Tetrahydrobiopterin in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Friend or Foe?
Abstract
1. Introduction
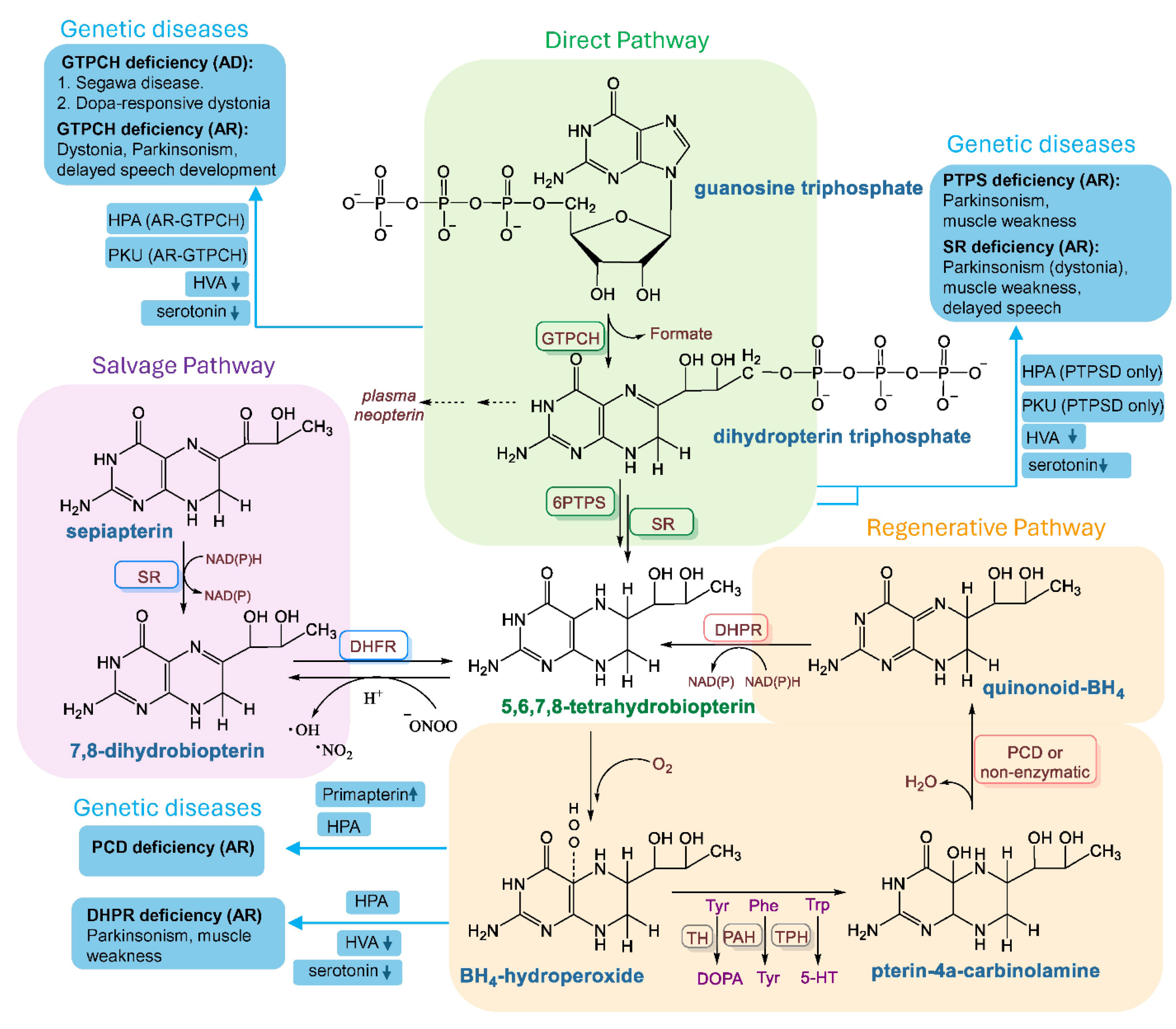
2. BH4 Biosynthesis in a Nutshell
3. Deficiency of BH4 in Disease
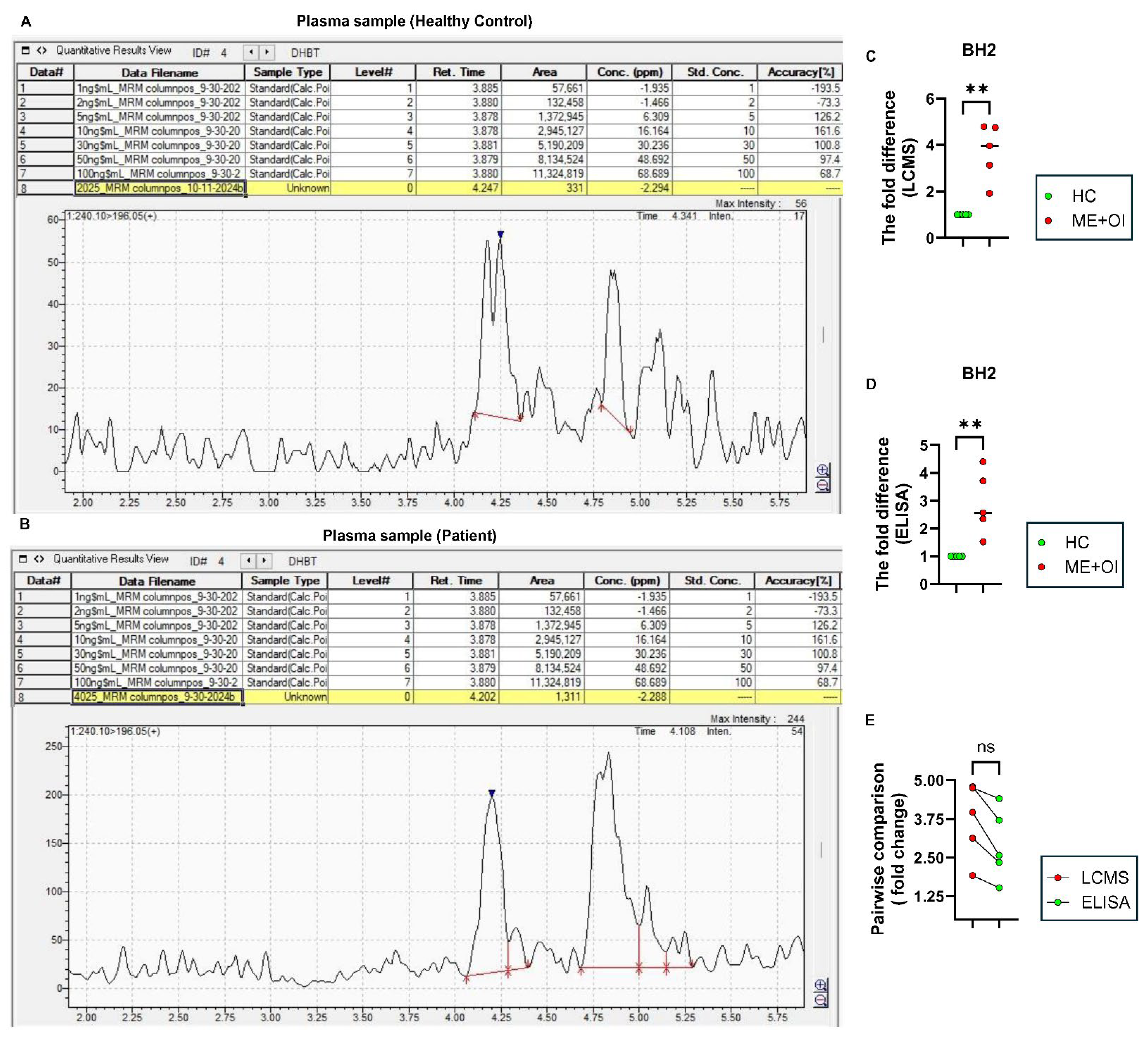
4. Detection of BH4: A Practical Challenge in ME/CFS Plasma Samples
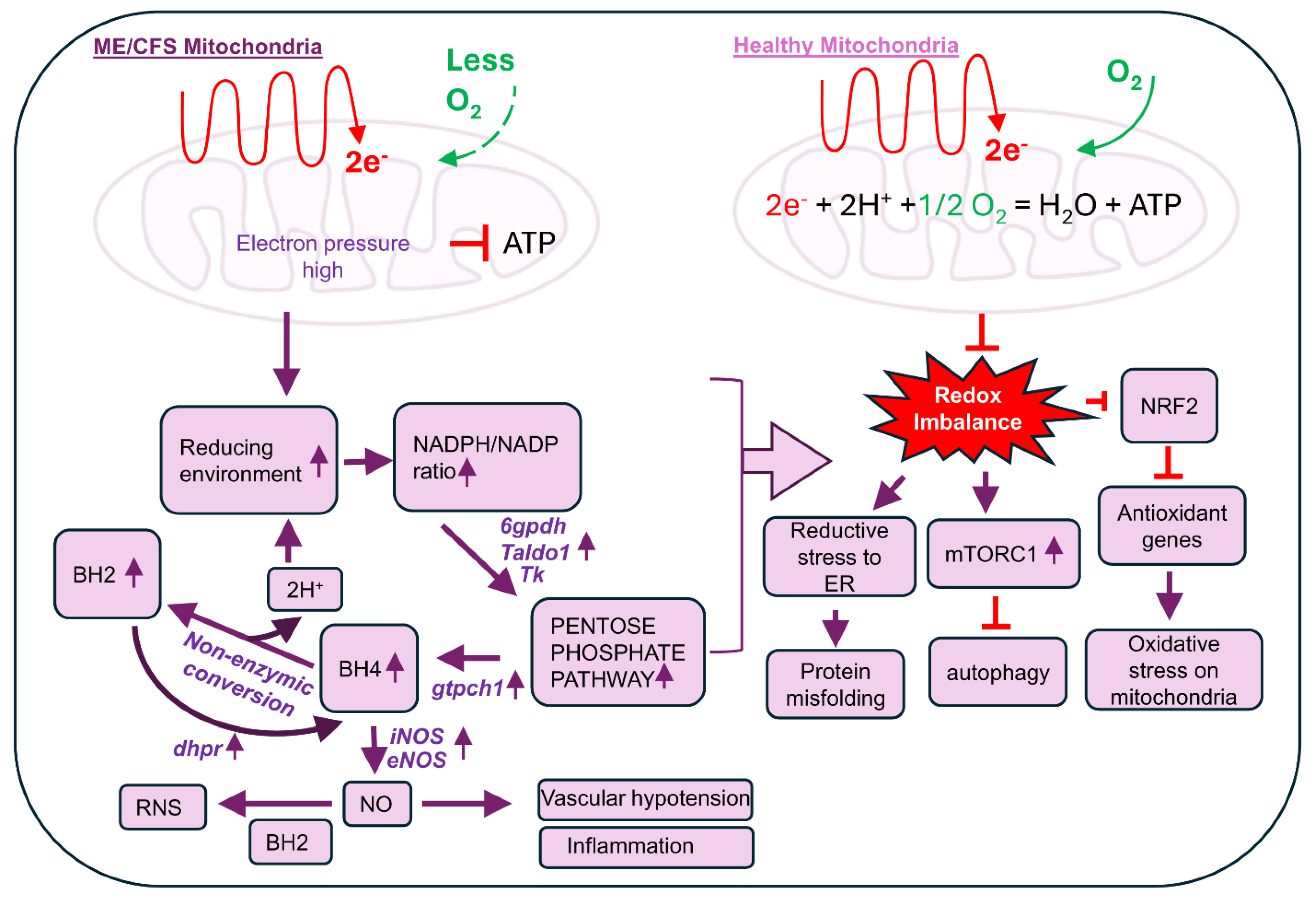
5. Regulation of BH4 Biosynthesis in ME/CFS

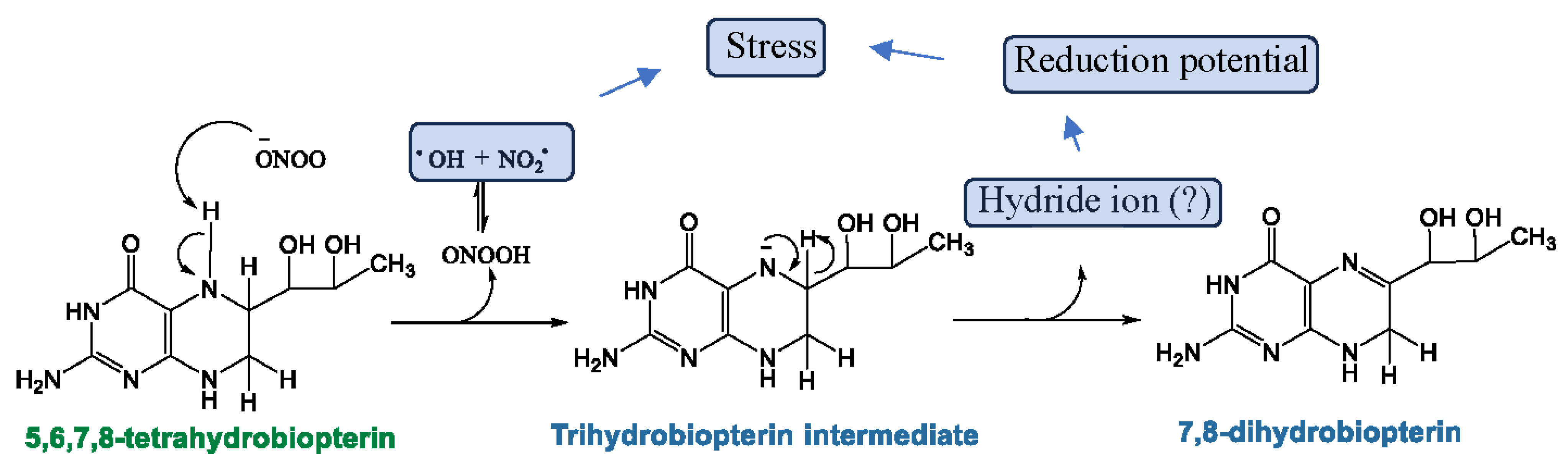
6. The Potential Role of Biopterins (BH4 and BH2) in the Pathogenesis of ME/CFS
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaufman, S. Properties of the pterin-dependent aromatic amino acid hydroxylases. In Ciba Foundation Symposium 22-Aromatic Amino Acids in the Brain; Wiley Online Library: Hoboken, NJ, USA, 1974; pp. 85–115. [Google Scholar]
- Tayeh, M.; Marletta, M. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate: Tetrahydrobiopterin is required as a cofactor. J. Biol. Chem. 1989, 264, 19654–19658. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, F.G. Note on a yellow pigment in butterflies. Nature 1889, 40, 335. [Google Scholar]
- Bendall, J.K.; Douglas, G.; McNeill, E.; Channon, K.M.; Crabtree, M.J. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid. Redox Signal. 2014, 20, 3040–3077. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S. The participation of tetra-hydrofolic acid in the enzymic conversion of phenylalanine to tyrosine. Biochim. Biophys. Acta 1958, 27, 428–429. [Google Scholar] [CrossRef]
- Fanet, H.; Capuron, L.; Castanon, N.; Calon, F.; Vancassel, S. Tetrahydrobioterin (BH4) Pathway: From Metabolism to Neuropsychiatry. Curr. Neuropharmacol. 2021, 19, 591–609. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.H.; Smith, R.M.; Nakane, M.; Murad, F. Ca2+/calmodulin-dependent NO synthase type I: A biopteroflavoprotein with Ca2+/calmodulin-independent diaphorase and reductase activities. Biochemistry 1992, 31, 3243–3249. [Google Scholar] [CrossRef] [PubMed]
- Marsden, P.A.; Schappert, K.T.; Chen, H.S.; Flowers, M.; Sundell, C.L.; Wilcox, J.N.; Lamas, S.; Michel, T. Molecular cloning and characterization of human endothelial nitric oxide synthase. FEBS Lett. 1992, 307, 287–293. [Google Scholar] [CrossRef]
- Moens, A.L.; Kass, D.A. Therapeutic potential of tetrahydrobiopterin for treating vascular and cardiac disease. J. Cardiovasc. Pharmacol. 2007, 50, 238–246. [Google Scholar] [CrossRef]
- Suschek, C.V.; Schnorr, O.; Kolb-Bachofen, V. The role of iNOS in chronic inflammatory processes in vivo: Is it damage-promoting, protective, or active at all? Curr. Mol. Med. 2004, 4, 763–775. [Google Scholar] [CrossRef]
- Knapp, S.; Irwin, M. Plasma levels of tetrahydrobiopterin and folate in major depression. Biol. Psychiatry 1989, 26, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Eichwald, T.; da Silva, L.d.B.; Staats Pires, A.C.; Niero, L.; Schnorrenberger, E.; Filho, C.C.; Espíndola, G.; Huang, W.-L.; Guillemin, G.J.; Abdenur, J.E.; et al. Tetrahydrobiopterin: Beyond Its Traditional Role as a Cofactor. Antioxidants 2023, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, D.; Annesley, S.J.; Fisher, P.R. Pathological Mechanisms Underlying Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Diagnostics 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Walitt, B.; Singh, K.; LaMunion, S.R.; Hallett, M.; Jacobson, S.; Chen, K.; Enose-Akahata, Y.; Apps, R.; Barb, J.J.; Bedard, P.; et al. Deep phenotyping of post-infectious myalgic encephalomyelitis/chronic fatigue syndrome. Nat. Commun. 2024, 15, 907. [Google Scholar] [CrossRef] [PubMed]
- Wirth, K.J.; Scheibenbogen, C. Pathophysiology of skeletal muscle disturbances in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). J. Transl. Med. 2021, 19, 162. [Google Scholar] [CrossRef]
- Hanson, M.R. The viral origin of myalgic encephalomyelitis/chronic fatigue syndrome. PLoS Pathog. 2023, 19, e1011523. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.; Hotopf, M.; Wessely, S. The prognosis of chronic fatigue and chronic fatigue syndrome: A systematic review. QJM Mon. J. Assoc. Physicians 1997, 90, 223–233. [Google Scholar] [CrossRef]
- Graves, B.S.; Patel, M.; Newgent, H.; Parvathy, G.; Nasri, A.; Moxam, J.; Gill, G.S.; Sawhney, V.; Gupta, M. Chronic Fatigue Syndrome: Diagnosis, Treatment, and Future Direction. Cureus 2024, 16, e70616. [Google Scholar] [CrossRef]
- Grach, S.L.; Seltzer, J.; Chon, T.Y.; Ganesh, R. Diagnosis and Management of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Mayo Clin. Proc. 2023, 98, 1544–1551. [Google Scholar] [CrossRef]
- Bernhoff, G.; Rasmussen-Barr, E.; Bunketorp Käll, L. A comparison of health-related factors between patients diagnosed with ME/CFS and patients with a related symptom picture but no ME/CFS diagnosis: A cross-sectional exploratory study. J. Transl. Med. 2022, 20, 577. [Google Scholar] [CrossRef]
- Bulbule, S.; Gottschalk, C.G.; Drosen, M.E.; Peterson, D.; Arnold, L.A.; Roy, A. Dysregulation of tetrahydrobiopterin metabolism in myalgic encephalomyelitis/chronic fatigue syndrome by pentose phosphate pathway. J. Cent. Nerv. Syst. Dis. 2024, 16, 11795735241271675. [Google Scholar] [CrossRef]
- Gottschalk, C.G.; Whelan, R.; Peterson, D.; Roy, A. Detection of Elevated Level of Tetrahydrobiopterin in Serum Samples of ME/CFS Patients with Orthostatic Intolerance: A Pilot Study. Int. J. Mol. Sci. 2023, 24, 8713. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.E.; Gross, S.S. Chapter 6—Tetrahydrobiopterin: An Essential Cofactor for Nitric Oxide Synthases and Amino Acid Hydroxylases. In Nitric Oxide, 2nd ed.; Ignarro, L.J., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 169–209. [Google Scholar]
- Naini, A.; Kaufmann, P.; Shanske, S.; Engelstad, K.; De Vivo, D.C.; Schon, E.A. Hypocitrullinemia in patients with MELAS: An insight into the “MELAS paradox”. J. Neurol. Sci. 2005, 229–230, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Opladen, T.; López-Laso, E.; Cortès-Saladelafont, E.; Pearson, T.S.; Sivri, H.S.; Yildiz, Y.; Assmann, B.; Kurian, M.A.; Leuzzi, V.; Heales, S.; et al. Consensus guideline for the diagnosis and treatment of tetrahydrobiopterin (BH4) deficiencies. Orphanet J. Rare Dis. 2020, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Sucher, R.; Gehwolf, P.; Oberhuber, R.; Hermann, M.; Margreiter, C.; Werner, E.R.; Obrist, P.; Schneeberger, S.; Ollinger, R.; Margreiter, R.; et al. Tetrahydrobiopterin protects the kidney from ischemia–reperfusion injury. Kidney Int. 2010, 77, 681–689. [Google Scholar] [CrossRef]
- Midhun, T.; Krishna, S.S.; Wilson, S.K. Tetrahydrobiopterin and Its Multiple Roles in Neuropsychological Disorders. Neurochem. Res. 2022, 47, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Villaume, W.A. Marginal BH4 deficiencies, iNOS, and self-perpetuating oxidative stress in post-acute sequelae of COVID-19. Med. Hypotheses 2022, 163, 110842. [Google Scholar] [CrossRef]
- Moens, A.L.; Kass, D.A. Tetrahydrobiopterin and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2439–2444. [Google Scholar] [CrossRef] [PubMed]
- Soula, M.; Weber, R.A.; Zilka, O.; Alwaseem, H.; La, K.; Yen, F.; Molina, H.; Garcia-Bermudez, J.; Pratt, D.A.; Birsoy, K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 2020, 16, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar] [PubMed]
- Antoniades, C.; Shirodaria, C.; Crabtree, M.; Rinze, R.; Alp, N.; Cunnington, C.; Diesch, J.; Tousoulis, D.; Stefanadis, C.; Leeson, P.; et al. Altered Plasma Versus Vascular Biopterins in Human Atherosclerosis Reveal Relationships Between Endothelial Nitric Oxide Synthase Coupling, Endothelial Function, and Inflammation. Circulation 2007, 116, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Michel, T. Thiol-metabolizing proteins and endothelial redox state: Differential modulation of eNOS and biopterin pathways. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H194–H201. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, C.; Van Assche, T.; Shirodaria, C.; Kylintireas, I.; Lindsay, A.C.; Lee, J.M.S.; Francis, J.M.; Sayeed, R.; Ratnatunga, C.; Pillai, R.; et al. B|Chronic oral tetrahydrobiopterin treatment in patients with coronary artery disease elevates total biopterin levels but does not improve biopterin redox status or vascular function: A randomised placebo-controlled trial. Heart 2010, 96, A1–A2. [Google Scholar] [CrossRef]
- Crabtree, M.J.; Tatham, A.L.; Al-Wakeel, Y.; Warrick, N.; Hale, A.B.; Cai, S.; Channon, K.M.; Alp, N.J. Quantitative Regulation of Intracellular Endothelial Nitric-oxide Synthase (eNOS) Coupling by Both Tetrahydrobiopterin-eNOS Stoichiometry and Biopterin Redox Status: Insights From Cells With TET-Regulated GTP Cyclohydrolase I Expression. J. Biol. Chem. 2009, 284, 1136–1144. [Google Scholar] [CrossRef]
- Schmidt, H.; Tegeder, I.; Geisslinger, G. Determination of Neopterin and Biopterin by Liquid Chromatography Coupled to Tandem Mass Spectrometry (LC-MS/MS) in Rat and Human Plasma, Cell Extracts and Tissue Homogenates. 2006. Available online: https://protocolexchange.researchsquare.com/article/nprot-86/v1 (accessed on 4 November 2024).
- Armstrong, C.W.; McGregor, N.R.; Lewis, D.P.; Butt, H.L.; Gooley, P.R. Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics 2015, 11, 1626–1639. [Google Scholar] [CrossRef]
- Fluge, Ø.; Mella, O.; Bruland, O.; Risa, K.; Dyrstad, S.E.; Alme, K.; Rekeland, I.G.; Sapkota, D.; Røsland, G.V.; Fosså, A.; et al. Metabolic profiling indicates impaired pyruvate dehydrogenase function in myalgic encephalopathy/chronic fatigue syndrome. JCI Insight 2016, 1, e89376. [Google Scholar] [CrossRef]
- Mandarano, A.H.; Maya, J.; Giloteaux, L.; Peterson, D.L.; Maynard, M.; Gottschalk, C.G.; Hanson, M.R. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J. Clin. Investig. 2020, 130, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, D.; Sanislav, O.; Allan, C.Y.; Smith, P.K.; Annesley, S.J.; Fisher, P.R. Dysregulated Provision of Oxidisable Substrates to the Mitochondria in ME/CFS Lymphoblasts. Int. J. Mol. Sci. 2021, 22, 2046. [Google Scholar] [CrossRef] [PubMed]
- Gumaa, K.; McLean, P. The pentose phosphate pathway of glucose metabolism. Enzyme profiles and transient and steady-state content of intermediates of alternative pathways of glucose metabolism in Krebs ascites cells. Biochem. J. 1969, 115, 1009–1029. [Google Scholar] [CrossRef]
- De Preter, G.; Neveu, M.-A.; Danhier, P.; Brisson, L.; Payen, V.L.; Porporato, P.E.; Jordan, B.F.; Sonveaux, P.; Gallez, B. Inhibition of the pentose phosphate pathway by dichloroacetate unravels a missing link between aerobic glycolysis and cancer cell proliferation. Oncotarget 2016, 7, 2910. [Google Scholar] [CrossRef] [PubMed]
- Abujrais, S.; Vallianatou, T.; Bergquist, J. Untargeted Metabolomics and Quantitative Analysis of Tryptophan Metabolites in Myalgic Encephalomyelitis Patients and Healthy Volunteers: A Comparative Study Using High-Resolution Mass Spectrometry. ACS Chem. Neurosci. 2024, 15, 3525–3534. [Google Scholar] [CrossRef]
- Perl, A. The pathogenesis of transaldolase deficiency. IUBMB Life 2007, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.C.; Dalby, P.A. The Two-Species Model of transketolase explains donor substrate-binding, inhibition and heat-activation. Sci. Rep. 2020, 10, 4148. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Kwon, J.J.; Chen, C.; Russell, J.; Acosta, K.; Burnaevskiy, N.; Crane, M.M.; Bitto, A.; Vander Wende, H.; Simko, M.; et al. Transaldolase inhibition impairs mitochondrial respiration and induces a starvation-like longevity response in Caenorhabditis elegans. PLoS Genet. 2017, 13, e1006695. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Meng, Q.; Sun, H.; Li, Y.; Li, Y.; Gu, L.; Liu, B.; Zhang, Y.; Zhou, H.; Xu, Z.; et al. The role of transketolase in human cancer progression and therapy. Biomed. Pharmacother. 2022, 154, 113607. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, N.M.; Zihlif, M.A.; Alqudah, D.A.; Alshaer, W.; Alqaraleh, M.; Sharab, A.; Abdalla, S.S. Effects of cyclic acute and chronic hypoxia on the expression levels of metabolism related genes in a pancreatic cancer cell line. Biomed. Rep. 2022, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Solaini, G.; Baracca, A.; Lenaz, G.; Sgarbi, G. Hypoxia and mitochondrial oxidative metabolism. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 1171–1177. [Google Scholar] [CrossRef]
- Tomas, C.; Elson, J.L.; Strassheim, V.; Newton, J.L.; Walker, M. The effect of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) severity on cellular bioenergetic function. PLoS ONE 2020, 15, e0231136. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, D.; Annesley, S.J.; Allan, C.Y.; Sanislav, O.; Lidbury, B.A.; Lewis, D.P.; Fisher, P.R. An isolated complex V inefficiency and dysregulated mitochondrial function in immortalized lymphocytes from ME/CFS patients. Int. J. Mol. Sci. 2020, 21, 1074. [Google Scholar] [CrossRef]
- Minchenko, O.; Garmash, I.; Kovalevska, O.; Tsymbal, D.; Minchenko, D. Expression of phosphoribosyl pyrophosphate synthetase genes in U87 glioma cells with ERN1 knockdown: Effect of hypoxia and endoplasmic reticulum stress. Ukr. Biochem. J. 2014, 86, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Campen, C.; Rowe, P.C.; Visser, F.C. Reductions in Cerebral Blood Flow Can Be Provoked by Sitting in Severe Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Healthcare 2020, 8, 394. [Google Scholar] [CrossRef]
- van Campen, C.L.M.; Verheugt, F.W.; Rowe, P.C.; Visser, F.C. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clin. Neurophysiol. Pract. 2020, 5, 50–58. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.M.; Rowe, P.C.; Visser, F.C. Blood volume status in ME/CFS correlates with the presence or absence of orthostatic symptoms: Preliminary results. Front. Pediatr. 2018, 6, 352. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, T.; Krohn, K.; Albers, S.; Meinertz, T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 2000, 43, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, T.; Brockhoff, C.; Mayer, B.; Warnholtz, A.; Mollnau, H.; Henne, S.; Meinertz, T.; Münzel, T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: Evidence for a dysfunctional nitric oxide synthase. Circ. Res. 2000, 86, e36–e41. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Kimura, M.; Noma, K.; Hara, K.; Jitsuiki, D.; Goto, C.; Oshima, T.; Chayama, K. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis 2006, 186, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Neubauer, A.; Kolesnik, B.; Stasch, J.-P.; Werner, E.R.; Gorren, A.C.; Mayer, B. Tetrahydrobiopterin protects soluble guanylate cyclase against oxidative inactivation. Mol. Pharmacol. 2012, 82, 420–427. [Google Scholar] [CrossRef]
- Guan, X.; Guan, X.; Lu, C.; Shang, B.; Zhao, Y.; Meng, Y.; Zhang, Z. Nebivolol combined with tetrahydrobiopterin affects diastolic function in spontaneously hypertensive rats via the nitric oxide/cyclic guanosine monophosphate signalling pathway. BMC Pharmacol. Toxicol. 2020, 21, 84. [Google Scholar] [CrossRef]
- Nakai, K.; Urushihara, M.; Kubota, Y.; Kosaka, H. Ascorbate enhances iNOS activity by increasing tetrahydrobiopterin in RAW 264.7 cells. Free Radic. Biol. Med. 2003, 35, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Dal Pra, I.; Gottardo, R.; Bortolotti, F.; Whitfield, J.F.; Armato, U. BH4 (tetrahydrobiopterin)-dependent activation, but not the expression, of inducible NOS (nitric oxide synthase)-2 in proinflammatory cytokine-stimulated, cultured normal human astrocytes is mediated by MEK–ERK kinases. J. Cell. Biochem. 2005, 94, 731–743. [Google Scholar] [CrossRef]
- Thoeni, G.; Stoitzner, P.; Brandacher, G.; Romani, N.; Heufler, C.; Werner-Felmayer, G.; Werner, E.R. Tetrahydro-4-aminobiopterin attenuates dendritic cell-induced T cell priming independently from inducible nitric oxide synthase. J. Immunol. 2005, 174, 7584–7591. [Google Scholar] [CrossRef] [PubMed]
- Csont, T.; Viappiani, S.; Sawicka, J.; Slee, S.; Altarejos, J.Y.; Batinić-Haberle, I.; Schulz, R. The involvement of superoxide and iNOS-derived NO in cardiac dysfunction induced by pro-inflammatory cytokines. J. Mol. Cell. Cardiol. 2005, 39, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Liu, H.-R.; Tao, L.; Liang, F.; Yan, L.; Zhao, R.-R.; Lopez, B.L.; Christopher, T.A.; Ma, X.-L. Role of iNOS-derived reactive nitrogen species and resultant nitrative stress in leukocytes-induced cardiomyocyte apoptosis after myocardial ischemia/reperfusion. Apoptosis 2007, 12, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Anavi, S.; Tirosh, O. iNOS as a metabolic enzyme under stress conditions. Free Radic. Biol. Med. 2020, 146, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Loscalzo, J. Metabolic Responses to Reductive Stress. Antioxid. Redox Signal. 2019, 32, 1330–1347. [Google Scholar] [CrossRef]
- Manford, A.G.; Rodríguez-Pérez, F.; Shih, K.Y.; Shi, Z.; Berdan, C.A.; Choe, M.; Titov, D.V.; Nomura, D.K.; Rape, M. A cellular mechanism to detect and alleviate reductive stress. Cell 2020, 183, 46–61.e21. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, A.; García-Aguilar, A.; Asahara, S.-I.; Kido, Y.; Guillén, C.; Pajvani, U.B.; Benito, M. MTORC1 regulates both general autophagy and mitophagy induction after oxidative phosphorylation uncoupling. Mol. Cell. Biol. 2017, 37, e00441-17. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, G.; Peterson, D.; Knox, K.; Maynard, M.; Whelan, R.J.; Roy, A. Elevated ATG13 in serum of patients with ME/CFS stimulates oxidative stress response in microglial cells via activation of receptor for advanced glycation end products (RAGE). Mol. Cell. Neurosci. 2022, 120, 103731. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, A.F.M.T.; Benko, A.; Bulbule, S.; Gottschalk, C.G.; Arnold, L.A.; Roy, A. Tetrahydrobiopterin in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Friend or Foe? Biomolecules 2025, 15, 102. https://doi.org/10.3390/biom15010102
Rahman AFMT, Benko A, Bulbule S, Gottschalk CG, Arnold LA, Roy A. Tetrahydrobiopterin in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Friend or Foe? Biomolecules. 2025; 15(1):102. https://doi.org/10.3390/biom15010102
Chicago/Turabian StyleRahman, A. F. M. Towheedur, Anna Benko, Sarojini Bulbule, Carl Gunnar Gottschalk, Leggy A. Arnold, and Avik Roy. 2025. "Tetrahydrobiopterin in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Friend or Foe?" Biomolecules 15, no. 1: 102. https://doi.org/10.3390/biom15010102
APA StyleRahman, A. F. M. T., Benko, A., Bulbule, S., Gottschalk, C. G., Arnold, L. A., & Roy, A. (2025). Tetrahydrobiopterin in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Friend or Foe? Biomolecules, 15(1), 102. https://doi.org/10.3390/biom15010102







