Molecular Identification and Engineering a Salt-Tolerant GH11 Xylanase for Efficient Xylooligosaccharides Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Reagents
2.2. Xylanase Activity Assay
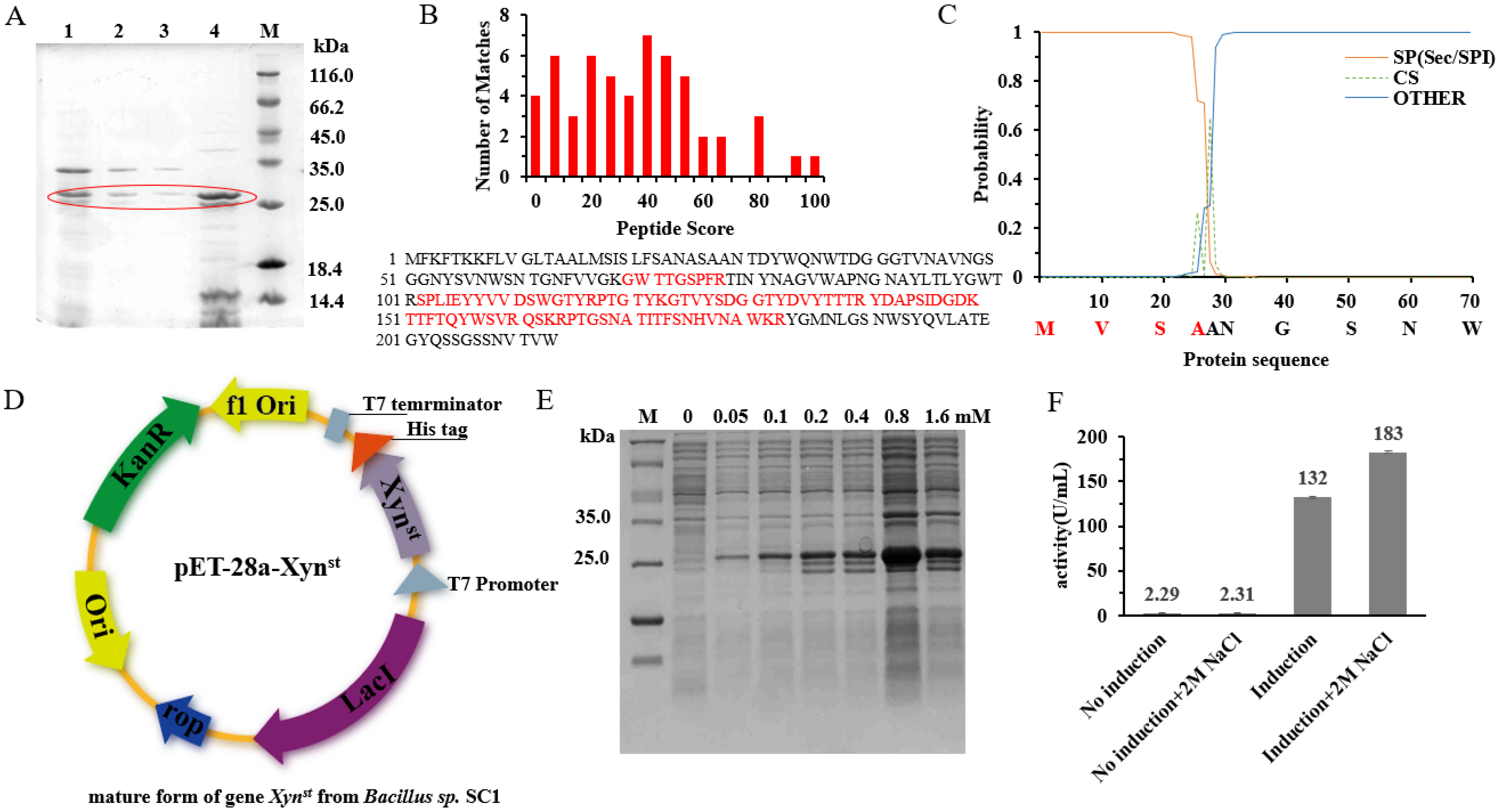
2.3. Isolation and Identification of a Salt-Resistant Xylanase
2.4. Cloning of Xynst and Its Heterologous Overexpression
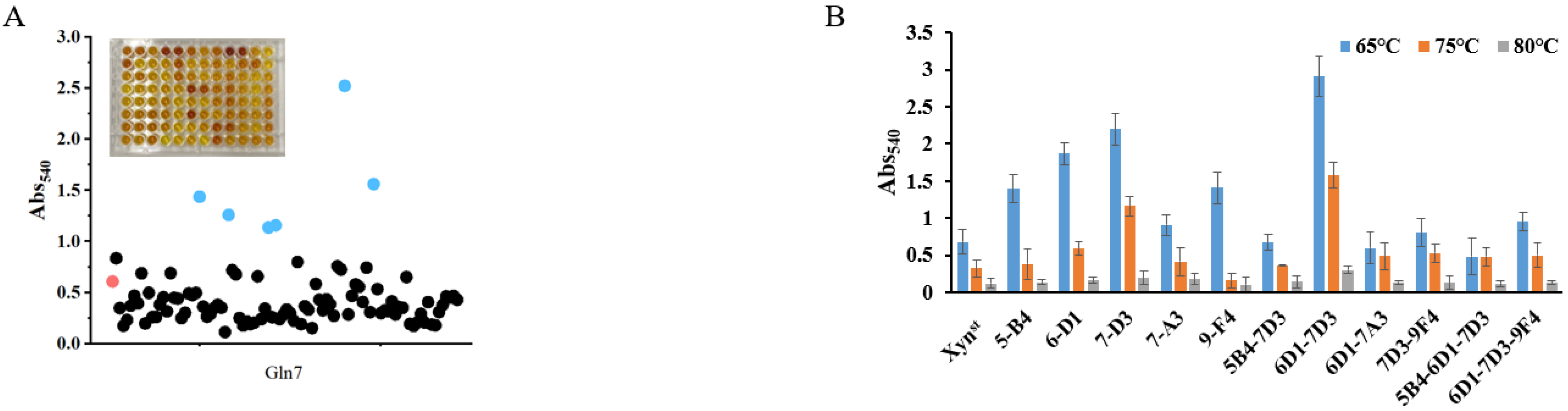
2.5. Rational Designing and Xylanase Mutant Library Construction
2.6. High-Throughput Screen of Mutant Library
2.7. Recombinant Xylanases Purification, Quantification, and Electrophoresis
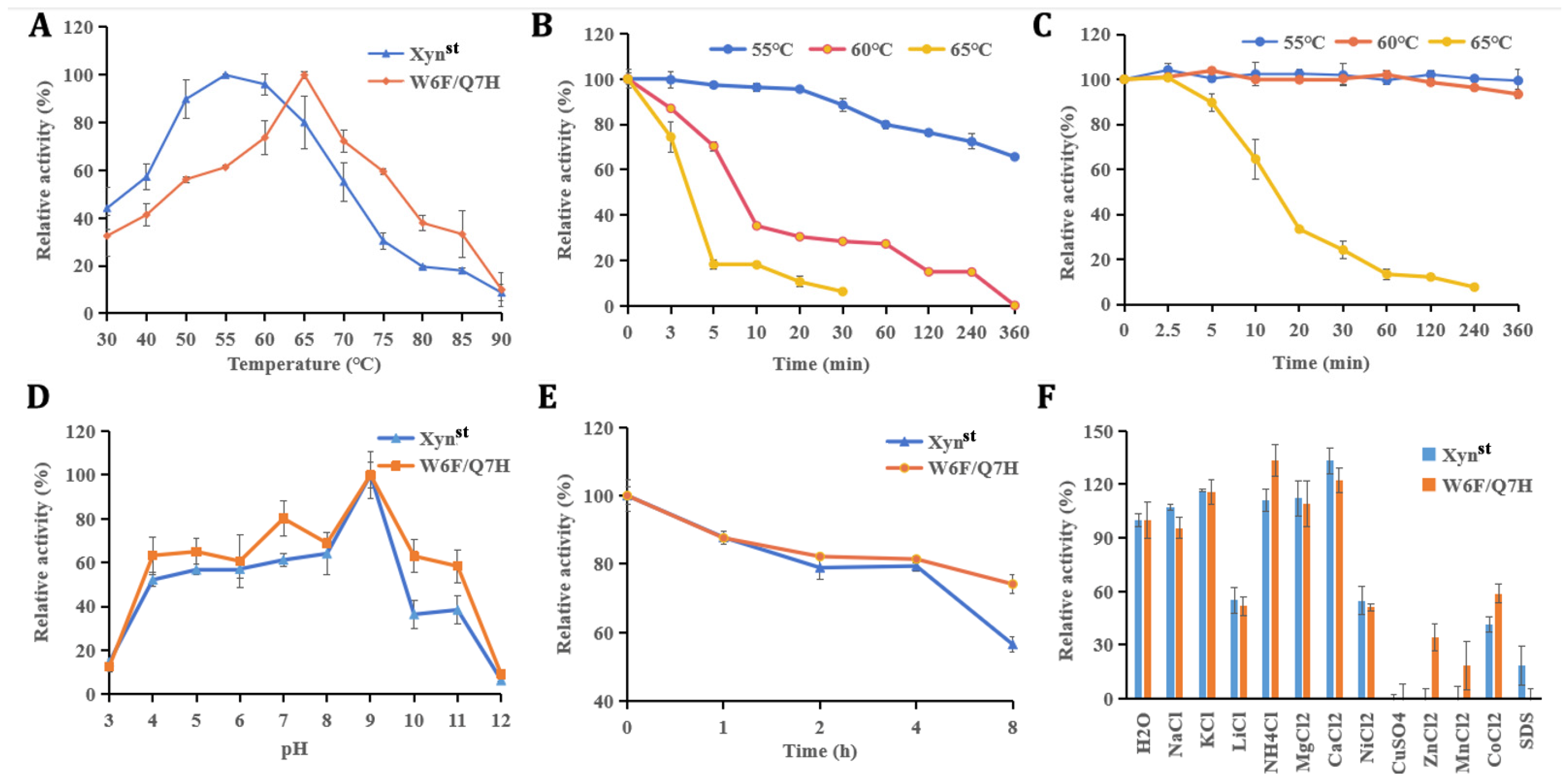
2.8. Biochemical Characterization of Xynst and Its Mutant W6F/Q7H
2.9. Test of Substrate Specificity and Kinetic Parameters
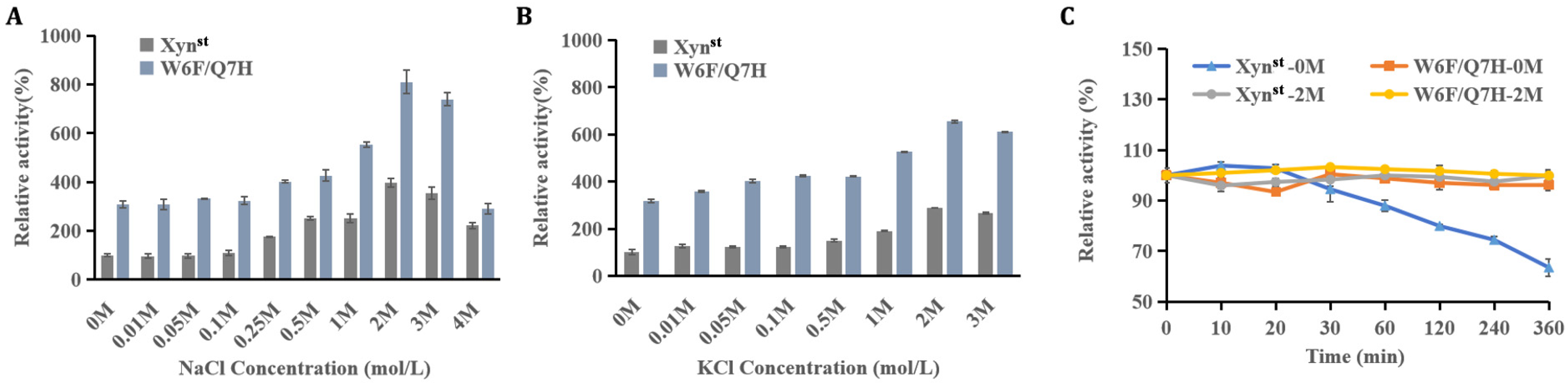
2.10. Effect of Salts on Xynst and Its Mutant W6F/Q7H
2.11. Molecular Dynamics Simulations of Xynst and Its Mutant W6F/Q7H
2.12. Hydrolysis Characteristics of Xynst and W6F/Q7H
2.13. Data Analysis and Statistics
3. Results and Discussion
3.1. Identification and Expression of the Salt-Tolerant GH11 Family Xylanase Xynst
3.2. Molecular Modification, Mutant Screen, and Characterization
3.3. Evaluation of Enzyme Activity and Stability in the Presence of Salt
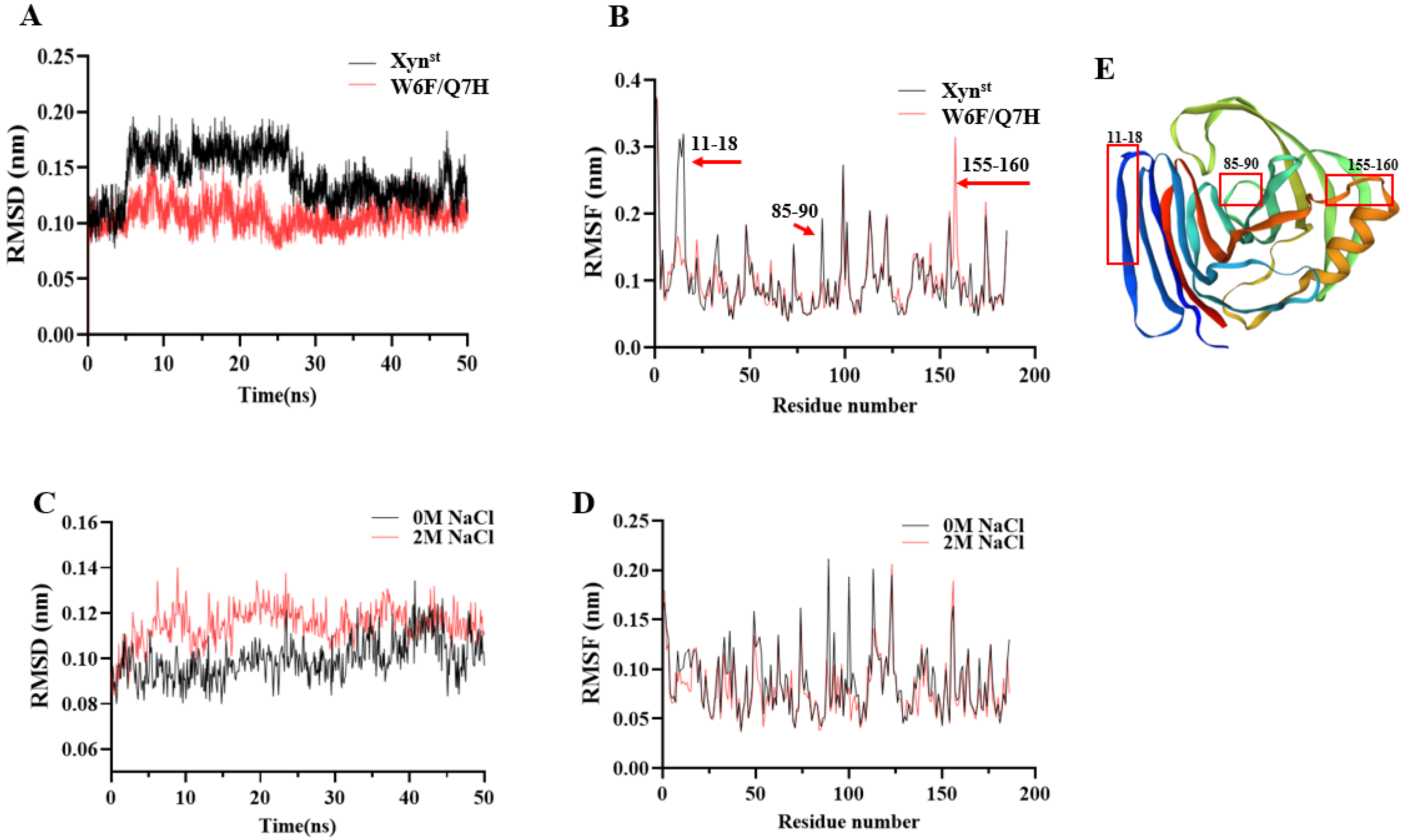
3.4. Molecular Dynamic Simulations in the Presence and Absence of Salt
3.5. Degradation of Xylans and Production of XOS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakamichi, Y.; Fouquet, T.; Ito, S.; Watanabe, M.; Matsushika, A.; Inoue, H. Structural and functional characterization of a bifunctional GH30-7 xylanase B from the filamentous fungus. J. Biol. Chem. 2019, 294, 4065–4078. [Google Scholar] [CrossRef]
- Subramaniyan, S.; Prema, P. Biotechnology of microbial xylanases: Enzymology, molecular biology, and application. Crit. Rev. Biotechnol. 2002, 22, 33–64. [Google Scholar] [CrossRef]
- Yan, S.; Xu, Y.; Yu, X.W. Rational engineering a xylanase hyper-producing system in Trichoderma reesei for efficient biomass degradation. Biotechnol. Biofuels Bioprod. 2021, 14, 90. [Google Scholar] [CrossRef]
- Törrönen, A.; Harkki, A.; Rouvinen, J. Three-dimensional structure of endo-1,4-beta-xylanase II from Trichoderma reesei: Two conformational states in the active site. EMBO J. 1994, 13, 2493–2501. [Google Scholar] [CrossRef]
- Davies, G.J.; Wilson, K.S.; Henrissat, B. Nomenclature for sugar-binding subsites in glycosyl hydrolases. Biochem. J. 1997, 321, 557–559. [Google Scholar] [CrossRef]
- Paës, G.; Tran, V.; Takahashi, M.; Boukari, I.; O’Donohue, M.J. New insights into the role of the thumb-like loop in GH-11 xylanases. Protein Eng. Des. Sel. 2007, 20, 15–23. [Google Scholar] [CrossRef]
- Verma, D. Extremophilic prokaryotic endoxylanases: Diversity, applicability, and molecular insights. Front. Microbiol. 2021, 12, 728475. [Google Scholar] [CrossRef]
- Motta, L.P.; Andrade, C.C.P.; Santana, M.H.A. A Review of Xylanase Production by the Fermentation of Xylan: Classification, Characterization and Applications; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Acevedo, J.P.; Reetz, M.T.; Asenjo, J.A.; Parra, L.P. One-step combined focused epPCR and saturation mutagenesis for thermostability evolution of a new cold-active xylanase. Enzym. Microb. Technol. 2017, 100, 60–70. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, R.; Zhou, J.; Huang, Z. Biotechnological aspects of salt-tolerant xylanases: A review. J. Agric. Food Chem. 2021, 69, 8610–8624. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Kim, H.; Park, H.J.; Lee, S.; Jung, Y.J.; Yoon, J.H.; Lee, J.S.; Park, K.; Yoo, Y.J.; Joo, J.C. Improving the catalytic performance of xylanase from Bacillus circulans through structure-based rational design. Bioresour. Technol. 2021, 340, 125737. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, K.; Pedroso, M.M.; Wu, B.; Gao, Z.; He, B.; Schenk, G. Sequence- and structure-guided improvement of the catalytic performance of a GH11 family xylanase from Bacillus subtilis. J. Biol. Chem. 2021, 297, 101262. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, L.; Xu, Y.; Lu, H.; Wu, Q.; Li, W.; Zhang, C.; Li, X. Three molecular modification strategies to improve the thermostability of xylanase XynA from Streptomyces rameus L2001. Foods 2023, 12, 879. [Google Scholar] [CrossRef]
- Bakry, M.M.; Salem, S.S.; Atta, H.M.; El-Gamal, M.S.; Fouda, A. Xylanase from thermotolerant Bacillus haynesii strain, synthesis, characterization, optimization using Box-Behnken Design, and biobleaching activity. Biomass Convers. Biorefinery 2022, 14, 9779–9792. [Google Scholar] [CrossRef]
- Beliën, T.; Joye, I.J.; Delcour, J.A.; Courtin, C.M. Computational design-based molecular engineering of the glycosyl hydrolase family 11 B. subtilis XynA endoxylanase improves its acid stability. Protein Eng. Des. Sel. 2009, 22, 587–596. [Google Scholar] [CrossRef]
- Baek, C.U.; Lee, S.G.; Chung, Y.R.; Cho, I.; Kim, J.H. Cloning of a family 11 xylanase gene from Bacillus amyloliquefaciens CH51 isolated from Cheonggukjang. Indian J. Microbiol. 2012, 52, 695–700. [Google Scholar] [CrossRef]
- Joshi, J.B.; Priyadharshini, R.; Uthandi, S. Glycosyl hydrolase 11 (xynA) gene with xylanase activity from thermophilic bacteria isolated from thermal springs. Microb. Cell Factories 2022, 21, 62. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, M.; Yang, Y.; Zhan, J.; Zhou, Y.; Zhao, X. Optimal secretion of alkali-tolerant xylanase in Bacillus subtilis by signal peptide screening. Appl. Microbiol. Biotechnol. 2016, 100, 8745–8756. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Rehman, A.U.; Luo, S.; Wang, Y.; Wei, H.; Zhang, K. Sensitivity of family GH11 Bacillus amyloliquefaciens xylanase A (BaxA) and the T33I mutant to Oryza sativa xylanase inhibitor protein (OsXIP): An experimental and computational study. Enzym. Microb. Technol. 2022, 156, 109998. [Google Scholar] [CrossRef]
- Rashid, R.; Sohail, M. Xylanolytic Bacillus species for xylooligosaccharides production: A critical review. Bioresour. Bioprocess. 2021, 8, 16. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Guleria, S.; Mehta, P.; Chauhan, A.; Parkash, J. Microbial xylanases and their industrial application in pulp and paper biobleaching: A review. Biotech 2017, 7, 11. [Google Scholar] [CrossRef]
- Dafchahi, M.N.; Acharya, B. Green extraction of xylan hemicellulose from wheat straw. Biomass Convers. Biorefinery 2024, 14, 21229–21243. [Google Scholar] [CrossRef]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Jiang, Z.; Liu, H.; Yang, S.; Yan, Q. Biochemical characterization of a novel xylanase from Paenibacillus barengoltzii and its application in xylooligosaccharides production from corncobs. Food Chem. 2018, 264, 310–318. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, Z.; Yang, C.; Li, P.; Xiao, J.; Wang, R.; Du, P.; Li, N.; Wang, J. Engineering mesophilic GH11 xylanase from Cellulomonas flavigena by rational design of N-terminus substitution. Front. Bioeng. Biotechnol. 2022, 3, 1044291. [Google Scholar] [CrossRef]
- Xu, X.; Liu, M.Q.; Huo, W.K.; Dai, X.J. Obtaining a mutant of Bacillus amyloliquefaciens xylanase A with improved catalytic activity by directed evolution. Enzym. Microb. Technol. 2016, 86, 59–66. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Li, P.; Wei, X.; Wang, Y.; Liu, H.; Xu, Y.; Zhang, Z.; Li, J.; Wang, J.; Guo, C.; Sui, S.; et al. Improvement of optimum pH and specific activity of pectate lyase from Bacillus RN.1 using loop replacement. Front. Bioeng. Biotechnol. 2023, 11, 1242123. [Google Scholar] [CrossRef]
- Chang, S.; Guo, Y.; Wu, B.; He, B. Extracellular expression of alkali tolerant xylanase from Bacillus subtilis Lucky9 in E. coli and application for xylooligosaccharides production from agro-industrial waste. Int. J. Biol. Macromol. 2017, 96, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, Y.; Zhang, L.; Xu, H.; Guo, X.; Yang, X.; Dong, B.; Cao, Y. Improved thermostability of an acidic xylanase from Aspergillus sulphureus by combined disulphide bridge introduction and proline residue substitution. Sci. Rep. 2017, 7, 1587. [Google Scholar] [CrossRef]
- Li, X.; She, Y.; Sun, B.; Song, H.; Zhu, Y.; Lv, Y.; Song, H. Purification and characterization of a cellulase-free, thermostable xylanase from Streptomyces rameus L2001 and its biobleaching effect on wheat straw pulp. Biochem. Eng. J. 2010, 52, 71–78. [Google Scholar] [CrossRef]
- Sheladiya, P.; Kapadia, C.; Prajapati, V.; Ali El Enshasy, H.; Abd Malek, R.; Marraiki, N.; Zaghloul, N.S.S.; Sayyed, R.Z. Production, statistical optimization, and functional characterization of alkali stable pectate lyase of Paenibacillus lactis PKC5 for use in juice clarification. Sci. Rep. 2022, 12, 7564. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, C.; Zhu, W.; Lu, H.; Li, X.; Yang, Y.; Xu, Y.; Li, W. Improved thermostability, acid tolerance as well as catalytic efficiency of Streptomyces rameus L2001 GH11 xylanase by N-terminal replacement. Enzym. Microb. Technol. 2023, 126, 110143. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Chen, Y.Y.; Zhuang, X.Y.; Xiao, Q.; Chen, J.; Chen, F.Q.; Yang, Q.M.; Weng, H.F.; Fang, B.S.; Xiao, A.F. A novel κ-Carrageenase from marine bacterium Rhodopirellula sallentina SM41: Heterologous expression, biochemical characterization and salt-tolerance mechanism investigation. Mar. Drugs 2022, 20, 783. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Ma, J.; Li, X.; Jiang, Z.; Yan, Q.; Yang, S. Heterologous expression and enzymatic properties of xylanase of GH11 family from Trichoderma asperellum. J. Food Sci. Technol. 2020, 38, 29–38. [Google Scholar]
- Chakdar, H.; Kumar, M.; Pandiyan, K.; Singh, A.; Nanjappan, K.; Kashyap, P.L.; Srivastava, A.K. Bacterial xylanases: Biology to biotechnology. 3 Biotech 2016, 6, 150. [Google Scholar] [CrossRef]
- Güler, F.; Özcelik, F. Screening of xylanase producing Bacillus species and optimization of xylanase process parameters in submerged fermentation. Biocatal. Agric. Biotechnol. 2023, 51, 102801. [Google Scholar] [CrossRef]
- Khandeparker, R.; Verma, P.; Deobagkar, D. A novel halotolerant xylanase from marine isolate Bacillus subtilis cho40: Gene cloning and sequencing. New Biotechnol. 2011, 28, 814–821. [Google Scholar] [CrossRef]
- Dao, T.M.A.; Cuong, N.T.; Nguyen, T.T.; Nguyen, N.P.D.; Tuyen, D.T. Purification, identification, and characterization of a glycoside hydrolase family 11-xylanase with high activity from Aspergillus niger VTCC 017. Mol. Biotechnol. 2022, 64, 187–198. [Google Scholar] [CrossRef]
- Saleem, M.; Aslam, F.; Akhtar, M.S.; Tariq, M.; Rajoka, M.I. Characterization of a thermostable and alkaline xylanase from Bacillus sp. and its bleaching impact on wheat straw pulp. World J. Microbiol. Biotechnol. 2012, 28, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Ventorim, R.Z.; de Oliveira Mendes, T.A.; Trevizano, L.M.; Dos Santos Camargos, A.M.; Guimarães, V.M. Impact of the removal of N-terminal non-structured amino acids on activity and stability of xylanases from Orpinomyces sp. PC-2. Int. J. Biol. Macromol. 2018, 106, 312–319. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, Y.; Zhong, H.; Huang, Y.; Li, X.; Wang, F. Cloning, over-expression and characterization of a thermo-tolerant xylanase from Thermotoga thermarum. Biotechnol. Lett. 2014, 36, 587–593. [Google Scholar] [CrossRef]
- Azouz, R.A.M.; Hegazy, U.M.; Said, M.M.; Bassuiny, R.I.; Salem, A.M.; Fahmy, A.S. Improving the catalytic efficiency of thermostable Geobacillus stearothermophilus xylanase XT6 by single-amino acid substitution. J. Biochem. 2020, 167, 203–215. [Google Scholar] [CrossRef]
- Heinen, P.R.; Henn, C.; Peralt, R.M.; Bracht, A.; Simão, R.C.G.; Silva, J.L.C.; Polizelo, M.L.T.M.; Kadowaki, M.K. Xylanase from Fusarium heterosporum: Properties and influence of thiol compounds on xylanase activity. Afr. J. Biotechnol. 2014, 13, 1047–1055. [Google Scholar] [CrossRef]
- Guan, G.Q.; Zhao, P.X.; Zhao, J.; Wang, M.J.; Huo, S.H.; Cui, F.J.; Jiang, J.X. Production and partial characterization of an alkaline xylanase from a novel fungus Cladosporium oxysporum. BioMed Res. Int. 2016, 2016, 4575024. [Google Scholar] [CrossRef]
- Kumar, S.; Haq, I.; Prakash, J.; Singh, S.K.; Mishra, S.; Raj, A. Purification, characterization and thermostability improvement of xylanase from Bacillus amyloliquefaciens and its application in pre-bleaching of kraft pulp. 3 Biotech 2017, 7, 20. [Google Scholar] [CrossRef]
- Salzano, F.; Aulitto, M.; Fiorentino, G.; Cannella, D.; Peeters, E.; Limauro, D. A novel endo-1,4-β-xylanase from Alicyclobacillus mali FL18: Biochemical characterization and its synergistic action with β-xylosidase in hemicellulose deconstruction. Int. J. Biol. Macromol. 2024, 264 Pt 1, 130550. [Google Scholar] [CrossRef]
- Wu, J.; Qiu, C.; Ren, Y.; Yan, R.; Ye, X.; Wang, G. Novel salt-tolerant xylanase from a mangrove-isolated fungus Phoma sp. MF13 and its application in chinese steamed bread. ACS Omega 2018, 3, 3708–3716. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Z.; Zhang, X.; Shao, Z.; Liu, Z. Cloning, expression and characterization of a novel cold-active and halophilic xylanase from Zunongwangia profunda. Extremophiles 2014, 18, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Sree Agash, S.G.; Rajasekaran, R. Exploring Bacillus species xylanases for industrial applications: Screening via thermostability and reaction modelling. J. Mol. Model. 2024, 30, 242. [Google Scholar] [CrossRef]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Abdella, A.; Ramadan, S.; Hamouda, R.A.; Saddiq, A.A.; Alhazmi, N.M.; Al-Saman, M.A. Paecilomyces variotii xylanase production, purification and characterization with antioxidant xylooligosaccharides production. Sci. Rep. 2021, 11, 16468. [Google Scholar] [CrossRef]
- Sun, Z.; Yue, Z.; Liu, E.; Li, X.; Li, C. Assessment of the bifidogenic and antibacterial activities of xylooligosaccharide. Front. Nutr. 2022, 9, 858949. [Google Scholar] [CrossRef]
- Zhou, M.; Fan, G.; Xia, H.; Zhang, X.; Teng, C.; Li, X. Ultrasound-assisted production of xylooligosaccharides from alkali-solubilized corncob bran using Penicillium janthinellum XAF01 acidic xylanase. Front. Bioeng. Biotechnol. 2021, 9, 755003. [Google Scholar] [CrossRef]
- Puițel, A.C.; Suditu, G.D.; Danu, M.; Ailiesei, G.L.; Nechita, M.T. An experimental study on the hot alkali extraction of xylan-based hemicelluloses from wheat straw and corn stalks and optimization methods. Agriculture 2022, 14, 1662. [Google Scholar] [CrossRef]


| Xylanase | Substrate | Vmax (μmol min−1 mg−1) | Km (mg mL−1) | Kcat (s−1) | Kcat/Km (mL mg−1 s−1) |
|---|---|---|---|---|---|
| Xynst | Wheat straw xylan | 2576 | 0.72 | 429.33 | 596.30 |
| Bagasse xylan | 3142 | 0.84 | 523.67 | 623.41 | |
| Beechwood xylan | 7123 | 0.30 | 1187.17 | 3957.22 | |
| Corncob xylan | 3142 | 0.84 | 523.67 | 623.41 | |
| W6F/Q7H | Wheat straw xylan | 5488 | 0.50 | 914.67 | 1829.33 |
| Bagasse xylan | 7862 | 0.44 | 1310.33 | 2978.03 | |
| Beechwood xylan | 16,722 | 0.18 | 2787.00 | 15,483.33 | |
| Corncob xylan | 4941 | 0.65 | 823.50 | 1266.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Sun, Z.; Ni, Z.; Qi, Y.; Sun, Q.; Hu, Y.; Li, C. Molecular Identification and Engineering a Salt-Tolerant GH11 Xylanase for Efficient Xylooligosaccharides Production. Biomolecules 2024, 14, 1188. https://doi.org/10.3390/biom14091188
Ma J, Sun Z, Ni Z, Qi Y, Sun Q, Hu Y, Li C. Molecular Identification and Engineering a Salt-Tolerant GH11 Xylanase for Efficient Xylooligosaccharides Production. Biomolecules. 2024; 14(9):1188. https://doi.org/10.3390/biom14091188
Chicago/Turabian StyleMa, Jiao, Zhongke Sun, Zifu Ni, Yanli Qi, Qianhui Sun, Yuansen Hu, and Chengwei Li. 2024. "Molecular Identification and Engineering a Salt-Tolerant GH11 Xylanase for Efficient Xylooligosaccharides Production" Biomolecules 14, no. 9: 1188. https://doi.org/10.3390/biom14091188
APA StyleMa, J., Sun, Z., Ni, Z., Qi, Y., Sun, Q., Hu, Y., & Li, C. (2024). Molecular Identification and Engineering a Salt-Tolerant GH11 Xylanase for Efficient Xylooligosaccharides Production. Biomolecules, 14(9), 1188. https://doi.org/10.3390/biom14091188






