L-Cysteine Upregulates Testosterone Biosynthesis and Blood–Testis Barrier Genes in Cultured Human Leydig Cells and THP-1 Monocytes and Increases Testosterone Secretion in Human Leydig Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture Treatment
2.2. Testosterone Assay
2.3. RNA Extraction and qPCR
2.4. Statistical Analysis
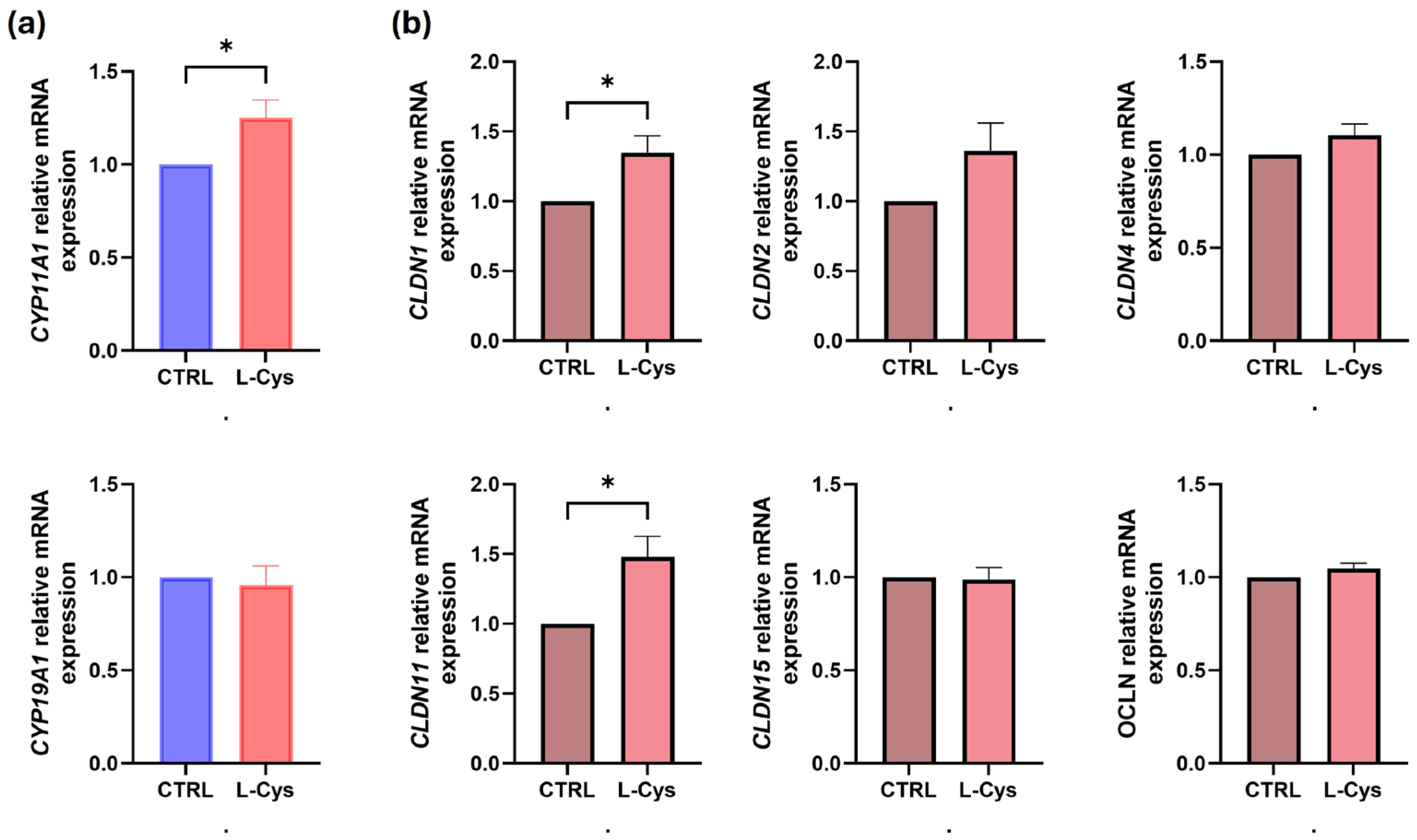
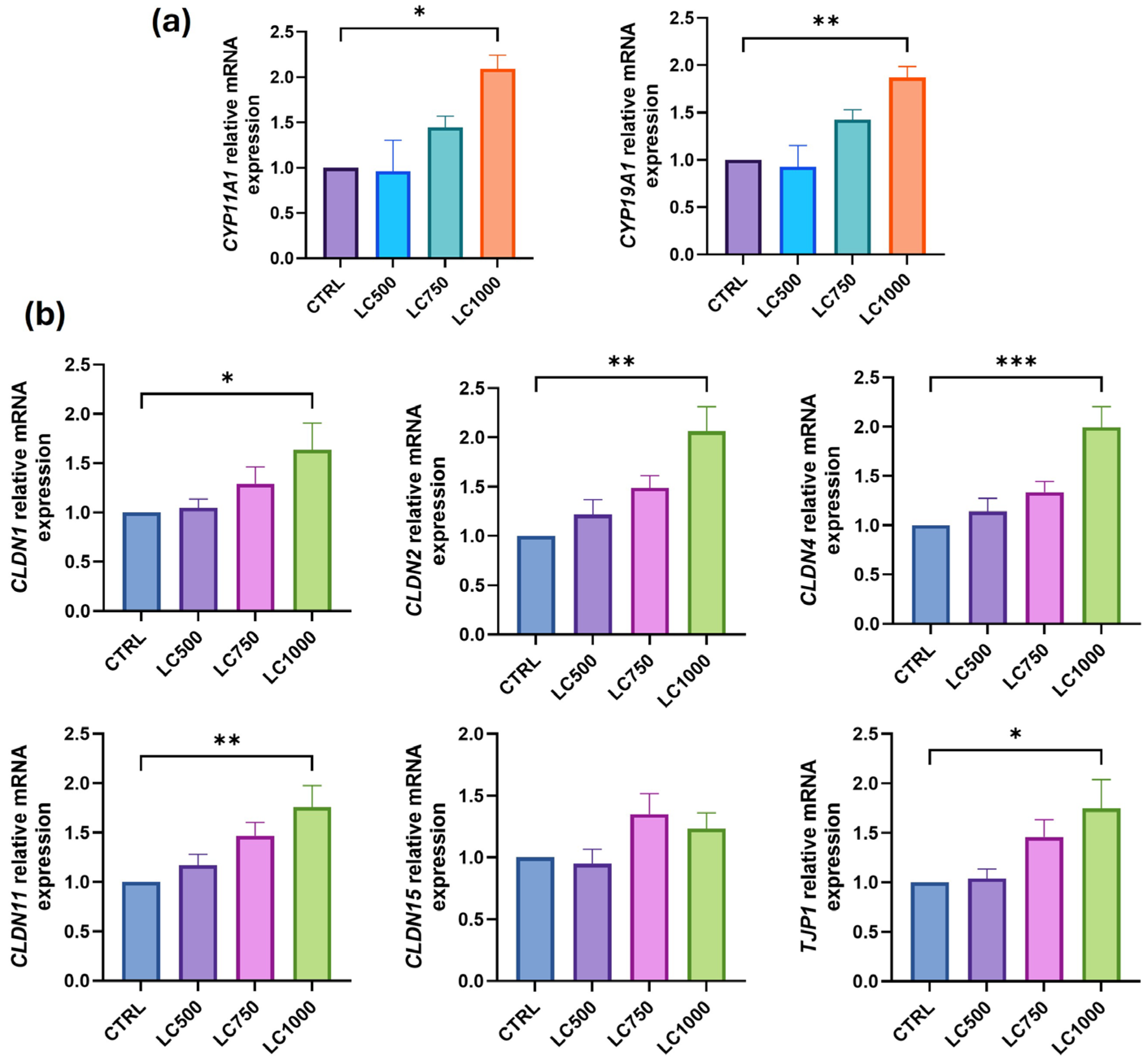
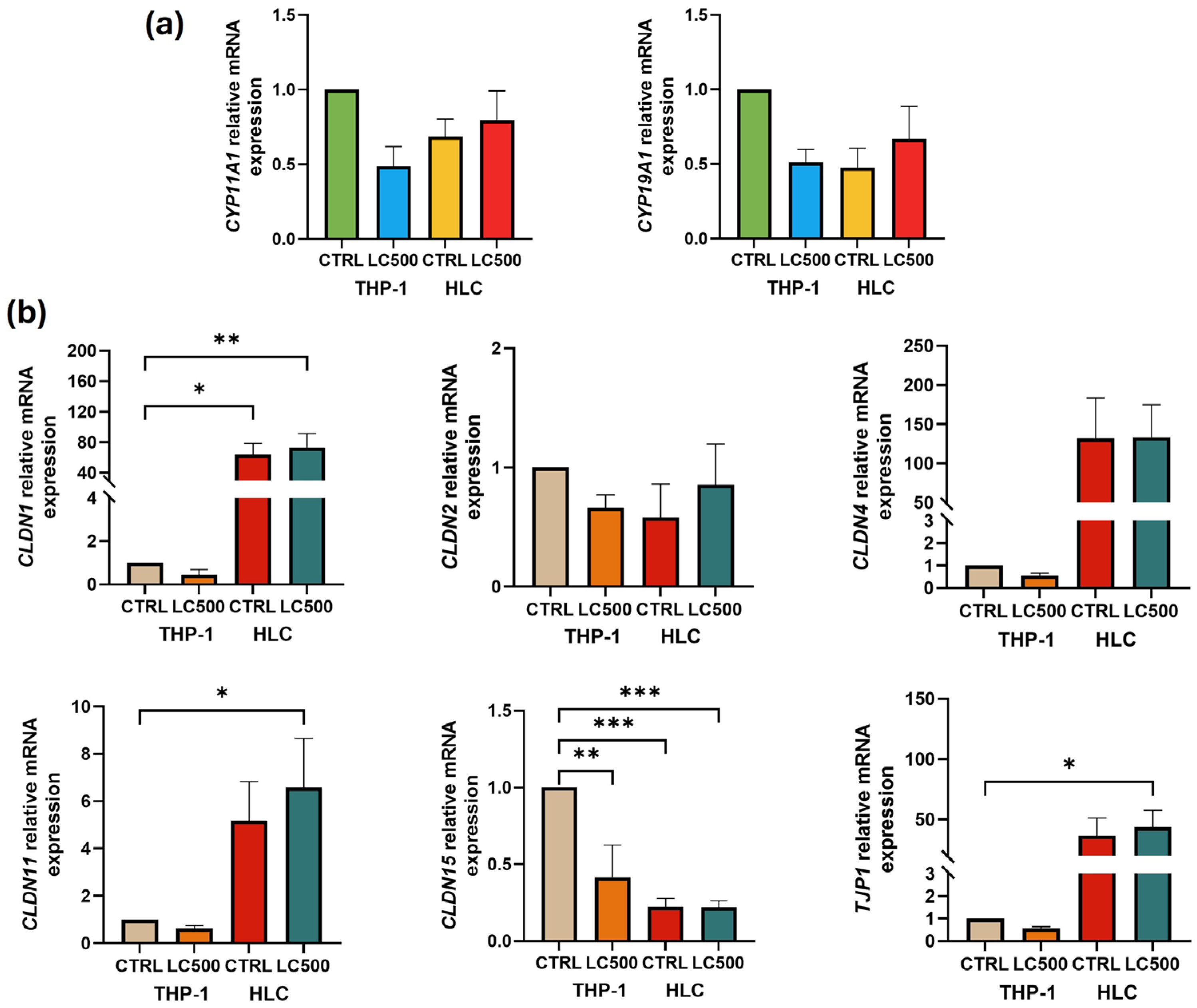
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Agha, O.M.; Axiotis, C.A. An in-depth look at Leydig cell tumor of the testis. Arch. Pathol. Lab. Med. 2007, 131, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kim, H.J.; Lee, C.H.; Choi, H.S.; Lee, K. Leydig Cell-Specific DAX1-Deleted Mice Has Higher Testosterone Level in the Testis during Pubertal Development. Reprod. Sci. 2022, 29, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Aladamat, N.; Tadi, P. Histology, Leydig Cells; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Griswold, S.L.; Behringer, R.R. Fetal Leydig cell origin and development. Sex. Dev. 2009, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, D.Z.; Medar, M.L.J.; Becin, A.P.; Andric, S.A.; Kostic, T.S. Growing up under Constant Light: A Challenge to the Endocrine Function of the Leydig Cells. Front. Endocrinol. 2021, 12, 653602. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Yang, G.; Wang, S.; Guo, M.; Lu, H.; Zhang, T. VDR promotes testosterone synthesis in mouse Leydig cells via regulation of cholesterol side chain cleavage cytochrome P450 (Cyp11a1) expression. Genes. Genom. 2023, 45, 1377–1387. [Google Scholar] [CrossRef]
- Katsumata, N.; Ohtake, M.; Hojo, T.; Ogawa, E.; Hara, T.; Sato, N.; Tanaka, T. Compound heterozygous mutations in the cholesterol side-chain cleavage enzyme gene (CYP11A) cause congenital adrenal insufficiency in humans. J. Clin. Endocrinol. Metab. 2002, 87, 3808–3813. [Google Scholar] [CrossRef]
- Richmond, E.J.; Flickinger, C.J.; McDonald, J.A.; Lovell, M.A.; Rogol, A.D. Lipoid congenital adrenal hyperplasia (CAH): Patient report and a mini-review. Clin. Pediatr. 2001, 40, 403–407. [Google Scholar] [CrossRef]
- Chen, H.; Ge, R.S.; Zirkin, B.R. Leydig cells: From stem cells to aging. Mol. Cell Endocrinol. 2009, 306, 9–16. [Google Scholar] [CrossRef]
- Acikel-Elmas, M.; Algilani, S.A.; Sahin, B.; Bingol Ozakpinar, O.; Gecim, M.; Koroglu, K.; Arbak, S. Apocynin Ameliorates Monosodium Glutamate Induced Testis Damage by Impaired Blood-Testis Barrier and Oxidative Stress Parameters. Life 2023, 13, 822. [Google Scholar] [CrossRef]
- Luaces, J.P.; Toro-Urrego, N.; Otero-Losada, M.; Capani, F. What do we know about blood-testis barrier? current understanding of its structure and physiology. Front. Cell Dev. Biol. 2023, 11, 1114769. [Google Scholar] [CrossRef]
- Bhat, A.A.; Syed, N.; Therachiyil, L.; Nisar, S.; Hashem, S.; Macha, M.A.; Yadav, S.K.; Krishnankutty, R.; Muralitharan, S.; Al-Naemi, H.; et al. Claudin-1, A Double-Edged Sword in Cancer. Int. J. Mol. Sci. 2020, 21, 569. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Billur, D.; Kizil, S.; Ozkavukcu, S.; Topal Celikkan, F.; Aydos, K.; Erdemli, E. Evaluation of blood-testis barrier integrity in terms of adhesion molecules in nonobstructive azoospermia. Andrologia 2020, 52, e13636. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.H.; Bukhari, I.; Zheng, W.; Yin, S.; Wang, Z.; Cooke, H.J.; Shi, Q.H. Blood-testis barrier and spermatogenesis: Lessons from genetically-modified mice. Asian J. Androl. 2014, 16, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pechenino, A.S.; Liu, J.; Beattie, M.C.; Brown, T.R.; Zirkin, B.R. Effect of glutathione depletion on Leydig cell steroidogenesis in young and old brown Norway rats. Endocrinology 2008, 149, 2612–2619. [Google Scholar] [CrossRef]
- Bosgelmez, I.I.; Guvendik, G. Beneficial Effects of N-Acetyl-L-cysteine or Taurine Pre- or Post-treatments in the Heart, Spleen, Lung, and Testis of Hexavalent Chromium-Exposed Mice. Biol. Trace Elem. Res. 2019, 190, 437–445. [Google Scholar] [CrossRef]
- Feng, D.; Huang, H.; Yang, Y.; Yan, T.; Jin, Y.; Cheng, X.; Cui, L. Ameliorative effects of N-acetylcysteine on fluoride-induced oxidative stress and DNA damage in male rats’ testis. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 792, 35–45. [Google Scholar] [CrossRef]
- Kemahli, E.; Uyeturk, U.; Cetinkaya, A.; Erimsah, S.; Uyeturk, U.; Gucuk, A. Protective Effects of N-Acetyl Cysteine on Undescended Testis after Orchiopexy: A Rat-model Study. J. Coll. Physicians Surg. Pak. 2023, 33, 319–324. [Google Scholar] [CrossRef]
- Abedi, B.; Tayefi-Nasrabadi, H.; Kianifard, D.; Basaki, M.; Shahbazfar, A.A.; Piri, A.; Dolatyarieslami, M. The effect of co-administration of artemisinin and N-acetyl cysteine on antioxidant status, spermatological parameters and histopathology of testis in adult male mice. Horm. Mol. Biol. Clin. Investig. 2023, 44, 207–214. [Google Scholar] [CrossRef]
- Acer-Demir, T.; Mammadov, M.; Ocbe, P.; Coruhlu, A.; Coskun, D.; Nazik, Y.; Tufekci, I.; Guney, L.H.; Hicsonmez, A. The long term effects of intrascrotal low dose and high dose N-acetylcysteine on testis damage in rat model of testicular torsion. J. Pediatr. Surg. 2020, 55, 672–680. [Google Scholar] [CrossRef]
- Bodur, A.; Alver, A.; Kahraman, C.; Altay, D.U.; Ince, I. Investigation of N-acetylcysteine on contralateral testis tissue injury by experimental testicular torsion: Long-term effect. Am. J. Emerg. Med. 2016, 34, 1069–1074. [Google Scholar] [CrossRef]
- Jannatifar, R.; Parivar, K.; Roodbari, N.H.; Nasr-Esfahani, M.H. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod. Biol. Endocrinol. 2019, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Monageng, E.; Offor, U.; Takalani, N.B.; Mohlala, K.; Opuwari, C.S. A Review on the Impact of Oxidative Stress and Medicinal Plants on Leydig Cells. Antioxidants 2023, 12, 1559. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. L-cysteine and hydrogen sulfide increase PIP3 and AMPK/PPARgamma expression and decrease ROS and vascular inflammation markers in high glucose treated human U937 monocytes. J. Cell Biochem. 2013, 114, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Kanikarla-Marie, P.; Jain, S.K. L-Cysteine supplementation reduces high-glucose and ketone-induced adhesion of monocytes to endothelial cells by inhibiting ROS. Mol. Cell Biochem. 2014, 391, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Surampudi, P.N.; Wang, C.; Swerdloff, R. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. Int. J. Endocrinol. 2012, 2012, 625434. [Google Scholar] [CrossRef]
- Hwang, K.; Walters, R.C.; Lipshultz, L.I. Contemporary concepts in the evaluation and management of male infertility. Nat. Rev. Urol. 2011, 8, 86–94. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef]
- Bosland, M.C. Testosterone treatment is a potent tumor promoter for the rat prostate. Endocrinology 2014, 155, 4629–4633. [Google Scholar] [CrossRef]
- Diemer, T.; Allen, J.A.; Hales, K.H.; Hales, D.B. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology 2003, 144, 2882–2891. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Su, P.K.; Wang, L.J.; Zheng, H.Q.; Bai, X.F.; Li, P.; Meng, X.P.; Yang, J.Y. T-2 toxin induces apoptosis via the Bax-dependent caspase-3 activation in mouse primary Leydig cells. Toxicol. Mech. Methods 2018, 28, 23–28. [Google Scholar] [CrossRef]
- Luo, L.; Chen, H.; Trush, M.A.; Show, M.D.; Anway, M.D.; Zirkin, B.R. Aging and the brown Norway rat leydig cell antioxidant defense system. J. Androl. 2006, 27, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cangello, D.; Benson, S.; Folmer, J.; Zhu, H.; Trush, M.A.; Zirkin, B.R. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: Relationship to reduced steroidogenic function? Exp. Gerontol. 2001, 36, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Mody, V.C., Jr.; Carlson, J.L.; Lynn, M.J.; Sternberg, P., Jr. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic. Biol. Med. 2002, 33, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Kaur, G. Alterations in oxidative stress scavenger system in aging rat brain and lymphocytes. Biogerontology 2002, 3, 161–173. [Google Scholar] [CrossRef]
- Liu, R.M. Down-regulation of gamma-glutamylcysteine synthetase regulatory subunit gene expression in rat brain tissue during aging. J. Neurosci. Res. 2002, 68, 344–351. [Google Scholar] [CrossRef]
- Larsson, S.C.; Hakansson, N.; Wolk, A. Dietary cysteine and other amino acids and stroke incidence in women. Stroke 2015, 46, 922–926. [Google Scholar] [CrossRef]
- Doosti, A.; Lotfi, Y.; Moossavi, A.; Bakhshi, E.; Talasaz, A.H.; Hoorzad, A. Comparison of the effects of N-acetyl-cysteine and ginseng in prevention of noise induced hearing loss in male textile workers. Noise Health 2014, 16, 223–227. [Google Scholar] [CrossRef]
- Xu, Y.J.; Tappia, P.S.; Neki, N.S.; Dhalla, N.S. Prevention of diabetes-induced cardiovascular complications upon treatment with antioxidants. Heart Fail. Rev. 2014, 19, 113–121. [Google Scholar] [CrossRef]
- Salaspuro, V.; Hietala, J.; Kaihovaara, P.; Pihlajarinne, L.; Marvola, M.; Salaspuro, M. Removal of acetaldehyde from saliva by a slow-release buccal tablet of L-cysteine. Int. J. Cancer 2002, 97, 361–364. [Google Scholar] [CrossRef]
- Ezzat, G.M.; Nassar, A.Y.; Bakr, M.H.; Mohamed, S.; Nassar, G.A.; Kamel, A.A. Acetylated Oligopeptide and N-acetyl cysteine Protected Against Oxidative Stress, Inflammation, Testicular-Blood Barrier Damage, and Testicular Cell Death in Iron-Overload Rat Model. Appl. Biochem. Biotechnol. 2023, 195, 5053–5071. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. l-Cysteine supplementation increases insulin sensitivity mediated by upregulation of GSH and adiponectin in high glucose treated 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2017, 630, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Micinski, D.; Parsanathan, R. l-Cysteine Stimulates the Effect of Vitamin D on Inhibition of Oxidative Stress, IL-8, and MCP-1 Secretion in High Glucose Treated Monocytes. J. Am. Coll. Nutr. 2021, 40, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Burgos, E.S.; Vuguin, P.M.; Manuel, C.R.; Pekson, R.; Munnangi, S.; Reznik, S.E.; Charron, M.J. N-Acetylcysteine Resolves Placental Inflammatory-Vasculopathic Changes in Mice Consuming a High-Fat Diet. Am. J. Pathol. 2019, 189, 2246–2257. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yang, Y.; Wei, S.; Spicer, L.J.; Kenez, A.; Xu, W.; Liu, Y.; Feng, T. Influence of N-acetylcysteine on steroidogenesis and gene expression in porcine placental trophoblast cells. Theriogenology 2021, 161, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, M.J.; Park, N.C.; Park, H.J. Effect of N-acetyl-L-cysteine on Testicular Tissue in Busulfan-Induced Dysfunction in the Male Reproductive System. World J. Mens. Health 2023, 41, 882–891. [Google Scholar] [CrossRef]
- Jana, K.; Dutta, A.; Chakraborty, P.; Manna, I.; Firdaus, S.B.; Bandyopadhyay, D.; Chattopadhyay, R.; Chakravarty, B. Alpha-lipoic acid and N-acetylcysteine protects intensive swimming exercise-mediated germ-cell depletion, pro-oxidant generation, and alteration of steroidogenesis in rat testis. Mol. Reprod. Dev. 2014, 81, 833–850. [Google Scholar] [CrossRef]
- Redouane, S.; Harmak, H.; Elkarhat, Z.; Charoute, H.; Malki, A.; Barakat, A.; Rouba, H. Exploring the impact of CYP11A1’s missense SNPs on the interaction between CYP11A1 and cholesterol: A comprehensive structural analysis and MD simulation study. Comput. Biol. Chem. 2023, 106, 107937. [Google Scholar] [CrossRef]
- Chen, H.; Hardy, M.P.; Huhtaniemi, I.; Zirkin, B.R. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J. Androl. 1994, 15, 551–557. [Google Scholar] [CrossRef]
- Wang, C.; Leung, A.; Sinha-Hikim, A.P. Reproductive aging in the male brown-Norway rat: A model for the human. Endocrinology 1993, 133, 2773–2781. [Google Scholar] [CrossRef]
- Culty, M.; Luo, L.; Yao, Z.X.; Chen, H.; Papadopoulos, V.; Zirkin, B.R. Cholesterol transport, peripheral benzodiazepine receptor, and steroidogenesis in aging Leydig cells. J. Androl. 2002, 23, 439–447. [Google Scholar] [CrossRef]
- Corona, G.; Rastrelli, G.; Di Pasquale, G.; Sforza, A.; Mannucci, E.; Maggi, M. Testosterone and Cardiovascular Risk: Meta-Analysis of Interventional Studies. J. Sex. Med. 2018, 15, 820–838. [Google Scholar] [CrossRef] [PubMed]
- Noyola-Martinez, N.; Halhali, A.; Zaga-Clavellina, V.; Olmos-Ortiz, A.; Larrea, F.; Barrera, D. A time-course regulatory and kinetic expression study of steroid metabolizing enzymes by calcitriol in primary cultured human placental cells. J. Steroid Biochem. Mol. Biol. 2017, 167, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Pandey, V.; Sahu, A.N.; Singh, A.; Dubey, P.K. Di-(2-ethylhexyl) phthalate (DEHP) inhibits steroidogenesis and induces mitochondria-ROS mediated apoptosis in rat ovarian granulosa cells. Toxicol. Res. 2019, 8, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Dankers, A.C.; Roelofs, M.J.; Piersma, A.H.; Sweep, F.C.; Russel, F.G.; van den Berg, M.; van Duursen, M.B.; Masereeuw, R. Endocrine disruptors differentially target ATP-binding cassette transporters in the blood-testis barrier and affect Leydig cell testosterone secretion in vitro. Toxicol. Sci. 2013, 136, 382–391. [Google Scholar] [CrossRef]
- Marettova, E.; Maretta, M.; Legath, J. Toxic effects of cadmium on testis of birds and mammals: A review. Anim. Reprod. Sci. 2015, 155, 1–10. [Google Scholar] [CrossRef]
- Shen, Y.; You, Y.; Zhu, K.; Fang, C.; Yu, X.; Chang, D. Bibliometric and visual analysis of blood-testis barrier research. Front. Pharmacol. 2022, 13, 969257. [Google Scholar] [CrossRef]
- Son, Y.; Heo, K.; Bae, M.J.; Lee, C.G.; Cho, W.S.; Kim, S.D.; Yang, K.; Shin, I.S.; Lee, M.Y.; Kim, J.S. Injury to the blood-testis barrier after low-dose-rate chronic radiation exposure in mice. Radiat. Prot. Dosim. 2015, 167, 316–320. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, M.; Tursi, N.J.; Yan, B.; Cao, X.; Che, Q.; Yang, N.; Dong, X. Uropathogenic Escherichia coli Infection Compromises the Blood-Testis Barrier by Disturbing mTORC1-mTORC2 Balance. Front. Immunol. 2021, 12, 582858. [Google Scholar] [CrossRef]
- Wang, R.; Song, B.; Wu, J.; Zhang, Y.; Chen, A.; Shao, L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018, 13, 8487–8506. [Google Scholar] [CrossRef]
- Paul, C.; Robaire, B. Impaired function of the blood-testis barrier during aging is preceded by a decline in cell adhesion proteins and GTPases. PLoS ONE 2013, 8, e84354. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, T.; Shao, J.; Sheng, C.; Hong, Y.; Ying, W.; Xia, W. Antioxidant protects blood-testis barrier against synchrotron radiation X-ray-induced disruption. Spermatogenesis 2015, 5, e1009313. [Google Scholar] [CrossRef] [PubMed]
- Mruk, D.D.; Cheng, C.Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, T.; Yu, H.; Shen, H.; Xia, W. Adjudin protects against cerebral ischemia reperfusion injury by inhibition of neuroinflammation and blood-brain barrier disruption. J. Neuroinflamm. 2014, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Bruewer, M.; Luegering, A.; Kucharzik, T.; Parkos, C.A.; Madara, J.L.; Hopkins, A.M.; Nusrat, A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 2003, 171, 6164–6172. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Biophys. Acta 2009, 1788, 864–871. [Google Scholar] [CrossRef]
- Liman, N. The abundance and localization of claudin-1 and -5 in the adult tomcats (Felis catus) testis, tubules rectus, rete testis, efferent ductules, and epididymis. Anat. Rec. 2023, 306, 2153–2169. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Wang, N.; Chen, M.; Gao, J.; Ji, X.; He, J.; Zhang, J.; Zhao, W. A series of BODIPY-based probes for the detection of cysteine and homocysteine in living cells. Talanta 2019, 195, 281–289. [Google Scholar] [CrossRef]
- Clemente Plaza, N.; Reig Garcia-Galbis, M.; Martinez-Espinosa, R.M. Effects of the Usage of l-Cysteine (l-Cys) on Human Health. Molecules 2018, 23, 575. [Google Scholar] [CrossRef]


| Gene | Orientation | Primer Sequence (5′–3′) |
|---|---|---|
| CYP11A1 | Forward | CGTCAGATCCATCGGGTTAATG |
| Reverse | CATTCCAACCATCCAGGTATCG | |
| CYP19A1 | Forward | GAGAACCAGGCTACAAGAGAAA |
| Reverse | TGGTGGAATCGGGTCTTTATG | |
| CLDN1 | Forward | CCAGTTAGAAGAGGTAGTGTGAAT |
| Reverse | CAGCCAGCTGAGCAAATAAAG | |
| CLDN2 | Forward | GGTGACATCCAGTGCAATCT |
| Reverse | CTACCGCCACTCTGTCTTTG | |
| CLDN4 | Forward | CAACTGCCTGGAGGATGAAA |
| Reverse | CACCGGCACTATCACCATAAG | |
| CLDN11 | Forward | GTGTGATCTCGGCTCATGTA |
| Reverse | GTAGTAGTGAACGCCTGTAGTC | |
| CLDN15 | Forward | TGACTCTGCCAAACAGCTAC |
| Reverse | GTCGGTGGCACAGCTAAA | |
| TJP1 | Forward | CCTTAGTGTCCAAACCAGACC |
| Reverse | AAAGCTGCCTGAGCAGTATC | |
| OCLN | Forward | CATTGCCATCTTTGCCTGTG |
| Reverse | CCAAAGCCACTTCCTCCATAA | |
| GAPDH | Forward | AGTATGACAACAGCCTCAAGAT |
| Reverse | GTCCTTCCACGATACCAAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Justin Margret, J.; Jain, S.K. L-Cysteine Upregulates Testosterone Biosynthesis and Blood–Testis Barrier Genes in Cultured Human Leydig Cells and THP-1 Monocytes and Increases Testosterone Secretion in Human Leydig Cells. Biomolecules 2024, 14, 1171. https://doi.org/10.3390/biom14091171
Justin Margret J, Jain SK. L-Cysteine Upregulates Testosterone Biosynthesis and Blood–Testis Barrier Genes in Cultured Human Leydig Cells and THP-1 Monocytes and Increases Testosterone Secretion in Human Leydig Cells. Biomolecules. 2024; 14(9):1171. https://doi.org/10.3390/biom14091171
Chicago/Turabian StyleJustin Margret, Jeffrey, and Sushil K. Jain. 2024. "L-Cysteine Upregulates Testosterone Biosynthesis and Blood–Testis Barrier Genes in Cultured Human Leydig Cells and THP-1 Monocytes and Increases Testosterone Secretion in Human Leydig Cells" Biomolecules 14, no. 9: 1171. https://doi.org/10.3390/biom14091171
APA StyleJustin Margret, J., & Jain, S. K. (2024). L-Cysteine Upregulates Testosterone Biosynthesis and Blood–Testis Barrier Genes in Cultured Human Leydig Cells and THP-1 Monocytes and Increases Testosterone Secretion in Human Leydig Cells. Biomolecules, 14(9), 1171. https://doi.org/10.3390/biom14091171






