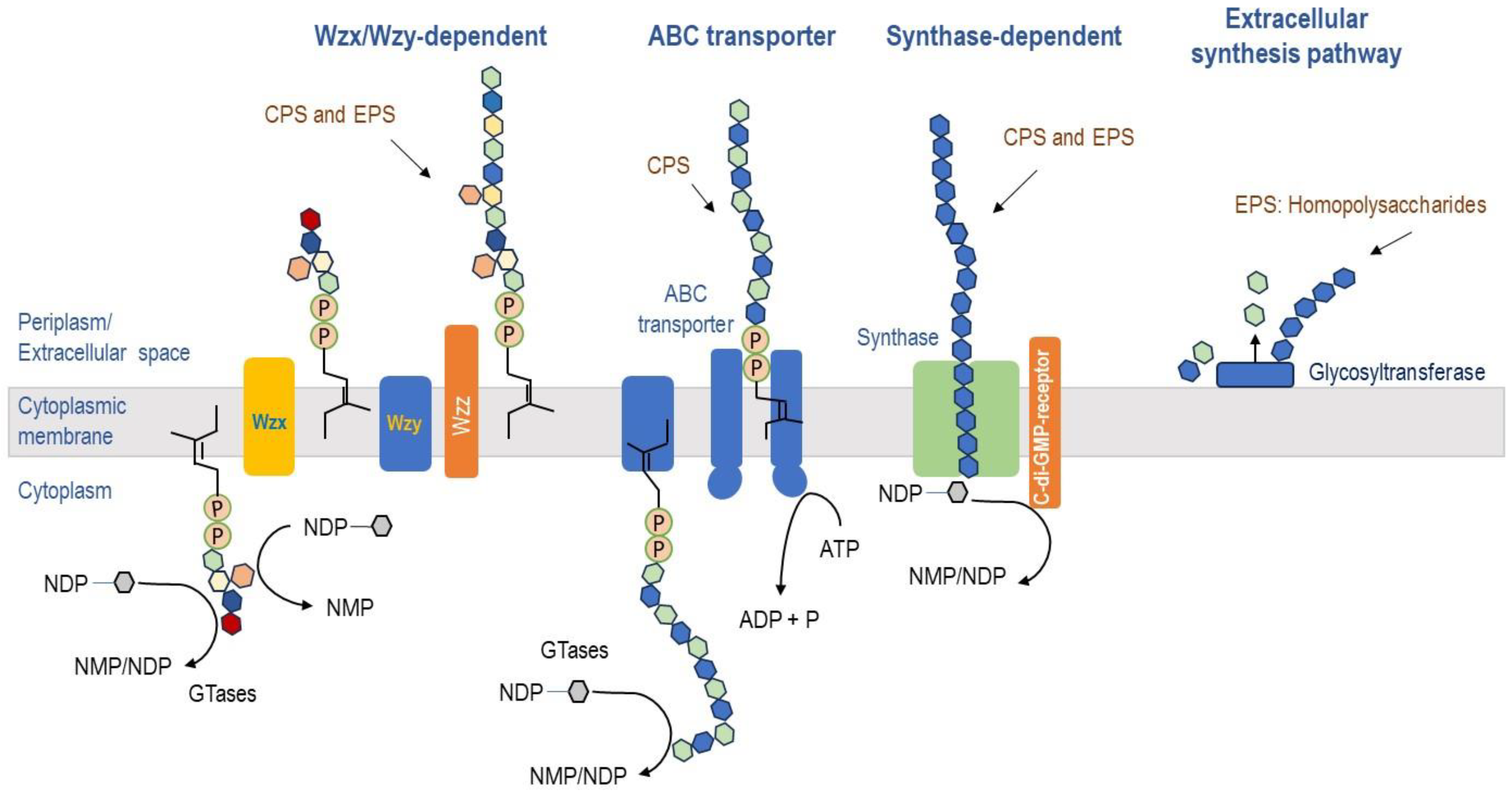
2.2. Biosynthesis and Producing Microorganisms
2.2.1. Biosynthesis of EPS
2.2.2. Producing Microorganisms
Bacteria
Fungi and Yeasts
Microalgae and Cyanobacteria
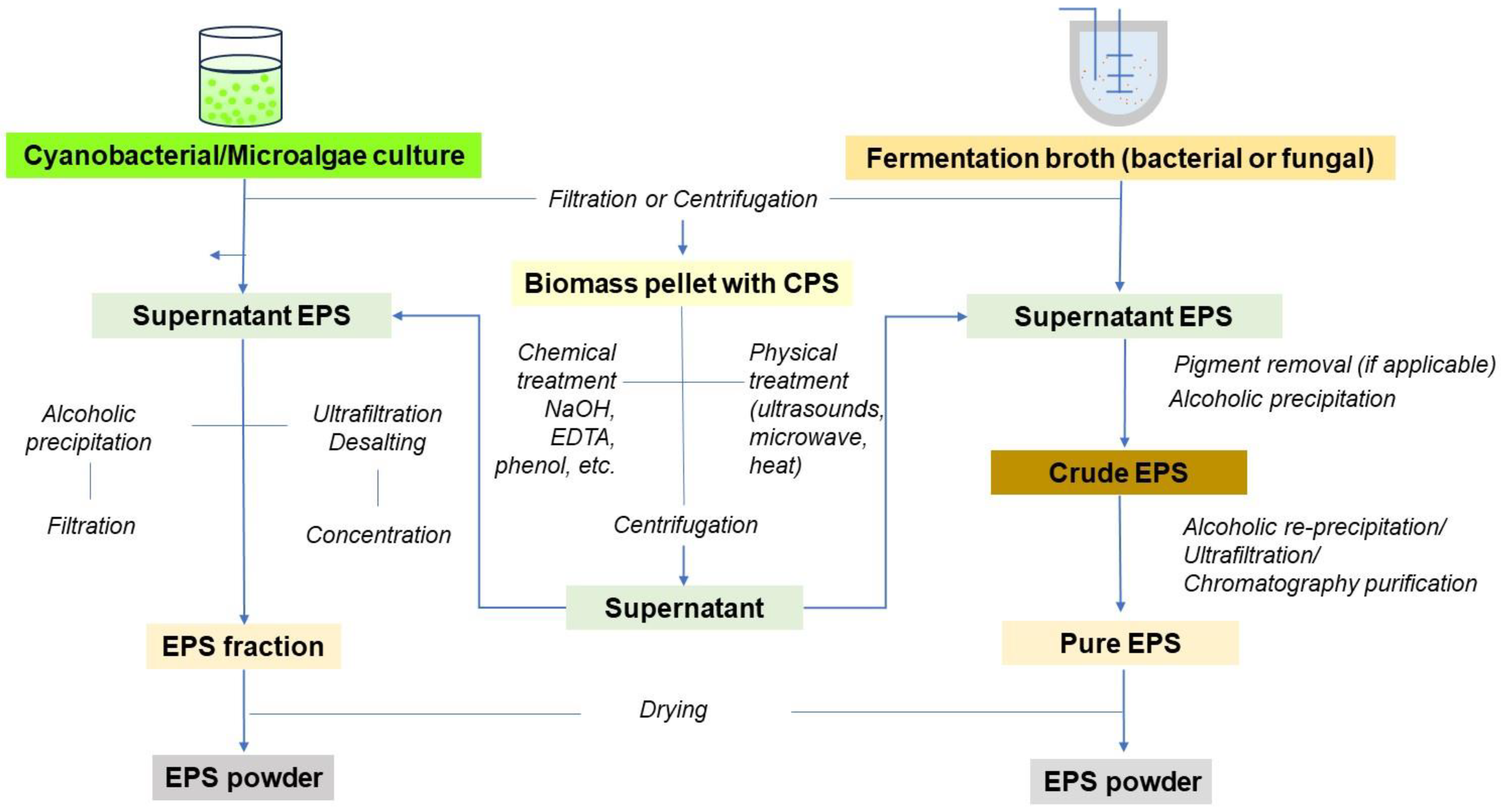
2.3. Production and Analytical Characterization
2.3.1. Production
2.3.2. Purification and Structural Identification
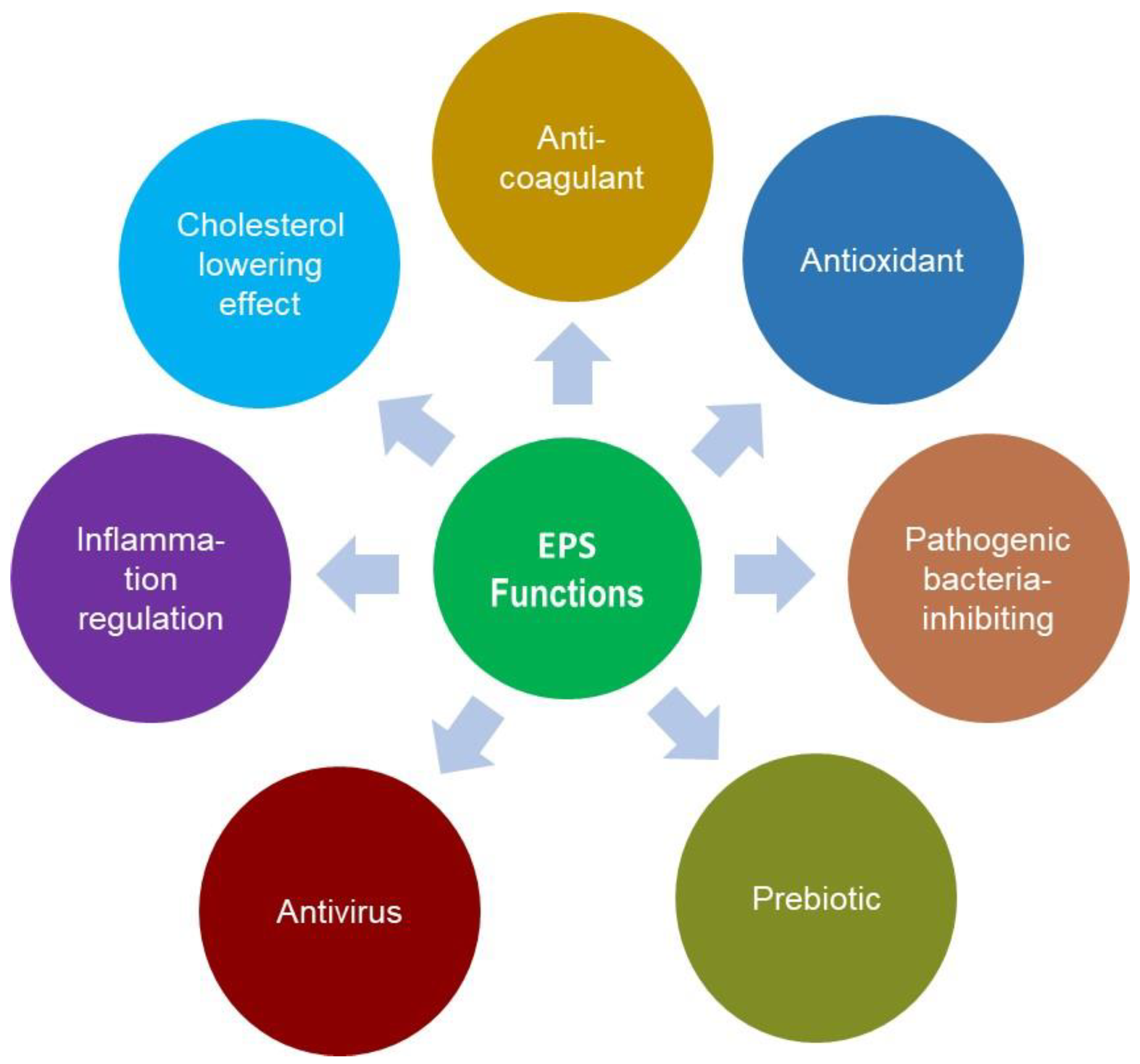
3. Properties and Functions
3.1. Physiological Functions
3.2. Physico-Chemical Properties and Functionalities
4. Current and Future Applications of EPSs
4.1. Applications in Pharmaceutical and Medical Fields
4.1.1. EPSs as Immunobiotic Agents
4.1.2. EPSs as Smart Delivery Systems
4.2. Other Industrial and Agricultural Applications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
ABC: ATP-Binding Cassette Ace: Acetate AIDS: Acquired Immunodeficiency Syndrome ALR: Air Lift Bioreactors Ara: Arabinose c-di-GMP: Cyclic Dimeric Guanosine Monophosphate CLIO: Cross-linked Dextran Coating CPS: Capsular Polysaccharide DSC: Differential Scanning Calorimetry EFSA : European Food Safety Authority EMA: European Medical Agency EOR: Enhanced Oil Recovery EPS: Exopolysaccharides FDA: Food Safety Authority and Fru: Fructose FT-IR: Fourier Transform—Infrared Fuc: Fucose Gal: Galactose GalA: Galacturonic acid Gal-N: Galactosamine GDP-Fruc: Guanosine Diphosphate Fructose Glc: Glucose Glc-1-P: Glucose-1-phosphate GlcA: Glucuronic acid GlcN: Glucosamine Gly: Glycerate GRAS: generally recognized as safe GulA: Guluronic acid HC: Heparin Cofactor HePS: Heteropolysaccharides HIV-1: Human Immunodeficiency Virus type 1 HoPS: Homopolysaccharides HPAEC: High performance Anion exchange chromatography HS: Heparin sulfate IgE: Immunoglobulin E IL: Interleukin INF: Interferon LAB: Lactic acid Bacteria MALS: Multi-Angle Light Scattering Man: Mannose (Man) Man-1-P: Mannose-1-phosphate ManA: Mannuronic acid MW: Molecular weight NIR: Near Infrared NMR: Near Magnetic Resonance NMP: Nucleoside Monophosphate NDP: Nucleoside Diphosphate OMA: Outer Membrane Auxiliary OPX: Outer Polysaccharide Export PCP: Polysaccharide co-polymerase Phosp: Phosphate Pyr: Pyruvate QPS: Qualified Presumption of Safety Rha: Rhamnose RI: Refractive Index Rib: Ribose (Rib) ROS: Reactive Oxygen Species SCFA: Short Chain Fatty acids SDS-PAGE: Sodium Dodecyl Sulfate—Polyacrylamide Gel SEC: Size Exclusion Chromatography SIV: Simian immunodeficiency virus STR: Stirred Tank Bioreactors Succ: Succinate Sulf: Sulfate TDP: Thymidine Diphosphate TGA: Thermogravimetric analysis UDP: Uridine Diphosphate UV: Ultraviolet XRD: X-Ray diffraction Xyl: Xylose |
References
- Costa, J.A.V.; Lucas, B.F.; Alvarenga, A.G.P.; Moreira, J.B.; de Morais, M.G. Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications. Polysaccharides 2021, 2, 759–772. [Google Scholar] [CrossRef]
- Hamidi, M.; Okoro, O.V.; Milan, P.B.; Khalili, M.R.; Samadian, H.; Nie, L.; Shavandi, A. Fungal Exopolysaccharides: Properties, Sources, Modifications, and Biomedical Applications. Carbohydr. Polym. 2022, 284, 119152. [Google Scholar] [CrossRef] [PubMed]
- Rahbar Saadat, Y.; Yari Khosroushahi, A.; Pourghassem Gargari, B. Yeast Exopolysaccharides and Their Physiological Functions. Folia Microbiol. 2021, 66, 171–182. [Google Scholar] [CrossRef]
- Sørensen, H.M.; Rochfort, K.D.; Maye, S.; MacLeod, G.; Brabazon, D.; Loscher, C.; Freeland, B. Exopolysaccharides of Lactic Acid Bacteria: Production, Purification and Health Benefits towards Functional Food. Nutrients 2022, 14, 2938. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Upadhyay, L.S.B. Microbial Exopolysaccharides: Synthesis Pathways, Types and Their Commercial Applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef]
- Branda, S.S.; Vik, Å.; Friedman, L.; Kolter, R. Biofilms: The Matrix Revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Y.; Yue, F.; Liu, L.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Exopolysaccharides Produced by Lactic Acid Bacteria and Bifidobacteria: Structures, Physiochemical Functions and Applications in the Food Industry. Food Hydrocoll. 2019, 94, 475–499. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef]
- Torino, M.I.; Font de Valdez, G.; Mozzi, F. Biopolymers from Lactic Acid Bacteria. Novel Applications in Foods and Beverages. Front. Microbiol. 2015, 6, 834. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Structural Features of Microbial Exopolysaccharides in Relation to Their Antioxidant Activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef]
- Moscovici, M. Present and Future Medical Applications of Microbial Exopolysaccharides. Front. Microbiol. 2015, 6, 1012. [Google Scholar] [CrossRef] [PubMed]
- Rahbar Saadat, Y.; Yari Khosroushahi, A.; Pourghassem Gargari, B. A Comprehensive Review of Anticancer, Immunomodulatory and Health Beneficial Effects of the Lactic Acid Bacteria Exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Eze, C.O.; Berebon, D.P.; Gugu, T.H.; Anazodo, F.I.; Okorie, J.E.; Eze, C.O.; Berebon, D.P.; Gugu, T.H.; Anazodo, F.I.; Okorie, J.E. Lactobacillus Exopolysaccharide: An Untapped Biopolymer. In Lactobacillus—A Multifunctional Genus; IntechOpen: London, UK, 2022; ISBN 978-1-80355-445-7. [Google Scholar]
- Amiri, S.; Rezaei Mokarram, R.; Sowti Khiabani, M.; Rezazadeh Bari, M.; Alizadeh Khaledabad, M. Exopolysaccharides Production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: Optimization of Fermentation Variables and Characterization of Structure and Bioactivities. Int. J. Biol. Macromol. 2019, 123, 752–765. [Google Scholar] [CrossRef]
- Harutoshi, T. Exopolysaccharides of Lactic Acid Bacteria for Food and Colon Health Applications. In Lactic Acid Bacteria-R & D for Food, Health and Livestock Purposes; InTech: London, UK, 2013. [Google Scholar]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial Exopolysaccharides: Biosynthesis Pathways and Engineering Strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef]
- Tan, K.-X.; Chamundeswari, V.N.; Loo, S.C.J. Prospects of Kefiran as a Food-Derived Biopolymer for Agri-Food and Biomedical Applications. RSC Adv. 2020, 10, 25339–25351. [Google Scholar] [CrossRef]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.; Øregaard, G.; Neves, A.R. Polysaccharide Production by Lactic Acid Bacteria: From Genes to Industrial Applications. FEMS Microbiol. Rev. 2017, 41, S168–S200. [Google Scholar] [CrossRef] [PubMed]
- Donot, F.; Fontana, A.; Baccou, J.C.; Schorr-Galindo, S. Microbial Exopolysaccharides: Main Examples of Synthesis, Excretion, Genetics and Extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- Badel, S.; Bernardi, T.; Michaud, P. New Perspectives for Lactobacilli Exopolysaccharides. Biotechnol. Adv. 2011, 29, 54–66. [Google Scholar] [CrossRef]
- Yates, L.E.; Mills, D.C.; DeLisa, M.P. Bacterial glycoengineering as a biosynthetic route to customized glycomolecules. In Advances in Glycobiotechnology; Rapp, E., Reichl, U., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 167–200. ISBN 978-3-030-69590-3. [Google Scholar]
- Sutherland, I.W. Biosynthesis of microbial exopolysaccharides. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 1982; Volume 23, pp. 79–150. ISBN 0065-2911. [Google Scholar]
- Singha, T.K. Microbial Extracellular Polymeric Substances: Production, Isolation and Applications. IOSR J. Pharm. 2012, 2, 271–281. [Google Scholar]
- Freitas, F.; Torres, C.A.; Reis, M.A. Engineering Aspects of Microbial Exopolysaccharide Production. Bioresour. Technol. 2017, 245, 1674–1683. [Google Scholar] [CrossRef]
- Chaudhari, V.; Buttar, H.S.; Bagwe-Parab, S.; Tuli, H.S.; Vora, A.; Kaur, G. Therapeutic and Industrial Applications of Curdlan with Overview on Its Recent Patents. Front. Nutr. 2021, 8, 646988. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nadzir, M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical Applications of Bacterial Exopolysaccharides: A Review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Panchal, R.; Prajapati, K.; Prajapati, M.; Sharma, S.; Saraf, M.S. Bacterial Exopolysaccharides: Types, Its Biosynthesis and Their Application in Different Fields. Acta Sci. Biotechnol. 2022, 3, 1–9. [Google Scholar]
- Zhang, P.; Yuan, L.; Zeng, J.; Zou, K.; Liu, B.; Qing, T.; Feng, B. Alginate Production of Pseudomonas Strains and Its Application in Preparation of Alginate-Biomass Hydrogel for Heavy Metal Adsorption. Int. J. Biol. Macromol. 2022, 222, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Benhadda, F.; Zykwinska, A.; Colliec-Jouault, S.; Sinquin, C.; Thollas, B.; Courtois, A.; Fuzzati, N.; Toribio, A.; Delbarre-Ladrat, C. Marine versus Non-Marine Bacterial Exopolysaccharides and Their Skincare Applications. Mar. Drugs 2023, 21, 582. [Google Scholar] [CrossRef]
- Mummaleti, G.; Sarma, C.; Kalakandan, S.K.; Gazula, H.; Sivanandham, V.; Anandharaj, A. Characterization of Levan Produced from Coconut Inflorescence Sap Using Bacillus Subtilis and Its Application as a Sweetener. LWT 2022, 154, 112697. [Google Scholar] [CrossRef]
- Baptista, S.; Torres, C.A.V.; Sevrin, C.; Grandfils, C.; Reis, M.A.M.; Freitas, F. Extraction of the Bacterial Extracellular Polysaccharide FucoPol by Membrane-Based Methods: Efficiency and Impact on Biopolymer Properties. Polymers 2022, 14, 390. [Google Scholar] [CrossRef]
- Wünsche, J.; Schmid, J. Acetobacteraceae as Exopolysaccharide Producers: Current State of Knowledge and Further Perspectives. Front. Bioeng. Biotechnol. 2023, 11, 1166618. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, G.; Tian, Y. Biological Activities and Applications of Exopolysaccharides Produced by Lactic Acid Bacteria: A Mini-Review. World J. Microbiol. Biotechnol. 2023, 39, 155. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef]
- Balkrishna, A.; Agarwal, V.; Kumar, G.; Gupta, A.K. Applications of Bacterial Polysaccharides with Special Reference to the Cosmetic Industry. In Microbial Bioprospecting for Sustainable Development; Springer: Singapore, 2018; pp. 189–202. [Google Scholar]
- Jeong, J.; Kim, Y.; Hu, Y.; Jung, S. Bacterial Succinoglycans: Structure, Physical Properties, and Applications. Polymers 2022, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lin, J.; Wang, W.; Li, S. Biopolymers Produced by Sphingomonas Strains and Their Potential Applications in Petroleum Production. Polymers 2022, 14, 1920. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Casas, A.; Toubarro, D.; Barros, A.N.; Teixeira, J.A. Microbial Hyaluronic Acid Production: A Review. Molecules 2023, 28, 2084. [Google Scholar] [CrossRef]
- Chen, C.C.; Nargotra, P.; Kuo, C.H.; Liu, Y.C. High-Molecular-Weight Exopolysaccharides Production from Tuber brochii Cultivated by Submerged Fermentation. Int. J. Mol. Sci. 2023, 24, 4875. [Google Scholar] [CrossRef]
- Santra, H.K.; Banerjee, D. Production, Optimization, Characterization and Drought Stress Resistance by β-Glucan-Rich Heteropolysaccharide From an Endophytic Fungi Colletotrichum Alatae LCS1 Isolated From Clubmoss (Lycopodium clavatum). Front. Fungal Biol. 2021, 2, 796010. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Liu, L.; Bai, R.; Zhang, S.; Hao, Y.; Xu, F.; Wei, B.; Zhao, H. Characteristic Analysis and Fermentation Optimization of a Novel Aureobasidium pullulans RM1603 with High Pullulan Yield. J. Biosci. Bioeng. 2024, 137, 335–343. [Google Scholar] [CrossRef]
- Shao, Z.; Tian, Y.; Liu, S.; Chu, X.; Mao, W. Anti-Diabetic Activity of a Novel Exopolysaccharide Produced by the Mangrove Endophytic Fungus Penicillium janthinellum N29. Mar. Drugs 2023, 21, 270. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Chen, X.; Dong, R.; Wang, G.; Xu, X.; Yu, Q.; Chen, Y.; Wang, X.; Xie, J. Characterization and Antioxidant Properties of Three Exopolysaccharides Produced by the Cyclocarya paliurus Endophytic Fungus. Int. J. Biol. Macromol. 2024, 271, 132110. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Z.; Wang, F.; Liu, L.; Wei, Y.; Li, J.; Zhang, L.; Zheng, K.; Wu, L.; Men, X. Extraction, Characterization, and Biological Activities of Exopolysaccharides from Plant Root Soil Fungus Fusarium merismoides A6. Braz. J. Microbiol. 2023, 54, 199–211. [Google Scholar] [CrossRef]
- Vadnerker, P.; Vyas, T.; Kapadia, C. Characterization of Exopolysaccharide Produced by Ganoderma sp. TP and Its Immunomodulatory Properties. Romanian Biotechnol. Lett. 2022, 28, 3527–3535. [Google Scholar] [CrossRef]
- Castro, S.; Garay, S.; Espinoza-Carhuancho, F.; Medina, J.; Mendoza, R.; Mauricio, F.; Mayta-Tovalino, F. Exploring the Potential of Probiotics in Dentistry: A Literature Review. Odovtos-Int. J. Dent. Sci. 2024, 26, 24–36. [Google Scholar] [CrossRef]
- Vanin, A.P.; Visentin, E.Z.; Fontana, R.C.; di Medeiros Leal, M.C.B.; de Avila e Silva, S.; Stokke, B.T.; Carbonero, E.R.; Camassola, M. β-(1 → 3)(1 → 6)Glucan from Schizophyllum commune 227E.32: High Yield Production via Glucose/Xylose Co-Metabolization. Carbohydr. Polym. 2023, 320, 121176. [Google Scholar] [CrossRef] [PubMed]
- Laroche, C. Exopolysaccharides from Microalgae and Cyanobacteria: Diversity of Strains, Production Strategies, and Applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef] [PubMed]
- Babiak, W.; Krzemińska, I. Extracellular Polymeric Substances (EPS) as Microalgal Bioproducts: A Review of Factors Affecting EPS Synthesis and Application in Flocculation Processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Moreira, J.B.; Kuntzler, S.G.; Bezerra, P.Q.M.; Cassuriaga, A.P.A.; Zaparoli, M.; da Silva, J.L.V.; Costa, J.A.V.; de Morais, M.G. Recent Advances of Microalgae Exopolysaccharides for Application as Bioflocculants. Polysaccharides 2022, 3, 264–276. [Google Scholar] [CrossRef]
- Visentin, T.G.; Guimarães, B.M.; Bastos, R.G. Effects of Temperature, pH, and C/N Ratio of Sugarcane Wastewater Processing (Vinasse) on Phormidium autumnale Heterotrophic Cultivation. Algal Res. 2024, 77, 103349. [Google Scholar] [CrossRef]
- Drira, M.; Elleuch, J.; Hlima, H.B.; Hentati, F.; Gardarin, C.; Rihouey, C.; Cerf, D.L.; Michaud, P.; Abdelkafi, S.; Fendri, I. Optimization of Exopolysaccharides Production by Porphyridium sordidum and Their Potential to Induce Defense Responses in Arabidopsis thaliana against Fusarium oxysporum. Biomolecules 2021, 11, 282. [Google Scholar] [CrossRef]
- Borjas Esqueda, A.; Gardarin, C.; Laroche, C. Exploring the Diversity of Red Microalgae for Exopolysaccharide Production. Mar. Drugs 2022, 20, 246. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium Spirulan, an Inhibitor of Enveloped Virus Replication, from a Blue-Green Alga Spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef]
- Uhliariková, I.; Šutovská, M.; Barboríková, J.; Molitorisová, M.; Kim, H.J.; Park, Y.I.; Matulová, M.; Lukavský, J.; Hromadková, Z.; Capek, P. Structural Characteristics and Biological Effects of Exopolysaccharide Produced by Cyanobacterium nostoc sp. Int. J. Biol. Macromol. 2020, 160, 364–371. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Mondal, A.; Bhunia, B.; Bandyopadhyay, T.K.; Jaladi, P.; Oinam, G.; Indrama, T. Purification, Characterization and Biotechnological Potential of New Exopolysaccharide Polymers Produced by Cyanobacterium Anabaena sp. CCC 745. Polymer 2019, 178, 121695. [Google Scholar] [CrossRef]
- Uhliariková, I.; Matulová, M.; Košťálová, Z.; Lukavský, J.; Capek, P. Lactylated Acidic Exopolysaccharide Produced by the Cyanobacterium Nostoc Cf. Linckia. Carbohydr. Polym. 2022, 276, 118801. [Google Scholar] [CrossRef] [PubMed]
- Gongi, W.; Gomez Pinchetti, J.L.; Cordeiro, N.; Ouada, H.B. Extracellular Polymeric Substances Produced by the Thermophilic Cyanobacterium Gloeocapsa gelatinosa: Characterization and Assessment of Their Antioxidant and Metal-Chelating Activities. Mar. Drugs 2022, 20, 227. [Google Scholar] [CrossRef]
- Patel, A.; Prajapat, J.B. Food and Health Applications of Exopolysaccharides Produced by Lactic Acid Bacteria. Adv. Dairy Res. 2013, 1, 107. [Google Scholar]
- De belder, A.N. CHAPTER 14—DEXTRAN. In Industrial Gums (Third Edition); Whistler, R.L., Bemiller, J.N., Eds.; Academic Press: London, UK, 1993; pp. 399–425. ISBN 978-0-08-092654-4. [Google Scholar]
- Yang, B.-K.; Ha, J.-Y.; Jeong, S.-C.; Das, S.; Yun, J.-W.; Lee, Y.-S.; Choi, J.-W.; Song, C.-H. Production of Exo-Polymers by Submerged Mycelial Culture of Cordyceps Militaris and Its Hypolipidemic Effect. J. Microbiol. Biotechnol. 2000, 10, 784–788. [Google Scholar]
- Shih, L.; Yu, J.-Y.; Hsieh, C.; Wu, J.-Y. Production and Characterization of Curdlan by Agrobacterium sp. Biochem. Eng. J. 2009, 43, 33–40. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, Y.; Li, Y.; Bao, J.; Fan, X.; Qu, Y.; Wang, Y.; Hu, Z.; Li, Q. Production and Optimization of Curdlan Produced by Pseudomonas sp. QL212. Int. J. Biol. Macromol. 2016, 89, 25–34. [Google Scholar] [CrossRef]
- Monsan, P.; Bozonnet, S.; Albenne, C.; Joucla, G.; Willemot, R.-M.; Remaud-Siméon, M. Homopolysaccharides from Lactic Acid Bacteria. Int. Dairy J. 2001, 11, 675–685. [Google Scholar] [CrossRef]
- Rosalam, S.; England, R. Review of Xanthan Gum Production from Unmodified Starches by Xanthomonas comprestris sp. Enzyme Microb. Technol. 2006, 39, 197–207. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Khanda, S.M. Pullulan: An Exopolysaccharide and Its Various Applications. Carbohydr. Polym. 2013, 95, 540–549. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M. Pullulan: Biosynthesis, Production, and Applications. Appl. Microbiol. Biotechnol. 2011, 92, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Kırtel, O.; Avşar, G.; Erkorkmaz, B.A.; Öner, E.T. Microbial Polysaccharides as Food Ingredients. In Microbial Production of Food Ingredients and Additives; Elsevier: Amsterdam, The Netherlands, 2017; pp. 347–383. [Google Scholar]
- Coviello, T.; Palleschi, A.; Grassi, M.; Matricardi, P.; Bocchinfuso, G.; Alhaique, F. Scleroglucan: A Versatile Polysaccharide for Modified Drug Delivery. Molecules 2005, 10, 6–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, H.; Tang, J.; Chen, J.; Zhang, X. Polysaccharides in Lentinus Edodes: Isolation, Structure, Immunomodulating Activity and Future Prospective. Crit. Rev. Food Sci. Nutr. 2014, 54, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Giavasis, I. Production of Microbial Polysaccharides for Use in Food. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 413–468. [Google Scholar]
- Giavasis, I.; Seviour, R.J.; Hudman, P.; McNeil, B. Fungal bioproducts for use in food: Polysaccharides, organic acids, and mycoprotein. In Advances in Food Bioproducts and Bioprocessing Technologies; CRC Press: Boca Raton, FL, USA, 2019; pp. 511–548. [Google Scholar]
- Babitskaya, V.G.; Shcherba, V.V.; Puchkova, T.A.; Smirnov, D.A. Polysaccharides of Ganoderma Lucidum: Factors Affecting Their Production. Appl. Biochem. Microbiol. 2005, 41, 169–173. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.; Luo, L. Antitumor Polysaccharides from Mushrooms: A Review on the Structural Characteristics, Antitumor Mechanisms and Immunomodulating Activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef]
- Yamanaka, D.; Liu, Y.; Motoi, M.; Ohno, N. Royal Sun Medicinal Mushroom, Agaricus brasiliensis Ka21 (Higher Basidiomycetes), as a Functional Food in Humans. Int. J. Med. Mushrooms 2013, 15, 335–343. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Wu, J.-Y. Effects of Tween 80 and pH on Mycelial Pellets and Exopolysaccharide Production in Liquid Culture of a Medicinal Fungus. J. Ind. Microbiol. Biotechnol. 2012, 39, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological Activities and Pharmaceutical Applications of Polysaccharide from Natural Resources: A Review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A Critical Review on the Impacts of β-Glucans on Gut Microbiota and Human Health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Mishima, T.; Murata, J.; Toyoshima, M.; Fujii, H.; Nakajima, M.; Hayashi, T.; Kato, T.; Saiki, I. Inhibition of Tumor Invasion and Metastasis by Calciumspirulan (Ca-SP), a Novel Sulfated Polysaccharide Derived from a Blue-Green Alga, Spirulina Platensis. Clin. Exp. Metastasis 1998, 16, 541–550. [Google Scholar] [CrossRef]
- De Philippis, R.; Vincenzini, M. Exocellular Polysaccharides from Cyanobacteria and Their Possible Applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, Extraction and Characterization of Microalgal and Cyanobacterial Exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of Cyanobacterial Exopolysaccharides: Composition, Structures, Inducing Factors and Putative Genes Involved in Their Biosynthesis and Assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Rajneesh, R.; Sonker, A.S.; Kannaujiya, V.K.; Sinha, R.P. Cyanobacterial extracellular polysaccharide sheath pigment, scytonemin: A novel multipurpose pharmacophore. In Marine Glycobiology; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-315-37139-9. [Google Scholar]
- Cruz, D.; Vasconcelos, V.; Pierre, G.; Michaud, P.; Delattre, C. Exopolysaccharides from Cyanobacteria: Strategies for Bioprocess Development. Appl. Sci. 2020, 10, 3763. [Google Scholar] [CrossRef]
- Diengdoh, O.L.; Syiem, M.B.; Pakshirajan, K.; Rai, A.N. Zn2+ Sequestration by Nostoc muscorum: Study of Thermodynamics, Equilibrium Isotherms, and Biosorption Parameters for the Metal. Environ. Monit. Assess. 2017, 189, 314. [Google Scholar] [CrossRef]
- Mota, R.; Rossi, F.; Andrenelli, L.; Pereira, S.B.; De Philippis, R.; Tamagnini, P. Released Polysaccharides (RPS) from Cyanothece sp. CCY 0110 as Biosorbent for Heavy Metals Bioremediation: Interactions between Metals and RPS Binding Sites. Appl. Microbiol. Biotechnol. 2016, 100, 7765–7775. [Google Scholar] [CrossRef]
- Shakeri, M.; Naji-Tabasi, S. Characterization and Optimization of Gellan Gum Production by Natural Sphingomonas sp. SM2. LWT 2024, 200, 116164. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Y.; Wang, Z.; Zhu, L.; Li, Z.; Jiang, Y.; Zhan, X.; Gao, M. Promoting Substrates Uptake and Curdlan Synthesis of Agrobacterium sp. by Attenuating the Exopolysaccharide Encapsulation. Carbohydr. Polym. 2023, 315, 120941. [Google Scholar] [CrossRef]
- Seviour, R.J.; McNeil, B.; Fazenda, M.L.; Harvey, L.M. Operating Bioreactors for Microbial Exopolysaccharide Production. Crit. Rev. Biotechnol. 2011, 31, 170–185. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, H.-J.; Xu, C.-P. Culture Characterization of Exopolysaccharides with Antioxidant Activity Produced by Pycnoporus sanguineus in Stirred-Tank and Airlift Reactors. J. Taiwan Inst. Chem. Eng. 2014, 45, 2075–2080. [Google Scholar] [CrossRef]
- Usuldin, S.R.A.; Ilham, Z.; Jamaludin, A.A.; Ahmad, R.; Wan-Mohtar, W.A.A.Q.I. Enhancing Biomass-Exopolysaccharides Production of Lignosus rhinocerus in a High-Scale Stirred-Tank Bioreactor and Its Potential Lipid as Bioenergy. Energies 2023, 16, 2330. [Google Scholar] [CrossRef]
- Jaswal, A.S.; Elangovan, R.; Mishra, S. Synthesis and Molecular Characterization of Levan Produced by Immobilized Microbacterium paraoxydans. J. Biotechnol. 2023, 373, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Namdeo, N.; Kumar, B.; Jha, H. Bioreactor Design for the Production of Microbial Polysaccharides. In Microbial Exopolysaccharides; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-00-334268-7. [Google Scholar]
- Finore, I.; Di Donato, P.; Mastascusa, V.; Nicolaus, B.; Poli, A. Fermentation Technologies for the Optimization of Marine Microbial Exopolysaccharide Production. Mar. Drugs 2014, 12, 3005–3024. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Anandapandian, K.T.K.; Parthiban, K. Production and Characterization of Exopolysaccharides (EPS) from Biofilm Forming Marine Bacterium. Braz. Arch. Biol. Technol. 2011, 54, 259–265. [Google Scholar] [CrossRef]
- Sanalibaba, P.; Cakmak, G.A. Exopolysaccharides Production by Lactic Acid Bacteria. Appl. Microbiol. Open Access 2016, 2, 1000115. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Advances in Production and Simplified Methods for Recovery and Quantification of Exopolysaccharides for Applications in Food and Health1. J. Dairy Sci. 2016, 99, 3229–3238. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Razafindralambo, H.; Blecker, C.; N’Yapo, C.; Thonart, P.; Delvigne, F. Stochastic Exposure to Sub-Lethal High Temperature Enhances Exopolysaccharides (EPS) Excretion and Improves Bifidobacterium bifidum Cell Survival to Freeze–Drying. Biochem. Eng. J. 2014, 88, 85–94. [Google Scholar] [CrossRef]
- Higgins, M.J.; Novak, J.T. Characterization of Exocellular Protein and Its Role in Bioflocculation. J. Environ. Eng. 1997, 123, 479–485. [Google Scholar] [CrossRef]
- Yegorenkova, I.V.; Tregubova, K.V.; Matora, L.Y.; Burygin, G.L.; Ignatov, V.V. Biofilm Formation by Paenibacillus polymyxa Strains Differing in the Production and Rheological Properties of Their Exopolysaccharides. Curr. Microbiol. 2011, 62, 1554–1559. [Google Scholar] [CrossRef]
- Poli, A.; Di Donato, P.; Abbamondi, G.R.; Nicolaus, B. Synthesis, Production, and Biotechnological Applications of Exopolysaccharides and Polyhydroxyalkanoates by Archaea. Archaea 2011, 2011, 693253. [Google Scholar] [CrossRef]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm Formation as Microbial Development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Schwarzmann, S.; Boring, J.R. Antiphagocytic Effect of Slime from a Mucoid Strain of Pseudomonas aeruginosa. Infect. Immun. 1971, 3, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An Overview of the Functionality of Exopolysaccharides Produced by Lactic Acid Bacteria. Int. Dairy J. 2002, 12, 163–171. [Google Scholar] [CrossRef]
- Li, W.; Ji, J.; Rui, X.; Yu, J.; Tang, W.; Chen, X.; Jiang, M.; Dong, M. Production of Exopolysaccharides by Lactobacillus helveticus MB2-1 and Its Functional Characteristics in Vitro. LWT Food Sci. Technol. 2014, 59, 732–739. [Google Scholar] [CrossRef]
- Khan, W.; Hosseinkhani, H.; Ickowicz, D.; Hong, P.-D.; Yu, D.-S.; Domb, A.J. Polysaccharide Gene Transfection Agents. Acta Biomater. 2012, 8, 4224–4232. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, H.; Fang, Y.; Nishinari, K.; Phillips, G.O. Schizophyllan: A Review on Its Structure, Properties, Bioactivities and Recent Developments. Bioact. Carbohydr. Diet. Fibre 2013, 1, 53–71. [Google Scholar] [CrossRef]
- Park, M.; Lee, D.; Hyun, J. Nanocellulose-Alginate Hydrogel for Cell Encapsulation. Carbohydr. Polym. 2015, 116, 223–228. [Google Scholar] [CrossRef]
- Albadran, H.A.; Chatzifragkou, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Development of Surfactant-Coated Alginate Capsules Containing Lactobacillus plantarum. Food Hydrocoll. 2018, 82, 490–499. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Li, D.; Chen, W.-J.; Ban, S.-N.; Liu, T.; Wen, H.; Jiang, M. Effects of Dietary Host-Associated Lactococcus lactis on Growth Performance, Disease Resistance, Intestinal Morphology and Intestinal Microbiota of Mandarin Fish (Siniperca chuatsi). Aquaculture 2021, 540, 736702. [Google Scholar] [CrossRef]
- Walker, A.W.; Duncan, S.H.; McWilliam Leitch, E.C.; Child, M.W.; Flint, H.J. pH and Peptide Supply Can Radically Alter Bacterial Populations and Short-Chain Fatty Acid Ratios within Microbial Communities from the Human Colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Cuesta, I.; Alvarez-Buylla, J.R.; Ruas-Madiedo, P.; Gueimonde, M.; de los Reyes-Gavilán, C.G. Bacteroides fragilis Metabolises Exopolysaccharides Produced by Bifidobacteria. BMC Microbiol. 2016, 16, 150. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.; Hall, L.J.; Cronin, M.; Zomer, A.; MacSharry, J.; Goulding, D.; O’Connell Motherway, M.; Shanahan, F.; Nally, K.; Dougan, G.; et al. Bifidobacterial Surface-Exopolysaccharide Facilitates Commensal-Host Interaction through Immune Modulation and Pathogen Protection. Proc. Natl. Acad. Sci. USA 2012, 109, 2108–2113. [Google Scholar] [CrossRef]
- Balzaretti, S.; Taverniti, V.; Guglielmetti, S.; Fiore, W.; Minuzzo, M.; Ngo, H.N.; Ngere, J.B.; Sadiq, S.; Humphreys, P.N.; Laws, A.P. A Novel Rhamnose-Rich Hetero-Exopolysaccharide Isolated from Lactobacillus paracasei DG Activates THP-1 Human Monocytic Cells. Appl. Environ. Microbiol. 2017, 83, e02702-16. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Llamas, M.G.; Iraporda, C.; Dueñas, M.T.; Abraham, A.G.; Garrote, G.L. Impact of Growth Temperature on Exopolysaccharide Production and Probiotic Properties of Lactobacillus paracasei Strains Isolated from Kefir Grains. Food Microbiol. 2018, 69, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, I.; Ktari, N.; Slima, S.B.; Triki, M.; Bardaa, S.; Mnif, H.; Salah, R.B. Evaluation of Dermal Wound Healing Activity and in Vitro Antibacterial and Antioxidant Activities of a New Exopolysaccharide Produced by Lactobacillus sp. Ca6. Int. J. Biol. Macromol. 2017, 103, 194–201. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, D.-H.; Kang, I.-B.; Kim, H.; Song, K.-Y.; Kim, H.-S.; Seo, K.-H. Characterization and Antibacterial Activity of a Novel Exopolysaccharide Produced by Lactobacillus kefiranofaciens DN1 Isolated from Kefir. Food Control 2017, 78, 436–442. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of Lactic Acid Bacteria: Structure, Bioactivity and Associations: A Review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.; Bajaj, B.K. Hypocholesterolemic and Bioactive Potential of Exopolysaccharide from a Probiotic Enterococcus faecium K1 Isolated from Kalarei. Bioresour. Technol. 2018, 254, 264–267. [Google Scholar] [CrossRef]
- Sasikumar, K.; Vaikkath, D.K.; Devendra, L.; Nampoothiri, K.M. An Exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with Potential Benefits for Making Functional Foods. Bioresour. Technol. 2017, 241, 1152–1156. [Google Scholar] [CrossRef]
- Ishimwe, N.; Daliri, E.B.; Lee, B.H.; Fang, F.; Du, G. The Perspective on Cholesterol-lowering Mechanisms of Probiotics. Mol. Nutr. Food Res. 2015, 59, 94–105. [Google Scholar] [CrossRef]
- Michael, D.R.; Davies, T.S.; Moss, J.W.E.; Calvente, D.L.; Ramji, D.P.; Marchesi, J.R.; Pechlivanis, A.; Plummer, S.F.; Hughes, T.R. The Anti-Cholesterolaemic Effect of a Consortium of Probiotics: An Acute Study in C57BL/6J Mice. Sci. Rep. 2017, 7, 2883. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mao, W.; Hou, Y.; Gao, Y.; Qi, X.; Zhao, C.; Chen, Y.; Chen, Y.; Li, N.; Wang, C. Preparation, Structure and Anticoagulant Activity of a Low Molecular Weight Fraction Produced by Mild Acid Hydrolysis of Sulfated Rhamnan from Monostroma latissimum. Bioresour. Technol. 2012, 114, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, X.; He, X.; Wang, S.; Cao, S.; Xia, Z.; Xian, H.; Qin, L.; Mao, W. Structure and Anticoagulant Property of a Sulfated Polysaccharide Isolated from the Green Seaweed Monostroma angicava. Carbohydr. Polym. 2017, 159, 195–206. [Google Scholar] [CrossRef]
- Rani, R.P.; Anandharaj, M.; Ravindran, A.D. Characterization of a Novel Exopolysaccharide Produced by Lactobacillus Gasseri FR4 and Demonstration of Its in Vitro Biological Properties. Int. J. Biol. Macromol. 2018, 109, 772–783. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, D.; Li, H.; Sun, Y.; Zeng, X.; Yan, B. Antioxidant and Immunomodulatory Activity of Selenium Exopolysaccharide Produced by Lactococcus lactis subsp. Lactis. Food Chem. 2013, 138, 84–89. [Google Scholar] [CrossRef]
- Pan, D.; Mei, X. Antioxidant Activity of an Exopolysaccharide Purified from Lactococcus lactis subsp. Lactis 12. Carbohydr. Polym. 2010, 80, 908–914. [Google Scholar] [CrossRef]
- Nácher-Vázquez, M.; Ballesteros, N.; Canales, Á.; Saint-Jean, S.R.; Pérez-Prieto, S.I.; Prieto, A.; Aznar, R.; López, P. Dextrans Produced by Lactic Acid Bacteria Exhibit Antiviral and Immunomodulatory Activity against Salmonid Viruses. Carbohydr. Polym. 2015, 124, 292–301. [Google Scholar] [CrossRef]
- Ren, W.; Xia, Y.; Wang, G.; Zhang, H.; Zhu, S.; Ai, L. Bioactive Exopolysaccharides from a S. thermophilus Strain: Screening, Purification and Characterization. Int. J. Biol. Macromol. 2016, 86, 402–407. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Characterization of a Novel Exopolysaccharide with Antitumor Activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 63, 133–139. [Google Scholar] [CrossRef]
- Singh, P.; Saini, P. Food and Health Potentials of Exopolysaccharides Derived from Lactobacilli. Microbiol. Res. J. Int. 2017, 22, 1–14. [Google Scholar] [CrossRef]
- Kim, K.; Lee, G.; Thanh, H.D.; Kim, J.-H.; Konkit, M.; Yoon, S.; Park, M.; Yang, S.; Park, E.; Kim, W. Exopolysaccharide from Lactobacillus plantarum LRCC5310 Offers Protection against Rotavirus-Induced Diarrhea and Regulates Inflammatory Response. J. Dairy Sci. 2018, 101, 5702–5712. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Thorp, S.C. Cell Surface Heparan Sulfate and Its Roles in Assisting Viral Infections. Med. Res. Rev. 2002, 22, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, R.; Qin, X.; Ma, X.; Liu, X.; Jia, S.; Zhong, C. A β-Glucan from Aureobasidium pullulans Enhanced the Antitumor Effect with Rituximab against SU-DHL-8. Int. J. Biol. Macromol. 2022, 220, 1356–1367. [Google Scholar] [CrossRef]
- Khalil, M.A.; Sonbol, F.I.; Al-Madboly, L.A.; Aboshady, T.A.; Alqurashi, A.S.; Ali, S.S. Exploring the Therapeutic Potentials of Exopolysaccharides Derived from Lactic Acid Bacteria and Bifidobacteria: Antioxidant, Antitumor, and Periodontal Regeneration. Front. Microbiol. 2022, 13, 803688. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Fu, Y.; Pan, N.; Zhang, H.; Xiu, L.; Liang, Y.; Liu, Y.; Liu, B.; Ma, C.; Du, R.; et al. Novel Exopolysaccharide Derived from Probiotic Lactobacillus pantheris TCP102 Strain with Immune-Enhancing and Anticancer Activities. Front. Microbiol. 2022, 13, 1015270. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, D.; Huang, Y. Exopolysaccharides from Lactiplantibacillus plantarum: Isolation, Purification, Structure–Function Relationship, and Application. Eur. Food Res. Technol. 2023, 249, 1431–1448. [Google Scholar] [CrossRef]
- Li, F.; Jiao, X.; Zhao, J.; Liao, X.; Wei, Y.; Li, Q. Antitumor Mechanisms of an Exopolysaccharide from Lactobacillus fermentum on HT-29 Cells and HT-29 Tumor-Bearing Mice. Int. J. Biol. Macromol. 2022, 209, 552–562. [Google Scholar] [CrossRef]
- Abdelnasser, S.M.; Abu-Shahba, N. Bacillus sonorinses Derived Exopolysaccharide Enhances Cell Cycle Arrest, Apoptosis, Necrosis, Autophagy and COX-2 down Regulation in Liver Cancer Cells. Biotechnol. Rep. 2024, 48, e00848. [Google Scholar] [CrossRef]
- Taşkaya, A.; Güvensen, N.C.; Güler, C.; Şancı, E.; Karabay, Ü. Exopolysaccharide from Rhodococcus pyridinivorans ZZ47 Strain: Evaluation of Biological Activity and Toxicity. J. Agric. Prod. 2023, 4, 63–71. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, G.; Li, F.; Fang, S.; Zhou, S.; Ishiwata, A.; Tonevitsky, A.G.; Shkurnikov, M.; Cai, H.; Ding, F. Immunomodulatory Effect and Biological Significance of β-Glucans. Pharmaceutics 2023, 15, 1615. [Google Scholar] [CrossRef]
- Notararigo, S.; Varela, E.; Otal, A.; Antolín, M.; Guarner, F.; López, P. Anti-Inflammatory Effect of an O-2-Substituted (1-3)-β-D-Glucan Produced by Pediococcus parvulus 2.6 in a Caco-2 PMA-THP-1 Co-Culture Model. Int. J. Mol. Sci. 2022, 23, 1527. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, X.; Peng, Q.; Ma, L.; Qiao, Y.; Shi, B. Exopolysaccharides from Lactic Acid Bacteria, as an Alternative to Antibiotics, on Regulation of Intestinal Health and the Immune System. Anim. Nutr. 2023, 13, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Shah, N.P.; Holley, R. Exopolysaccharides as Antimicrobial Agents: Mechanism and Spectrum of Activity. Front. Microbiol. 2021, 12, 664395. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ju, Y.; Liu, N.; Shi, S.; Hao, L. Structural Characteristics of Microbial Exopolysaccharides in Association with Their Biological Activities: A Review. Chem. Biol. Technol. Agric. 2023, 10, 137. [Google Scholar] [CrossRef]
- Kiššová, Z.; Schusterová, P.; Mudroňová, D.; Novotný, J.; Tkáčiková, Ľ. Exopolysaccharides from Limosilactobacillus reuteri: Their Influence on in Vitro Activation of Porcine Monocyte-Derived Dendritic Cells-Brief Report. Vet. Res. Commun. 2024, 1–7. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Wu, Y.; Mehwish, H.M.; Bansal, M.; Zhao, L. Lactobacillus Exopolysaccharides: New Perspectives on Engineering Strategies, Physiochemical Functions, and Immunomodulatory Effects on Host Health. Trends Food Sci. Technol. 2020, 103, 36–48. [Google Scholar] [CrossRef]
- Domingos-Lopes, M.F.P.; Nagy, A.; Stanton, C.; Ross, P.R.; Gelencsér, E.; Silva, C.C.G. Immunomodulatory Activity of Exopolysaccharide Producing Leuconostoc citreum Strain Isolated from Pico Cheese. J. Funct. Foods 2017, 33, 235–243. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Wang, H.; Zhang, H.; Chen, W.; Lu, W. Lactic Acid Bacteria-Derived Exopolysaccharide: Formation, Immunomodulatory Ability, Health Effects, and Structure-Function Relationship. Microbiol. Res. 2023, 274, 127432. [Google Scholar] [CrossRef]
- Dargahi, N.; Johnson, J.C.; Apostolopoulos, V. Immune Modulatory Effects of Probiotic Streptococcus Thermophilus on Human Monocytes. Biologics 2021, 1, 396–415. [Google Scholar] [CrossRef]
- Hickey, A.; Stamou, P.; Udayan, S.; Ramón-Vázquez, A.; Esteban-Torres, M.; Bottacini, F.; Woznicki, J.A.; Hughes, O.; Melgar, S.; Ventura, M.; et al. Bifidobacterium Breve Exopolysaccharide Blocks Dendritic Cell Maturation and Activation of CD4+ T Cells. Front. Microbiol. 2021, 12, 653587. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, S.; Cui, J.; Guo, T.; Zhang, J.; Li, B. Alleviative Effects of Exopolysaccharide Produced by Lactobacillus helveticus KLDS1. 8701 on Dextran Sulfate Sodium-Induced Colitis in Mice. Microorganisms 2021, 9, 2086. [Google Scholar] [CrossRef] [PubMed]
- Young, I.D.; Latousakis, D.; Juge, N. The Immunomodulatory Properties of β-2, 6 Fructans: A Comprehensive Review. Nutrients 2021, 13, 1309. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yajima, T.; Li, W.; Saito, K.; Ohshima, Y.; Yoshikai, Y. Levan (β-2, 6-Fructan), a Major Fraction of Fermented Soybean Mucilage, Displays Immunostimulating Properties via Toll-like Receptor 4 Signalling: Induction of Interleukin-12 Production and Suppression of T-Helper Type 2 Response and Immunoglobulin E Production. Clin. Exp. Allergy 2006, 36, 94–101. [Google Scholar] [CrossRef]
- Wahab, W.A.A.; Shafey, H.I.; Mahrous, K.F.; Esawy, M.A.; Saleh, S.A.A. Coculture of Bacterial Levans and Evaluation of Its Anti-Cancer Activity against Hepatocellular Carcinoma Cell Lines. Sci. Rep. 2024, 14, 3173. [Google Scholar] [CrossRef]
- Aquinas, N.; Bhat, M.R.; Selvaraj, S. A Review Presenting Production, Characterization, and Applications of Biopolymer Curdlan in Food and Pharmaceutical Sectors. Polym. Bull. 2022, 79, 6905–6927. [Google Scholar] [CrossRef]
- Ganie, S.A.; Rather, L.J.; Assiri, M.A.; Li, Q. Recent Innovations (2020–2023) in the Approaches for the Chemical Functionalization of Curdlan and Pullulan: A Mini-Review. Int. J. Biol. Macromol. 2024, 260, 129412. [Google Scholar] [CrossRef]
- Andreu, S.; von Kobbe, C.; Delgado, P.; Ripa, I.; Buzón, M.J.; Genescà, M.; Gironès, N.; del Moral-Salmoral, J.; Ramírez, G.A.; Zúñiga, S. Dextran Sulfate from Leuconostoc mesenteroides B512F Exerts Potent Antiviral Activity against SARS-CoV-2 in Vitro and in Vivo. Front. Microbiol. 2023, 14, 1185504. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Kujawowicz, K.; Witkowska, A.M. Beta-Glucans from Fungi: Biological and Health-Promoting Potential in the COVID-19 Pandemic Era. Nutrients 2021, 13, 3960. [Google Scholar] [CrossRef]
- Wu, N.; Ge, X.; Yin, X.; Yang, L.; Chen, L.; Shao, R.; Xu, W. A Review on Polysaccharide Biosynthesis in Cordyceps militaris. Int. J. Biol. Macromol. 2024, 260, 129336. [Google Scholar] [CrossRef]
- Afreen, A.; Ahmed, Z.; Khalid, N.; Ferheen, I.; Ahmed, I. Optimization and Cholesterol-Lowering Activity of Exopolysaccharide from Lactiplantibacillus paraplantarum NCCP 962. Appl. Microbiol. Biotechnol. 2023, 107, 1189–1204. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, X.; Liu, X.; Wang, X.; Gao, X. Therapeutic and Improving Function of Lactobacilli in the Prevention and Treatment of Cardiovascular-Related Diseases: A Novel Perspective from Gut Microbiota. Front. Nutr. 2021, 8, 693412. [Google Scholar]
- Al-Nabulsi, A.A.; Jaradat, Z.W.; Qudsi, F.R.A.; Elsalem, L.; Osaili, T.M.; Olaimat, A.N.; Esposito, G.; Liu, S.-Q.; Ayyash, M.M. Characterization and Bioactive Properties of Exopolysaccharides Produced by Streptococcus thermophilus and Lactobacillus bulgaricus Isolated from Labaneh. LWT 2022, 167, 113817. [Google Scholar] [CrossRef]
- Aloraini, G.S.; Albureikan, M.O.I.; Shahlol, A.M.A.; Shamrani, T.; Daghistani, H.; El-Nablaway, M.; Tharwat, N.A.; Elazzazy, A.M.; Basyony, A.F.; Ghareeb, A. Biomedical and Therapeutic Potential of Marine-Derived Pseudomonas sp. Strain AHG22 Exopolysaccharide: A Novel Bioactive Microbial Metabolite. Rev. Adv. Mater. Sci. 2024, 63, 20240016. [Google Scholar] [CrossRef]
- Ge, Z.; Wang, D.; Azi, F.; Zhao, W.; Wang, P.; Dong, M.; Wang, J.; Zhao, Y.; Zhao, X. The Optimization of in Situ Exopolysaccharides Production in Lactobacillus helveticus MB2-1 Fermented Milk and Its Functional Characteristics in Vitro. Int. Dairy J. 2024, 155, 105969. [Google Scholar] [CrossRef]
- Carvalho, F.M.; Teixeira-Santos, R.; Mergulhao, F.J.M.; Gomes, L.C. The Use of Probiotics to Fight Biofilms in Medical Devices: A Systematic Review and Meta-Analysis. Microorganisms 2020, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Giordani, B.; Parolin, C.; Vitali, B. Lactobacilli as Anti-Biofilm Strategy in Oral Infectious Diseases: A Mini-Review. Front. Med. Technol. 2021, 3, 769172. [Google Scholar] [CrossRef]
- Song, Y.; Sun, M.; Feng, L.; Liang, X.; Song, X.; Mu, G.; Tuo, Y.; Jiang, S.; Qian, F. Antibiofilm Activity of Lactobacillus plantarum 12 Exopolysaccharides against Shigella flexneri. Appl. Environ. Microbiol. 2020, 86, e00694-20. [Google Scholar] [CrossRef] [PubMed]
- Aliouche, N.; Sifour, M.; Kebsa, W.; Khennouf, T.; Ercan, F.; Ouled-Haddar, H. Prophylactic Effect and Antiulcerogenic Potential of Probiotic Lactiplantibacillus plantarum E1K2R2 and Its Exopolysaccharide against Ibuprofen-Induced Acute Gastric Ulcer. Probiotics Antimicrob. Proteins 2024, 2024, 1–16. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Z.; Zhou, Q.; Li, P.; Wu, S.; Zhou, T.; Gu, Q. Exopolysaccharide from Lacticaseibacillus paracasei Alleviates Gastritis in Helicobacter Pylori-Infected Mice by Regulating Gastric Microbiota. Front. Nutr. 2024, 11, 1426358. [Google Scholar] [CrossRef]
- Al-Qaysi, S.A.S.; Al-Haideri, H.; Al-Shimmary, S.M.; Abdulhameed, J.M.; Alajrawy, O.I.; Al-Halbosiy, M.M.; Moussa, T.A.A.; Farahat, M.G. Bioactive Levan-Type Exopolysaccharide Produced by Pantoea agglomerans ZMR7: Characterization and Optimization for Enhanced Production. J. Microbiol. Biotechnol. 2021, 31, 696. [Google Scholar] [CrossRef]
- Erdal Altıntaş, Ö.; Toksoy Öner, E.; Çabuk, A.; Aytar Çelik, P. Biosynthesis of Levan by Halomonas elongata 153B: Optimization for Enhanced Production and Potential Biological Activities for Pharmaceutical Field. J. Polym. Environ. 2023, 31, 1440–1455. [Google Scholar] [CrossRef]
- Cao, C.; Bian, Y.; Cang, W.; Wu, J.; Wu, R. Structural Characterization and Hepatoprotective Activity of Exopolysaccharide from Bacillus velezensis SN-1. J. Sci. Food Agric. 2023, 103, 738–749. [Google Scholar] [CrossRef]
- Lee, M.-G.; Joeng, H.; Shin, J.; Kim, S.; Lee, C.; Song, Y.; Lee, B.-H.; Park, H.-G.; Lee, T.-H.; Jiang, H.-H. Potential Probiotic Properties of Exopolysaccharide-Producing Lacticaseibacillus paracasei EPS DA-BACS and Prebiotic Activity of Its Exopolysaccharide. Microorganisms 2022, 10, 2431. [Google Scholar] [CrossRef] [PubMed]
- Bisson, G.; Comuzzi, C.; Giordani, E.; Poletti, D.; Boaro, M.; Marino, M. An Exopolysaccharide from Leuconostoc mesenteroides Showing Interesting Bioactivities versus Foodborne Microbial Targets. Carbohydr. Polym. 2023, 301, 120363. [Google Scholar] [CrossRef]
- Kavitake, D.; Tiwari, S.; Shah, I.A.; Devi, P.B.; Delattre, C.; Reddy, G.B.; Shetty, P.H. Antipathogenic Potentials of Exopolysaccharides Produced by Lactic Acid Bacteria and Their Food and Health Applications. Food Control 2023, 152, 109850. [Google Scholar] [CrossRef]
- Ma, L.; Xu, X.; Peng, Q.; Yang, S.; Zhang, Y.; Tian, D.; Shi, L.; Qiao, Y.; Shi, B. Exopolysaccharide from Lactobacillus casei NA-2 Attenuates Escherichia coli O157:H7 Surface Adhesion via Modulation of Membrane Surface Properties and Adhesion-Related Gene Expression. Microb. Pathog. 2022, 173, 105863. [Google Scholar] [CrossRef] [PubMed]
- Zammuto, V.; Spanò, A.; Agostino, E.; Macrì, A.; De Pasquale, C.; Ferlazzo, G.; Rizzo, M.G.; Nicolò, M.S.; Guglielmino, S.; Gugliandolo, C. Anti-Bacterial Adhesion on Abiotic and Biotic Surfaces of the Exopolysaccharide from the Marine Bacillus Licheniformis B3-15. Mar. Drugs 2023, 21, 313. [Google Scholar] [CrossRef]
- IS, W.B. Biofilm Development and Approaches to Biofilm Inhibition by Exopolysaccharides. New Microbiol. 2022, 45, 227–236. [Google Scholar]
- Giordani, B.; Naldi, M.; Croatti, V.; Parolin, C.; Erdoğan, Ü.; Bartolini, M.; Vitali, B. Exopolysaccharides from Vaginal lactobacilli Modulate Microbial Biofilms. Microb. Cell Factories 2023, 22, 45. [Google Scholar] [CrossRef]
- Tao, T.; Zhang, L.; Yu, T.; Ma, J.; Lu, S.; Ren, J.; Li, X.; Guo, X. Exopolysaccharide Production by Lactobacillus Plantarum T10 Is Responsible for the Probiotic Activity in Enhancing Intestinal Barrier Function in Vitro and in Vivo. Food Funct. 2024, 15, 3583–3599. [Google Scholar] [CrossRef]
- Xie, Y.; Pei, F.; Liu, Y.; Liu, Z.; Chen, X.; Xue, D. Fecal Fermentation and High-Fat Diet-Induced Obesity Mouse Model Confirmed Exopolysaccharide from Weissella cibaria PFY06 Can Ameliorate Obesity by Regulating the Gut Microbiota. Carbohydr. Polym. 2023, 318, 121122. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rivero, C.; López-Gómez, J.P. Unlocking the Potential of Fermentation in Cosmetics: A Review. Fermentation 2023, 9, 463. [Google Scholar] [CrossRef]
- Mahmoud, M.G.; Awady, M.E.E.; Selim, M.S.; Ibrahim, A.Y.; Ibrahim, F.M.; Mohamed, S.S. Characterization of Biologically Active Exopolysaccharide Produced by Streptomyces sp. NRCG4 and Its Anti-Alzheimer Efficacy: In-Vitro Targets. J. Genet. Eng. Biotechnol. 2023, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Sirin, S.; Aslim, B. Characterization of Lactic Acid Bacteria Derived Exopolysaccharides for Use as a Defined Neuroprotective Agent against Amyloid Beta1–42-Induced Apoptosis in SH-SY5Y Cells. Sci. Rep. 2020, 10, 8124. [Google Scholar] [CrossRef]
- Darilmaz, D.O.; Beyatli, Y. Investigating Hydrophobicity and the Effect of Exopolysaccharide on Aggregation Properties of Dairy Propionibacteria Isolated from Turkish Homemade Cheeses. J. Food Prot. 2012, 75, 359–365. [Google Scholar] [CrossRef]
- Hooshdar, P.; Kermanshahi, R.K.; Ghadam, P.; Khosravi-Darani, K. A Review on Production of Exopolysaccharide and Biofilm in Probiotics Like Lactobacilli and Methods of Analysis. Biointerface Res. Appl. Chem. 2020, 10, 6058–6075. [Google Scholar] [CrossRef]
- Jahr, H.; Bahro, R.; Eichenlaub, R. Genetics of phytopathology: Phytopathogenic bacteria. In Progress in Botany: Genetics Cell Biology and Physiology Systematics and Comparative Morphology Ecology and Vegetation Science; Esser, K., Kadereit, J.W., Lüttge, U., Runge, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 119–138. ISBN 978-3-642-59940-8. [Google Scholar]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, Bioemulsifiers and Exopolysaccharides from Marine Microorganisms. Biotechnol. Adv. 2010, 28, 436–450. [Google Scholar] [CrossRef]
- d’Abzac, P.; Bordas, F.; Joussein, E.; Van Hullebusch, E.D.; Piet, N.L.; Guibaud, G. Metal Binding Properties of Extracellular Polymeric Substances Extracted from Anaerobic Granular Sludges. Environ. Sci. Pollut. Res. 2013, 20, 4509–4519. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Yang, N.; Jin, Z.; Xu, X. Comparison of Dextran Molecular Weight on Wheat Bread Quality and Their Performance in Dough Rheology and Starch Retrogradation. LWT 2018, 98, 39–45. [Google Scholar]
- Du, R.; Yu, L.; Yu, N.; Ping, W.; Song, G.; Ge, J. Characterization of Exopolysaccharide Produced by Levilactobacillus brevis HDE-9 and Evaluation of Its Potential Use in Dairy Products. Int. J. Biol. Macromol. 2022, 217, 303–311. [Google Scholar] [CrossRef]
- Kavitake, D.; Tiwari, S.; Devi, P.B.; Shah, I.A.; Reddy, G.B.; Shetty, P.H. Production, Purification, and Functional Characterization of Glucan Exopolysaccharide Produced by Enterococcus Hirae Strain OL616073 of Fermented Food Origin. Int. J. Biol. Macromol. 2024, 259, 129105. [Google Scholar] [CrossRef] [PubMed]
- Mummaleti, G.; Sarma, C.; Yarrakula, S.; Urla, R.; Gazula, H. Production, Properties and Applications of Levan Polysaccharide. Food Humanit. 2024, 3, 100369. [Google Scholar] [CrossRef]
- Tintoré, M.; Cuñé, J.; Vu, L.D.; Poppe, J.; Van den Abbeele, P.; Baudot, A.; de Lecea, C. A Long-Chain Dextran Produced by Weissella cibaria Boosts the Diversity of Health-Related Gut Microbes Ex Vivo. Biology 2024, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Besrour-Aouam, N.; Fhoula, I.; Hernández-Alcántara, A.M.; Mohedano, M.L.; Najjari, A.; Prieto, A.; Ruas-Madiedo, P.; López, P.; Ouzari, H.-I. The Role of Dextran Production in the Metabolic Context of Leuconostoc and Weissella Tunisian Strains. Carbohydr. Polym. 2021, 253, 117254. [Google Scholar]
- Georgalaki, M.; Zoumpopoulou, G.; Anastasiou, R.; Kazou, M.; Tsakalidou, E. Lactobacillus kefiranofaciens: From Isolation and Taxonomy to Probiotic Properties and Applications. Microorganisms 2021, 9, 2158. [Google Scholar] [CrossRef]
- Bibi, A.; Xiong, Y.; Rajoka, M.S.R.; Mehwish, H.M.; Radicetti, E.; Umair, M.; Shoukat, M.; Khan, M.K.I.; Aadil, R.M. Recent Advances in the Production of Exopolysaccharide (EPS) from Lactobacillus spp. and Its Application in the Food Industry: A Review. Sustainability 2021, 13, 12429. [Google Scholar] [CrossRef]
- Kavitake, D.; Balyan, S.; Devi, P.B.; Shetty, P.H. Evaluation of Oil-in-Water (O/W) Emulsifying Properties of Galactan Exopolysaccharide from Weissella confusa KR780676. J. Food Sci. Technol. 2020, 57, 1579–1585. [Google Scholar] [CrossRef]
- Kermanshahi, R.K.; Khaniki, G.J.; Goudarzi, L. Biosorption of Cd+2 and Pb+2 by Exopolysaccharide Extracted from Lactobacillus Fermentum 6b; Adsorption Isotherm and Kinetic Studies. Iran. J. Public Health 2023, 52, 622–632. [Google Scholar] [CrossRef]
- Werning, M.L.; Hernández-Alcántara, A.M.; Ruiz, M.J.; Soto, L.P.; Dueñas, M.T.; López, P.; Frizzo, L.S. Biological Functions of Exopolysaccharides from Lactic Acid Bacteria and Their Potential Benefits for Humans and Farmed Animals. Foods 2022, 11, 1284. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Liu, P.; Ahmed, Z.; Xiao, P.; Bai, X. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohydrate Polymers. 2010, 82, 895–903. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Li, D.; Tian, J.; Xiao, L.; Kwok, L.-Y.; Li, W.; Sun, Z. Structure Characterization, Antioxidant Capacity, Rheological Characteristics and Expression of Biosynthetic Genes of Exopolysaccharides Produced by Lactococcus lactis subsp. Lactis IMAU11823. Food Chem. 2022, 384, 132566. [Google Scholar] [CrossRef] [PubMed]
- Tarique, M.; Ali, A.H.; Kizhakkayil, J.; Gan, R.-Y.; Liu, S.-Q.; Kamal-Eldin, A.; Ayyash, M. Investigating the Biological Activities and Prebiotic Potential of Exopolysaccharides Produced by Lactobacillus delbrueckii and Lacticaseibacillus rhamnosus: Implications for Gut Microbiota Modulation and Rheological Properties in Fermented Milk. Food Hydrocoll. Health 2023, 4, 100162. [Google Scholar] [CrossRef]
- Hu, X.; Pang, X.; Wang, P.G.; Chen, M. Isolation and Characterization of an Antioxidant Exopolysaccharide Produced by Bacillus sp. S-1 from Sichuan Pickles. Carbohydr. Polym. 2019, 204, 9–16. [Google Scholar] [CrossRef]
- Yang, J.; Yang, H. Recent Development in Se-Enriched Yeast, Lactic Acid Bacteria and Bifidobacteria. Crit. Rev. Food Sci. Nutr. 2023, 63, 411–425. [Google Scholar] [CrossRef]
- Kodali, V.P.; Perali, R.S.; Sen, R. Purification and Partial Elucidation of the Structure of an Antioxidant Carbohydrate Biopolymer from the Probiotic Bacterium Bacillus coagulans RK-02. J. Nat. Prod. 2011, 74, 1692–1697. [Google Scholar] [CrossRef]
- Abdl Aali, R.A.K.; Al-Sahlany, S.T.G. Gellan Gum as a Unique Microbial Polysaccharide: Its Characteristics, Synthesis, and Current Application Trends. Gels 2024, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Gniewosz, M.; Pobiega, K.; Kraśniewska, K.; Synowiec, A.; Chaberek, M.; Galus, S. Characterization and Antifungal Activity of Pullulan Edible Films Enriched with Propolis Extract for Active Packaging. Foods 2022, 11, 2319. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial Exopolysaccharides: Functionality and Prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Chapter 4—Natural polymers: Polysaccharides and their derivatives for biomedical applications. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 67–89. ISBN 978-0-12-396983-5. [Google Scholar]
- McHugh, D.J. Production, Properties and Uses of Alginates. Prod. Util. Prod. Commer. Seaweeds FAO Fish. Tech. Pap. 1987, 288, 58–115. [Google Scholar]
- Mocanu, G.; Mihai, D.; Dulong, V.; Picton, L.; Lecerf, D. New Anionic Amphiphilic Thermosensitive Pullulan Derivatives. Carbohydr. Polym. 2011, 84, 276–281. [Google Scholar] [CrossRef]
- CP Kelco Ingredients & Products. Available online: https://www.cpkelco.com/products/ (accessed on 29 August 2024).
- Borschiver, S.; Vasconcelos, R.C.; Silva, F.C.; Freitas, G.C.; Santos, P.E.; do Bomfim, R.O. Technology Roadmap for Hyaluronic Acid and Its Derivatives Market. Biofuels Bioprod. Biorefining 2019, 13, 435–444. [Google Scholar] [CrossRef]
- PULLULAN|Research and Development Stories|Research and Development|Nagase Viita Co., Ltd. Available online: https://group.nagase.com/viita/en/rd/story/03/ (accessed on 29 August 2024).
- Emre Oz, Y.; Keskin-Erdogan, Z.; Safa, N.; Esin Hames Tuna, E. A Review of Functionalised Bacterial Cellulose for Targeted Biomedical Fields. J. Biomater. Appl. 2021, 36, 648–681. [Google Scholar] [CrossRef] [PubMed]
- Meito Sangyo | Dextrans and Dextran Derivatives. Available online: https://www.meito-sangyo.co.jp/kaseihin/index_e/dextran/ (accessed on 29 August 2024).
- Dahiya, D.; Nigam, P.S. Dextran of Diverse Molecular-Configurations Used as a Blood-Plasma Substitute, Drug-Delivery Vehicle and Food Additive Biosynthesized by Leuconostoc, Lactobacillus and Weissella. Appl. Sci. 2023, 13, 12526. [Google Scholar] [CrossRef]
- Singhvi, G.; Hans, N.; Shiva, N.; Kumar Dubey, S. Chapter 5—Xanthan gum in drug delivery applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 121–144. ISBN 978-0-12-817055-7. [Google Scholar]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Urtuvia, V.; Maturana, N.; Acevedo, F.; Peña, C.; Díaz-Barrera, A. Bacterial Alginate Production: An Overview of Its Biosynthesis and Potential Industrial Production. World J. Microbiol. Biotechnol. 2017, 33, 198. [Google Scholar] [CrossRef]
- Feketshane, Z.; Alven, S.; Aderibigbe, B.A. Gellan Gum in Wound Dressing Scaffolds. Polymers 2022, 14, 4098. [Google Scholar] [CrossRef]
- Muthukumar, T.; Song, J.E.; Khang, G. Biological Role of Gellan Gum in Improving Scaffold Drug Delivery, Cell Adhesion Properties for Tissue Engineering Applications. Mol. Basel Switz. 2019, 24, 4514. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Kaur, N.; Singh, D.; Purewal, S.S.; Kennedy, J.F. Pullulan in Pharmaceutical and Cosmeceutical Formulations: A Review. Int. J. Biol. Macromol. 2023, 231, 123353. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qi, X.; Zhang, Z. Intra-Articular Oxygen-Ozone versus Hyaluronic Acid in Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Int. J. Surg. 2018, 58, 3–10. [Google Scholar] [CrossRef]
- Gershon, R.K.; Kondo, K. Infectious Immunological Tolerance. Immunology 1971, 21, 903. [Google Scholar] [PubMed]
- Gershon, R.K.; Kondo, K. Cell Interactions in the Induction of Tolerance: The Role of Thymic Lymphocytes. Immunology 1970, 18, 723. [Google Scholar] [PubMed]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic Self-Tolerance Maintained by Activated T Cells Expressing IL-2 Receptor Alpha-Chains (CD25). Breakdown of a Single Mechanism of Self-Tolerance Causes Various Autoimmune Diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [CrossRef]
- Kanmani, P.; Albarracin, L.; Kobayashi, H.; Iida, H.; Komatsu, R.; Humayun Kober, A.K.M.; Ikeda-Ohtsubo, W.; Suda, Y.; Aso, H.; Makino, S.; et al. Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 Modulate Innate Antiviral Immune Response in Porcine Intestinal Epithelial Cells. Mol. Immunol. 2018, 93, 253–265. [Google Scholar] [CrossRef]
- Makino, S.; Sato, A.; Goto, A.; Nakamura, M.; Ogawa, M.; Chiba, Y.; Hemmi, J.; Kano, H.; Takeda, K.; Okumura, K.; et al. Enhanced Natural Killer Cell Activation by Exopolysaccharides Derived from Yogurt Fermented with Lactobacillus Delbrueckii ssp. Bulgaricus OLL1073R-1. J. Dairy Sci. 2016, 99, 915–923. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Fang, X.; Min, W.; Yang, Z. Characterization and Immunomodulatory Activity of an Exopolysaccharide Produced by Lactobacillus Plantarum JLK0142 Isolated from Fermented Dairy Tofu. Int. J. Biol. Macromol. 2018, 115, 985–993. [Google Scholar] [CrossRef]
- Hougaard, A.B.; Pindstrup, H.; Arneborg, N.; Andersen, M.L.; Skibsted, L.H. Free Radical Formation by Lactobacillus Acidophilus NCFM Is Enhanced by Antioxidants and Decreased by Catalase. Food Res. Int. 2016, 79, 81–87. [Google Scholar] [CrossRef]
- Hsieh, F.-C.; Lan, C.-C.E.; Huang, T.-Y.; Chen, K.-W.; Chai, C.-Y.; Chen, W.-T.; Fang, A.-H.; Chen, Y.-H.; Wu, C.-S. Heat-Killed and Live Lactobacillus Reuteri GMNL-263 Exhibit Similar Effects on Improving Metabolic Functions in High-Fat Diet-Induced Obese Rats. Food Funct. 2016, 7, 2374–2388. [Google Scholar]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory effects of probiotics on cytokine profiles. BioMed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef]
- Wang, A.N.; Cai, C.J.; Zeng, X.F.; Zhang, F.R.; Zhang, G.L.; Thacker, P.A.; Wang, J.J.; Qiao, S.Y. Dietary Supplementation with L Actobacillus Fermentum I5007 Improves the Anti-oxidative Activity of Weanling Piglets Challenged with Diquat. J. Appl. Microbiol. 2013, 114, 1582–1591. [Google Scholar]
- Lu, W.; Chen, S.; Lai, C.; Guo, W.; Fu, L.; Andrieu, J.-M. Induction of CD8+ Regulatory T Cells Protects Macaques against SIV Challenge. Cell Rep. 2012, 2, 1736–1746. [Google Scholar] [PubMed]
- Xiu, L.; Zhang, H.; Hu, Z.; Liang, Y.; Guo, S.; Yang, M.; Du, R.; Wang, X. Immunostimulatory Activity of Exopolysaccharides from Probiotic Lactobacillus Casei WXD030 Strain as a Novel Adjuvant in Vitro and in Vivo. Food Agric. Immunol. 2018, 29, 1086–1105. [Google Scholar] [CrossRef]
- Hongying, F.; Xianbo, W.; Fang, Y.; Yang, B.; Beiguo, L. Oral Immunization with Recombinant Lactobacillus Acidophilus Expressing the Adhesin Hp0410 of Helicobacter Pylori Induces Mucosal and Systemic Immune Responses. Clin. Vaccine Immunol. 2014, 21, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, A.; Liu, X.; Zhang, W.; Dimitrov, A.S.; Jia, L.; Lee, P.P.; Fouts, T.R.; Parks, T.P.; Lagenaur, L.A. Expression of Human Immunodeficiency Virus Type 1 Neutralizing Antibody Fragments Using Human Vaginal Lactobacillus. AIDS Res. Hum. Retroviruses 2016, 32, 964–971. [Google Scholar] [CrossRef]
- Suebwongsa, N.; Lulitanond, V.; Mayo, B.; Yotpanya, P.; Panya, M. Development of an Escherichia coli–Lactobacillus casei Shuttle Vector for Heterologous Protein Expression in Lactobacillus casei. Springerplus 2016, 5, 169. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.S.; Syed Hassan, S.; Yap, W.B. Expression of Surface-bound Nonstructural 1 (NS 1) Protein of Influenza Virus A H5N1 on Lactobacillus casei Strain C1. Lett. Appl. Microbiol. 2017, 64, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, X.; Wu, T.; Fang, L.; Liu, C.; Min, W. In Vitro Immunomodulatory Effects of Acidic Exopolysaccharide Produced by Lactobacillus planetarium JLAU103 on RAW264.7 Macrophages. Int. J. Biol. Macromol. 2020, 156, 1308–1315. [Google Scholar] [CrossRef]
- Yu, M.; Qi, R.; Chen, C.; Yin, J.; Ma, S.; Shi, W.; Wu, Y.; Ge, J.; Jiang, Y.; Tang, L. Immunogenicity of Recombinant Lactobacillus casei-expressing F4 (K88) Fimbrial Adhesin FaeG in Conjunction with a Heat-labile Enterotoxin A (LTAK 63) and Heat-labile Enterotoxin B (LTB) of Enterotoxigenic Escherichia Coli as an Oral Adjuvant in Mice. J. Appl. Microbiol. 2017, 122, 506–515. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Iqbal, H.M.N. Microbial Exopolysaccharide-Based Nano-Carriers with Unique Multi-Functionalities for Biomedical Sectors. Biologia 2021, 76, 673–685. [Google Scholar] [CrossRef]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-Coated Iron Oxide Nanoparticles: A Versatile Platform for Targeted Molecular Imaging, Molecular Diagnostics, and Therapy. Acc. Chem. Res. 2011, 44, 842–852. [Google Scholar] [CrossRef]
- Topal, M.; Arslan Topal, E.I. Extracellular polymeric substances in textile industry. In Sustainable Approaches in Textiles and Fashion: Fibres, Raw Materials and Product Development; Muthu, S.S., Ed.; Springer Nature: Singapore, 2022; pp. 23–40. ISBN 978-981-19087-8-1. [Google Scholar]
- Altamira-Algarra, B.; Rueda, E.; Lage, A.; San León, D.; Martínez-Blanch, J.F.; Nogales, J.; García, J.; Gonzalez-Flo, E. New Strategy for Bioplastic and Exopolysaccharides Production: Enrichment of Field Microbiomes with Cyanobacteria. New Biotechnol. 2023, 78, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Abou-alfitooh, S.A.M.; El-hoshoudy, A.N. Eco-Friendly Modified Biopolymers for Enhancing Oil Production: A Review. J. Polym. Environ. 2024, 32, 2457–2483. [Google Scholar] [CrossRef]
- Gao, C. Potential of Welan Gum to Enhance Oil Recovery. J. Pet. Explor. Prod. Technol. 2015, 5, 197–200. [Google Scholar] [CrossRef]
- He, S.; Zhang, M.; Chen, B.; Wei, X.; Su, X. Modification of Welan Gum with Poly(2-Oxazoline) to Obtain Thermoviscosifying Polymer for Enhanced Oil Recovery. Int. J. Biol. Macromol. 2024, 263, 130193. [Google Scholar] [CrossRef]
- Sengupta, S.; Dey, S. Microbial Exo-Polysaccharides (EPS): Role in Agriculture and Environment. Agric. Food 2019, 1, 4–8. [Google Scholar]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Banerjee, A.; Sarkar, S.; Govil, T.; González-Faune, P.; Cabrera-Barjas, G.; Bandopadhyay, R.; Salem, D.R.; Sani, R.K. Extremophilic Exopolysaccharides: Biotechnologies and Wastewater Remediation. Front. Microbiol. 2021, 12, 721365. [Google Scholar] [CrossRef]
- Costa, J.; Baratto, M.C.; Spinelli, D.; Leone, G.; Magnani, A.; Pogni, R. A Novel Bio-Adhesive Based on Chitosan-Polydopamine-Xanthan Gum for Glass, Cardboard and Textile Commodities. Polymers 2024, 16, 1806. [Google Scholar] [CrossRef]
- Ahmed, F.; Hutton-Prager, B. Xanthan Gum Modification to Surface and Interfacial Properties between Soil-Based Matrixes and Petroleum Oils to Minimize Soil Pollution. Appl. Res. 2024, 3, e202400096. [Google Scholar] [CrossRef]
- Fu, X.; Qin, F.; Liu, T.; Zhang, X. Enhanced Oil Recovery Performance and Solution Properties of Hydrophobic Associative Xanthan Gum. Energy Fuels 2022, 36, 181–194. [Google Scholar] [CrossRef]
- De Melo Teixeira, L.; da Silva Santos, É.; dos Santos, R.S.; Ramos, A.V.G.; Baldoqui, D.C.; Bruschi, M.L.; Gonçalves, J.E.; Gonçalves, R.A.C.; de Oliveira, A.J.B. Production of Exopolysaccharide from Klebsiella oxytoca: Rheological, Emulsifying, Biotechnological Properties, and Bioremediation Applications. Int. J. Biol. Macromol. 2024, 278, 134400. [Google Scholar] [CrossRef]
- Fortuna, B.; Logar, J.; Sorze, A.; Valentini, F.; Smolar, J. Influence of Xanthan Gum-Based Soil Conditioners on the Geotechnical Properties of Soils. Appl. Sci. 2024, 14, 4044. [Google Scholar] [CrossRef]
- Garmasheva, I.; Tomila, T.; Kharkhota, M.; Oleschenko, L. Exopolysaccharides of Lactic Acid Bacteria as Protective Agents against Bacterial and Viral Plant Pathogens. Int. J. Biol. Macromol. 2024, 276, 133851. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Liu, Q.; Wang, Y.; Long, C. Pullulan-Based Coatings Carrying Biocontrol Yeast Mixed with NaCl to Control Citrus Postharvest Disease Decays. Pestic. Biochem. Physiol. 2024, 205, 106108. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Martinez-Rodriguez, S.; Md Fadilah, N.I.; Looi Qi Hao, D.; Markey, G.; Shukla, P.; Fauzi, M.B.; Panetsos, F. Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues. Polymers 2024, 16, 1280. [Google Scholar] [CrossRef]
- Barros De Medeiros, V.P.; Da Costa, W.K.A.; Da Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as Source of Functional Ingredients in New-Generation Foods: Challenges, Technological Effects, Biological Activity, and Regulatory Issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 4929–4950. [Google Scholar] [CrossRef]
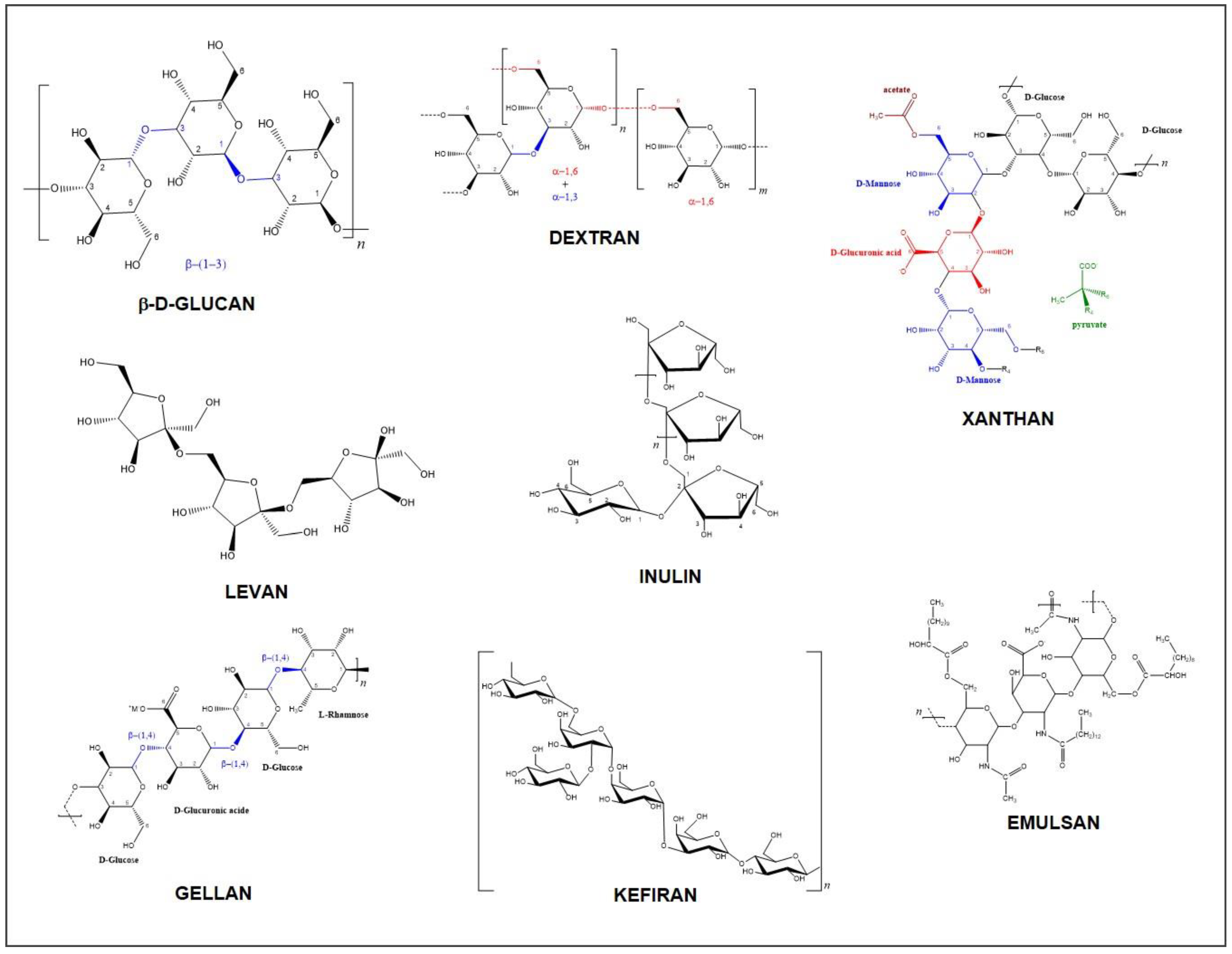

| Monomers | Substituents | Linkage | Branching | Charge | HoPS | HePS | |
|---|---|---|---|---|---|---|---|
| Bacterial EPS | |||||||
| LAB EPS | |||||||
| Glc | β(1→3) | Linear | Neutral | β-D-glucan | |||
| α(1→3) | Linear | Mutan | |||||
| α(1→3) | Linear | Neutran | |||||
| α(1→6); α(1→4) | Branched | Reuteran | |||||
| α(1→6); α(1→3) | Branched | Dextran | |||||
| α(1→6); α(1→3) | Linear | Alternan | |||||
| Fru | β(2→1) | Linear | Fructan | ||||
| Gal | Linear | Polygalactan | |||||
| Fru | β(2→6); β(2→1) | Branched | Levan | ||||
| Glc; Gal | Branched | Kefiran | |||||
| Non-LAB EPS | |||||||
| Glc | Neutral | Curdulan | |||||
| Glc; Man; GlcA | Ace; Pyr | β(1→4) | Branched | Anionic | Xanthan | ||
| GulA; ManA | Ace | Branched | Alginate | ||||
| Glc; Rha; GlcA | Ace; Gly | Linear | Anionic | Gellan | |||
| GlcN | β(1→4); β(1→3) | Linear | Anionic | Hyaluronic acid | |||
| Man | Neutral | Xylinan | |||||
| Glc | β(1→4) | Branched | Neutral | Cellulose | |||
| Fungi EPS | |||||||
| Glc | α(1→4); α(1→6) | Linear | Neutral | Pullulan | |||
| Amino sugar | Ace; Gal-N, R-COOH | Anionic | Emulsan | ||||
| Microorganism | Polymer | Sugar Monomers | Non-Sugar Residues | Mw (Da) | Reference |
|---|---|---|---|---|---|
| BACTERIA | |||||
| Agrobacterium sp. | Curdlan | Glc | --- | 5.3 × 104–2.0 × 106 | [25] |
| Azotobacter vinelandii; Pseudomonas aeruginosa | Alginate | GulA, ManA | Ace | (0.3–1.3) × 106 | [26,27,28] |
| Bacillus subtilis; Halomonas sp.; Zymomonas sp. | Levan | Fru | --- | 2 × 106 | [29,30] |
| Enterobacter A47 | FucoPol | Fuc, Gal, Glc, GlcA | Ace, Pyr, Succ | (1.7–5.8) × 106 | [31] |
| Klebsiella pneumoniae | Fucogel | GalA, Fuc, Gal | Ace | 4 × 104 | [29] |
| Acetobacter sp.; Glucanoacetobacter sp.; Rhizobium sp.; Sarcina sp. | Bacterial cellulose | Glc | --- | ~106 | [29,32,33] |
| Lactobacillus sp.; Leuconostoc sp.; Streptococcus sp. | Dextran | Glc | --- | 103–107 | [34] |
| Pseudomonas oleovorans | GalactoPol | Gal, Glc, Man, Rha | Ace, Pyr, Succ | (1.0–5.0) × 106 | [35,36] |
| Sphingomonas paucimobilis | Gellan | Glc, Rha, GlcA | Gly, Ace | 5.2 × 105 | [37] |
| Streptococcus zooepidemicus | Hyaluronic acid | GlcNAc, GlcA | --- | (2.0–3.0) × 103 | [38] |
| Xanthomonas sp. | Xanthan | Glc, Man, GlcA | Pyr, Ace | 2.0 × 106–5.0 × 107 | [27] |
| FUNGI | |||||
| Tuber borchii | --- | Glc | --- | 92 × 103 | [39] |
| Colletotrichum alatae LCS1 | --- | Man, Gal, Rha, Ara, Glc, Fuc | --- | --- | [40] |
| Aureobasidium pullulans | Pullulan | Glc | --- | 4.8 × 104–2.2 × 106 | [2,41] |
| Penicillium janthinellumN29 | --- | Gal, Man | --- | 10.24 × 103 | [42] |
| Monascus purpureus | --- | Fuc, Gal, Glc, Man, GalA, GlcA | --- | 3.2 × 105 | [43] |
| Penicillium citrinum | --- | Ara, Gal, Glc, Man, GalA, GlcA | --- | 1.58 × 105 | |
| Aspergillus versicolor | --- | Ara, Gal, Glc, Man, Xyl, GalA, GlcA | --- | 1.14 × 105 | |
| Fusarium merismoides A6 | --- | Man, Glc, Gal, Rib | --- | (5.14–6.50) × 104 | [44] |
| Ganoderma lucidum | --- | Gal, Man, Glc, Ara, Rha | --- | 2.08 × 104 | [45] |
| Sclerotium sp. | Scleroglucan | Glc | --- | 1.3 × 105–6.0 × 106 | [46] |
| Schizophyllum commune 227E.32 | Schizophyllan | Glc | --- | 1.1 × 106 | [47] |
| MICROALGAE | |||||
| Anabaena augstmalis | --- | Glc, Gal, Man, Xyl, Fuc, Rha, Gal-N, GlcN, GalA, GlcA | Sulf | n.a. | [48] |
| Dunaliella tertiolecta | --- | Glc | --- | n.a. | [49] |
| Scenedesmus acuminatus | --- | Gal, GlcN, Man | --- | High (>50 × 103) and low molecular weight (<3 × 103) | [50] |
| Phormidium autumnale | --- | Rha, Rib, Man, Glc, Fuc, Gal, Ara, GalA, GlcA | Sulf | n.a. | [48,51] |
| Porphyridium sordidum | --- | Fuc, Rha, Ara, Gal, Glc, Xyl, GlcA | Sulf | 14 × 105 | [52] |
| Rhodella sp. | --- | Xyl, Gal, Glc, Rha, Ara, GlcA | Sulf | n.a. | [53] |
| Synechocystis aquatilis | --- | Fuc, Glc, Rha, Xyl, Man, GlcN, GalA, GlcA | Sulf | n.a. | [48] |
| CYANOBACTERIA | |||||
| Spirulina platensis | --- | Fru, Rha, Rib, Man, Gal, GalA, Glc, Xyl | Sulf, Ca | [54] | |
| Nostoc sp. | --- | Ara, Glc, Man, Xyl, GlcA | lactyl | 214 × 103 | [55] |
| Anabaena sp. CCC 745 | --- | Glc, Rha, GlcA | 19.57 × 103 30.29 × 103 | [56] | |
| Nostoc cf. linckia | --- | Glc, Gal, Xyl, Man, GlcA | lactyl | 1.31 × 105 | [57] |
| Gloeocapsa gelatinosa | --- | Glc, Gal, Ara, Fuc, Xyl, Rha, Man, GlcA, GalA | 67.2 × 103 598.3 × 103 | [58] | |
| Recovery/Purification | Functions | Reference |
|---|---|---|
| Heating—Sonication | Recovery of CPS | [97] |
| Precipitation | ||
| Dialysis | Removing simple carbohydrates | |
| Ion-exchange chromatography | Final purification before quantification | |
| Size Exclusion Chromatography (SEC) | ||
| Preparative Sodium Dodecyl Sulfate—Polyacrylamide Gel (SDS-PAGE) | ||
| Qualitative analysis | ||
| Ultraviolet (UV) spectroscopy | Detection of nucleic acids and proteins | [7] |
| Fourier Transform—Infrared (FT-IR) spectroscopy | Detection of functional group; configuration α or β; fingerprints | [5] |
| Gel permeation chromatography or SEC-Multi-Angle Light Scattering (MALS) | Molecular mass detection | |
| Gas chromatography coupled to mass spectrometry | Monosaccharide composition | |
| High performance Anion exchange chromatography (HPAEC) | Linkage and composition | [5] |
| Near Magnetic Resonance (NMR) spectroscopy | linkage pattern | |
| Confocal laser scanning microscopy | Microstructure analysis | [97] |
| Scanning electron microscopy | ||
| Transmission electron microscopy | ||
| Atomic force microscopy | [7] | |
| Differential scanning calorimetry (DSC) | Structural analysis | |
| Thermogravimetric analysis (TGA) | [5] | |
| X-Ray diffraction (XRD) | ||
| Laser light scattering/electrophoretic analysis | Physico-chemical properties | [98] |
| Quantitative analysis | ||
| Gravimetrics | [97] | |
| Colorimetrics | ||
| SEC-Refractive Index (RI) | ||
| Near Infrared (NIR) spectroscopy |
| Biological Properties and Health Benefits | EPS | Source | Reference |
|---|---|---|---|
| Anticancer activity and Anticancer adjuvant | |||
| Antitumor activity by the activation of defender cells against cancer cells | β-glucans-based EPS | Aureobasidium pullulans | [134] |
| Anticancer activity against human colon, liver, embryonic kidney, breast cancer cell lines | Levans | Lactobacili, Bifidobacteria | [135] |
| Antiproliferative effect | Lactobacillus pantheris TCP102 | [136] | |
| Antitumor activity on HepG-2, BGC-823, HT-29 cancerous cells | L. plantarum 70810 | [137] | |
| Induced cytotoxicity in colon cancer cell lines | Limosilactobacillus fermentum YL-11 | [138] | |
| Apoptotic, antiangiogenic effects, and autophagy | Bacillus sonorensis, Rhodococcus pyridinivorans ZZ47 | [139,140] | |
| Immunomodulatory activity | |||
| Immunomodulation effects through interaction with macrophage receptors | β-glucans-based EPS | [141] | |
| Immunomodulation by human macrophage activation (cytokine production) | β-glucans | P. parvulus | [142] |
| Modulate the immune system (innate and adaptive response) | LAB | [143] | |
| Suppressors of the immune response | Lactobacillus confusus TISTR 1498 | [144,145] | |
| Stimulation of antigen presenting cells (e.g., dendritic cells) | Limosilactobacillus reuteri L26, L. reuteri DSM17938 | [146] | |
| Stimulate production of cytokines by macrophages | Nostoc sp. | [55] | |
| Maintaining the immune balance in states of inflammation and/or infection | Lactobacilli | [147] | |
| Improving allergic responses and suppressing allergen specific IgE synthesis | Leuconostoc citreum L3C1E7 | [148] | |
| Suppression the pro-inflammation and promotion of regulatory cytokine | S. thermophilus, Bacillus licheniformis, Leu.mesenteroides | [149] | |
| Activation of T lymphocytes and monocytes | S. thermophilus, Bifidobacterium breve | [150,151] | |
| Evasion of potentially damaging immune responses | Bifidobacterium breve | [151] | |
| Restoration of the mucosal barrier | Lactobacillus helveticus KLDS1. 8701 | [152] | |
| Immunostimulator | Levan (β-2, 6-fructan) | B. subtilis natto | [153,154] |
| Antiviral effects | |||
| Antiviral activity on avian influenza and adenovirus | Levans | B. subtilis (honey) | [155] |
| Anti-AIDS | Curdlan | Agrobacterium sp. | [156] |
| Antiviral against human hepatitis B | Curdulan sulfate | [157] | |
| Effects against enveloped viruses | Dextran sulfate | Leu. mesenteroides B512F | [158] |
| Antiviral and antibacterial activities | β-glucans-based EPS | Fungi | [159] |
| Cholesterol lowering and anti-hypertensive properties | |||
| Hypoglycemic and hypolipidemic activities | Cordyceps militaris. | [160] | |
| Lowering blood cholesterol | Lactiplantibacillus paraplantarum NCCP 962 | [161] | |
| Modulation of lipid metabolism | Lactobacilli | [162] | |
| Cholesterol-lowering properties and inhibit α-amylase | L. plantarum RJF4 | [137] | |
| Antihypertensive effects | S. thermophilus and Lactobacillus bulgaricus | [163] | |
| Anti-diabetes type 2 and hypocholesterolemia | |||
| Anti-diabetes | Pseudomonas sp. strain AHG22 | [164] | |
| Antibiofilm agents | |||
| Ability to repress biofilm | Lactobacillus helveticus MB2-1 | [165] | |
| Limiting the biofilm formation on medical devices | L. fermentum, Leu. citreum, Leu.Mesenteroides, Leu. Pseudomesenteroides, Ped. pentosaceus | [166] | |
| Antiadhesive and antibiofilm activities against oral S. Aureus strains | Lactobacilli | [167] | |
| Antibiofilm activity | L. plantarum-12 | [168] | |
| Anti-ulcer effects | |||
| Gastro-protective effect | L. plantarum E1K2R2 | [169] | |
| Inhibition of the adhesion of H. pylori | Lacticaseibacillus paracasei | [170] | |
| Antioxidant activities | |||
| Antioxidant and antiproliferative activities against human gastric | Levan | Pantoea agglomerans ZMR7 | [171] |
| Removal of free radicals scavenging activities | Halomonas elongata | [172] | |
| Scavenging of reactive oxygen species (ROS) and reduction in lipid peroxidation | Bacillus velezensis SN-1 | [173] | |
| Inhibition of H2O2 induced apoptosis | L. plantarum C88 | [137] | |
| Ferrous ion chelation | Gloeocapsa gelatinosa | [58] | |
| Prebiotic activities | |||
| Prebiotic effect | HePS | L. paracasei | [174] |
| Bifidogenic effect | Dextran | Leu. mesenteroides | [175] |
| Pathogen Antagonism | |||
| Prevent binding of pathogenic bacteria to mucus | HePS | Probiotic LAB | [176] |
| Reduce the adherence of pathogen to Caco-2 cells surfaces | L. casei NA-2 | [177] | |
| Inhibit the biofilm formation of a number of pathogens | B. licheniformis, Leu. mesenteroides | [175,178,179] | |
| Modulate microbial biofilms | Man-Glu | Lactobacillus | [180] |
| Enhancing intestinal barrier function | Man-Glu-Rib | L. plantarum | [181] |
| Anti-obesity effects | |||
| Improved human gut microbiota | Glucan | Weissella cibaria | [182] |
| Anti-skin irritation | |||
| Anti-inflammation | Dextran | Leuconostocaceae | [183] |
| Anti-Alzheimer’s disease | |||
| Treating Alzheimer’s illness to obviate side effects of synthetic drugs | ManA-Glc-Man-Rha | Streptomyces | [184] |
| Neuroprotective agent against amyloid beta1–42-induced apoptosis in SH-SY5Y cells | Man-Glc-Fru-Ace-Glc-N | Lactobacillus delbrueckii ssp. bulgaricus B3 | [185] |
| EPS | Activities | Producers | Reference |
|---|---|---|---|
| α-D-glucan | Viscosifier | Levilactobacillus brevis HDE-9 | [192] |
| Water holding capacity | Enterococcus hirae OL616073 | [193] | |
| Levan | Enhancer of Bifidobacteria growth | Lactobacillus sanfranciscensis | [194] |
| Dextran | Enhancer of probiotic growth | W. cibaria | [195] |
| Promote colonization of strains | Leuconostoc lactis | [196] | |
| Kefiran | Enhancer of Bifidobacteria growth | Lactobacillus kefiranofaciens | [197] |
| Emulsifying and flocculating activities | L. kefiranofaciens | [197] | |
| Galactan | Emulsifying capacity and stability; flocculating activity at a wide range of pH | Weissella confusa | [198,199] |
| Anionic EPS | Heavy metal absorption activity (binding agents, bioabsorbents) | Lactobacilli | [200] |
| [Man:Glc]-EPS | Antibiofilm against pathogens | L. fermentum LB-69 | [201] |
| [Gal:Glc:Man]-EPS | Probiofilm | L. plantarum KF5 | [202] |
| [Glc:Man 1:7.01] [Man:Glc:Rha 7.45: 1.00: 2.34] | a protector against oxidative stresses or reducing power or agent against free radicals | L. lactis subsp. lactis IMAU11823 | [203] |
| [Glu:Rib:Man:Xyl 1.0:16.4:6.6:6.5] [Rib:Man:Xyl:GA:Ara 7.1:1.6:4.8:1.0:9.0] | Inhibitor of lipid peroxidation | Lactobacillus sp. | [204] |
| [Uronic acid]-EPS | Protector of cell membrane against lipid peroxidation | L. plantarum LP6 | [137] |
| [Gal-Glc-Man]-EPS | Inhibition of H2O2 | Bacillus sp. S-1 | [205] |
| [Se]-EPS | Antioxidant activity | L. lactis | [206] |
| [Glc-Man-Gal-Fuc-Glc-NH2]-EPS | Antioxidant effects | B. coagulans RK-02 | [207] |
| Gellan | Gelling (flexible elastic gel) and emulsifying activities | Sphingomonas elodea | [208] |
| Pullulan | Coating protector effect against mold | Aureobasidium pullulans | [209] |
| EPS | Microorganism Producer | Manufacturer | Brand Name | Reference |
|---|---|---|---|---|
| Xanthan | Xanthomonas campestris | CPKelco, San Diego, CA, USA | Keldent | [214] |
| Xantural | ||||
| Gellan | Pseudomonas elodea | CPKelco, San Diego, CA, USA | Gelzan | |
| Kelcogel | ||||
| Hyaluronic acid | Streptococcus zooepidemicus Equi | Contipro Biotech, Dolni Dobrouc, Czech Republic | Sodium hyaluronate | [215] |
| Pullulan | Aureabasidium pullulans | Nagase Viita (Hayashibara Co., Ltd., Okayama, Japan) | Pullulan | [216] |
| Cellulose | Gluconacetobacter xylinum | fzmb GmbH, Research Centre of Medical Technology and Biotechnology | Nanomasque | [217] |
| Axcelon Demacare Inc, North York, ON, Canada | Nanoderm | |||
| Dextran | Leu. mesenteroides | Meito Sangyo, Nagoya, Japan | Dextran sulfate Na | [218] |
| Medical and Pharmaceutical Applications | EPS | Source | Reference |
|---|---|---|---|
| Blood plasma volume expander, blood plasma substitute | Dextran | Leu. mesenteroides | [219] |
| Excipients, thickener and suspension stabilizer, drug-controlled release carrier | Xanthan | Xanthomonas campestris | [220] |
| Tablets disintegrant, thickener, emulsion stabilizing agents | Alginate | Pseudomonas aeruginosa Azotobacter vinelandii | [221,222] |
| Antiacid protector in capsules | Na-alginate | ||
| Disintegrating agent, drug-controlled release, gelling agent, wound dressing | Gellan | Sphingomonas elodea Sphingomonas paucimobilis | [223,224] |
| Binding and film-forming properties, coating agent for oxygen impermeability | Pullulan | Aurebasidium pullulans | [225] |
| Vitreous substitution during eye surgery, intraarticular injections in osteoarthritis | Hyaluronic acid | Streptococcus equi | [226,227] |
| Mechanism | EPS Producing LAB | References |
|---|---|---|
| Interaction with dendritic cells and macrophages | Lactobacillus bulgaricus | [232] |
| Enhancing the proliferation of lymphocytes | ||
| Induction of nitric oxide secretion in vitro | L. plantarum JLK0142 | [233] |
| Enhancing the phagocytic potential of macrophages | ||
| Increasing IgA concentrations in the intestinal mucosa | ||
| Stimulating lymphocyte proliferation |
| Uses | EPS | Properties | Reference |
|---|---|---|---|
| Textiles | Xanthan | Improve adhesive properties of textile | [256] |
| Oil Recovery | Xanthan | Reduce oil spreading | [257] |
| Modified xanthan | Enhance oil recovery | [258] | |
| Bioremediation | KO-EPS | Chelate iron | [259] |
| Agricultural | Xanthan | Improve water absorption and water retention capacity | [260] |
| EPS | Antifungal activity | [261] | |
| Packaging | Pullulan | Antifungal activity | [262] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.-T.; Pham, T.-T.; Nguyen, P.-T.; Le-Buanec, H.; Rabetafika, H.N.; Razafindralambo, H.L. Advances in Microbial Exopolysaccharides: Present and Future Applications. Biomolecules 2024, 14, 1162. https://doi.org/10.3390/biom14091162
Nguyen H-T, Pham T-T, Nguyen P-T, Le-Buanec H, Rabetafika HN, Razafindralambo HL. Advances in Microbial Exopolysaccharides: Present and Future Applications. Biomolecules. 2024; 14(9):1162. https://doi.org/10.3390/biom14091162
Chicago/Turabian StyleNguyen, Huu-Thanh, Thuy-Trang Pham, Phu-Tho Nguyen, Hélène Le-Buanec, Holy N. Rabetafika, and Hary L. Razafindralambo. 2024. "Advances in Microbial Exopolysaccharides: Present and Future Applications" Biomolecules 14, no. 9: 1162. https://doi.org/10.3390/biom14091162
APA StyleNguyen, H.-T., Pham, T.-T., Nguyen, P.-T., Le-Buanec, H., Rabetafika, H. N., & Razafindralambo, H. L. (2024). Advances in Microbial Exopolysaccharides: Present and Future Applications. Biomolecules, 14(9), 1162. https://doi.org/10.3390/biom14091162







